Advances in the Strategic Approaches of Pre- and Post-Harvest Treatment Technologies for Peach Fruits (Prunus persica)
Abstract
:1. Introduction
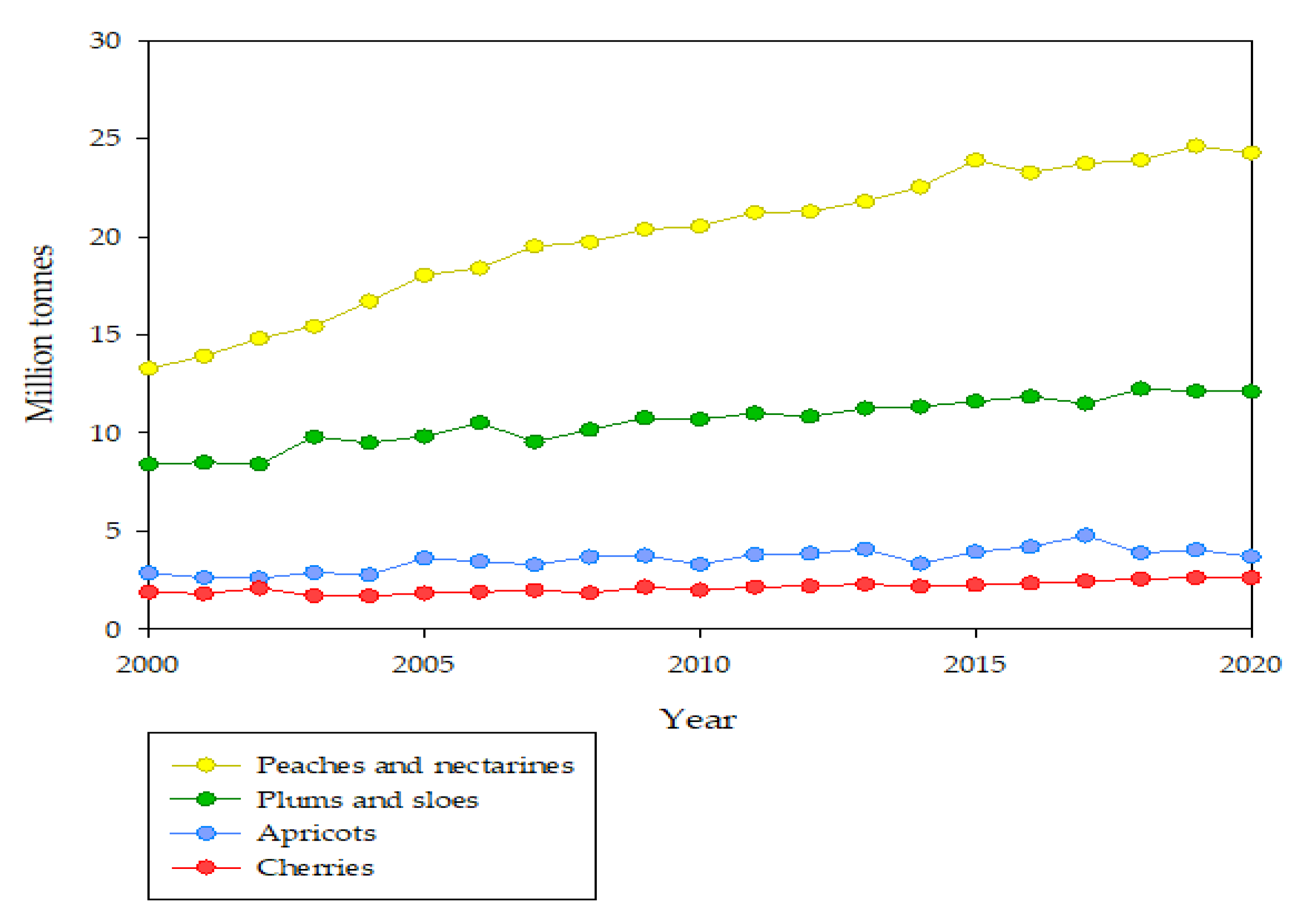
2. Quality Factors of the Pre- and Post-Harvest Treatment of Peach Fruits
3. Strategic Approach to the Application of Pre-Harvest Treatment Technologies for Peach Fruits
3.1. Spraying and Spreading Chemical Agents to Fruits
3.1.1. Calcium Salts
3.1.2. Acids
3.1.3. Other Chemical Agents for Pre-Harvest Treatment
3.2. The Use of Fertilizers
| Method | Cultivar | Method and Treatment Conditions | Results and Implications | Reference |
|---|---|---|---|---|
| Foliar spray of salts six times with 2-week intervals before harvest followed by cold storage after harvest | Scarlett O’Hara |
|
| [48] |
| Foliar spray of salts three times with 15-day intervals before harvest followed by cold storage after harvest | Dessert Red |
|
| [49] |
| Foliar spray of salts three times with 2-week intervals before harvest followed by cold storage after harvest | Florida Prince |
|
| [50] |
| Foliar spray of calcium supplemented with other activating nutrients 10 days after anthesis followed by cold storage after harvest | Sevilla 2 |
|
| [51] |
| Foliar spray of salts 6 weeks after blooming followed by cold storage after harvest | Medium Sultani |
|
| [52] |
| Spreading of the salicylic acid solution 23 and 15 days before harvest followed by cold storage | Cresthaven |
|
| [53] |
| Spray of oxalic acid on fruit surface 15 days before harvest followed by cold storage | Anjiry maleki |
|
| [55] |
| Spray of gibberellic acid before harvest (at the beginning or the end of pit hardening) followed by cold storage | Chiripa |
|
| [54] |
| Spray of sodium nitroprusside (SNP) 14 days before harvest followed by cold storage | GH Hill |
|
| [56] |
| Spray of putrescine (PUT) three times before harvest followed by cold storage | Flordaking |
|
| [57] |
| Application of fertilizer 10–15 days before flowering and 15 days before harvest | Beijing 2 |
|
| [60] |
4. Strategic Approach of the Application of Post-Harvest Treatment Technologies for Peach Fruits
4.1. Physical Treatments
4.1.1. Temperature Control
4.1.2. Modified Atmosphere
4.1.3. Irradiation
Light Irradiation
Gamma Irradiation
Microwave Irradiation
| Method | Cultivar | Treatment Conditions | Results and Implications | Reference |
|---|---|---|---|---|
| Cold storage | August Flame |
|
| [75] |
| Hydrocooling followed by cold storage | Spring Belle |
|
| [76] |
| Hot air or hot water treatment followed by cold storage | Xiahui 5 |
|
| [77] |
| Hot water treatment followed by room temperature storage | Huiyulu |
|
| [78] |
| Hot water treatment followed by room temperature storage | June Prince |
|
| [79] |
| Hot water treatment followed by cold storage | Roig |
|
| [80] |
| Controlled air treatment during cold storage | Hujingmilu |
|
| [83] |
| Hypobaric treatment during cold storage | Xiahui-8 |
|
| [84] |
| Short-term hypobaric treatment followed by cold storage | Yingshuanghong |
|
| [85] |
| Short-term hypobaric treatment followed by cold storage | Feicheng |
|
| [86] |
| UV-C irradiation followed by cold storage | Xiahui 5 |
|
| [87] |
| UV-B irradiation followed by room temperature storage | Fairtime |
|
| [88] |
| UV-C and UV-Birradiation followed by cold storage | - |
|
| [89] |
| Gamma irradiation followed by room temperature storage | - |
|
| [91] |
| Gamma irradiation followed by cold storage | Mid pride |
|
| [92] |
| Microwave irradiation followed by cold storage |
|
| [93] |
4.2. Chemical Treatments
4.2.1. Spraying or Dipping Treatment Methods Using Solutions of Chemical Agents
Spraying Treatment
Dipping Treatment
Sequential Dipping and Spraying Treatments
4.2.2. Gas Treatment of Vaporized Chemical Agents
| Method | Cultivar | Method and Treatment Conditions | Results and Implications | Reference |
|---|---|---|---|---|
| Spraying citric acid followed by room temperature storage | Hujingmilu |
|
| [108] |
| Spraying glucose oxidase immobilized on ZnO nanoparticles (GOx/ZnONPs) followed by room temperature storage | - |
|
| [110] |
| Spraying the essential oil (EO) compounds emulsion followed by room temperature storage | Springcrest |
|
| [114] |
| Dipping in EO solution followed by cold or room temperature storage | Chimarrita |
|
| [115] |
| Dipping in EO and/or chitosan followed by cold storage | Zaferani |
|
| [118] |
| Dipping in glycine betaine (GB) solution followed by cold storage | YuhuRa No.2 |
|
| [120] |
| Dipping in melatonin solution followed by room temperature storage | Shahong, Qinmi |
|
| [121] |
| Dipping in melatonin followed by cold storage | Hujing |
|
| [122] |
| Dipping in putrescine (PUT) solution followed by room temperature storage | Monley |
|
| [123] |
| Dipping in GABA solution followed by cold storage | Baifeng |
|
| [124] |
| Dipping in salicylic acid solution followed by cold storage | Anjiry maleky |
|
| [126] |
| Dipping in salicylic acid solution followed by cold storage | Flordaking |
|
| [125] |
| Dipping in CaCl2 solution followed by ambient temperature storage after cold storage | Earli Grande |
|
| [128] |
| Dipping in yeast saccharide solution followed by room temperature storage | Baifeng |
|
| [129] |
| Dipping in benzo-thiadiazole-7-carbothioic acid S-methyl ester (BTH) solution followed by room temperature storage | Jiubao |
|
| [130] |
| Soaking in calcium nanoparticles with ascorbic acid followed by cold storage | Florida Prince |
|
| [131] |
| Dipping in nano-chitosan followed by cold storage | Florida Prince |
|
| [132] |
| Dipping in gum followed by refrigerated storage | Jinxiu |
|
| [134] |
| Dipping and daily spraying of electrolyzed oxidizing water followed by room temperature storage | - |
|
| [32] |
| Fumigation of volatile organic compounds from Bacillus subtilis followed by room temperature storage | Zhaohui |
|
| [136] |
| Fumigation of NO gas followed by cold storage | Feicheng |
|
| [139] |
| Fumigation of NO gas followed by cold or room temperature storage | Feicheng |
|
| [138] |
| Fumigation of NO gas followed by room temperature storage | Rojo Rito |
