Effects of Sewage Treatment Water Supply on Leaf Development and Yield of Tuberous Roots in Multilayered Sweet Potato Cultivation
Abstract
1. Introduction
2. Materials and Methods
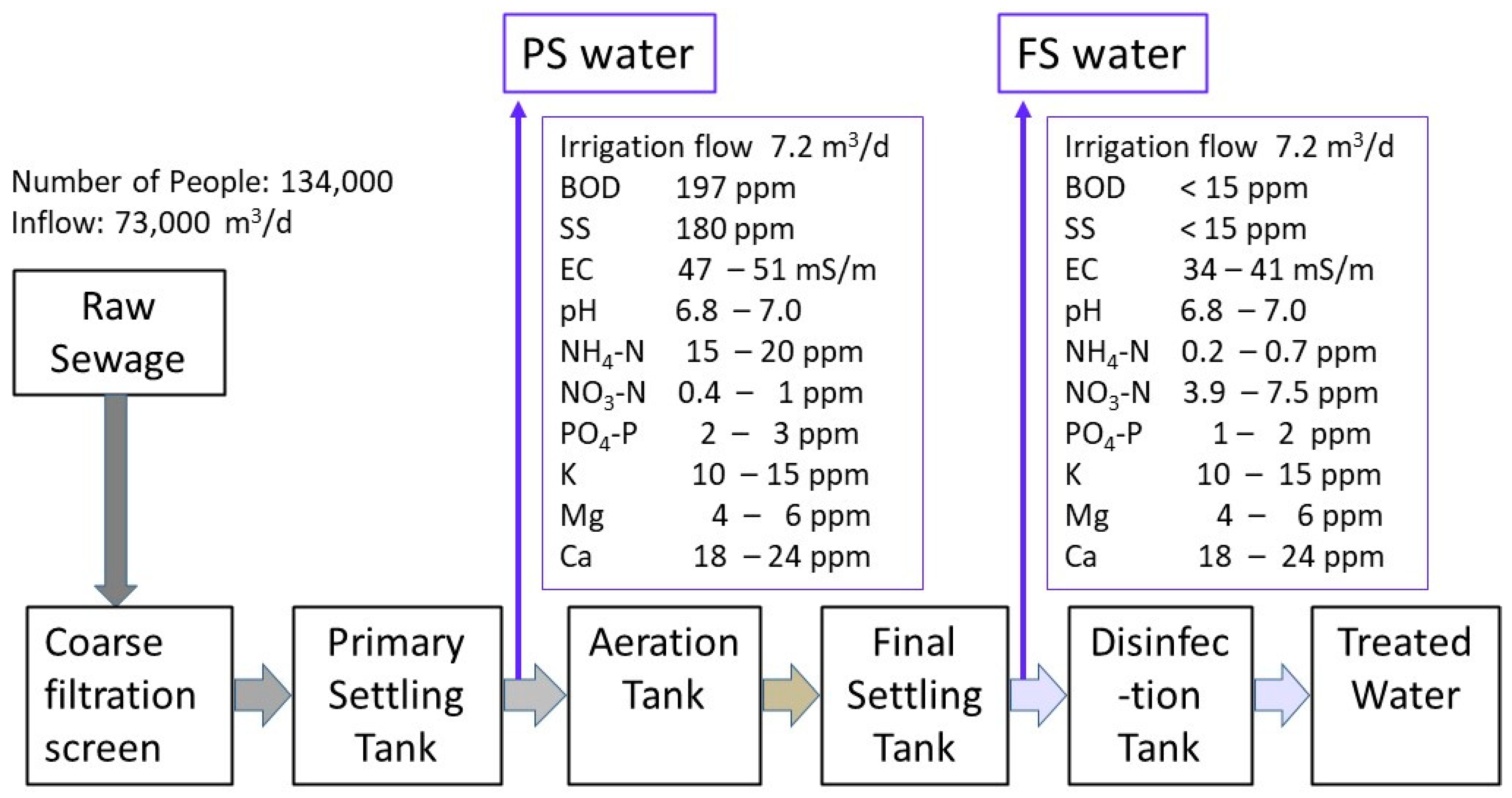
2.1. Rhizosphere Irrigation Supply of Sewage and Treated Sewage
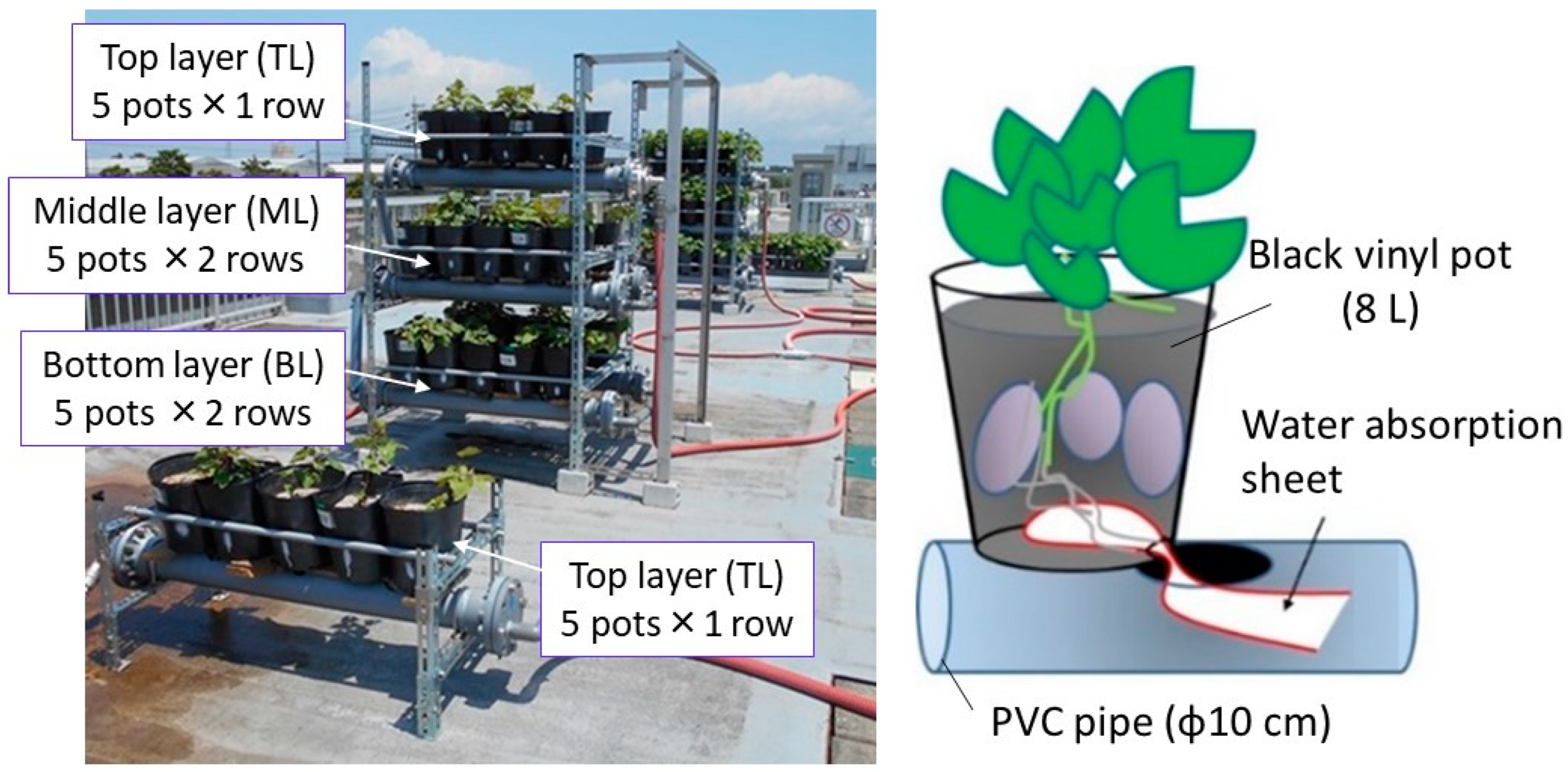
2.2. Three-Layer Cultivation System
2.3. Winter Cultivation Using Greenhouse Supplied with Sewage Treatment Water
2.4. Solar Radiation Ratio in Three-Layer Cultivation
2.5. Photosynthetic Efficiency in Three-Layer Cultivation
2.6. Statical Analysis
3. Results
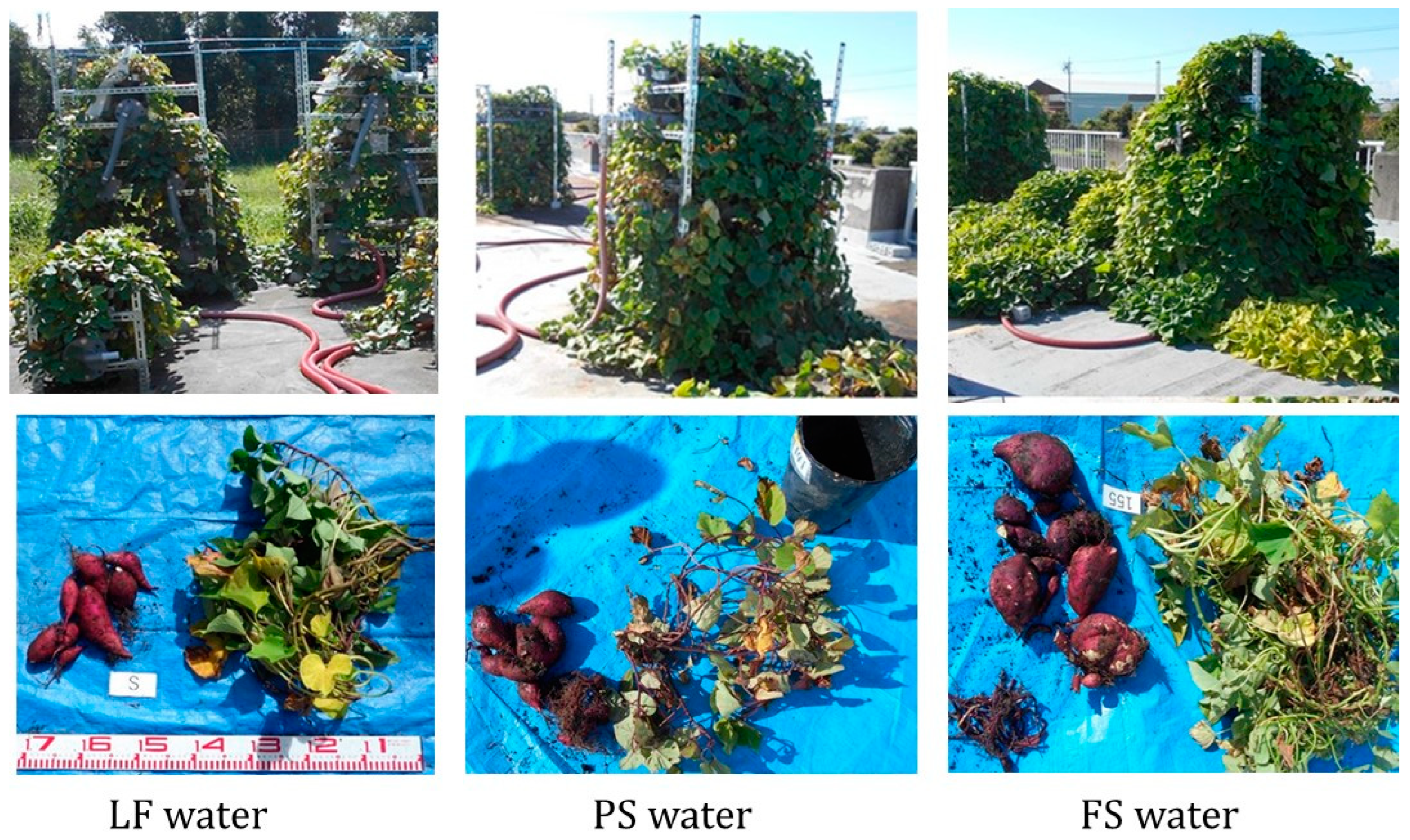
