Effects of Exogenously Applied Copper in Tomato Plants’ Oxidative and Nitrogen Metabolisms under Organic Farming Conditions
Abstract
1. Introduction
2. Materials and Methods
2.1. Copper Quantification
2.2. Plant Material and Copper Exposition
2.3. Nitrate Quantification
2.4. Protein Extraction and Quantification
2.5. Nitrate Reductase Quantification
2.6. Glutamine Synthetase Quantification
2.7. Glutamate Dehydrogenase Quantification
2.8. Proline Quantification
2.9. Hydrogen Peroxide Quantification
2.10. Ascorbate Quantification
2.11. Semi-Quantitative Polymerase Chain Reaction (PCR)
2.12. Statistical Analysis
3. Results
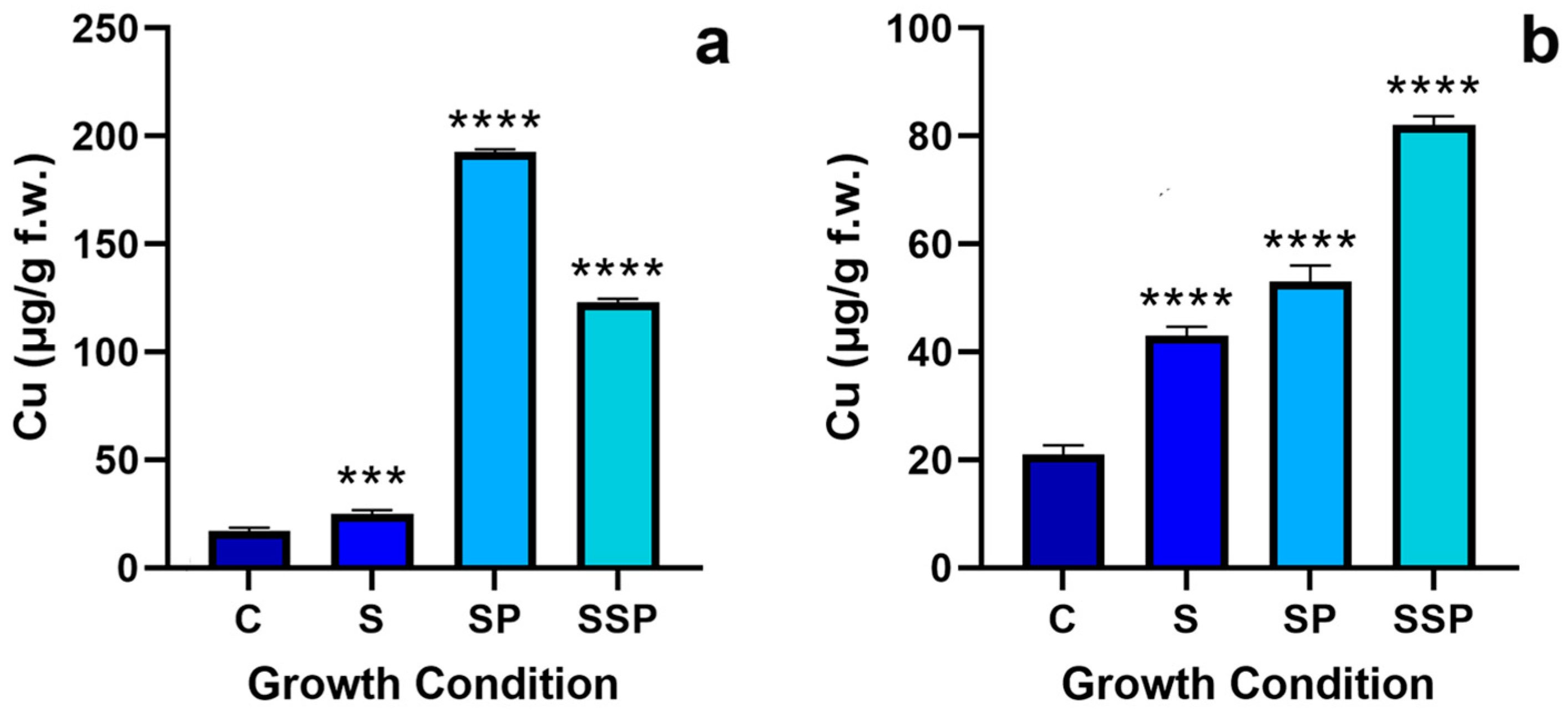
3.1. Copper Quantification
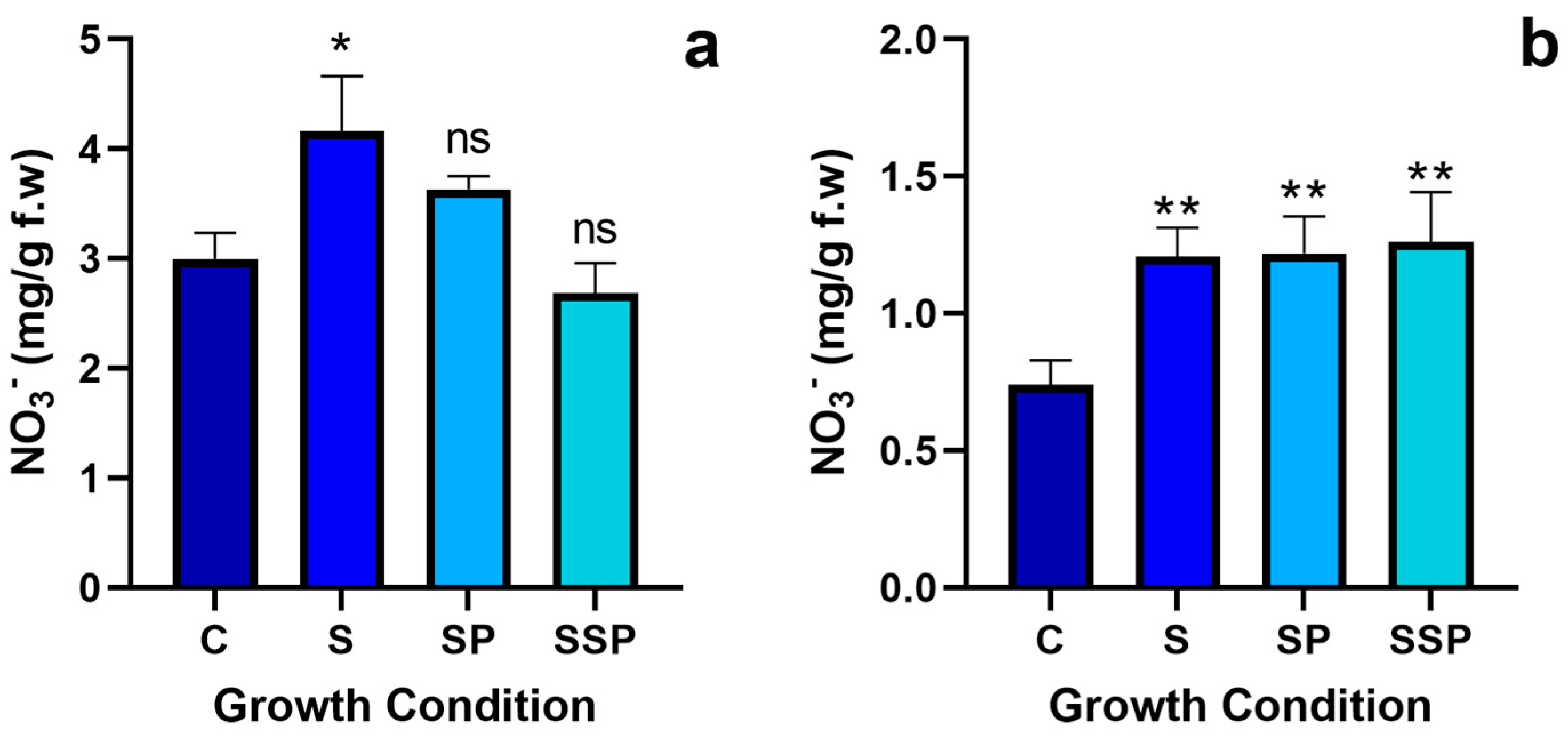
3.2. Nitrate Quantification
3.3. Nitrate Reductase Activity
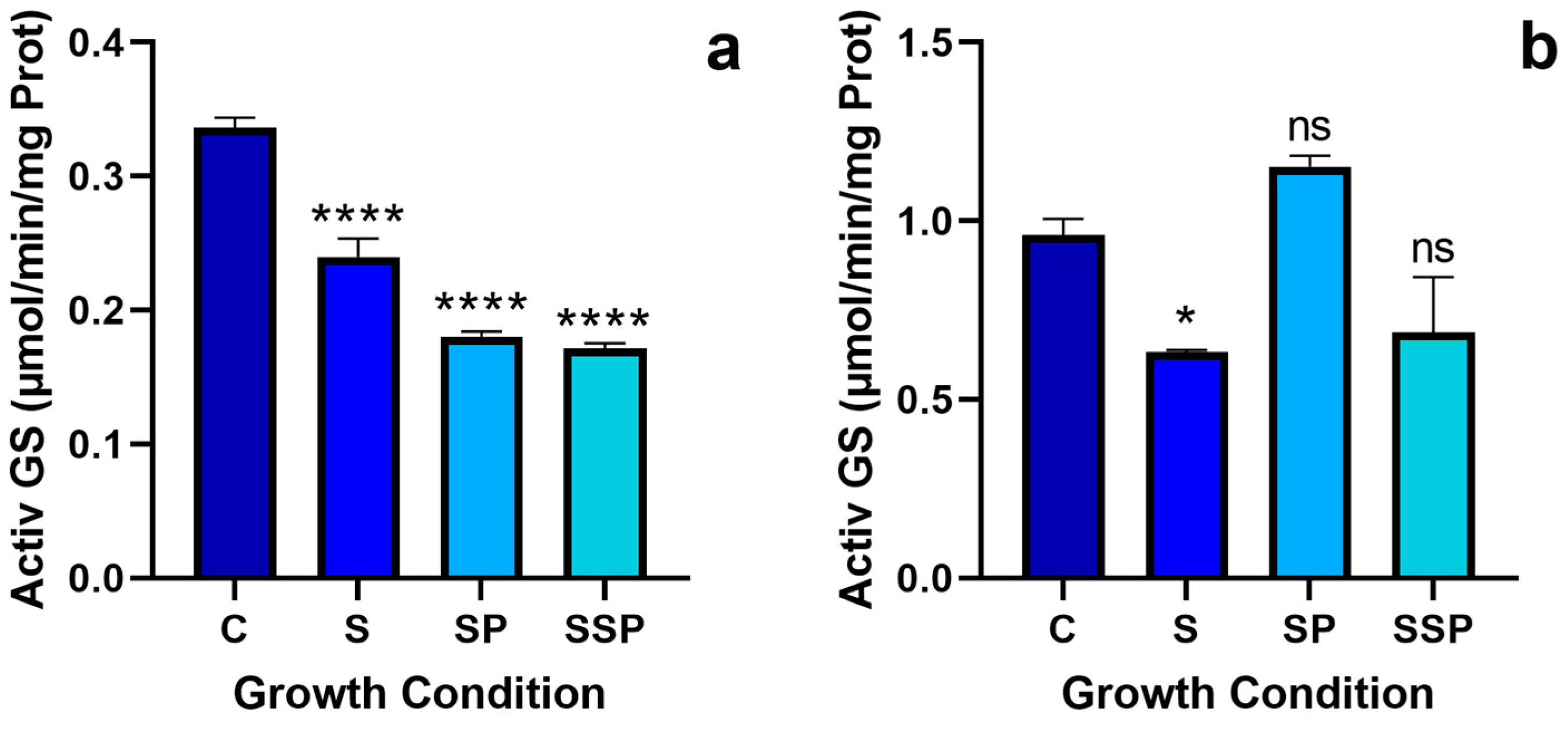
3.4. Glutamine Synthetase Activity
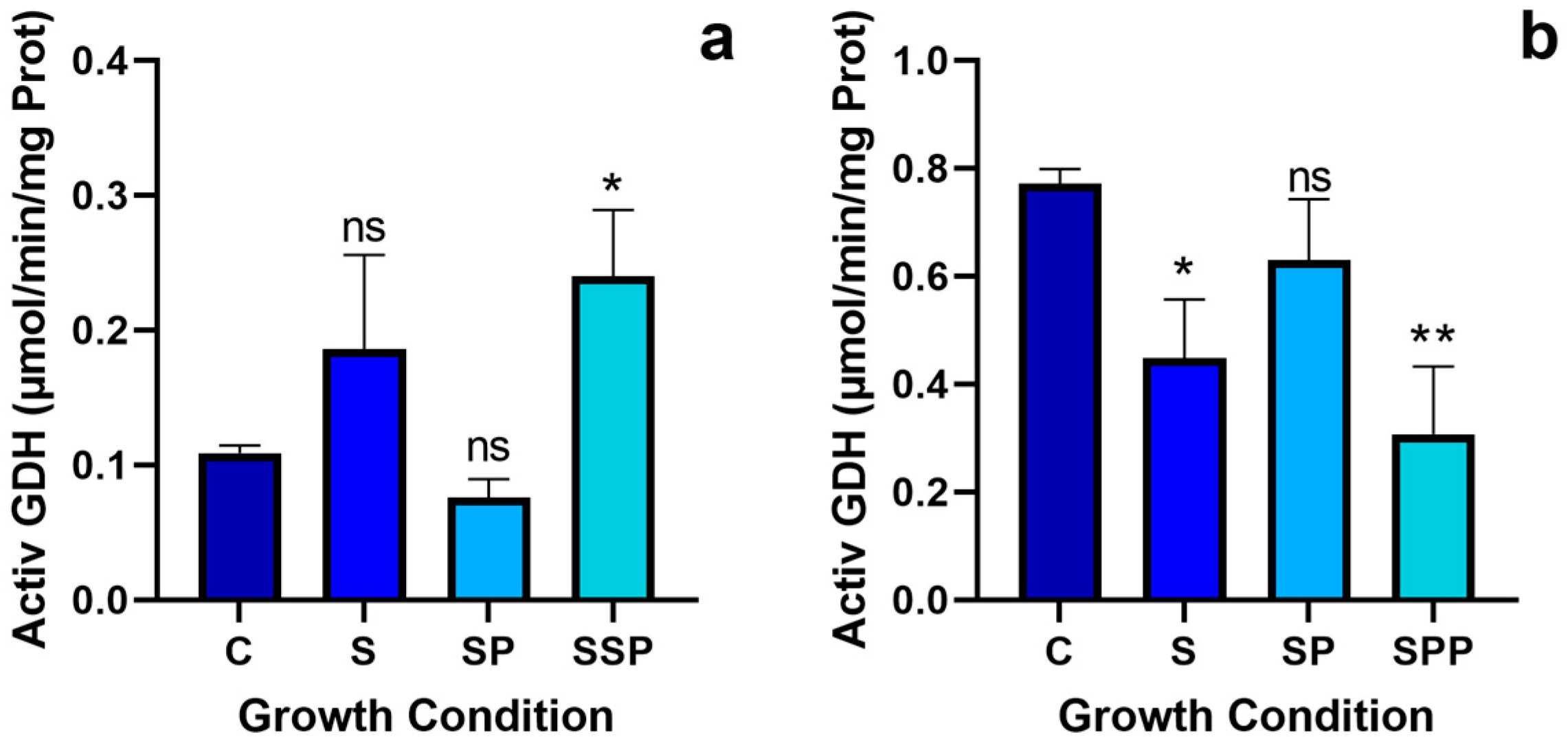
3.5. Glutamate Dehydrogenase Activity
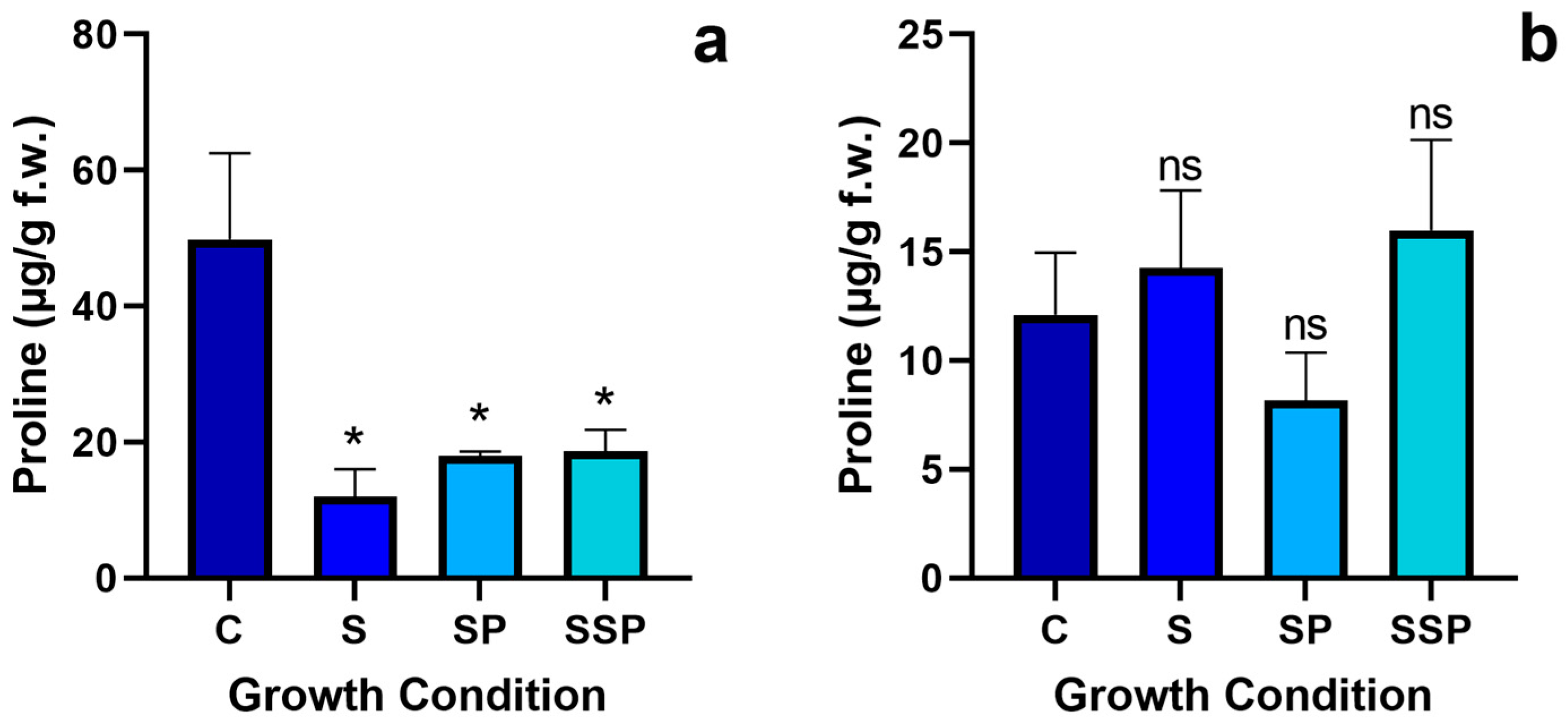
3.6. Proline Levels
3.7. Hydrogen Peroxide Levels
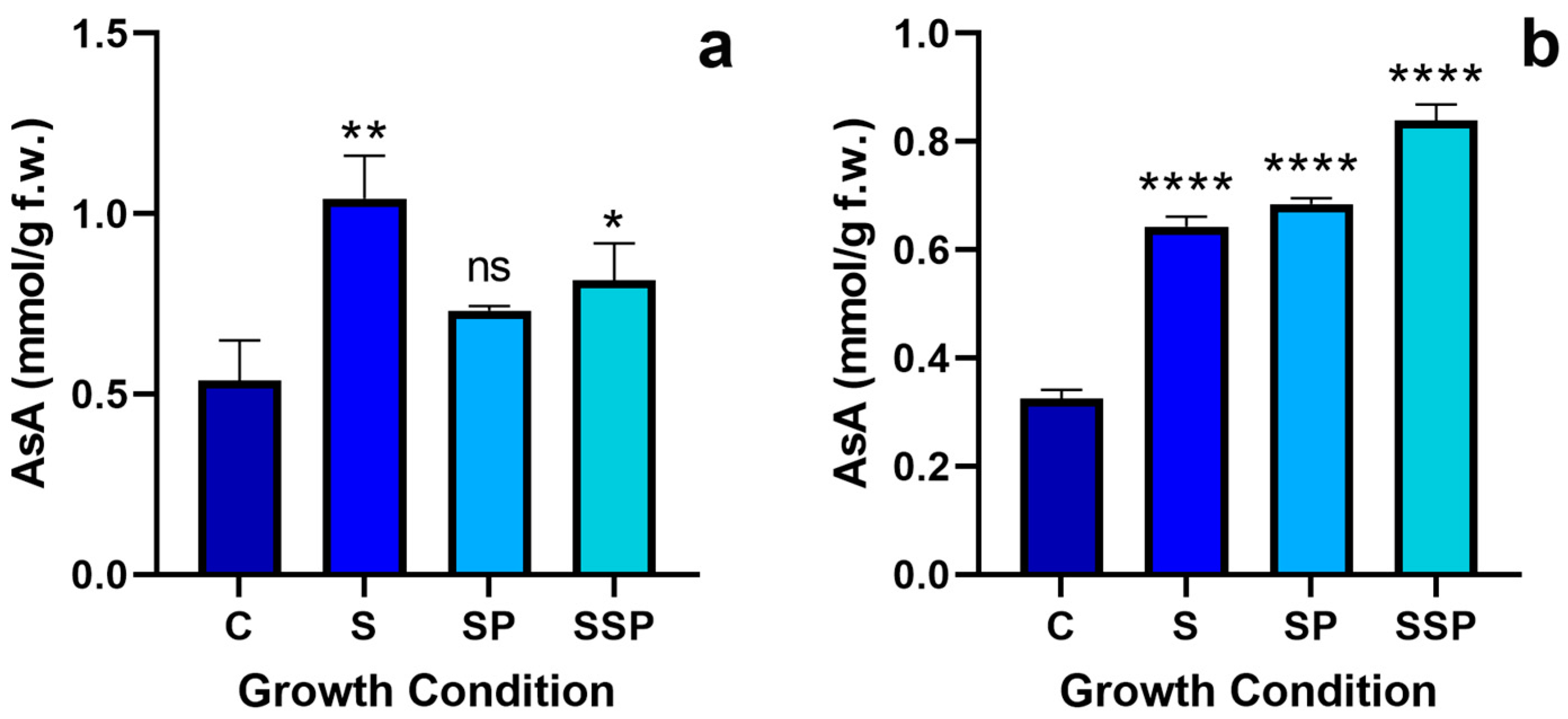
3.8. Ascorbate Quantification
3.8.1. Total Ascorbate Quantification
3.8.2. Reduced Ascorbate Quantification
3.8.3. Oxidised Ascorbate
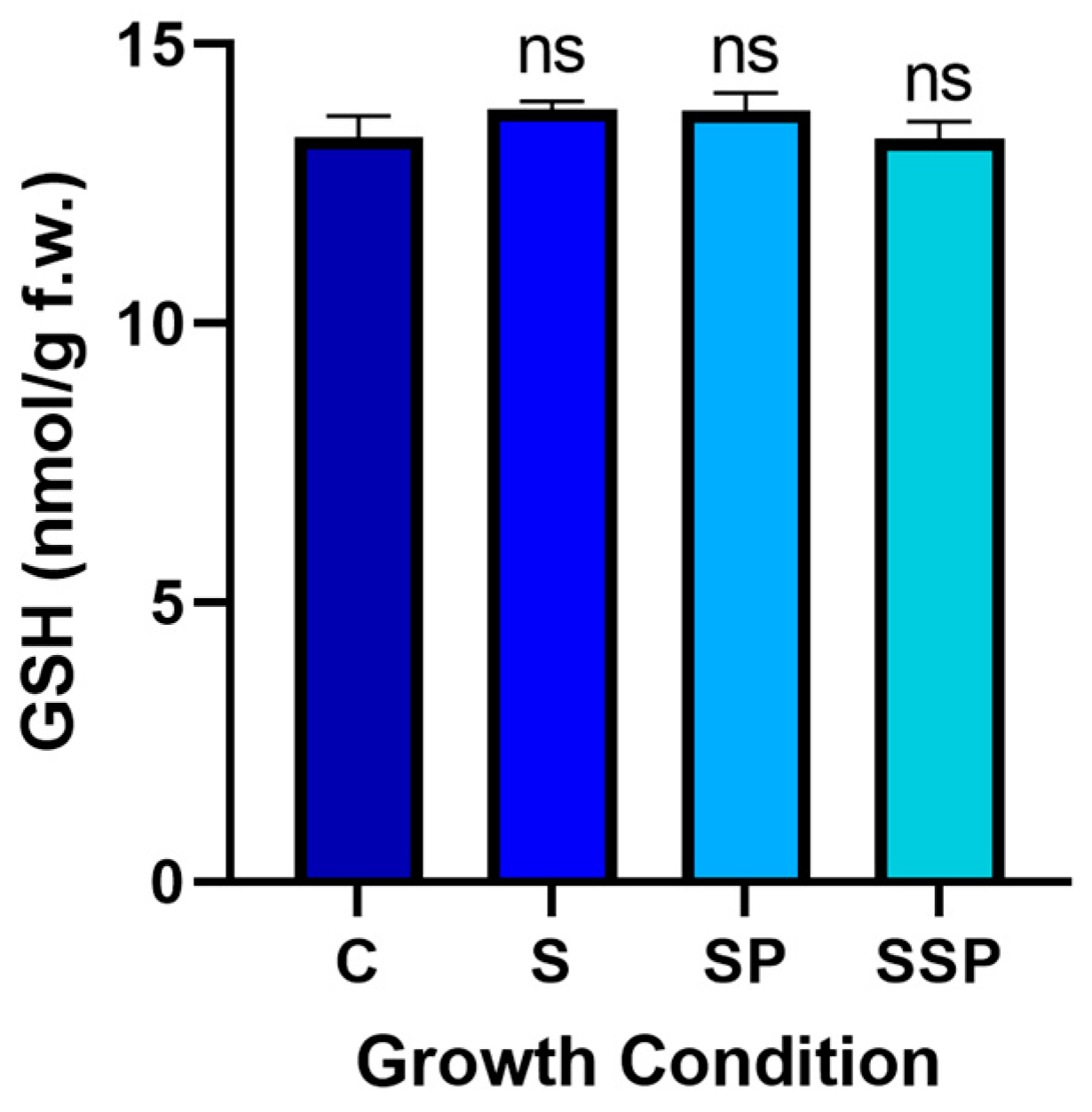
3.9. Glutathione Levels
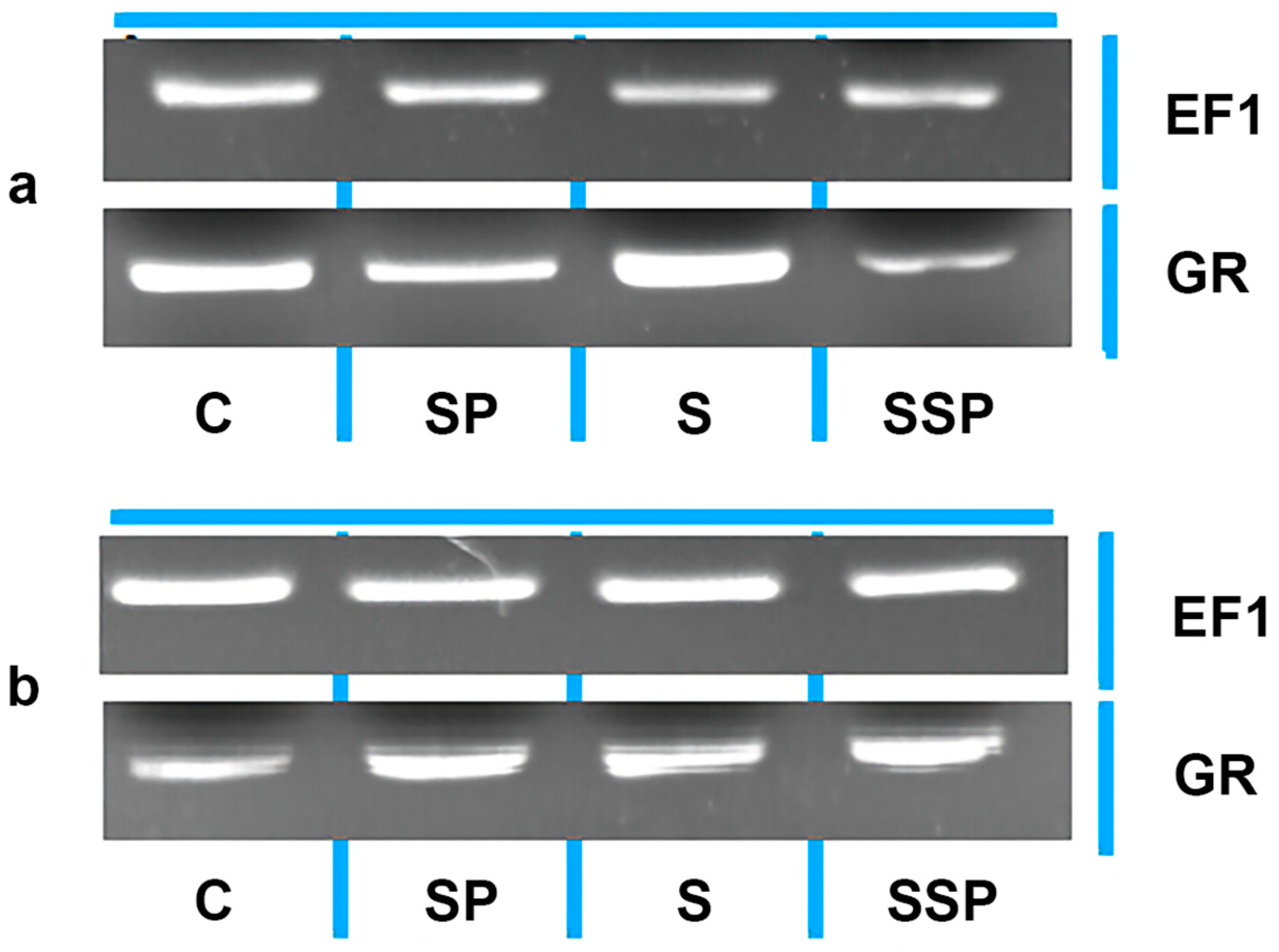
3.10. Semi-Quantitative Polymerase Chain Reaction (PCR) for GR
4. Discussion
4.1. Copper’s Effect on Nitrogen Use Efficiency
4.2. Copper’s Effect on the Ascorbate–Glutathione Pathway
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAOSTAT. Available online: https://www.fao.org/faostat/en/#data/QV (accessed on 10 January 2022).
- Eurostat. Key Figures on the European Food Chain; Eutostat: Luxembourg, 2021; pp. 1–105. [Google Scholar] [CrossRef]
- Panthee, D.R.; Chen, F. Genomics of Fungal Disease Resistance in Tomato. Curr. Genom. 2009, 11, 30–39. [Google Scholar] [CrossRef]
- Gatahi, D.M. Challenges and Opportunities in Tomato Production Chain and Sustainable Standards. Int. J. Hortic. Sci. Technol. 2020, 7, 235–262. [Google Scholar] [CrossRef]
- Lamichhane, J.R.; Osdaghi, E.; Behlau, F.; Köhl, J.; Jones, J.B.; Aubertot, J.N. Thirteen Decades of Antimicrobial Copper Compounds Applied in Agriculture. A Review. Agron. Sustain. Dev. 2018, 38, 28. [Google Scholar] [CrossRef]