|
| [137] |
| Fumigation of NO gas followed by room temperature storage | Xiahui no.5 |
|
| [140] |
| Fumigation of EO followed by room temperature storage | Early grand |
|
| [141] |
| Exposure to volatile EO (microencapsulated) and/or 1-methylcyclopropene (1-MCP) followed by cold storage | Yanhong |
|
| [142] |
| Fumigation of 1-MCP followed by cold storage with the flow microcirculation of ozone (O3) | Jinqiuhong |
|
| [143] |
4.3. Biological Treatments
| Method | Cultivar | Treatment Conditions | Results and Implications | Reference |
|---|---|---|---|---|
| Inoculation of antagonist on peach followed by room temperature storage | Dajiubao |
|
| [147] |
| Inoculation of antagonist on peach followed by room temperature storage | Baifeng |
|
| [148] |
| Inoculation of antagonist on peach followed by room temperature storage | Baihua |
|
| [150] |
| Inoculation of antagonist on peach followed by cold storage | Redhaven |
|
| [151] |
4.4. Combined Treatments
4.4.1. Combination of Physical and Chemical Treatment
4.4.2. Combination of Physical and Biological Treatment
4.4.3. Combination of Chemical and Biological Treatment
| Method | Cultivar | Treatment Conditions | Results and Implications | Reference |
|---|---|---|---|---|
| Dipping in salicylic acid solution with ultrasound treatment followed by room temperature storage | Baifeng |
|
| [161] |
| Dipping in salicylic acid solution after hot air treatment followed by the cold storage | Baifeng |
|
| [162] |
| Gamma irradiation treatment after carboxymethyl cellulose coating followed by cold or room temperature storage | - |
|
| [169] |
| Inoculation of antagonist after microwave treatment | Baihua |
|
| [170] |
| Inoculation of antagonist after hot air treatment | Baihua |
|
| [171] |
| Treatment of salicylic acid with antagonist | Jiubao |
|
| [174] |
| Treatment of essential oils (EOs) with antagonist followed by room temperature and cold storage | Transvaal |
|
| [37] |
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hussain, S.Z.; Naseer, B.; Qadri, T.; Fatima, T.; Bhat, T.A. Peach (Prunus Persica)—Morphology, taxonomy, composition and health benefits. In Fruits Grown in Highland Regions of the Himalayas; Springer: Cham, Switzerland, 2021; pp. 207–217. [Google Scholar]
- Bento, C.; Gonçalves, A.C.; Silva, B.; Silva, L.R. Peach (Prunus persica): Phytochemicals and health benefits. Food Rev. Int. 2020, 38, 1703–1734. [Google Scholar] [CrossRef]
- Busatto, N.; Tadiello, A.; Trainotti, L.; Costa, F. Climacteric ripening of apple fruit is regulated by transcriptional circuits stimulated by cross-talks between ethylene and auxin. Plant Signal. Behav. 2017, 12, e1268312. [Google Scholar] [CrossRef] [Green Version]
- Flore, J.A. Stone fruit. In Handbook of Environmental Physiology of Fruit Crops; CRC Press: Boca Raton, FL, USA, 2018; pp. 233–270. [Google Scholar]
- Lurie, S.; Crisosto, C.H. Chilling injury in peach and nectarine. Postharvest Biol. Technol. 2005, 37, 195–208. [Google Scholar] [CrossRef]
- Ozkan, Y.; Ucar, M.; Yildiz, K.; Ozturk, B. Pre-harvest gibberellic acid (GA3) treatments play an important role on bioactive compounds and fruit quality of sweet cherry cultivars. Sci. Hortic. 2016, 211, 358–362. [Google Scholar] [CrossRef]
- Salazar, J.A.; Pacheco, I.; Zapata, P.; Shinya, P.; Ruiz, D.; Martínez-Gómez, P.; Infante, R. Identification of loci controlling phenology, fruit quality and post-harvest quantitative parameters in Japanese plum (Prunus salicina Lindl.). Postharvest Biol. Technol. 2020, 169, 111292. [Google Scholar] [CrossRef]
- Hua, X.; Li, T.; Wu, C.; Zhou, D.; Fan, G.; Li, X.; Cong, K.; Yan, Z.; Cheng, X. Pulsed light improved the shelf life of apricot (after simulated long-distance air transportation) by regulating cell wall metabolism. Postharvest Biol. Technol. 2023, 196, 112187. [Google Scholar] [CrossRef]
- Gradziel, T.M. Wild and related species as a breeding source for biotic stress resistance of peach cultivars and rootstocks. In Genomic Designing for Biotic Stress Resistant Fruit Crops; Springer: Cham, Switzerland, 2022; pp. 257–274. [Google Scholar]
- Mele, M.A.; Islam, M.Z.; Kang, H.-M.; Giuffrè, A.M. Pre-and post-harvest factors and their impact on oil composition and quality of olive fruit. Emir. J. Food Agric. 2018, 30, 592–603. [Google Scholar]
- Munhuweyi, K.; Mpai, S.; Sivakumar, D. Extension of avocado fruit postharvest quality using non-chemical treatments. Agronomy 2020, 10, 212. [Google Scholar] [CrossRef] [Green Version]
- Musacchi, S.; Serra, S. Apple fruit quality: Overview on pre-harvest factors. Sci. Hortic. 2018, 234, 409–430. [Google Scholar] [CrossRef]
- Fischer, G.; Melgarejo, L.M.; Cutler, J. Pre-harvest factors that influence the quality of passion fruit: A review. Agron. Colomb. 2018, 36, 217–226. [Google Scholar] [CrossRef]
- Correia, S.; Schouten, R.; Silva, A.P.; Gonçalves, B. Sweet cherry fruit cracking mechanisms and prevention strategies: A review. Sci. Hortic. 2018, 240, 369–377. [Google Scholar] [CrossRef]
- Nor, S.M.; Ding, P. Trends and advances in edible biopolymer coating for tropical fruit: A review. Food Res. Int. 2020, 134, 109208. [Google Scholar] [CrossRef] [PubMed]
- Minas, I.S.; Tanou, G.; Molassiotis, A. Environmental and orchard bases of peach fruit quality. Sci. Hortic. 2018, 235, 307–322. [Google Scholar] [CrossRef]
- Ali, A.; Rasool, K.; Ganai, N.A.; Wani, A.H. Role of salicylic acid in postharvest management of peach and plum fruits: A review. Pharma Innov. J. 2022, 11, 659–663. [Google Scholar]
- Zhisheng, M.; Yunyun, J.; Yuehui, W.; Ruixia, B. Review on effects of nitrogen on the internal quality of peach fruit. Agric. Biotechnol. 2020, 9, 2164–4993. [Google Scholar]
- Bose, S.K.; Howlader, P.; Wang, W.; Yin, H. Oligosaccharide is a promising natural preservative for improving postharvest preservation of fruit: A review. Food Chem. 2021, 341, 128178. [Google Scholar] [CrossRef] [PubMed]
- Food and Agriculture Organization of the United Nations (FAO), FAOSTAT. Available online: https://www.fao.org/faostat/en/#compare (accessed on 8 January 2023).