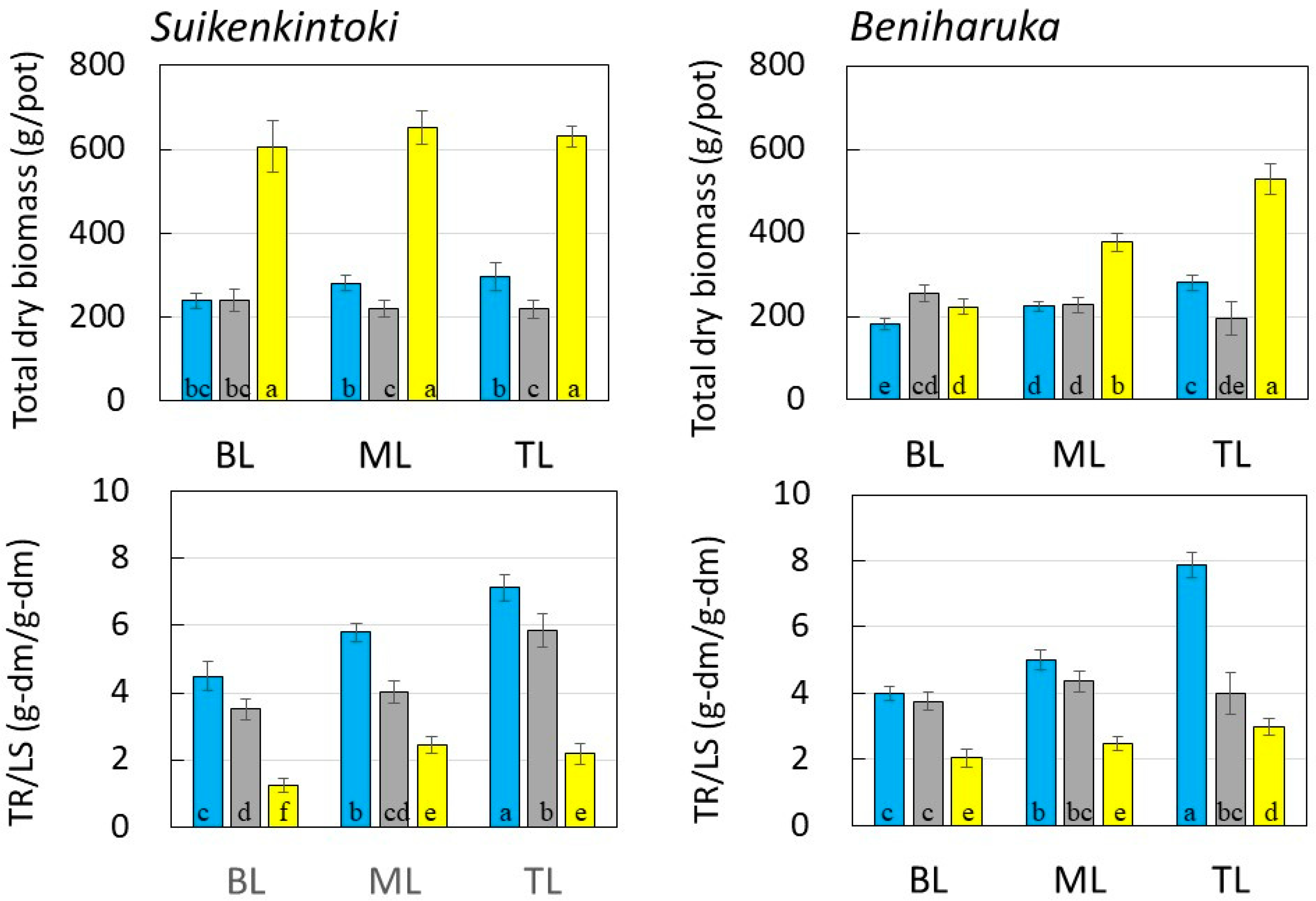
3.1. Sweet Potato Production Using Sewage and Treated Sewage
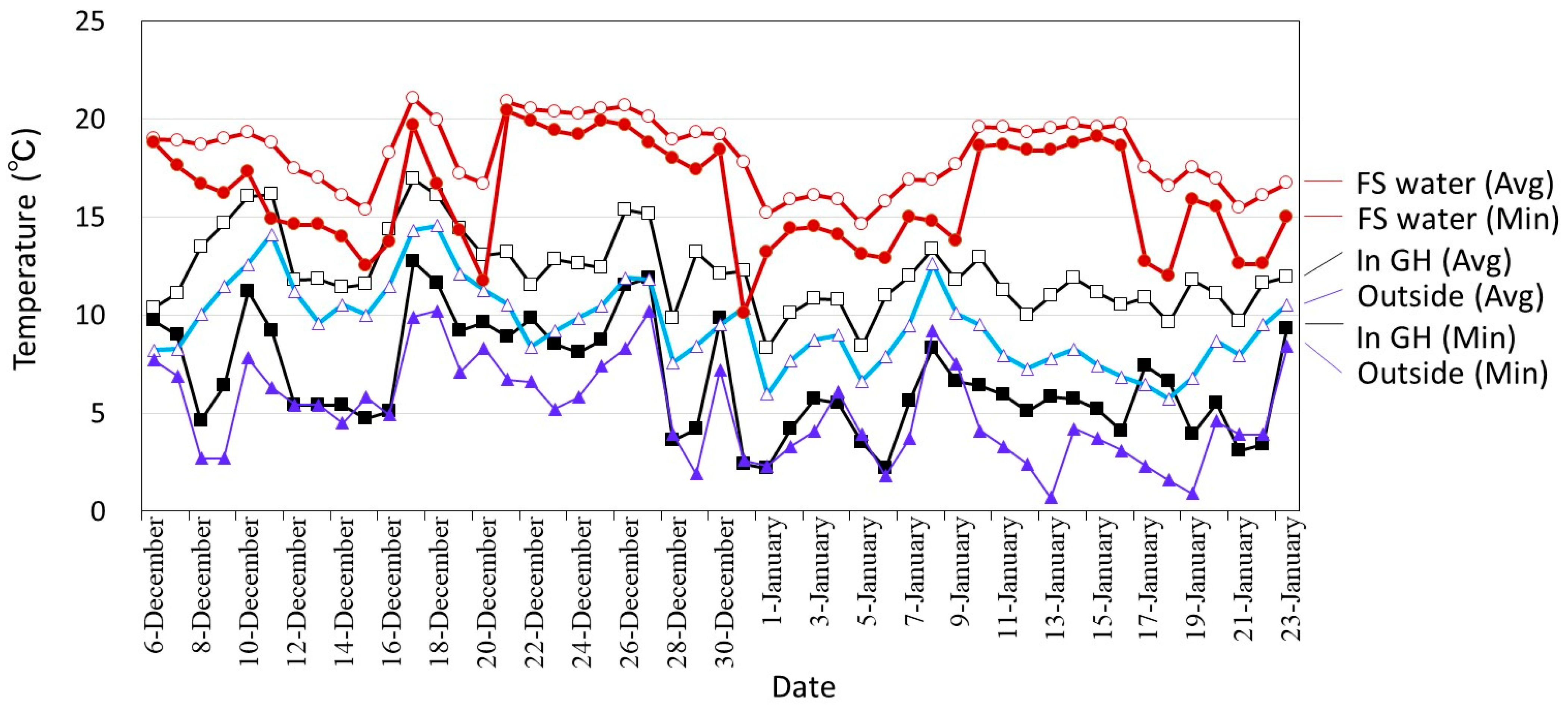
3.2. Winter Cultivation Supplied with Sewage Treatment Water
3.3. Photosynthetic Efficiency in Summer and Winter Cultivations
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Petsakos, A.; Prager, S.D.; Gonzalez, C.E.; Gama, A.C.; Sulser, T.B.; Gbegbelegbe, S.; Kikulwe, E.M.; Hareau, G. Understanding the consequences of changes in the production frontiers for roots, tubers and bananas. Glob. Food Secur. 2019, 20, 180–188. [Google Scholar] [CrossRef]