- La Torre, A.; Iovino, V.; Caradonia, F. Copper in Plant Protection: Current Situation and Prospects. Phytopathol. Mediterr. 2018, 57, 201–236. [Google Scholar] [CrossRef]
- Ballabio, C.; Panagos, P.; Lugato, E.; Huang, J.-H.; Orgiazzi, A.; Jones, A.; Fernández-Ugalde, O.; Borrelli, P.; Montanarella, L. Copper Distribution in European Topsoils: An Assessment Based on LUCAS Soil Survey. Sci. Total Environ. 2018, 636, 282–298. [Google Scholar] [CrossRef]
- Kühne, S.; Strassemeyer, J.; Rossberg, D. Anwendung Kupferhaltiger Pflanzenschutzmittel in Deutschland. Nachr. Dtsch. Pflanz. 2009, 61, 126–130. [Google Scholar]
- Speiser, B.; Schärer, H.-J.; Tamm, L. Direct Plant Protection in Organic Farming. In Improving Organic Crop Cultivation; Burleigh Dodds Science Publishing: Cambridge, UK, 2018; pp. 1–21. ISBN 178676184X. [Google Scholar]
- Vavoulidou, E.; Avramides, E.J.; Papadopoulos, P.; Dimirkou, A.; Charoulis, A.; Konstantinidou-Doltsinis, S. Copper Content in Agricultural Soils Related to Cropping Systems in Different Regions of Greece. Commun. Soil Sci. Plant Anal. 2005, 36, 759–773. [Google Scholar] [CrossRef]
- Chaignon, V.; Sanchez-Neira, I.; Herrmann, P.; Jaillard, B.; Hinsinger, P. Copper Bioavailability and Extractability as Related to Chemical Properties of Contaminated Soils from a Vine-Growing Area. Environ. Pollut. 2003, 123, 229–238. [Google Scholar] [CrossRef]
- Fernández-Calviño, D.; Nóvoa-Muñoz, J.C.; López-Periago, E.; Arias-Estéves, M. Changes in Copper Content and Distribution in Young, Old and Abandoned Vineyard Acid Soils Due to Land Use Changes. Land Degrad. Dev. 2008, 19, 165–177. [Google Scholar] [CrossRef]
- Mirlean, N.; Roisenberg, A.; Chies, J.O. Metal Contamination of Vineyard Soils in Wet Subtropics (Southern Brazil). Environ. Pollut. 2007, 149, 10–17. [Google Scholar] [CrossRef]
- Deluisa, A.; Giandon, P.; Aichner, M.; Bortolami, P.; Bruna, L.; Lupetti, A.; Nardelli, F.; Stringari, G. Copper Pollution in Italian Vineyard Soils. Commun. Soil Sci. Plant Anal. 2008, 27, 1537–1548. [Google Scholar] [CrossRef]
- Gessler, C.; Pertot, I.; Perazzolli, M. Plasmopara Viticola: A Review of Knowledge on Downy Mildew of Grapevine and Effective Disease Management. Phytopathol. Mediterr. 2011, 50, 3–44. [Google Scholar]
- Tamm, L.; Thuerig, B.; Apostolov, S.; Blogg, H.; Borgo, E.; Corneo, P.E.; Fittje, S.; de Palma, M.; Donko, A.; Experton, C.; et al. Use of Copper-Based Fungicides in Organic Agriculture in Twelve European Countries. Agronomy 2022, 12, 673. [Google Scholar] [CrossRef]