- Abbott, J.A. Quality measurement of fruits and vegetables. Postharvest Biol. Technol. 1999, 15, 207–225. [Google Scholar] [CrossRef]
- de Oliveira Anese, R.; Monteiro, T.M.; Pless, G.Z.; Brackmann, A.; Thewes, F.R.; Wendt, L.M. Pre-harvest silicon spraying: Impact on decay, metabolism, and overall quality of ‘Galaxy’apples after harvest and cold storage. Sci. Hortic. 2022, 301, 111122. [Google Scholar] [CrossRef]
- Tyagi, S.; Sahay, S.; Imran, M.; Rashmi, K.; Mahesh, S.S. Pre-harvest factors influencing the postharvest quality of fruits: A review. Curr. J. Appl. Sci. Technol. 2017, 23, 12. [Google Scholar] [CrossRef]
- Khan, A.; Hussain, K.; Shah, H.; Malik, A.; Anwar, R.; Rehman, R.; Bakhsh, A. Cold storage influences postharvest chilling injury and quality of peach fruits. J. Hortic. Sci. Technol. 2018, 1, 28–34. [Google Scholar] [CrossRef]
- Hadiwijaya, Y.; Putri, I.; Mubarok, S.; Hamdani, J. Rapid and non-destructive prediction of total soluble solids of guava fruits at various storage periods using handheld near-infrared instrument. Earth Environ. Sci. 2020, 1, 012022. [Google Scholar]
- Yang, B.; Gao, Y.; Yan, Q.; Qi, L.; Zhu, Y.; Wang, B.J.S. Estimation method of soluble solid content in peach based on deep features of hyperspectral imagery. Sensors 2020, 20, 5021. [Google Scholar] [CrossRef] [PubMed]
- Tyl, C.; Sadler, G.D. pH and titratable acidity. In Food Analysis; Springer: Berlin/Heidelberg, Germany, 2017; pp. 389–406. [Google Scholar]
- Gasic, K.; Abdelghafar, A.; Reighard, G.; Windham, J.; Ognjanov, M. Fruit maturity affects fruit quality and bioactive compound accumulation in peach. Sustain. Lives Livelihoods Landsc. 2016, 1119, 197–202. [Google Scholar] [CrossRef]
- Lim, S.; Lee, J.G.; Lee, E.J. Comparison of fruit quality and GC–MS-based metabolite profiling of kiwifruit ‘Jecy green’: Natural and exogenous ethylene-induced ripening. Food Chem. 2017, 234, 81–92. [Google Scholar] [CrossRef]
- Ali, S.; Anjum, M.A.; Nawaz, A.; Naz, S.; Ejaz, S.; Sardar, H.; Saddiq, B. Tragacanth gum coating modulates oxidative stress and maintains quality of harvested apricot fruits. Int. J. Biol. Macromol. 2020, 163, 2439–2447. [Google Scholar] [CrossRef]
- Cömert, E.D.; Mogol, B.A.; Gökmen, V. Relationship between color and antioxidant capacity of fruits and vegetables. Curr. Res. Food Sci. 2020, 2, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Guentzel, J.L.; Lam, K.L.; Callan, M.A.; Emmons, S.A.; Dunham, V.L. Postharvest management of gray mold and brown rot on surfaces of peaches and grapes using electrolyzed oxidizing water. Int. J. Food Microbiol. 2010, 143, 54–60. [Google Scholar] [CrossRef]
- Karabulut, O.A.; Baykal, N. Integrated control of postharvest diseases of peaches with a yeast antagonist, hot water and modified atmosphere packaging. Crop Prot. 2004, 23, 431–435. [Google Scholar] [CrossRef]
- Karabulut, O.A.; Cohen, L.; Wiess, B.; Daus, A.; Lurie, S.; Droby, S. Control of brown rot and blue mold of peach and nectarine by short hot water brushing and yeast antagonists. Postharvest Biol. Technol. 2002, 24, 103–111. [Google Scholar] [CrossRef]
- Salem, E.A.; Youssef, K.; Sanzani, S.M. Evaluation of alternative means to control postharvest Rhizopus rot of peaches. Sci. Hortic. 2016, 198, 86–90. [Google Scholar] [CrossRef]
- Hu, M.J.; Cox, K.D.; Schnabel, G.; Luo, C.-X. Monilinia species causing brown rot of peach in China. PLoS ONE 2011, 6, e24990. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arrebola, E.; Sivakumar, D.; Bacigalupo, R.; Korsten, L. Combined application of antagonist Bacillus amyloliquefaciens and essential oils for the control of peach postharvest diseases. Crop Prot. 2010, 29, 369–377. [Google Scholar] [CrossRef] [Green Version]
- Escarpa, A.; González, M. Approach to the content of total extractable phenolic compounds from different food samples by comparison of chromatographic and spectrophotometric methods. Anal. Chim. Acta 2001, 427, 119–127. [Google Scholar] [CrossRef]
- Tehrani, H.S.; Moosavi-Movahedi, A.A. Catalase and its mysteries. Prog. Biophys. Mol. Biol. 2018, 140, 5–12. [Google Scholar] [CrossRef]
- Stephenie, S.; Chang, Y.P.; Gnanasekaran, A.; Esa, N.M.; Gnanaraj, C. An insight on superoxide dismutase (SOD) from plants for mammalian health enhancement. J. Funct. Foods 2020, 68, 103917. [Google Scholar] [CrossRef]
- Yoshimura, K.; Yabuta, Y.; Ishikawa, T.; Shigeoka, S. Expression of spinach ascorbate peroxidase isoenzymes in response to oxidative stresses. Plant Physiol. 2000, 123, 223–234. [Google Scholar] [CrossRef] [Green Version]
- Passardi, F.; Theiler, G.; Zamocky, M.; Cosio, C.; Rouhier, N.; Teixera, F.; Margis-Pinheiro, M.; Ioannidis, V.; Penel, C.; Falquet, L. PeroxiBase: The peroxidase database. Phytochemistry 2007, 68, 1605–1611. [Google Scholar] [CrossRef]
- Kohli, P.; Kalia, M.; Gupta, R. Pectin methylesterases: A review. J. Bioprocess. Biotech. 2015, 5, 1. [Google Scholar]
- MacDonald, M.J.; D’Cunha, G.B. A modern view of phenylalanine ammonia lyase. Biochem. Cell Biol. 2007, 85, 273–282. [Google Scholar] [CrossRef]
- Wang, J.; Zeng, Q.; Zhu, J.; Liu, G.; Tang, H. Dissimilarity of ascorbate–glutathione (AsA–GSH) cycle mechanism in two rice (Oryza sativa L.) cultivars under experimental free-air ozone exposure. Agric. Ecosyst. Environ. 2013, 165, 39–49. [Google Scholar] [CrossRef]
- Yoruk, R.; Marshall, M.R. Physicochemical properties and function of plant polyphenol oxidase: A review. J. Food Biochem. 2003, 27, 361–422. [Google Scholar] [CrossRef]
- Feng, B.; Dong, Z.; Xu, Z.; An, X.; Qin, H.; Wu, N.; Wang, D.; Wang, T. Molecular analysis of lipoxygenase (LOX) genes in common wheat and phylogenetic investigation of LOX proteins from model and crop plants. J. Cereal Sci. 2010, 52, 387–394. [Google Scholar] [CrossRef]
- Elmer, P.; Spiers, T.; Wood, P. Effects of pre-harvest foliar calcium sprays on fruit calcium levels and brown rot of peaches. Crop Prot. 2007, 26, 11–18. [Google Scholar] [CrossRef]
- Aziz, M.; Soliman, M.; Ennab, H. Effect of potassium silicate and chelated calcium sprays on yield, quality and storage of peach fruits cv. “Dessert red”. Menoufia J. Plant Prod. 2021, 6, 119–135. [Google Scholar] [CrossRef]
- El-Badawy, H. Effect of chitosan and calcium chloride spraying on fruits quality of Florida prince peach under cold storage. Res. J. Agric. Biol. Sci. 2012, 8, 272–281. [Google Scholar]
