Exogenous β-Aminobutyric Acid (BABA) Improves the Growth, Essential Oil Content, and Composition of Grapefruit Mint (Mentha suaveolens × piperita) under Water Deficit Stress Conditions
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Appling Water Deficit Stress and BABA Spraying
2.3. Growth Parameters and Photosynthetic Pigments Assay
2.4. Total Soluble Protein Content (TSPC)
2.5. Enzymatic Antioxidants Activity
2.6. Total Proline Content
2.7. Malondialdehyde (MDA) Content
2.8. Essential Oil (EO) Extraction
2.9. GC-FID and GC-MS Analysis
2.10. Statistical Analysis
3. Results and Discussion
3.1. Growth Characteristics
3.2. Photosynthetic Pigments
3.3. Antioxidant Enzymes Activity
3.4. Proline and MDA Content
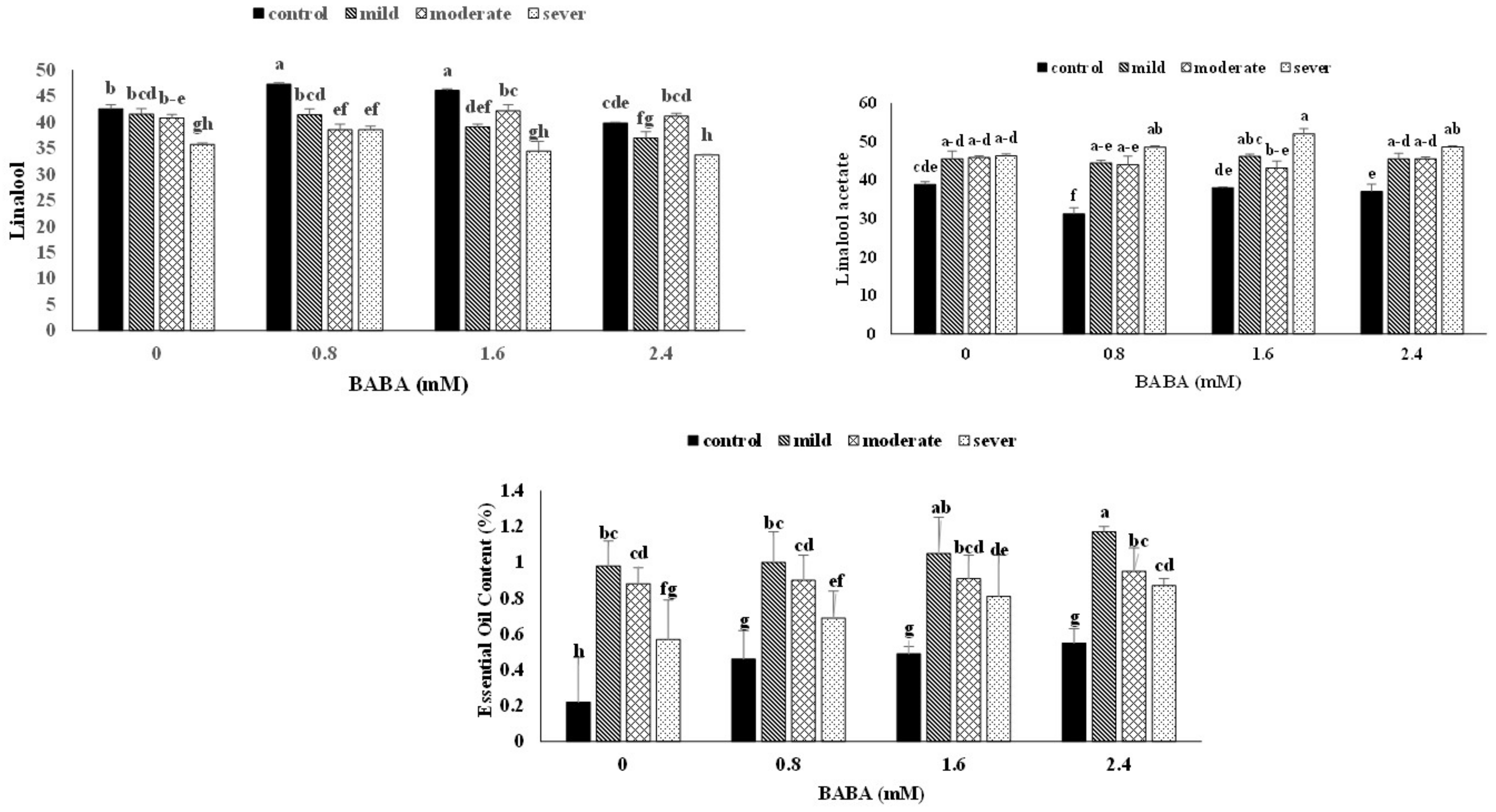
3.5. Essential Oil (EO) Content and Compositions
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kapp, K.; Püssa, T.; Orav, A.; Roasto, M.; Raal, A.; Vuorela, P.; Vuorela, H.; Tammela, P. Chemical composition and antibacterial effect of Mentha spp. grown in Estonia. Nat. Prod. Commun. 2020, 15, 1934578X20977615. [Google Scholar] [CrossRef]
- Mamadalieva, N.Z.; Hussain, H.; Xiao, J. Recent advances in genus Mentha: Phytochemistry, antimicrobial effects, and food applications. Food Front. 2020, 1, 435–458. [Google Scholar] [CrossRef]
- Silva, D.; Vieira, R.; Alves, R.; Mendes, R.; Cardoso, L.; Queiroz, L.; Santos, I. Mint (Mentha spp.) germplasm conservation in Brazil. Rev. Bras. Pl. Med. 2006, 8, 27–31. [Google Scholar]
- Ahmadi, H.; Morshedloo, M.R.; Emrahi, R.; Javanmard, A.; Rasouli, F.; Maggi, F.; Kumar, M.; Lorenzo, J.M. Introducing three new fruit-scented mints to farmlands: Insights on drug yield, essential-oil quality, and antioxidant properties. Antioxidants 2022, 11, 866. [Google Scholar] [CrossRef] [PubMed]
- Le, Y.; Wu, Y.; Zhang, Y.; Yao, L. ISSR analysis of genetic relationships between eleven varieties of mentha. J. Shanghai Jiaotong Univ. (Agric. Sci.) 2008, 26, 29–32. [Google Scholar]
- De Sousa Barros, A.; de Morais, S.M.; Ferreira, P.A.T.; Vieira, Í.G.P.; Craveiro, A.A.; dos Santos Fontenelle, R.O.; de Menezes, J.E.S.A.; da Silva, F.W.F.; de Sousa, H.A. Chemical composition and functional properties of essential oils from Mentha species. Ind. Crops Prod. 2015, 76, 557–564. [Google Scholar] [CrossRef]
- Yamasaki, K.; Nakano, M.; Kawahata, T.; Mori, H.; Otake, T.; Ueda, N.; Oishi, I.; Inami, R.; Yamane, M.; Nakamura, M. Anti-HIV-1 activity of herbs in Labiatae. Biol. Pharm. Bull. 1998, 21, 829–833. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, H.; Jin, P.; Guo, X.; Li, Y.; Fan, C.; Wang, J.; Zheng, Y. Enhancement of storage quality and antioxidant capacity of harvested sweet cherry fruit by immersion with β-aminobutyric acid. Postharvest Biol. Technol. 2016, 118, 71–78. [Google Scholar] [CrossRef]