- Kandeler, F.; Kampichler, C.; Horak, O. Influence of Heavy Metals on the Functional Diversity of Soil Microbial Communities. Biol. Fertil. Soils 1996, 23, 299–306. [Google Scholar] [CrossRef]
- Tugbaeva, A.; Ermoshin, A.; Wuriyanghan, H.; Maleva, M.; Borisova, G.; Kiseleva, I. Copper Stress Enhances the Lignification of Axial Organs in Zinnia Elegans. Horticulturae 2022, 8, 558. [Google Scholar] [CrossRef]
- European Commission. Commission Implementing Regulation (EU) 2018/1981—Of 13 December 2018—Renewing the Approval of the Active Substances Copper Compounds, as Candidates for Substitution, in Accordance with Regulation (EC) No 1107/2009 of the European Parliament and of the Council Concerning the Placing of Plant Protection Products on the Market, and Amending the Annex to Commission Implementing Regulation (EU) No 540/2011. Off. J. Eur. Union 2018, 317, 1–5. [Google Scholar]
- European Commission. Commission Regulation (EC) No 889/2008 of 5 September 2008 Laying down Detailed Rules for the Implementation of Council Regulation (EC) No 834/2007 on Organic Production and Labelling of Organic Products with Regard to Organic Production, Labelling and Co. Off. J. Eur. Union 2008, 250, 1–84. [Google Scholar]
- EFSA Panel on Additives and Products or Substances used in Animal Feed. Revision of the Currently Authorised Maximum Copper Content in Complete Feed. EFSA J. 2016, 14, e04563. [Google Scholar] [CrossRef]
- Clarkson, D.T.; Hanson, J.B. The Mineral Nutrition of Higher Plants. Annu. Rev. Plant Physiol. 1980, 31, 239–298. [Google Scholar] [CrossRef]
- Alaoui-Sossé, B.; Genet, P.; Vinit-Dunand, F.; Toussaint, M.L.; Epron, D.; Badot, P.M. Effect of Copper on Growth in Cucumber Plants (Cucumis sativus) and Its Relationships with Carbohydrate Accumulation and Changes in Ion Contents. Plant Sci. 2004, 166, 1213–1218. [Google Scholar] [CrossRef]
- Navari-Izzo, F.; Cestone, B.; Cavallini, A.; Natali, L.; Giordani, T.; Quartacci, M.F. Copper Excess Triggers Phospholipase D Activity in Wheat Roots. Phytochemistry 2006, 67, 1232–1242. [Google Scholar] [CrossRef] [PubMed]
- Bernal, M.; Ramiro, M.V.; Cases, R.; Picorel, R.; Yruela, I. Excess Copper Effect on Growth, Chloroplast Ultrastructure, Oxygen-Evolution Activity and Chlorophyll Fluorescence in Glycine Max Cell Suspensions. Physiol. Plant 2006, 127, 312–325. [Google Scholar] [CrossRef]