- Serrano, M.; Martínez-Romero, D.; Castillo, S.; Guillén, F.; Valero, D. Effect of preharvest sprays containing calcium, magnesium and titanium on the quality of peaches and nectarines at harvest and during postharvest storage. J. Sci. Food Agric. 2004, 84, 1270–1276. [Google Scholar] [CrossRef]
- El-Dengawy, E.; El-Shobaky, M.; Serag, T. Effect of pre-harvest treatments of peach trees on fruits quality characters during cold storage. J. Plant Prod. 2019, 10, 217–222. [Google Scholar] [CrossRef] [Green Version]
- Erogul, D.; Özsoydan, İ. Effect of pre-harvest salicylic acid treatments on the quality and shelf life of the ‘Cresthaven’ peach cultivar. Folia Hortic. 2020, 32, 221–227. [Google Scholar] [CrossRef]
- Pegoraro, C.; Zanuzo, M.R.; Chaves, F.C.; Brackmann, A.; Girardi, C.L.; Lucchetta, L.; Nora, L.; Silva, J.A.; Rombaldi, C.V. Physiological and molecular changes associated with prevention of woolliness in peach following pre-harvest application of gibberellic acid. Postharvest Biol. Technol. 2010, 57, 19–26. [Google Scholar] [CrossRef]
- Razavi, F.; Hajilou, J. Enhancement of postharvest nutritional quality and antioxidant capacity of peach fruits by preharvest oxalic acid treatment. Sci. Hortic. 2016, 200, 95–101. [Google Scholar] [CrossRef]
- Saba, M.K.; Moradi, S. Sodium nitroprusside (SNP) spray to maintain fruit quality and alleviate postharvest chilling injury of peach fruit. Sci. Hortic. 2017, 216, 193–199. [Google Scholar] [CrossRef]
- Abbasi, N.A.; Ali, I.; Hafiz, I.A.; Alenazi, M.M.; Shafiq, M. Effects of putrescine application on peach fruit during storage. Sustainability 2019, 11, 2013. [Google Scholar] [CrossRef] [Green Version]
- Zhu, L.; Yu, H.; Dai, X.; Yu, M.; Yu, Z.J.S.H. Effect of methyl jasmonate on the quality and antioxidant capacity by modulating ascorbate-glutathione cycle in peach fruit. Sci. Hortic. 2022, 303, 111216. [Google Scholar] [CrossRef]
- Xiao, Q.; Wang, J.; Liang, D.; Xia, H.; Wang, T.; Lyu, X. Effects of formulated fertilization on soil physical and chemical characteristics of early ripe peach Orchard. Earth Environ. Sci. 2019, 295, 012086. [Google Scholar] [CrossRef]
- Liang, D.; Wang, J.; Xiao, Q.; Xia, H.; Liu, L.; Lyu, X. Effects of formulated fertilization on fruit quality of early ripe peach ‘Beijing 2’. Earth Environ. Sci. 2019, 295, 012085. [Google Scholar] [CrossRef]
- Ortiz, A.; Graell, J.; Lara, I. Preharvest calcium applications inhibit some cell wall-modifying enzyme activities and delay cell wall disassembly at commercial harvest of ‘Fuji Kiku-8′ apples. Postharvest Biol. Technol. 2011, 62, 161–167. [Google Scholar] [CrossRef]
- Liu, H.; Chen, F.; Lai, S.; Tao, J.; Yang, H.; Jiao, Z. Effects of calcium treatment and low temperature storage on cell wall polysaccharide nanostructures and quality of postharvest apricot (Prunus armeniaca). Food Chem. 2017, 225, 87–97. [Google Scholar] [CrossRef]
- Jiang, B.; Liu, R.; Fang, X.; Tong, C.; Chen, H.; Gao, H. Effects of salicylic acid treatment on fruit quality and wax composition of blueberry (Vaccinium virgatum Ait). Food Chem. 2022, 368, 130757. [Google Scholar] [CrossRef]
- Pérez-Llorca, M.; Muñoz, P.; Müller, M.; Munné-Bosch, S. Biosynthesis, metabolism and function of auxin, salicylic acid and melatonin in climacteric and non-climacteric fruits. Front. Plant Sci. 2019, 10, 136. [Google Scholar] [CrossRef] [Green Version]
- Zheng, X.; Tian, S.; Meng, X.; Li, B. Physiological and biochemical responses in peach fruit to oxalic acid treatment during storage at room temperature. Food Chem. 2007, 104, 156–162. [Google Scholar] [CrossRef]
- Zheng, X.; Tian, S. Effect of oxalic acid on control of postharvest browning of litchi fruit. Food Chem. 2006, 96, 519–523. [Google Scholar] [CrossRef]
- Prasad, R.; Shivay, Y.S. Oxalic acid/oxalates in plants: From self-defence to phytoremediation. Curr. Sci. 2017, 112, 1665–1667. [Google Scholar] [CrossRef]
- Camara, M.C.; Vandenberghe, L.P.; Rodrigues, C.; de Oliveira, J.; Faulds, C.; Bertrand, E.; Soccol, C.R. Current advances in gibberellic acid (GA3) production, patented technologies and potential applications. Planta 2018, 248, 1049–1062. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Jiang, H.; Bi, Y.; He, X.; Wang, Y.; Li, Y.; Zheng, X.; Prusky, D. Preharvest multiple sprays with sodium nitroprusside promote wound healing of harvested muskmelons by activation of phenylpropanoid metabolism. Postharvest Biol. Technol. 2019, 158, 110988. [Google Scholar] [CrossRef]
- Ge, Y.; Chen, Y.; Li, C.; Zhao, J.; Wei, M.; Li, X.; Yang, S.; Mi, Y. Effect of sodium nitroprusside treatment on shikimate and phenylpropanoid pathways of apple fruit. Food Chem. 2019, 290, 263–269. [Google Scholar] [CrossRef] [PubMed]
- Khaliq, G.; Ullah, M.; Memon, S.A.; Ali, A.; Rashid, M. Exogenous nitric oxide reduces postharvest anthracnose disease and maintains quality of custard apple (Annona squamosa L.) fruit during ripening. J. Food Meas. Charact. 2021, 15, 707–716. [Google Scholar] [CrossRef]
- Razzaq, K.; Khan, A.S.; Malik, A.U.; Shahid, M.; Ullah, S. Role of putrescine in regulating fruit softening and antioxidative enzyme systems in ‘Samar bahisht chaunsa’ mango. Postharvest Biol. Technol. 2014, 96, 23–32. [Google Scholar] [CrossRef]
- Mirdehghan, S.; Rahemi, M.; Castillo, S.; Martínez-Romero, D.; Serrano, M.; Valero, D. Pre-storage application of polyamines by pressure or immersion improves shelf-life of pomegranate stored at chilling temperature by increasing endogenous polyamine levels. Postharvest Biol. Technol. 2007, 44, 26–33. [Google Scholar] [CrossRef]
- Zhao, X.; Zhao, C.; Du, X.; Dong, D. Detecting and mapping harmful chemicals in fruit and vegetables using nanoparticle-enhanced laser-induced breakdown spectroscopy. Sci. Rep. 2019, 9, 906. [Google Scholar] [CrossRef] [Green Version]
- Ceccarelli, A.; Farneti, B.; Frisina, C.; Allen, D.; Donati, I.; Cellini, A.; Costa, G.; Spinelli, F.; Stefanelli, D. Harvest maturity stage and cold storage length influence on flavour development in peach fruit. Agronomy 2018, 9, 10. [Google Scholar] [CrossRef] [Green Version]
- Caprioli, I.; Lafuente, M.T.; Rodrigo, M.J.; Mencarelli, F. Influence of postharvest treatments on quality, carotenoids, and abscisic acid content of stored “Spring Belle” peach (Prunus persica) fruit. J. Agric. Food Chem. 2009, 57, 7056–7063. [Google Scholar] [CrossRef] [PubMed]