- Woolfe, J. Sweet Potato: An Untapped Food Resource, 1st ed.; Cambridge University Press: New York, NY, USA, 1992; p. 643. [Google Scholar]
- Pimentel, D.; Doughty, R.; Carothers, C.; Lamberson, S.; Bora, N.; Lee, K. Energy inputs in crop production in developing and developed countries. In Food Security and Environmental Quality in the Developing World; CRC Press: Boca Raton, FL, USA, 2002; pp. 129–151. [Google Scholar]
- Koçar, G.; Civas, N. An overview of biofuels from energy crops: Current status and future prospects. Renew. Sust. Energy Rev. 2013, 28, 900–916. [Google Scholar] [CrossRef]
- Lareo, C.; Ferrari, M.D. Sweet potato as a bioenergy crop for fuel ethanol production. Perspectives and Challenges. In Bioethanol Production from Food Crops; Academic Press: New York, NY, USA, 2019; pp. 115–147. [Google Scholar] [CrossRef]
- Crop Survey (Rice, Barley, Beans, Sweet Potatoes, Forage Crops and Industrial Crops). Annual Statistics on Agriculture, Forestry and Fisheries FY2021 (in Japanese); Ministry of Agriculture, Forestry and Fisheries: Tokyo, Japan, 2022. Available online: https://www.maff.go.jp/j/tokei/kouhyou/sakumotu/sakkyou_kome/index.html#y17 (accessed on 30 November 2022).