- Yruela, I.; Pueyo, J.J.; Alonso, P.J.; Picorel, R. Photoinhibition of Photosystem II from Higher Plants. J. Biol. Chem. 1996, 271, 27408–27415. [Google Scholar] [CrossRef]
- Saleem, M.H.; Ali, S.; Irshad, S.; Hussaan, M.; Rizwan, M.; Rana, M.S.; Hashem, A.; Abd_allah, E.F.; Ahmad, P. Copper Uptake and Accumulation, Ultra-Structural Alteration, and Bast Fibre Yield and Quality of Fibrous Jute (Corchorus capsularis L.) Plants Grown under Two Different Soils of China. Plants 2020, 9, 404. [Google Scholar] [CrossRef] [PubMed]
- Chrysargyris, A.; Papakyriakou, E.; Petropoulos, S.A.; Tzortzakis, N. The Combined and Single Effect of Salinity and Copper Stress on Growth and Quality of Mentha Spicata Plants. J. Hazard. Mater. 2019, 368, 584–593. [Google Scholar] [CrossRef]
- Pádua, M.; Aubert, S.; Casimiro, A.; Bligny, R.; Millar, A.H.; Day, D.A. Induction of Alternative Oxidase by Excess Copper in Sycamore Cell Suspensions. Plant Physiol. Biochem. 1999, 37, 131–137. [Google Scholar] [CrossRef]
- Nazir, F.; Hussain, A.; Fariduddin, Q. Hydrogen Peroxide Modulate Photosynthesis and Antioxidant Systems in Tomato (Solanum lycopersicum L.) Plants under Copper Stress. Chemosphere 2019, 230, 544–558. [Google Scholar] [CrossRef]
- Hippler, F.W.R.; Mattos-Jr, D.; Boaretto, R.M.; Williams, L.E. Copper Excess Reduces Nitrate Uptake by Arabidopsis Roots with Specific Effects on Gene Expression. J. Plant Physiol. 2018, 228, 158–165. [Google Scholar] [CrossRef]
- Mcallister, C.H.; Beatty, P.H.; Good, A.G. Engineering Nitrogen Use Efficient Crop Plants: The Current Status. Plant Biotechnol. J. 2012, 10, 1011–1025. [Google Scholar] [CrossRef]
- Zhang, Z.; Xiong, S.; Wei, Y.; Meng, X.; Wang, X.; Ma, X. The Role of Glutamine Synthetase Isozymes in Enhancing Nitrogen Use Efficiency of N-Efficient Winter Wheat. Sci. Rep. 2017, 7, 1000. [Google Scholar] [CrossRef]
- Chattha, M.S.; Ali, Q.; Haroon, M.; Afzal, M.J.; Javed, T.; Hussain, S.; Mahmood, T.; Solanki, M.K.; Umar, A.; Abbas, W.; et al. Enhancement of Nitrogen Use Efficiency through Agronomic and Molecular Based Approaches in Cotton. Front. Plant Sci. 2022, 13, 1–24. [Google Scholar] [CrossRef]
- Rajput, V.D.; Harish; Singh, R.K.; Verma, K.K.; Sharma, L.; Quiroz-Figueroa, F.R.; Meena, M.; Gour, V.S.; Minkina, T.; Sushkova, S.; et al. Recent Developments in Enzymatic Antioxidant Defence Mechanism in Plants with Special Reference to Abiotic Stress. Biology 2021, 10, 267. [Google Scholar] [CrossRef]