- Deschamps, C.; Zanatta, J.L.; Bizzo, H.R.; Oliveira, M.d.C.; Roswalka, L.C. Seasonal evaluation of essential oil yield of mint species. Ciência E Agrotecnologia 2008, 32, 725–730. [Google Scholar] [CrossRef]
- Mircioaga, N.; Calinescu, I. Extraction and identification of active principles from Mentha piperita L. Rev. De Chim. 2011, 62, 1073–1076. [Google Scholar]
- Park, K.-W.; Kim, D.-Y.; Lee, S.-Y.; Kim, J.-H.; Yang, D.-S. A multivariate statistical approach to comparison of essential oil composition from three mentha species. Hortic. Sci. Technol. 2011, 29, 382–387. [Google Scholar]
- Schmidt, E.; Bail, S.; Buchbauer, G.; Stoilova, I.; Atanasova, T.; Stoyanova, A.; Krastanov, A.; Jirovetz, L. Chemical composition, olfactory evaluation and antioxidant effects of essential oil from Mentha × piperita. Nat. Prod. Commun. 2009, 4, 1934578X0900400819. [Google Scholar] [CrossRef]
- Morshedloo, M.R.; Ebadi, A.; Maggi, F.; Fattahi, R.; Yazdani, D.; Jafari, M. Chemical characterization of the essential oil compositions from Iranian populations of Hypericum perforatum L. Ind. Crops Prod. 2015, 76, 565–573. [Google Scholar] [CrossRef]
- Yang, L.; Wen, K.-S.; Ruan, X.; Zhao, Y.-X.; Wei, F.; Wang, Q. Response of plant secondary metabolites to environmental factors. Molecules 2018, 23, 762. [Google Scholar] [CrossRef]
- Elansary, H.O.; Szopa, A.; Kubica, P.; Ekiert, H.; Ali, H.M.; Elshikh, M.S.; Abdel-Salam, E.M.; El-Esawi, M.; El-Ansary, D.O. Bioactivities of traditional medicinal plants in Alexandria. Evid.-Based Complement. Altern. Med. 2018, 2018, 1463579. [Google Scholar] [CrossRef]
- Bistgani, Z.E.; Hashemi, M.; DaCosta, M.; Craker, L.; Maggi, F.; Morshedloo, M.R. Effect of salinity stress on the physiological characteristics, phenolic compounds and antioxidant activity of Thymus vulgaris L. and Thymus daenensis Celak. Ind. Crops Prod. 2019, 135, 311–320. [Google Scholar] [CrossRef]
- Amani Machiani, M.; Javanmard, A.; Ostadi, A.; Morshedloo, M.; Chabokpour, J. Effects of harvest time and mycorrhiza fungus application on quantitative and qualitative yield of thyme (Thymus vulgaris L.) essential oil at different irrigation levels. Iran. J. Med. Aromat. Plants Res. 2021, 36, 1022–1037. [Google Scholar]
- Imahori, Y. Postharvest stress treatments in fruits and vegetables. In Abiotic Stress Responses in Plants; Ahmad, P., Prasad, M., Eds.; Springer: New York, NY, USA, 2012; pp. 347–358. [Google Scholar]
- Morshedloo, M.R.; Salami, S.A.; Nazeri, V.; Craker, L.E. Prolonged water stress on growth and constituency of Iranian of Oregano (Origanum vulgare L.). J. Med. Act. Plants 2017, 5, 7–19. [Google Scholar]
- Hazzoumi, Z.; Moustakime, Y.; Joutei, K.A. Effect of arbuscular mycorrhizal fungi (AMF) and water stress on growth, phenolic compounds, glandular hairs, and yield of essential oil in basil (Ocimum gratissimum L.). Chem. Biol. Technol. Agric. 2015, 2, 10. [Google Scholar] [CrossRef]