- Huan, C.; Han, S.; Jiang, L.; An, X.; Yu, M.; Xu, Y.; Ma, R.; Yu, Z. Postharvest hot air and hot water treatments affect the antioxidant system in peach fruit during refrigerated storage. Postharvest Biol. Technol. 2017, 126, 1–14. [Google Scholar] [CrossRef]
- Zhang, L.; Yu, Z.; Jiang, L.; Jiang, J.; Luo, H.; Fu, L. Effect of post-harvest heat treatment on proteome change of peach fruit during ripening. J. Proteom. 2011, 74, 1135–1149. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Sui, Y.; Wisniewski, M.; Droby, S.; Tian, S.; Norelli, J.; Hershkovitz, V. Effect of heat treatment on inhibition of Monilinia fructicola and induction of disease resistance in peach fruit. Postharvest Biol. Technol. 2012, 65, 61–68. [Google Scholar] [CrossRef]
- Jemric, T.; Ivic, D.; Fruk, G.; Matijas, H.S.; Cvjetkovic, B.; Bupic, M.; Pavkovic, B. Reduction of postharvest decay of peach and nectarine caused by Monilinia laxa using hot water dipping. Food Bioprocess Technol. 2011, 4, 149–154. [Google Scholar] [CrossRef]
- Koukounaras, A.; Diamantidis, G.; Sfakiotakis, E. The effect of heat treatment on quality retention of fresh-cut peach. Postharvest Biol. Technol. 2008, 48, 30–36. [Google Scholar] [CrossRef]
- Murray, R.; Lucangeli, C.; Polenta, G.; Budde, C. Combined pre-storage heat treatment and controlled atmosphere storage reduced internal breakdown of ‘Flavorcrest’peach. Postharvest Biol. Technol. 2007, 44, 116–121. [Google Scholar] [CrossRef]
- Liu, H.; He, H.; Liu, C.; Wang, C.; Qiao, Y.; Zhang, B. Changes of sensory quality, flavor-related metabolites and gene expression in peach fruit treated by controlled atmosphere (CA) under cold storage. Int. J. Mol. Sci. 2022, 23, 7141. [Google Scholar] [CrossRef]
- Wu, X.; Wu, H.; Yu, M.; Ma, R.; Yu, Z. Effect of combined hypobaric and cold storage on defense-related enzymes in postharvest peach fruit during ripening. Acta Physiol. Plant. 2022, 44, 93. [Google Scholar] [CrossRef]
- Zhang, X.; Xin, L.; Wang, C.; Sun, S.; Lyu, Y. Short-term hypobaric treatment enhances chilling tolerance in peaches. J. Food Process. Preserv. 2021, 45, e15362. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, T.; Zhu, S.; Wang, D.; Sun, S.; Xin, L. Short-term hypobaric treatment alleviates chilling injury by regulating membrane fatty acids metabolism in peach fruit. J. Food Biochem. 2022, 46, e14113. [Google Scholar] [CrossRef] [PubMed]
- Kan, J.; Hui, Y.; Lin, X.; Liu, Y.; Jin, C. Postharvest ultraviolet-C treatment of peach fruit: Changes in transcriptome profile focusing on genes involved in softening and senescence. J. Food Process. Preserv. 2021, 45, e15813. [Google Scholar] [CrossRef]
- Santin, M.; Lucini, L.; Castagna, A.; Chiodelli, G.; Hauser, M.; Ranieri, A. Post-harvest UV-B radiation modulates metabolite profile in peach fruit. Postharvest Biol. Technol. 2018, 139, 127–134. [Google Scholar] [CrossRef]
- Abdipour, M.; Hosseinifarahi, M.; Naseri, N. Combination method of UV-B and UV-C prevents post-harvest decay and improves organoleptic quality of peach fruit. Sci. Hortic. 2019, 256, 108564. [Google Scholar] [CrossRef]
- Jia, X.; Li, J.; Du, M.; Zhao, Z.; Song, J.; Yang, W.; Zheng, Y.; Chen, L.; Li, X. Combination of low fluctuation of temperature with TiO2 photocatalytic/ozone for the quality maintenance of postharvest peach. Foods 2020, 9, 234. [Google Scholar] [CrossRef] [Green Version]
- Khan, Q.U.; Mohammadzai, I.; Shah, Z.; Khattak, T.N.; Noreen, H.; Hassan, W. Effect of gamma irradiation on nutrients and shelf life of peach (Prunus persical) stored at ambient temperature. Open Conf. Proc. J. 2018, 9, 8–15. [Google Scholar] [CrossRef]
- Melo, A.A.M.; Olabode, P.N.; Michael, B.C.; Prakash, A. Causes of irradiation-induced softening in peaches. Radiat. Phys. Chem. 2018, 152, 107–113. [Google Scholar] [CrossRef]
- Wang, K.; Zhu, G.; Li, Y.; Chen, S.; Rashid, A.; Wang, X.; Wu, X. Non-thermal effects of microwave irradiation alleviates postharvest chilling injury of peach fruit by retarding phenolic accumulation and enhancing membrane stability. Food Chem. 2023, 411, 135448. [Google Scholar] [CrossRef]
- Park, Y.S.; Im, M.H.; Choi, J.H.; Lee, H.C.; Ham, K.S.; Kang, S.G.; Park, Y.K.; Suhaj, M.; Namiesnik, J.; Gorinstein, S. Effect of long-term cold storage on physicochemical attributes and bioactive components of kiwi fruit cultivars. CyTA-J. Food 2014, 12, 360–368. [Google Scholar] [CrossRef]
- Fagundes, C.; Moraes, K.; Pérez-Gago, M.B.; Palou, L.; Maraschin, M.; Monteiro, A. Effect of active modified atmosphere and cold storage on the postharvest quality of cherry tomatoes. Postharvest Biol. Technol. 2015, 109, 73–81. [Google Scholar] [CrossRef]
- Riudavets, J.; Alonso, M.; Gabarra, R.; Arnó, J.; Jaques, J.A.; Palou, L. The effects of postharvest carbon dioxide and a cold storage treatment on Tuta absoluta mortality and tomato fruit quality. Postharvest Biol. Technol. 2016, 120, 213–221. [Google Scholar] [CrossRef]
- Piccolo, E.L.; Martìnez Garcìa, L.; Landi, M.; Guidi, L.; Massai, R.; Remorini, D. Influences of postharvest storage and processing techniques on antioxidant and nutraceutical properties of Rubus idaeus L.: A mini-review. Horticulturae 2020, 6, 105. [Google Scholar] [CrossRef]
- Zhang, M.; Liu, W.; Li, C.; Shao, T.; Jiang, X.; Zhao, H.; Ai, W. Postharvest hot water dipping and hot water forced convection treatments alleviate chilling injury for zucchini fruit during cold storage. Sci. Hortic. 2019, 249, 219–227. [Google Scholar] [CrossRef]
- Endo, H.; Miyazaki, K.; Ose, K.; Imahori, Y. Hot water treatment to alleviate chilling injury and enhance ascorbate-glutathione cycle in sweet pepper fruit during postharvest cold storage. Sci. Hortic. 2019, 257, 108715. [Google Scholar] [CrossRef]
- Pinela, J.; Ferreira, I.C. Nonthermal physical technologies to decontaminate and extend the shelf-life of fruits and vegetables: Trends aiming at quality and safety. Crit. Rev. Food Sci. Nutr. 2017, 57, 2095–2111. [Google Scholar] [CrossRef]
- Zhang, W.; Jiang, W. UV treatment improved the quality of postharvest fruits and vegetables by inducing resistance. Trends Food Sci. Technol. 2019, 92, 71–80. [Google Scholar] [CrossRef]
- Gong, D.; Cao, S.; Sheng, T.; Shao, J.; Song, C.; Wo, F.; Chen, W.; Yang, Z. Effect of blue light on ethylene biosynthesis, signalling and fruit ripening in postharvest peaches. Sci. Hortic. 2015, 197, 657–664. [Google Scholar] [CrossRef]
- Turtoi, M. Ultraviolet light treatment of fresh fruits and vegetables surface: A review. J. Agroaliment. Process. Technol. 2013, 19, 325–337. [Google Scholar]