- Apel, K.; Hirt, H. Reactive Oxygen Species: Metabolism, Oxidative Stress, and Signal Transduction. Annu. Rev. Plant Biol. 2004, 55, 373–399. [Google Scholar] [CrossRef] [PubMed]
- Miotto, A.; Ceretta, C.A.; Brunetto, G.; Nicoloso, F.T.; Girotto, E.; Farias, J.G.; Tiecher, T.L.; De Conti, L.; Trentin, G. Copper Uptake, Accumulation and Physiological Changes in Adult Grapevines in Response to Excess Copper in Soil. Plant Soil 2014, 374, 593–610. [Google Scholar] [CrossRef]
- Martins, M.L.R. Metabolic Pinpointing of Solanum lycopersicum L. Response to High Zn Levels—The Relevance of Metallothioneins, GSH Metabolism and the Antioxidant System. Master’s Thesis, Faculdade de Ciências da Universidade do Porto, Porto, Portugal, 2017. [Google Scholar]
- Taiz, L.; Zeiger, E. Plant Physiology and Development; Sinauer Associates Incorporated: Sunderland, MA, USA, 2019; Volume 53, ISBN 9788578110796. [Google Scholar]
- Bradford, M.M. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Martins, M.; Lopes, J.; Sousa, B.; Soares, C.; Valente, I.M.; Rodrigues, J.A.; Fidalgo, F.; Teixeira, J. Cr (VI)-Induced Oxidative Damage Impairs Ammonia Assimilation into Organic Forms in Solanum lycopersicum L. Plant Stress 2021, 2, 100034. [Google Scholar] [CrossRef]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid Determination of Free Proline for Water-Stress Studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Gillespie, K.M.; Ainsworth, E.A. Measurement of Reduced, Oxidized and Total Ascorbate Content in Plants. Nat. Protoc. 2007, 2, 871–874. [Google Scholar] [CrossRef]
- Løvdal, T.; Lillo, C. Reference Gene Selection for Quantitative Real-Time PCR Normalization in Tomato Subjected to Nitrogen, Cold, and Light Stress. Anal. Biochem. 2009, 387, 238–242. [Google Scholar] [CrossRef]
- Good, A.G.; Shrawat, A.K.; Muench, D.G. Can Less Yield More? Is Reducing Nutrient Input into the Environment Compatible with Maintaining Crop Production? Trends Plant Sci. 2004, 9, 597–605. [Google Scholar] [CrossRef]
- Zhang, L.L.; He, X.-J.; Chen, M.; An, R.-D.; An, X.-L.; Li, J. Responses of Nitrogen Metabolism to Copper Stress in Luffa Cylindrica Roots. J. Soil Sci. Plant Nutr. 2014, 14, 616–624. [Google Scholar] [CrossRef]