- Borges, C.V.; Minatel, I.O.; Gomez-Gomez, H.A.; Lima, G.P.P. Medicinal plants: Influence of environmental factors on the content of secondary metabolites. In Medicinal Plants and Environmental Challenges; Ghorbanpour, M., Varma, A., Eds.; Springer: Cham, Switzerland, 2017; pp. 259–277. [Google Scholar]
- Kleinwächter, M.; Selmar, D. New insights explain that drought stress enhances the quality of spice and medicinal plants: Potential applications. Agron. Sustain. Dev. 2015, 35, 121–131. [Google Scholar] [CrossRef]
- Alhaithloul, H.A.; Soliman, M.H.; Ameta, K.L.; El-Esawi, M.A.; Elkelish, A. Changes in ecophysiology, osmolytes, and secondary metabolites of the medicinal plants of Mentha piperita and Catharanthus roseus subjected to drought and heat stress. Biomolecules 2019, 10, 43. [Google Scholar] [CrossRef]
- Halder, M.; Sarkar, S.; Jha, S. Elicitation: A biotechnological tool for enhanced production of secondary metabolites in hairy root cultures. Eng. Life Sci. 2019, 19, 880–895. [Google Scholar] [CrossRef] [PubMed]
- Naik, P.M.; Al-Khayri, J.M. Abiotic and biotic elicitors-role in secondary metabolites production through in vitro culture of medicinal plants. In Abiotic and Biotic Stress in Plants—Recent Advances and Future Perspectives; InTech: Rijeka, Croatia, 2016; pp. 247–277. [Google Scholar]
- Creelman, R.A.; Mullet, J.E. Biosynthesis and action of jasmonates in plants. Annu. Rev. Plant Biol. 1997, 48, 355–381. [Google Scholar] [CrossRef] [PubMed]
- Daneshmand, F.; Arvin, M.J.; Kalantari, K.M. Effect of acetylsalicylic acid (Aspirin) on salt and osmotic stress tolerance in Solanum bulbocastanum in vitro: Enzymatic antioxidants. Am.-Eurasian J. Agric. Environ. Sci. 2009, 6, 92–99. [Google Scholar]
- Radman, R.; Saez, T.; Bucke, C.; Keshavarz, T. Elicitation of plants and microbial cell systems. Biotechnol. Appl. Biochem. 2003, 37, 91–102. [Google Scholar] [CrossRef]
- Thakur, M.; Bhattacharya, S.; Khosla, P.K.; Puri, S. Improving production of plant secondary metabolites through biotic and abiotic elicitation. J. Appl. Res. Med. Aromat. Plants 2019, 12, 1–12. [Google Scholar] [CrossRef]
- Oka, Y.; Cohen, Y.; Spiegel, Y. Local and systemic induced resistance to the root-knot nematode in tomato by DL-β-amino-n-butyric acid. Phytopathology 1999, 89, 1138–1143. [Google Scholar] [CrossRef]
- Cohen, Y.; Vaknin, M.; Mauch-Mani, B. BABA-induced resistance: Milestones along a 55-year journey. Phytoparasitica 2016, 44, 513–538. [Google Scholar] [CrossRef]
- Baccelli, I.; Mauch-Mani, B. Beta-aminobutyric acid priming of plant defense: The role of ABA and other hormones. Plant Mol. Biol. 2016, 91, 703–711. [Google Scholar] [CrossRef]