- Lante, A.; Tinello, F.; Nicoletto, M. UV-A light treatment for controlling enzymatic browning of fresh-cut fruits. Innov. Food Sci. Emerg. Technol. 2016, 34, 141–147. [Google Scholar] [CrossRef]
- Khan, S.; Dar, A.H.; Shams, R.; Aga, M.B.; Siddiqui, M.W.; Mir, S.A.; Rizvi, Q.U.; Khan, S.A.; Altaf, A. Applications of ultraviolet light–emitting diode technology in horticultural produce: A systematic review and meta-analysis. Food Bioprocess Technol. 2022, 15, 487–497. [Google Scholar] [CrossRef]
- Bisht, B.; Bhatnagar, P.; Gururani, P.; Kumar, V.; Tomar, M.S.; Sinhmar, R.; Rathi, N.; Kumar, S. Food irradiation: Effect of ionizing and non-ionizing radiations on preservation of fruits and vegetables—A review. Trends Food Sci. Technol. 2021, 114, 372–385. [Google Scholar] [CrossRef]
- Soni, A.; Smith, J.; Thompson, A.; Brightwell, G. Microwave-induced thermal sterilization—A review on history, technical progress, advantages and challenges as compared to the conventional methods. Trends Food Sci. Technol. 2020, 97, 433–442. [Google Scholar] [CrossRef]
- Yang, C.; Chen, T.; Shen, B.; Sun, S.; Song, H.; Chen, D.; Xi, W. Citric acid treatment reduces decay and maintains the postharvest quality of peach (Prunus persica L.) fruit. Food Sci. Nutr. 2019, 7, 3635–3643. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, X.; Wang, Y.; Wang, Y.; Huang, H.; Bai, Y.; Su, X.; Zhang, J.; Yao, B.; Tu, T.; Luo, H. Exploiting the activity–stability trade-off of glucose oxidase from Aspergillus niger using a simple approach to calculate thermostability of mutants. Food Chem. 2021, 342, 128270. [Google Scholar] [CrossRef]
- Batool, R.; Kazmi, S.A.R.; Khurshid, S.; Saeed, M.; Ali, S.; Adnan, A.; Altaf, F.; Hameed, A.; Batool, F.; Fatima, N. Postharvest shelf life enhancement of peach fruit treated with glucose oxidase immobilized on ZnO nanoparticles. Food Chem. 2022, 366, 130591. [Google Scholar] [CrossRef]
- Sivakumar, D.; Bautista-Baños, S. A review on the use of essential oils for postharvest decay control and maintenance of fruit quality during storage. Crop Prot. 2014, 64, 27–37. [Google Scholar] [CrossRef]
- Spadaro, D.; Gullino, M.L. Use of essential oils to control postharvest rots on pome and stone fruit. In Post-Harvest Pathology; Springer: Berlin/Heidelberg, Germany, 2014; pp. 101–110. [Google Scholar]
- Cho, T.J.; Park, S.M.; Yu, H.; Seo, G.H.; Kim, H.W.; Kim, S.A.; Rhee, M.S. Recent advances in the application of antibacterial complexes using essential oils. Molecules 2020, 25, 1752. [Google Scholar] [CrossRef]
- Elshafie, H.S.; Mancini, E.; Sakr, S.; De Martino, L.; Mattia, C.A.; De Feo, V.; Camele, I. Antifungal activity of some constituents of Origanum vulgare L. essential oil against postharvest disease of peach fruit. J. Med. Food 2015, 18, 929–934. [Google Scholar] [CrossRef]
- Pansera, M.R.; Conte, R.I.; e Silva, S.M.; Sartori, V.C.; silva Ribeiro, R.T. Strategic control of postharvest decay in peach caused by Monilinia fructicola and Colletotrichum gloeosporioides. Appl. Res. Agrotechnol. 2015, 8, 7–14. [Google Scholar]
- Moon, H.; Kim, N.H.; Kim, S.H.; Kim, Y.; Ryu, J.H.; Rhee, M.S. Teriyaki sauce with carvacrol or thymol effectively controls Escherichia coli O157: H7, Listeria monocytogenes, Salmonella Typhimurium, and indigenous flora in marinated beef and marinade. Meat Sci. 2017, 129, 147–152. [Google Scholar] [CrossRef]
- Eduardo, I.; Chietera, G.; Bassi, D.; Rossini, L.; Vecchietti, A. Identification of key odor volatile compounds in the essential oil of nine peach accessions. J. Sci. Food Agric. 2010, 90, 1146–1154. [Google Scholar] [CrossRef] [PubMed]
- Rahimi, R.; ValizadehKaji, B.; Khadivi, A.; Shahrjerdi, I. Effect of chitosan and thymol essential oil on quality maintenance and shelf life extension of peach fruits cv.‘Zaferani’. J. Hortic. Postharvest Res. 2019, 2, 143–156. [Google Scholar]
- Mansour, M.M.F. Protection of plasma membrane of onion epidermal cells by glycinebetaine and proline against NaCl stress. Plant Physiol. Biochem. 1998, 36, 767–772. [Google Scholar] [CrossRef]
- Shan, T.; Jin, P.; Zhang, Y.; Huang, Y.; Wang, X.; Zheng, Y. Exogenous glycine betaine treatment enhances chilling tolerance of peach fruit during cold storage. Postharvest Biol. Technol. 2016, 114, 104–110. [Google Scholar] [CrossRef]
- Gao, H.; Zhang, Z.K.; Chai, H.K.; Cheng, N.; Yang, Y.; Wang, D.N.; Yang, T.; Cao, W. Melatonin treatment delays postharvest senescence and regulates reactive oxygen species metabolism in peach fruit. Postharvest Biol. Technol. 2016, 118, 103–110. [Google Scholar] [CrossRef]
- Cao, S.; Shao, J.; Shi, L.; Xu, L.; Shen, Z.; Chen, W.; Yang, Z. Melatonin increases chilling tolerance in postharvest peach fruit by alleviating oxidative damage. Sci. Rep. 2018, 8, 806. [Google Scholar] [CrossRef] [Green Version]
- Kibar, H.; Taş, A.; Gündoğdu, M. Evaluation of biochemical changes and quality in peach fruit: Effect of putrescine treatments and storage. J. Food Compos. Anal. 2021, 102, 104048. [Google Scholar] [CrossRef]
- Shang, H.; Cao, S.; Yang, Z.; Cai, Y.; Zheng, Y. Effect of exogenous γ-aminobutyric acid treatment on proline accumulation and chilling injury in peach fruit after long-term cold storage. J. Agric. Food Chem. 2011, 59, 1264–1268. [Google Scholar] [CrossRef]
- Tareen, M.J.; Abbasi, N.A.; Hafiz, I.A. Postharvest application of salicylic acid enhanced antioxidant enzyme activity and maintained quality of peach cv.‘Flordaking’ fruit during storage. Sci. Hortic. 2012, 142, 221–228. [Google Scholar] [CrossRef]
- Razavi, F.; Hajilou, J.; Dehgan, G.; Nagshi, R.; Turchi, M. Enhancement of postharvest quality of peach fruit by salicylic acid treatment. Int. J. Biosci. 2014, 4, 177–184. [Google Scholar]
- Elham, S.; Vali, R.; Yavar, S. Effect of calcium chloride (CaCl2) on postharvest quality of apple fruits. Afr. J. Agric. Res. 2011, 6, 5139–5143. [Google Scholar]
- Gupta, N.; Jawandha, S.K.; Gill, P.S. Effect of calcium on cold storage and post-storage quality of peach. J. Food Sci. Technol. 2011, 48, 225–229. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, Q.; Chen, Q.; Chen, Z.; Xu, H.; Fu, M.; Li, S.; Wang, H.; Xu, M. Activating defense responses and reducing postharvest blue mold decay caused by Penicillium expansum in peach fruit by yeast saccharide. Postharvest Biol. Technol. 2012, 74, 100–107. [Google Scholar] [CrossRef]