- Eguchi, T.; Moriyama, S.; Miyama, I.; Yoshida, S.; Chikushi, J. A hydroponic method suitable for tops production of a sweetpotato cultivar ‘Suioh’. J. Sci. High Technol. Agric. 2007, 19, 167–174. [Google Scholar] [CrossRef]
- Montoro, S.B.; Lucas, J., Jr.; Santos, D.F.L.; Costa, M.S.S.M. Anaerobic co-digestion of sweet potato and dairy cattle manure: A technical and economic evaluation for energy and biofertilizer production. J. Clean. Prod. 2019, 226, 1082–1091. [Google Scholar] [CrossRef]
- Catherine, C.; Twizerimana, M. Biogas production from thermochemically pretreated sweet potato root waste. Heliyon 2022, 8, e10376. [Google Scholar] [CrossRef] [PubMed]
- Daniel-Gromke, J.; Rensberg, N.; Denysenko, V.; Stinner, W.; Schmalfuß, T.; Scheftelowitz, M.; Nelles, M.; Liebetrau, J. Current Developments in Production and Utilization of Biogas and Biomethane in Germany. Chem. Ing. Tech. 2018, 90, 17–35. [Google Scholar] [CrossRef]
- Forestry Agency, Ministry of Agriculture, Forestry and Fisheries. Annual Report on Forest and Forestry in Japan FY 2021 (in Japanese); Forestry Agency, Ministry of Agriculture, Forestry and Fisheries: Tokyo, Japan, 2022. Available online: https://www.rinya.maff.go.jp/j/kikaku/hakusyo/r3hakusyo/attach/pdf/index-2.pdf (accessed on 30 November 2022).
- Overview of Japan’s Energy Balance Flow (in Japanese). In Energy White Paper; Agency for Natural Resource and Energy: Tokyo, Japan, 2022; p. 73. Available online: https://www.enecho.meti.go.jp/about/whitepaper/2022/pdf/2_1.pdf (accessed on 30 November 2022).
- Yanagida, T.; Yoshida, T.; Kuboyama, H.; Jinkawa, M. Relationship between feedstock price and break-even point of woody. biomass power generation under FIT program. J. Jpn. Inst. Energy 2015, 94, 311–320. [Google Scholar] [CrossRef]
- Li, G.; Lin, Z.; Xu, Y.; Liu, Z.; Zhang, H.; Li, H.; Qiu, Y. Photosynthesis-light response models for varieties of sweet potato. Fujian J. Agri. Sci. 2018, 33, 687–690. [Google Scholar]
- He, D.; Yan, Z.; Sun, X.; Yang, P. Leaf development and energy yield of hydroponic sweetpotato seedlings using single-node cutting as influenced by light intensity and LED spectrum. J. Plant Physiol. 2020, 254, 153274. [Google Scholar] [CrossRef]
- Suzuki, T.; Sakamoto, M.; Kubo, H.; Miyabe, Y.; Hiroshima, D. Effects of solar radiation on leaf development and yield of tu-berous roots in multilayered sweet potato cultivation. Plants 2023, 12, 287. [Google Scholar] [CrossRef]
- The Russia-Ukraine War’s Impact on Global Fertilizer Markets, Research Rabobank, April 2022. Available online: https://research.rabobank.com/far/en/sectors/farm-inputs/the-russia-ukraine-war-impact-on-global-fertilizer-markets.html (accessed on 5 January 2023).
- Ghorbani, M.; Petr Konvalina, P.; Walkiewicz, A.; Reinhard, W.; Neugschwandtner, R.W.; Kopecký, M.; Zamanian, K.; Chen, W.H.; Bucur, D. Feasibility of biochar derived from sewage sludge to promote sustainable agriculture and mitigate GHG emissions—A review. Environ. Res. Public Health 2022, 19, 12983. [Google Scholar] [CrossRef] [PubMed]
- Ragonezi, C.; Nunes, N.; Oliveira, M.C.O.; Freitas, J.G.R.; Ganança, J.F.T.; Carvalho, M.A.A.P. Sewage sludge fertilization-A case study of sweet potato yield and heavy metal accumulation. Agronomy 2022, 12, 1902. [Google Scholar] [CrossRef]
- Japan Keen to Use Sewage Sludge as Fertilizer. The Japan News (yomiuri.co.jp). 9 October 2022. Available online: https://japannews.yomiuri.co.jp/society/general-news/20221009-63512/ (accessed on 5 January 2023).