- Tavallali, V.; Karimi, S.; Mohammadi, S.; Hojati, S. Effects of 5-aminobutyric acid on the induction of resistance to Penicillium italicum. World Appl. Sci. J. 2008, 5, 345–351. [Google Scholar]
- Wang, L.; Jin, P.; Wang, J.; Jiang, L.; Shan, T.; Zheng, Y. Effect of β-aminobutyric acid on cell wall modification and senescence in sweet cherry during storage at 20 °C. Food Chem. 2015, 175, 471–477. [Google Scholar] [CrossRef]
- Jakab, G.; Ton, J.; Flors, V.; Zimmerli, L.; Métraux, J.-P.; Mauch-Mani, B. Enhancing Arabidopsis salt and drought stress tolerance by chemical priming for its abscisic acid responses. Plant Physiol. 2005, 139, 267–274. [Google Scholar] [CrossRef]
- Abid, G.; Ouertani, R.N.; Jebara, S.H.; Boubakri, H.; Muhovski, Y.; Ghouili, E.; Abdelkarim, S.; Chaieb, O.; Hidri, Y.; Kadri, S. Alleviation of drought stress in faba bean (Vicia faba L.) by exogenous application of β-aminobutyric acid (BABA). Physiol. Mol. Biol. Plants 2020, 26, 1173–1186. [Google Scholar] [CrossRef]
- Morshedloo, M.R.; Craker, L.E.; Salami, A.; Nazeri, V.; Sang, H.; Maggi, F. Effect of prolonged water stress on essential oil content, compositions and gene expression patterns of mono-and sesquiterpene synthesis in two oregano (Origanum vulgare L.) subspecies. Plant Physiol. Biochem. 2017, 111, 119–128. [Google Scholar] [CrossRef]
- Hoagland, D.R.; Arnon, D.I. The water-culture method for growing plants without soil. Circular. Calif. Agric. Exp. Stn. 1950, 347, 1–32. [Google Scholar]
- Arnon, D.I. Copper enzymes in isolated chloroplasts. Polyphenoloxidase in Beta vulgaris. Plant Physiol. 1949, 24, 1. [Google Scholar] [CrossRef]
- Sutour, S.; Bradesi, P.; Casanova, J.; Tomi, F. Composition and chemical variability of Mentha suaveolens ssp. suaveolens and M. suaveolens ssp. insularis from Corsica. Chem. Biodivers. 2010, 7, 1002–1008. [Google Scholar] [CrossRef]
- Miyake, C.; Asada, K. Inactivation mechanism of ascorbate peroxidase at low concentrations of ascorbate; hydrogen peroxide decomposes compound I of ascorbate peroxidase. Plant Cell Physiol. 1996, 37, 423–430. [Google Scholar] [CrossRef]
- Beauchamp, C.; Fridovich, I. Superoxide dismutase: Improved assays and an assay applicable to acrylamide gels. Anal. Biochem. 1971, 44, 276–287. [Google Scholar] [CrossRef]
- Bates, L.S.; Waldren, R.P.; Teare, I. Rapid determination of free proline for water-stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Heath, R.L.; Packer, L. Photoperoxidation in isolated chloroplasts: I. Kinetics and stoichiometry of fatty acid peroxidation. Arch. Biochem. Biophys. 1968, 125, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Pavela, R.; Morshedloo, M.R.; Mumivand, H.; Khorsand, G.J.; Karami, A.; Maggi, F.; Desneux, N.; Benelli, G. Phenolic monoterpene-rich essential oils from Apiaceae and Lamiaceae species: Insecticidal activity and safety evaluation on non-target earthworms. Entomol. Gen. 2020, 40, 421–435. [Google Scholar] [CrossRef]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry, 5th online ed.; Texensis Publishing: Gruver, TX, USA, 2017. [Google Scholar]