- Liu, H.; Jiang, W.; Bi, Y.; Luo, Y. Postharvest BTH treatment induces resistance of peach (Prunus persica L. cv. Jiubao) fruit to infection by Penicillium expansum and enhances activity of fruit defense mechanisms. Postharvest Biol. Technol. 2005, 35, 263–269. [Google Scholar] [CrossRef]
- AA, L.a.; Ismail, H.; Kassem, H.S. Postharvest treatment of ‘Florida Prince’ peaches with a calcium nanoparticle–ascorbic acid mixture during cold storage and its effect on antioxidant enzyme activities. Horticulturae 2021, 7, 499. [Google Scholar] [CrossRef]
- Gad, M.; Ibrahim, M. Coating Florida prince peach fruits with nano-chitosan for increasing storage and shelf life. Agric Env. 2018, 18, 27–33. [Google Scholar]
- Fallah, Z.P.; Motamedzadegan, A.; Haghighi, M.M.; Latifi, Z.; Khesht, S.G. Comparing the effect of arabic, basil seed and salvia macrosiphon gums-based coatings on the shelf-life of tomatoes. Prev. Nutr. Food Sci. 2021, 26, 469. [Google Scholar] [CrossRef]
- Zhang, L.; Kou, X.; Huang, X.; Li, G.; Liu, J.; Ye, J. Peach-gum: A promising alternative for retarding the ripening and senescence in postharvest peach fruit. Postharvest Biol. Technol. 2020, 161, 111088. [Google Scholar] [CrossRef]
- Iram, A.; Wang, X.; Demirci, A. Electrolyzed oxidizing water and its applications as sanitation and cleaning agent. Food Eng. Rev. 2021, 13, 411–427. [Google Scholar] [CrossRef]
- Zhou, M.; Li, P.; Wu, S.; Zhao, P.; Gao, H. Bacillus subtilis CF-3 volatile organic compounds inhibit Monilinia fructicola growth in peach fruit. Front. Microbiol. 2019, 10, 1804. [Google Scholar] [CrossRef] [Green Version]
- Flores, F.B.; Sánchez-Bel, P.; Valdenegro, M.; Romojaro, F.; Martínez-Madrid, M.C.; Egea, M.I. Effects of a pretreatment with nitric oxide on peach (Prunus persica L.) storage at room temperature. Eur. Food Res. Technol. 2008, 227, 1599–1611. [Google Scholar] [CrossRef]
- Zhu, S.; Liu, M.; Zhou, J. Inhibition by nitric oxide of ethylene biosynthesis and lipoxygenase activity in peach fruit during storage. Postharvest Biol. Technol. 2006, 42, 41–48. [Google Scholar] [CrossRef]
- Zhu, S.; Zhou, J. Effects of nitric oxide on fatty acid composition in peach fruits during storage. J. Agric. Food Chem. 2006, 54, 9447–9452. [Google Scholar] [CrossRef] [PubMed]
- Kang, R.; Zhang, L.; Jiang, L.; Yu, M.; Ma, R.; Yu, Z. Effect of postharvest nitric oxide treatment on the proteome of peach fruit during ripening. Postharvest Biol. Technol. 2016, 112, 277–289. [Google Scholar] [CrossRef]
- Ziedan, E.; Farrag, E.S. Fumigation of peach fruits with essential oils to control postharvest decay. Res. J. Agric. Biol. Sci. 2008, 4, 512–519. [Google Scholar]
- Yang, W.; Wang, L.; Ban, Z.; Yan, J.; Lu, H.; Zhang, X.; Wu, Q.; Aghdam, M.S.; Luo, Z.; Li, L. Efficient microencapsulation of syringa essential oil; the valuable potential on quality maintenance and storage behavior of peach. Food Hydrocoll. 2019, 95, 177–185. [Google Scholar] [CrossRef]
- Du, M.; Jia, X.; Li, J.; Li, X.; Jiang, J.; Li, H.; Zheng, Y.; Liu, Z.; Zhang, X.; Fan, J. Regulation effects of 1-MCP combined with flow microcirculation of sterilizing medium on peach shelf quality. Sci. Hortic. 2020, 260, 108867. [Google Scholar] [CrossRef]
- Oikeh, E.I.; Ayevbuomwan, M.; Irabor, F.; Oikeh, A.O.; Oviasogie, F.E.; Omoregie, E.S. Evaluation of the phenolic content, antioxidant and antimicrobial activities of oil and non-oil extracts of Citrus sinensis (L.) Osbeck seeds. Prev. Nutr. Food Sci. 2020, 25, 280. [Google Scholar] [CrossRef]
- Sun, Y.; Lu, R.; Pan, L.; Wang, X.; Tu, K. Assessment of the optical properties of peaches with fungal infection using spatially-resolved diffuse reflectance technique and their relationships with tissue structural and biochemical properties. Food Chem. 2020, 321, 126704. [Google Scholar] [CrossRef]
- Sellitto, V.M.; Zara, S.; Fracchetti, F.; Capozzi, V.; Nardi, T. Microbial biocontrol as an alternative to synthetic fungicides: Boundaries between pre-and postharvest applications on vegetables and fruits. Fermentation 2021, 7, 60. [Google Scholar] [CrossRef]
- Xu, B.; Zhang, H.; Chen, K.; Xu, Q.; Yao, Y.; Gao, H. Biocontrol of postharvest Rhizopus decay of peaches with Pichia caribbica. Curr. Microbiol. 2013, 67, 255–261. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Zheng, Q.; Xu, B.; Liu, J. Identification of the fungal pathogens of postharvest disease on peach fruits and the control mechanisms of Bacillus subtilis JK-14. Toxins 2019, 11, 322. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Z.; Sui, Y.; Li, J.; Tian, X.; Wang, Q. Biological control of postharvest fungal decays in citrus: A review. Crit. Rev. Food Sci. Nutr. 2022, 62, 861–870. [Google Scholar] [CrossRef]
- Zhang, H.; Zheng, X.; Yu, T. Biological control of postharvest diseases of peach with Cryptococcus laurentii. Food Control 2007, 18, 287–291. [Google Scholar] [CrossRef]
- Zhang, D.; Spadaro, D.; Garibaldi, A.; Gullino, M.L. Efficacy of the antagonist Aureobasidium pullulans PL5 against postharvest pathogens of peach, apple and plum and its modes of action. Biol. Control 2010, 54, 172–180. [Google Scholar] [CrossRef]
- Khan, N.; Bhat, Z.; Bhat, M. Diseases of Stone Fruit Crops. In Production Technology of Stone Fruits; Springer: Berlin/Heidelberg, Germany, 2021; pp. 359–395. [Google Scholar]
- Martínez-Blay, V.; Taberner, V.; Pérez-Gago, M.B.; Palou, L. Postharvest treatments with sulfur-containing food additives to control major fungal pathogens of stone fruits. Foods 2021, 10, 2115. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Li, B.; Zhang, Z.; Chen, Y.; Tian, S. Antagonistic yeasts: A promising alternative to chemical fungicides for controlling postharvest decay of fruit. J. Fungi 2020, 6, 158. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Yang, Q.; Zhao, Q.; Dhanasekaran, S.; Ahima, J.; Zhang, X.; Zhou, S.; Droby, S.; Zhang, H. Aureobasidium pullulans S-2 reduced the disease incidence of tomato by influencing the postharvest microbiome during storage. Postharvest Biol. Technol. 2022, 185, 111809. [Google Scholar] [CrossRef]
- Rueda-Mejia, M.P.; Nägeli, L.; Lutz, S.; Hayes, R.D.; Varadarajan, A.R.; Grigoriev, I.V.; Ahrens, C.H.; Freimoser, F.M. Genome, transcriptome and secretome analyses of the antagonistic, yeast-like fungus Aureobasidium pullulans to identify potential biocontrol genes. Microb. Cell 2021, 8, 184. [Google Scholar] [CrossRef]