- Huo, K.; Shangguan, X.; Xia, Y.; Shen, Z.; Chen, C. Excess Copper Inhibits the Growth of Rice Seedlings by Decreasing Uptake of Nitrate. Ecotoxicol. Environ. Saf. 2020, 190, 110105. [Google Scholar] [CrossRef] [PubMed]
- Burzyński, M.; Burzyński, M. The Uptake and Accumulation of Phosphorous and Nitrates and the Activity of Nitrate Reductase in Cucumber Seedlings Treated with PbCl2 or CdCl2. Acta Soc. Bot. Pol. 2014, 57, 349–359. [Google Scholar] [CrossRef]
- Xiong, Z.-T.; Liu, C.; Geng, B. Phytotoxic Effects of Copper on Nitrogen Metabolism and Plant Growth in Brassica Pekinensis Rupr. Ecotoxicol. Environ. Saf. 2006, 64, 273–280. [Google Scholar] [CrossRef] [PubMed]
- Vajpayee, P.; Tripathi, R.D.; Rai, U.N.; Ali, M.B.; Singh, S.N. Chromium (VI) Accumulation Reduces Chlorophyll Biosynthesis, Nitrate Reductase Activity and Protein Content in Nymphaea alba L. Chemosphere 2000, 41, 1075–1082. [Google Scholar] [CrossRef]
- Hussain, S.; Khaliq, A.; Noor, M.A.; Tanveer, M.; Hussain, H.A.; Hussain, S.; Shah, T.; Mehmood, T. Metal Toxicity and Nitrogen Metabolism in Plants: An Overview. In Carbon and Nitrogen Cycling in Soil; Springer: Singapore, 2020; pp. 221–248. [Google Scholar]
- Yu, C.C.; Hung, K.T.; Kao, C.H. Nitric Oxide Reduces Cu Toxicity and Cu-Induced NH4+ Accumulation in Rice Leaves. J. Plant Physiol. 2005, 162, 1319–1330. [Google Scholar] [CrossRef]
- Miflin, B.J.; Lea, P.J.; Wallsgrove, R.M. The Role of Glutamine in Ammonia Assimilation and Reassimilation in Plants. In Glutamine: Metabolism, Enzymology, and Regulation; Elsevier: Amsterdam, The Netherlands, 1980; pp. 213–234. [Google Scholar]
- Burzyński, M.; Buczek, J. Uptake and Assimilation of Ammonium Ions by Cucumber Seedlings from Solutions with Different PH and Addition of Heavy Metals. Acta Soc. Bot. Pol. 1998, 67, 197–200. [Google Scholar] [CrossRef]
- Chaffei, C.; Pageau, K.; Suzuki, A.; Gouia, H.; Ghorbel, M.H.; Masclaux-Daubresse, C. Cadmium Toxicity Induced Changes in Nitrogen Management in Lycopersicon esculentum Leading to a Metabolic Safeguard Through an Amino Acid Storage Strategy. Plant Cell Physiol. 2004, 45, 1681–1693. [Google Scholar] [CrossRef]
- Jha, A.B.; Dubey, R.S. Arsenic Exposure Alters Activity Behaviour of Key Nitrogen Assimilatory Enzymes in Growing Rice Plants. Plant Growth Regul. 2004, 43, 259–268. [Google Scholar] [CrossRef]