- Morshedloo, M.R.; Maggi, F.; Neko, H.T.; Aghdam, M.S. Sumac (Rhus coriaria L.) fruit: Essential oil variability in Iranian populations. Ind. Crops Prod. 2018, 111, 1–7. [Google Scholar] [CrossRef]
- Farooq, M.; Hussain, M.; Wahid, A.; Siddique, K. Drought stress in plants: An overview. In Plant Responses to Drought Stress; Aroca, R., Ed.; Springer: Berlin/Heidelberg, Germany, 2012; pp. 1–33. [Google Scholar]
- Levitt, J. Responses of Plants to Environmental Stresses. Volume II. Water, Radiation, Salt, and Other Stresses; Academic Press: New York, NY, USA, 1980. [Google Scholar]
- Wu, C.-C.; Singh, P.; Chen, M.-C.; Zimmerli, L. L-Glutamine inhibits beta-aminobutyric acid-induced stress resistance and priming in Arabidopsis. J. Exp. Bot. 2010, 61, 995–1002. [Google Scholar] [CrossRef]
- Mohammadi, H.; Amirikia, F.; Ghorbanpour, M.; Fatehi, F.; Hashempour, H. Salicylic acid induced changes in physiological traits and essential oil constituents in different ecotypes of Thymus kotschyanus and Thymus vulgaris under well-watered and water stress conditions. Ind. Crops Prod. 2019, 129, 561–574. [Google Scholar] [CrossRef]
- Baghbani-Arani, A.; Modarres-Sanavy, S.A.M.; Mashhadi-Akbar-Boojar, M.; Mokhtassi-Bidgoli, A. Towards improving the agronomic performance, chlorophyll fluorescence parameters and pigments in fenugreek using zeolite and vermicompost under deficit water stress. Ind. Crops Prod. 2017, 109, 346–357. [Google Scholar] [CrossRef]
- Navari-Izzo, F.; Quartacci, M.; Izzo, R. Water-stress induced changes in protein and free amino acids in field grown maize and sunflower. Plant Physiol. Biochem. 1990, 28, 531–537. [Google Scholar]
- Egert, M.; Tevini, M. Influence of drought on some physiological parameters symptomatic for oxidative stress in leaves of chives (Allium schoenoprasum). Environ. Exp. Bot. 2002, 48, 43–49. [Google Scholar] [CrossRef]
- Oliveira Neto, C.F.d.; Lobato, A.K.d.S.; Gonçalves-Vidigal, M.C.; Costa, R.C.L.d.; Santos Filho, B.G.d.; Alves, G.A.R.; Maia, W.; Cruz, F.; Neves, H.; Lopes, M.S. Carbon compounds and chlorophyll contents in sorghum submitted to water deficit during three growth stages. J. Food Agric. Environ. 2009, 7, 588–593. [Google Scholar]
- Piekkielek, W.; Fox, R. Use of a chlorophyll meter to predict sidedress nitrogen requirements for maize. Agron. J. 1992, 84, 59–65. [Google Scholar] [CrossRef]
- Selim, S.; Akhtar, N.; El Azab, E.; Warrad, M.; Alhassan, H.H.; Abdel-Mawgoud, M.; Al Jaouni, S.K.; Abdelgawad, H. Innovating the synergistic assets of β-amino butyric acid (BABA) and selenium nanoparticles (SeNPs) in improving the growth, nitrogen metabolism, biological activities, and nutritive value of Medicago interexta sprouts. Plants 2022, 11, 306. [Google Scholar] [CrossRef]