- Bernat, M.; Segarra, J.; Xu, X.-M.; Casals, C.; Usall, J. Influence of temperature on decay, mycelium development and sporodochia production caused by Monilinia fructicola and M. laxa on stone fruits. Food Microbiol. 2017, 64, 112–118. [Google Scholar] [CrossRef] [Green Version]
- Di Francesco, A.; Mari, M.; Ugolini, L.; Baraldi, E. Effect of Aureobasidium pullulans strains against Botrytis cinerea on kiwifruit during storage and on fruit nutritional composition. Food Microbiol. 2018, 72, 67–72. [Google Scholar] [CrossRef] [PubMed]
- Abbey, J.A.; Percival, D.; Abbey, L.; Asiedu, S.K.; Prithiviraj, B.; Schilder, A. Biofungicides as alternative to synthetic fungicide control of grey mould (Botrytis cinerea)–prospects and challenges. Biocontrol Sci. Technol. 2019, 29, 207–228. [Google Scholar] [CrossRef]
- Zhang, H.; Godana, E.A.; Sui, Y.; Yang, Q.; Zhang, X.; Zhao, L. Biological control as an alternative to synthetic fungicides for the management of grey and blue mould diseases of table grapes: A review. Crit. Rev. Microbiol. 2020, 46, 450–462. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Cao, S.; Cai, Y.; Zheng, Y. Combination of salicylic acid and ultrasound to control postharvest blue mold caused by Penicillium expansum in peach fruit. Innov. Food Sci. Emerg. Technol. 2011, 12, 310–314. [Google Scholar] [CrossRef]
- Cao, S.; Hu, Z.; Zheng, Y.; Lu, B. Synergistic effect of heat treatment and salicylic acid on alleviating internal browning in cold-stored peach fruit. Postharvest Biol. Technol. 2010, 58, 93–97. [Google Scholar] [CrossRef]
- Fan, K.; Wu, J.; Chen, L. Ultrasound and its combined application in the improvement of microbial and physicochemical quality of fruits and vegetables: A review. Ultrason. Sonochemistry 2021, 80, 105838. [Google Scholar] [CrossRef]
- Wisniewski, M.; Droby, S.; Norelli, J.; Liu, J.; Schena, L. Alternative management technologies for postharvest disease control: The journey from simplicity to complexity. Postharvest Biol. Technol. 2016, 122, 3–10. [Google Scholar] [CrossRef]
- Zhao, B.; Hu, S.; Wang, D.; Chen, H.; Huang, M. Inhibitory effect of gamma irradiation on Penicillium digitatum and its application in the preservation of Ponkan fruit. Sci. Hortic. 2020, 272, 109598. [Google Scholar] [CrossRef]
- Panahirad, S.; Dadpour, M.; Peighambardoust, S.H.; Soltanzadeh, M.; Gullón, B.; Alirezalu, K.; Lorenzo, J.M. Applications of carboxymethyl cellulose-and pectin-based active edible coatings in preservation of fruits and vegetables: A review. Trends Food Sci. Technol. 2021, 110, 663–673. [Google Scholar] [CrossRef]
- Jafarzadeh, S.; Nafchi, A.M.; Salehabadi, A.; Oladzad-Abbasabadi, N.; Jafari, S.M. Application of bio-nanocomposite films and edible coatings for extending the shelf life of fresh fruits and vegetables. Adv. Colloid Interface Sci. 2021, 291, 102405. [Google Scholar] [CrossRef]
- Al-Tayyar, N.A.; Youssef, A.M.; Al-Hindi, R.R. Edible coatings and antimicrobial nanoemulsions for enhancing shelf life and reducing foodborne pathogens of fruits and vegetables: A review. Sustain. Mater. Technol. 2020, 26, e00215. [Google Scholar] [CrossRef]
- Hussain, P.R.; Suradkar, P.P.; Wani, A.M.; Dar, M.A. Potential of carboxymethyl cellulose and γ-irradiation to maintain quality and control disease of peach fruit. Int. J. Biol. Macromol. 2016, 82, 114–126. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Fu, C.; Zheng, X.; Xi, Y.; Jiang, W.; Wang, Y. Control of postharvest Rhizopus rot of peach by microwave treatment and yeast antagonist. Eur. Food Res. Technol. 2004, 218, 568–572. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, L.; Zheng, X.; Dong, Y. Effect of yeast antagonist in combination with heat treatment on postharvest blue mold decay and Rhizopus decay of peaches. Int. J. Food Microbiol. 2007, 115, 53–58. [Google Scholar] [CrossRef]
- Zhang, H.; Ge, L.; Ma, L.; Chen, K.; Jiang, S. Effects of microwave treatment on postharvest decay of fruits: A review. Stewart Postharvest Rev. 2009, 5, 1–7. [Google Scholar]
- Sisquella, M.; Viñas, I.; Teixidó, N.; Picouet, P.; Usall, J. Continuous microwave treatment to control postharvest brown rot in stone fruit. Postharvest Biol. Technol. 2013, 86, 1–7. [Google Scholar] [CrossRef]
- Zhang, H.; Ma, L.; Wang, L.; Jiang, S.; Dong, Y.; Zheng, X. Biocontrol of gray mold decay in peach fruit by integration of antagonistic yeast with salicylic acid and their effects on postharvest quality parameters. Biol. Control 2008, 47, 60–65. [Google Scholar] [CrossRef]
| Category | Examples of the Factors |
|---|---|
| Stability of fruit quality | Weight, volume, length, width or diameter, total soluble solid (TSS), soluble solid content (SSC), titratable acidity (TA), ethylene emission (production), malondialdehyde (MDA) content, firmness, color, vitamin C content, pectin content |
| Microbial deterioration and damage | Infected wounds, decay, disease incidence, lesion diameter |
| Antioxidant capacity | Antioxidants content (phenolics, flavonoids), activity of enzymes (catalase (CAT), superoxide dismutase (SOD), ascorbate peroxidase (APX), peroxidase (POD), pectin methyl esterase (PME), phenylalanine ammonia-lyase (PAL), enzymes of ascorbate-glutathione (AsA-GSH) cycle, polyphenol oxidase (PPO), lipoxygenase (LOX)) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shin, J.S.; Park, H.S.; Lee, K.W.; Song, J.S.; Han, H.Y.; Kim, H.W.; Cho, T.J. Advances in the Strategic Approaches of Pre- and Post-Harvest Treatment Technologies for Peach Fruits (Prunus persica). Horticulturae 2023, 9, 315. https://doi.org/10.3390/horticulturae9030315
Shin JS, Park HS, Lee KW, Song JS, Han HY, Kim HW, Cho TJ. Advances in the Strategic Approaches of Pre- and Post-Harvest Treatment Technologies for Peach Fruits (Prunus persica). Horticulturae. 2023; 9(3):315. https://doi.org/10.3390/horticulturae9030315
Chicago/Turabian StyleShin, Jin Song, Han Sol Park, Ki Won Lee, Ji Seop Song, Hea Yeon Han, Hye Won Kim, and Tae Jin Cho. 2023. "Advances in the Strategic Approaches of Pre- and Post-Harvest Treatment Technologies for Peach Fruits (Prunus persica)" Horticulturae 9, no. 3: 315. https://doi.org/10.3390/horticulturae9030315
APA StyleShin, J. S., Park, H. S., Lee, K. W., Song, J. S., Han, H. Y., Kim, H. W., & Cho, T. J. (2023). Advances in the Strategic Approaches of Pre- and Post-Harvest Treatment Technologies for Peach Fruits (Prunus persica). Horticulturae, 9(3), 315. https://doi.org/10.3390/horticulturae9030315