- The State of the Sewage Treatment Population at the End of Fiscal 2021 (in Japanese), Press Release by the Ministry of Land, Infrastructure, Transport and Tourism, Tokyo, Japan. 25 August 2022. Available online: https://www.mlit.go.jp/report/press/content/001497947.pdf (accessed on 5 January 2023).
- Bashana, L.E.; Bashana, Y. Recent advances in removing phosphorus from wastewater and its future use as fertilizer (1997-2003). Water Res. 2004, 38, 4222–4246. [Google Scholar] [CrossRef]
- Rusanescu, C.O.; Rusanescu, M.; Constantin, G.A. Wastewater management in agriculture. Water 2022, 14, 3351. [Google Scholar] [CrossRef]
- Chojnacka, K.; Skrzypczak, K.; Szopa, D.; Izydorczyk, G.; Moustakas, K.; Witek-Krowiak, A. Management of biological sewage sludge: Fertilizer nitrogen recovery as the solution to fertilizer crisis. J. Environ. Manag. 2023, 326, 116602. [Google Scholar] [CrossRef] [PubMed]
- Herzel, H.; Krüger, O.; Hermann, L.; Adam, C. Sewage sludge ash—A promising secondary phosphorus source for fertilizer production. Sci. Total Environ. 2016, 542, 1136–1143. [Google Scholar] [CrossRef] [PubMed]
- Kehrein, P.; Loosdrecht, M.; Osseweijer, P.; Garfí, M.; Dewulf, J.; Posada, J. A critical review of resource recovery from municipal wastewater treatment plants—market supply potentials, technologies and bottlenecks. Environ. Sci. Water Res. Technol. 2020, 6, 877–910. [Google Scholar] [CrossRef]
- Mackowiak, C.L.; Garland, J.L.; Strayer, R.F.; Finger, B.W.; Wheeler, R.M. Comparison of aerobically-treated and untreated crop residue as a source of recycled nutrients in a recirculating hydroponic system. Adv. Space Res. 1996, 18, 281–287. [Google Scholar] [CrossRef]
- Rufí-Salís, M.; Calvo, M.J.; Anna Petit-Boix, A.; Gara Villalba, G.; Gabarrell, X. Exploring nutrient recovery from hydroponics in urban agriculture: An environmental assessment. Resour. Conser. Recyc. 2020, 155, 104683. [Google Scholar] [CrossRef]
- Stutte, G.W. Process and product: Recirculating hydroponics and bioactive compounds in a controlled environment. Am. Soc. Hortic. Sci. 2006, 41, 526–530. [Google Scholar] [CrossRef]
- Halbert-Howard, A.; Häfner, F.; Karlowsky, S.; Schwarz, D.; Krause, A. Evaluating recycling fertilizers for tomato cultivation in hydroponics, and their impact on greenhouse gas emissions. Environ. Sci. Pollut. Res. 2021, 28, 59284–59303. [Google Scholar] [CrossRef] [PubMed]
- Shin, C.; McCarty, P.L.; Kim, J.; Bae, J. Pilot-scale temperate-climate treatment of domestic wastewater with a staged anaerobic fluidized membrane bioreactor (SAF-MBR). Bioresour. Technol. 2014, 159, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Tassou, S.A. Heat recovery from sewage effluent using heat pumps. Heat Recov. Sys. CHP 1988, 8, 141–148. [Google Scholar] [CrossRef]
- Noda, T.; Kobayashi, T.; Suda, I. Effect of soil temperature on starch properties of sweet potatoes. Carbohydr. Polym. 2001, 44, 239–246. [Google Scholar] [CrossRef]
- Dumbuya, G.; Alemayehu, H.A.; Hasan, M.M.; Matsuyama, M.; Shimono, H. Effect of soil temperature on growth and yield of sweet potato (Ipomoea batatas L.) under cool climate. J. Agri. Meteorol. 2021, 77, 118–127. [Google Scholar] [CrossRef]
- Shankar, A.B.; Kaushik, P. Visiting sweet potato from a breeding perspective: An update. Preprints 2022, 1, 2022040149. [Google Scholar] [CrossRef]
- Nhuchhen, D.R.; Salam, P.A. Estimation of higher heating value of biomass from proximate analysis: A. new approach. Fuel 2012, 90, 55–63. [Google Scholar] [CrossRef]
- Valentine, J.; Clifton-Brown, J.; Hastings, A.; Robson, P.; Allison, G.; Smith, P. Food vs. fuel: The use of land for lignocellulosic ‘next generation’ energy crops that minimize competition with primary food production. Bioenergy 2012, 4, 1–19. [Google Scholar] [CrossRef]
- Lal, R. World crop residues production and implications of its use as a biofuel. Environ. Int. 2005, 31, 575–584. [Google Scholar] [CrossRef]
- Sarkar, N.; Ghosh, S.K.; Bannerjee, S.; Aikat, K. Bioethanol production from agricultural wastes: An overview. Renew. Energy 2012, 37, 19–27. [Google Scholar] [CrossRef]
- BP p.l.c. bp Statistical Review of World Energy 2022, 71st ed.; BP p.l.c.: London, UK, 2022; pp. 9–10. Available online: https://www.bp.com/content/dam/bp/business-sites/en/global/corporate/pdfs/energy-economics/statistical-review/bp-stats-review-2022-full-report.pdf (accessed on 5 January 2023).