- Jisha, K.C.; Puthur, J.T. Seed priming with beta-amino butyric acid improves abiotic stress tolerance in rice seedlings. Rice Sci. 2016, 23, 242–254. [Google Scholar] [CrossRef]
- Wang, X.; Liu, H.; Yu, F.; Hu, B.; Jia, Y.; Sha, H.; Zhao, H. Differential activity of the antioxidant defence system and alterations in the accumulation of osmolyte and reactive oxygen species under drought stress and recovery in rice (Oryza sativa L.) tillering. Sci. Rep. 2019, 9, 8543. [Google Scholar] [CrossRef]
- Ahmadi, H.; Babalar, M.; Sarcheshmeh, M.A.A.; Morshedloo, M.R.; Shokrpour, M. Effects of exogenous application of citrulline on prolonged water stress damages in hyssop (Hyssopus officinalis L.): Antioxidant activity, biochemical indices, and essential oils profile. Food Chem. 2020, 333, 127433. [Google Scholar] [CrossRef]
- Sánchez, F.J.; Manzanares, M.a.; de Andres, E.F.; Tenorio, J.L.; Ayerbe, L. Turgor maintenance, osmotic adjustment and soluble sugar and proline accumulation in 49 pea cultivars in response to water stress. Field Crops Res. 1998, 59, 225–235. [Google Scholar] [CrossRef]
- Gagneul, D.; Aïnouche, A.; Duhazé, C.; Lugan, R.; Larher, F.R.; Bouchereau, A. A reassessment of the function of the so-called compatible solutes in the halophytic Plumbaginaceae Limonium latifolium. Plant Physiol. 2007, 144, 1598–1611. [Google Scholar] [CrossRef]
- Kuznetsov, V.V.; Shevyakova, N. Proline under stress: Biological role, metabolism, and regulation. Russ. J. Plant Physiol. 1999, 46, 274–287. [Google Scholar]
- Singh, P.K.; Wu, C.-C.; Zimmerli, L. β-aminobutyric acid priming by stress imprinting. Plant Signal. Behav. 2010, 5, 878–880. [Google Scholar] [CrossRef]
- Jawahar, G.; Rajasheker, G.; Maheshwari, P.; Punita, D.L.; Jalaja, N.; Kumari, P.H.; Kumar, S.A.; Afreen, R.; Karumanchi, A.R.; Rathnagiri, P. Osmolyte diversity, distribution, and their biosynthetic pathways. In Plant Signaling Molecules; Elsevier: Amsterdam, The Netherlands, 2019; pp. 449–458. [Google Scholar]
- Mahmud, J.A.; Hasanuzzaman, M.; Khan, M.I.R.; Nahar, K.; Fujita, M. β-Aminobutyric acid pretreatment confers salt stress tolerance in Brassica napus L. by modulating reactive oxygen species metabolism and methylglyoxal detoxification. Plants 2020, 9, 241. [Google Scholar] [CrossRef]
- Du, Z.; Hu, B.; Shi, A.; Ma, X.; Cheng, Y.; Chen, P.; Liu, Y.; Lin, X.; Ruan, R. Cultivation of a microalga Chlorella vulgaris using recycled aqueous phase nutrients from hydrothermal carbonization process. Bioresour. Technol. 2012, 126, 354–357. [Google Scholar] [CrossRef]
- Raut, J.S.; Karuppayil, S.M. A status review on the medicinal properties of essential oils. Ind. Crops Prod. 2014, 62, 250–264. [Google Scholar] [CrossRef]