- Miflin, B.J.; Habash, D.Z. The Role of Glutamine Synthetase and Glutamate Dehydrogenase in Nitrogen Assimilation and Possibilities for Improvement in the Nitrogen Utilization of Crops. J. Exp. Bot. 2002, 53, 979–987. [Google Scholar] [CrossRef]
- Gouia, H.; Habib Ghorbal, M.; Meyer, C. Effects of Cadmium on Activity of Nitrate Reductase and on Other Enzymes of the Nitrate Assimilation Pathway in Bean. Plant Physiol. Biochem. 2000, 38, 629–638. [Google Scholar] [CrossRef]
- Boussama, N.; Ouariti, O.; Suzuki, A.; Ghorbal, M.H. Cd-Stress on Nitrogen Assimilation. J. Plant Physiol. 1999, 155, 310–317. [Google Scholar] [CrossRef]
- Mittal, S.; Sawhney, S.K. Influence of Lead on Enzymes of Nitrogen Metabolism in Germinating Pea Seeds. Plant Physiol. Biochem. 1990, 17, 75–81. [Google Scholar]
- Szabados, L.; Savouré, A. Proline: A Multifunctional Amino Acid. Trends Plant Sci. 2010, 15, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.K.; Tewari, R.K. Cadmium Toxicity Induced Changes in Plant Water Relations and Oxidative Metabolism of Brassica juncea L. Plants. J. Environ. Biol. 2003, 24, 107–112. [Google Scholar] [PubMed]
- Yang, Y.; Zhang, Y.; Wei, X.; You, J.; Wang, W.; Lu, J.; Shi, R. Comparative Antioxidative Responses and Proline Metabolism in Two Wheat Cultivars under Short Term Lead Stress. Ecotoxicol. Environ. Saf. 2011, 74, 733–740. [Google Scholar] [CrossRef] [PubMed]
- Hare, P.D.; Cress, W.A. Metabolic Implications of Stress-Induced Proline Accumulation in Plants. Plant Growth Regul. 1997, 21, 79–102. [Google Scholar] [CrossRef]
- Delauney, A.J.; Verma, D.P.S. Proline Biosynthesis and Osmoregulation in Plants. Plant J. 1993, 4, 215–223. [Google Scholar] [CrossRef]
- Brugière, N.; Dubois, F.; Limami, A.M.; Lelandais, M.; Roux, Y.; Sangwan, R.S.; Hirel, B. Glutamine Synthetase in the Phloem Plays a Major Role in Controlling Proline Production. Plant Cell 1999, 11, 1995–2011. [Google Scholar] [CrossRef] [PubMed]
- Szalai, G.; Kellos, T.; Galiba, G.; Kocsy, G. Glutathione as an Antioxidant and Regulatory Molecule in Plants Under Abiotic Stress Conditions. J. Plant Growth Regul. 2009, 28, 66–80. [Google Scholar] [CrossRef]
- Ullah Qadir, S.; Raja, V.; Siddiqui, W.A.; Shah, T.; Alansi, S.; El-Sheikh, M.A. Ascorbate Glutathione Antioxidant System Alleviates Fly Ash Stress by Modulating Growth Physiology and Biochemical Responses in Solanum Lycopersicum. Saudi J. Biol. Sci. 2022, 29, 1322–1336. [Google Scholar] [CrossRef] [PubMed]