- Schwerz, F.; Neto, D.D.; Caron, B.O.; Nardini, C.; Sgarbossa, J.; Eloy, E.; Behling, A.; Elli, E.F.; Reichardt, K. Biomass and potential energy yield of perennial woody energy crops under reduced planting spacing. Renew. Energy 2020, 153, 1238–1250. [Google Scholar] [CrossRef]
- Lewandowski, I.; Scurlock, J.M.O.; Lindvall, E.; Christou, M. The development and current status of perennial rhizomatous grasses as energy crops in the US and Europe. Biomass Bioenergy 2003, 25, 335–361. [Google Scholar] [CrossRef]
- Matsunami, H.; Kobayashi, M.; Ando, S.; Terajima, Y.; Tsuruta, S.; Sato, H. Effect of planting density and fertilizer application level on dry matter yield of Erianthus arundinaceus (L.). Jpn. J. Grassl. Sci. 2016, 61, 224–233. [Google Scholar] [CrossRef]
- Sawasdee, V.; Pisutpaisal, N. Feasibility of biogas production from Napier grass. Energy Procedia 2014, 61, 1229–1233. [Google Scholar] [CrossRef]
- Waramit, N.; Chaugool, J. Napier grass: A novel energy crop development and the current status in Thailand. J. Int. Soc. Asian Agric. Sci. 2014, 20, 139–150. [Google Scholar]
- Hou, R.; Zhang, N.; Yang, C.; Zhao, J.; Li, P.; Sun, B. Proposal of a novel power and methanol cogeneration through biogas upgrading, liquefied natural gas cooling, and combined natural gas reforming integration. Energy 2023, 126842, in press. [Google Scholar] [CrossRef]
- Liew, L.N.; Shi, J.; Li, Y. Methane production from solid-state anaerobic digestion of lignocellulosic biomass. Biomass Bioenergy 2012, 46, 125–132. [Google Scholar] [CrossRef]
- Pomdaeng, P.; Chen-Yeon Chu, C.Y.; Sripraphaa, K.; Sintuya, H. An accelerated approach of biogas production through a two-stage BioH2/CH4 continuous anaerobic digestion system from Napier grass. Bioresource Technol. 2022, 361, 127709. [Google Scholar] [CrossRef]
- Intasit, R.; Khunrae, P.; Weeradej Meeinkuirt, M.; Soontorngun, N. Fungal pretreatments of Napier grass and sugarcane leaves for high recovery of lignocellulosic enzymes and methane production. Ind. Crop. Prod. 2022, 180, 114706. [Google Scholar] [CrossRef]
- Ito, K.; Oi, S.; Takeda, T.; Okubo, T.; Hoshimo, M.; Miyagi, Y.; Numaguchi, H.; Inanaga, S.; Toyama, N.; Nagai, S.; et al. Availability of napiergrass as a raw material for methane production. Jpn. J. Crop. Sci. 1990, 59, 239–244. [Google Scholar] [CrossRef]
- Suzuki, T.; Sakamoto, M.; Kawakami, T.; Kubo, H.; Miyabe, Y.; Makita, Y.; Hiroshima, D. Methane production efficiency in anaerobic digestion reaction using sweet potatoes and wastewater treatment residues (in Japanese). Proc. JSES Mtg. 2021, 1, 209–212. Available online: https://researchmap.jp/tksuzuki-waka-kindai/published_papers/35733731 (accessed on 10 February 2023).
- Coelho, R.D.; Lizcano, J.V.; Barros, T.H.S.; Barbosa, F.S.; Leal, D.P.V.; Santos, L.C.S.; Ribeiro, N.L.; Fraga Júnior, E.F.; Derrel, L.; Martin, D.L. Effect of water stress on renewable energy from sugarcane biomass. Renew. Sust. Energy Rev. 2019, 103, 399–407. [Google Scholar] [CrossRef]
- Leal, M.R.L.V.; Walter, A.S.; Seabra, J.E.A. Sugarcane as an energy source. Biomass Conver. Biorefin. 2013, 3, 17–26. [Google Scholar] [CrossRef]
- Waclawovsky, A.J.; Sato, P.M.; Lembke, C.G.; Moore, P.H.; Souza, G.M. Sugarcane for bioenergy production: An assessment of yield and regulation of sucrose content. Plant Biotechnol. J. 2010, 8, 263–276. [Google Scholar] [CrossRef]
- Silva, V.P.R.; Silva, R.A.; Cavalcanti, E.P.; Braga, C.C.; Azevedo, P.V.; Singh, V.P.; Pereira, E.R.R. Trends in solar radiation in NCEP/NCAR database and measurements in northeastern Brazil. Solar Energy 2010, 84, 1852–1862. [Google Scholar] [CrossRef]
- Trejo-Tellez, L.I.; Gomez-Merino, F.C. Nutrient solution for hydroponic systems. In Hydroponics: A Standard Methodology for Plant Biological Researches; Asao, T., Ed.; InTech: Rijeka, Croatia, 2012; pp. 1–22. Available online: https://www.researchgate.net/publication/221928014 (accessed on 10 February 2023).