- Morshedloo, M.R.; Mumivand, H.; Craker, L.E.; Maggi, F. Chemical composition and antioxidant activity of essential oils in Origanum vulgare subsp. gracile at different phenological stages and plant parts. J. Food Process. Preserv. 2018, 42, e13516. [Google Scholar] [CrossRef]
- Ghanbari, M.; Ariafar, S. The effect of water deficit and zeolite application on Growth Traits and Oil Yield of Medicinal Peppermint (Mentha piperita L.). Int. J. Med. Aromat. Plants 2013, 3, 32–39. [Google Scholar]
- Lange, B.M.; Ahkami, A. Metabolic engineering of plant monoterpenes, sesquiterpenes and diterpenes—Current status and future opportunities. Plant Biotechnol. J. 2013, 11, 169–196. [Google Scholar] [CrossRef]
- Selmar, D.; Kleinwächter, M. Influencing the product quality by deliberately applying drought stress during the cultivation of medicinal plants. Ind. Crops Prod. 2013, 42, 558–566. [Google Scholar] [CrossRef]
- Delfine, S.; Loreto, F.; Pinelli, P.; Tognetti, R.; Alvino, A. Isoprenoids content and photosynthetic limitations in rosemary and spearmint plants under water stress. Agric. Ecosyst. Environ. 2005, 106, 243–252. [Google Scholar] [CrossRef]
- Croteau, R.; Martinkus, C. Metabolism of monoterpenes: Demonstration of (+)-neomenthyl-β-d-glucoside as a major metabolite of (−)-menthone in peppermint (Mentha piperita). Plant Physiol. 1979, 64, 169–175. [Google Scholar] [CrossRef]
- Govahi, M.; Ghalavand, A.; Nadjafi, F.; Sorooshzadeh, A. Comparing different soil fertility systems in Sage (Salvia officinalis) under water deficiency. Ind. Crops Prod. 2015, 74, 20–27. [Google Scholar] [CrossRef]
- Islam, S.; Mohammad, F. Triacontanol as a dynamic growth regulator for plants under diverse environmental conditions. Physiol. Mol. Biol. Plants 2020, 26, 871–883. [Google Scholar] [CrossRef]
- Abdul-Hafeez, E.Y.; Ibrahim, O.H. Effects of chitosan and BABA foliar application on flowering and chemical characteristics of German chamomile ‘Bode-gold’. S. Afr. J. Bot. 2021, 139, 241–245. [Google Scholar] [CrossRef]
- Prins, C.L.; Vieira, I.J.; Freitas, S.P. Growth regulators and essential oil production. Braz. J. Plant Physiol. 2010, 22, 91–102. [Google Scholar] [CrossRef]
| Water Stress | BABA (mM) | Fresh Weight (g Pot−1) | Chlorophyll a (mg g−1 Fresh Weight) | Chlorophyll b (mg g−1 Fresh Weight) | Total Chlorophyll (mg g−1 Fresh Weight) | Carotenoid (mg g−1 Fresh Weight) | MDA (nmol g−1 Fresh Weight) | APX (μmol asc. Min−1 mg−1 Protein) | SOD (Units min−1 mg−1 Protein) |
| 0 | 100 ± 0.58 c * | 5.03 ± 0.01 a | 1.88 ± 0.01 abc | 6.91 ± 0.01 abc | 1.79 ± 0.03 d | 0.33 ± 0.01 hij | 0.2 ± 0.02 j | 15.13 ± 1.17 h | |
| 100% FC | 0.8 | 100 ± 0.00 c | 5.06 ± 0.01 a | 1.91 ± 0.01 ab | 6.91 ± 0.01 ab | 2.06 ± 0.11 c | 0.31 ± 0.04 ij | 0.25 ± 0.01 ij | 21.49 ± 1.44 g |