- Gupta, M.; Cuypers, A.; Vangronsveld, J.; Clijsters, H. Copper Affects the Enzymes of the Ascorbate-Glutathione Cycle and Its Related Metabolites in the Roots of Phaseolus vulgaris. Physiol. Plant 1999, 106, 262–267. [Google Scholar] [CrossRef]
- Cuypers, A.; Vangronsveld, J.; Clijsters, H. Biphasic Effect of Copper on the Ascorbate-Glutathione Pathway in Primary Leaves of Phaseolus Vulgaris Seedlings during the Early Stages of Metal Assimilation. Physiol. Plant 2000, 110, 512–517. [Google Scholar] [CrossRef]
- Latowski, D.; Surówka, E.; Strzałka, K. Regulatory Role of Components of Ascorbate-Glutathione Pathway in Plant Stress Tolerance. In Ascorbate-Glutathione Pathway and Stress Tolerance in Plants; Springer: Dordrecht, The Netherlands, 2010; pp. 1–53. [Google Scholar] [CrossRef]
- Shalata, A.; Neumann, P.M. Exogenous Ascorbic Acid (Vitamin C) Increases Resistance to Salt Stress and Reduces Lipid Peroxidation. J. Exp. Bot. 2001, 52, 2207–2211. [Google Scholar] [CrossRef] [PubMed]




| Gene | Primer | Sequence | Amplicon Size (bp) |
|---|---|---|---|
| GR | Forward | 5′-AAAGACCGAGGAGATTGTACG-3′ | 322 |
| Reverse | 5′-CATTCCTCGCCATATAGAAGC-3′ | ||
| EF1 | Forward | 5′-GGAACTTGAGAAGGAGCCTAAG-3′ | 158 |
| Reverse | 5′-CAACACCAACAGCAACAGTCT-3′ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alves, A.; Ribeiro, R.; Azenha, M.; Cunha, M.; Teixeira, J. Effects of Exogenously Applied Copper in Tomato Plants’ Oxidative and Nitrogen Metabolisms under Organic Farming Conditions. Horticulturae 2023, 9, 323. https://doi.org/10.3390/horticulturae9030323
Alves A, Ribeiro R, Azenha M, Cunha M, Teixeira J. Effects of Exogenously Applied Copper in Tomato Plants’ Oxidative and Nitrogen Metabolisms under Organic Farming Conditions. Horticulturae. 2023; 9(3):323. https://doi.org/10.3390/horticulturae9030323
Chicago/Turabian StyleAlves, Alexandre, Rafael Ribeiro, Manuel Azenha, Mário Cunha, and Jorge Teixeira. 2023. "Effects of Exogenously Applied Copper in Tomato Plants’ Oxidative and Nitrogen Metabolisms under Organic Farming Conditions" Horticulturae 9, no. 3: 323. https://doi.org/10.3390/horticulturae9030323
APA StyleAlves, A., Ribeiro, R., Azenha, M., Cunha, M., & Teixeira, J. (2023). Effects of Exogenously Applied Copper in Tomato Plants’ Oxidative and Nitrogen Metabolisms under Organic Farming Conditions. Horticulturae, 9(3), 323. https://doi.org/10.3390/horticulturae9030323