- Wortman, S.E. Crop physiological response to nutrient solution electrical conductivity and pH in an ebb-and-flow hydroponic system. Sci. Hortic. 2015, 194, 34–42. [Google Scholar] [CrossRef]
- Sharma, N.; Achrya, S.; Kumar, K.; Singh, N.; Chaurasia, O.P. Hydroponics as an advanced technique for vegetable production: An overview. J. Soil Water Conserv. 2018, 17, 364–371. [Google Scholar] [CrossRef]
- Sawicka, B.; Krochmal-Marczak, B.; Pszczółkowski, P.; Bielinska, E.J.; Wójcikowska-Kapusta, A.; Barbas, P.; Skiba, D. Effect of differentiated nitrogen fertilization on the enzymatic activity of the soil for sweet potato (Ipomoea batatas L. [Lam.]) cultivation. Agronomy 2020, 10, 1970. [Google Scholar] [CrossRef]
- Li, S.; Zhao, L.; Zhang, S.; Liu, Q.; Li, H. Effects of nitrogen level and soil moisture on sweet potato root distribution and soil chemical properties. J. Soil Sci. Plant Nutr. 2021, 21, 536–546. [Google Scholar] [CrossRef]
- Adnane Bargaz, A.; Lyamlouli, K.; Chtouki, M.; Zeroual, Y.; Dhiba, D. Soil microbial resources for improving fertilizers efficiency in an integrated plant nutrient management system. Front. Microbiol. 2018, 9, 1606. [Google Scholar] [CrossRef]
- Suzuki, T.; Sakamoto, M. Sponge fault structural model to elucidate the effects of snow load, sea level and air pressure on crustal pumping movement activated by global warming (in Japanese). Proc. JSES Mtg. 2022, 1, 209–212. Available online: https://researchmap.jp/tksuzuki-waka-kindai/published_papers/40516708 (accessed on 10 February 2023).




| Cultivation Period | Summer Cultivation June–November (160 d) | Winter Cultivation December–July (233 d) | Total (393 d) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Tuberous Roots | Leaves and Stems | Tuberous Roots | Leaves and Stems | Total Biomass | |||||||
| Layer | Fresh weight | SK | BH | SK | BH | SK | BH | SK | BH | SK | BH |
| TL | g-fm/pot | 1493 | 1364 | 1656 | 1106 | 989 | 1037 | 971 | 2565 | 5110 | 6072 |
| ML | g-fm/pot | 1595 | 930 | 1578 | 905 | 581 | 543 | 1027 | 1873 | 4782 | 4252 |
| BL | g-fm/pot | 1162 | 517 | 2250 | 608 | 433 | 454 | 1352 | 859 | 5197 | 2438 |
| Total yield * | kg-fm/25 pots | 35.0 | 21.3 | 46.6 | 20.7 | 15.1 | 15.2 | 28.7 | 40.1 | 125 | 97 |
| Productivity ** | kg-fm/m2 | 19.5 | 11.8 | 25.9 | 11.5 | 8.4 | 8.4 | 15.9 | 22.3 | 70 | 54 |
| Layer | Dry weight | SK | BH | SK | BH | SK | BH | SK | BH | SK | BH |
| TL | g-dm/pot | 433 | 396 | 199 | 133 | 287 | 301 | 117 | 308 | 1035 | 1137 |
| ML | g-dm/pot | 463 | 270 | 189 | 109 | 169 | 158 | 123 | 225 | 944 | 761 |
| BL | g-dm/pot | 337 | 150 | 270 | 73 | 125 | 132 | 162 | 103 | 895 | 458 |
| Total yield * | kg-dm/25 pots | 10.2 | 6.2 | 5.6 | 2.5 | 4.4 | 4.4 | 3.4 | 4.8 | 23.6 | 17.9 |
| Energy yield *** | MJ-dm/MJ-solar | 3.9% | 2.4% | 2.1% | 0.9% | 1.2% | 1.2% | 1.0% | 1.3% | 3.8% | 2.9% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suzuki, T.; Sakamoto, M.; Kubo, H.; Miyabe, Y.; Hiroshima, D. Effects of Sewage Treatment Water Supply on Leaf Development and Yield of Tuberous Roots in Multilayered Sweet Potato Cultivation. Horticulturae 2023, 9, 309. https://doi.org/10.3390/horticulturae9030309
Suzuki T, Sakamoto M, Kubo H, Miyabe Y, Hiroshima D. Effects of Sewage Treatment Water Supply on Leaf Development and Yield of Tuberous Roots in Multilayered Sweet Potato Cultivation. Horticulturae. 2023; 9(3):309. https://doi.org/10.3390/horticulturae9030309
Chicago/Turabian StyleSuzuki, Takahiro, Masaru Sakamoto, Hiroshi Kubo, Yui Miyabe, and Daisuke Hiroshima. 2023. "Effects of Sewage Treatment Water Supply on Leaf Development and Yield of Tuberous Roots in Multilayered Sweet Potato Cultivation" Horticulturae 9, no. 3: 309. https://doi.org/10.3390/horticulturae9030309
APA StyleSuzuki, T., Sakamoto, M., Kubo, H., Miyabe, Y., & Hiroshima, D. (2023). Effects of Sewage Treatment Water Supply on Leaf Development and Yield of Tuberous Roots in Multilayered Sweet Potato Cultivation. Horticulturae, 9(3), 309. https://doi.org/10.3390/horticulturae9030309