| (Control) | 1.6 | 103.33 ± 0.88 b | 5.09 ± 0.02 a | 1.94 ± 0.01 a | 7.04 ± 0.02 ab | 2.48 ± 0.05 b | 0.31 ± 0.01 ij | 0.23 ± 0.01 ij | 23.15 ± 1.45 g |
| 2.4 | 111.66 ± 0.88 a | 5.23 ± 0.05 a | 1.95 ± 0.00 a | 7.18 ± 0.05 a | 2.64 ± 0.06 a | 0.26 ± 0.01 j | 0.31 ± 0.05 ij | 26.22 ± 1.8 g | |
| 0 | 80 ± 0.00 g | 4.96 ± 0.00 a | 1.65 ± 0.01 de | 6.62 ± 0.01 abcd | 1.62 ± 0.01 efg | 0.65 ± 0.01 d | 0.51 ± 0.05 hi | 26.82 ± 1.53 g | |
| 75% FC | 0.8 | 90 ± 0.58 f | 4.97 ± 0.00 a | 1.72 ± 0.03 d | 6.7 ± 0.03 abcd | 1.67 ± 0.02 def | 0.43 ± 0.05 gh | 0.75 ± 0.08 gh | 40.65 ± 1.67 f |
| (Mild) | 1.6 | 95 ± 0.58 e | 4.99 ± 0.01 a | 1.81 ± 0.00 c | 6.8 ± 0.01 abc | 1.72 ± 0.01 de | 0.38 ± 0.05 hi | 0.83 ± 0.07 g | 51.62 ± 1.23 de |
| 2.4 | 96.66 ± 0.33 d | 5.02 ± 0.00 a | 1.85 ± 0.01 bc | 6.86 ± 0.01 abc | 1.77 ± 0.00 d | 0.36 ± 0.02 hi | 1.14 ± 0.03 f | 38.88 ± 1.21 f | |
| 0 | 56.66 ± 0.33 k | 4.9 ± 0.01 a | 1.28 ± 0.05 h | 6.18 ± 0.06 d | 1.49 ± 0.01 hi | 0.87 ± 0.02 c | 1.23 ± 0.03 ef | 40.79 ± 1.68 f | |
| 55% FC | 0.8 | 63.33 ± 0.33 j | 4.93 ± 0.00 a | 1.46 ± 0.02 g | 6.38 ± 0.02 cd | 1.51 ± 0.01 ghi | 0.6 ± 0.04 de | 1.39 ± 0.04 def | 53.43 ± 1.63 d |
| (Moderate) | 1.6 | 66.66 ± 0.33 i | 4.93 ± 0.00 a | 1.54 ± 0.02 fg | 6.47 ± 0.02 bcd | 1.54 ± 0.00 ghi | 0.34 ± 0.05 hij | 1.59 ± 0.02 cd | 60.3 ± 1.57 c |
| 2.4 | 75 ± 0.58 h | 4.95 ± 0.00 a | 1.6 ± 0.02 ef | 6.56 ± 0.02 bcd | 1.58 ± 0.01 fgh | 0.49 ± 0.05 fg | 1.63 ± 0.22 cd | 51.39 ± 0.28 de | |
| 0 | 30 ± 0.58 o | 1.12 ± 0.27 c | 0.13 ± 0.01 l | 1.26 ± 0.29 g | 0.63 ± 0.06 k | 1.55 ± 0.04 a | 1.48 ± 0.1 de | 47.43 ± 3.9 e | |
| 35% FC | 0.8 | 39 ± 1 n | 3.96 ± 0.7 b | 0.22 ± 0.06 k | 4.18 ± 0.74 f | 1.22 ± 0.09 j | 1.06 ± 0.03 b | 1.8 ± 0.04 bc | 64.49 ± 2.37 bc |
| (Severe) | 1.6 | 41.66 ± 0.33 m | 4.83 ± 0.02 a | 0.44 ± 0.03 j | 5.27 ± 0.05 e | 1.44 ± 0.00 i | 0.69 ± 0.03 d | 2.28 ± 0.29 a | 78.33 ± 3.49 a |
| 2.4 | 46.66 ± 0.33 l | 4.87 ± 0.00 a | 0.62 ± 0.07 i | 5.49 ± 0.07 e | 1.45 ± 0.00 i | 0.54 ± 0.01 ef | 1.98 ± 0.03 b | 66.19 ± 2.99 b | |
| LSD | 1.59 | 0.54 | 0.08 | 0.57 | 0.12 | 0.09 | 0.29 | 5.88 |
| Water Stress | Dry Weight (g Pot−1) | Total Proline Content (µmol g−1 Fresh Weight) | Total Protein Content (mg g-1 Fresh Weight) | GPX (μmol H2O2. min-1 mg-1 protein) |
|---|---|---|---|---|
| Drought | ** | ** | ** | ** |
| 100%FC | 31.25 ± 0.79 a* | 14.33 ± 0.72 c | 2.51 ± 0.1 a | 0.11 ± 0.01 c |
| 75%FC | 26.58 ± 0.83 b | 17.15 ± 0.68 b | 2.19 ± 0.1 b | 0.14 ± 0.02 c |
| 55%FC | 18.41 ± 0.42 c | 18.49 ± 0.38 a | 2.06 ± 0.09 b | 0.27 ± 0.02 b |
| 35% FC | 11.54 ± 0.51 d | 19.28 ± 0.75 a | 1.62 ± 0.14 c | 0.39 ± 0.03 a |
| BABA | ** | ** | * | ns |
| 0(mM) | 20.04 ± 2.14 c | 14.9 ± 0.81 b | 1.85 ± 0.15 b | 0.18 ± 0.03 |
| 0.8(mM) | 20.83 ± 2.23 c | 17.76 ± 0.69 a | 2.03 ± 0.12 ab | 0.23 ± 0.04 |
| 1.6(mM) | 22.25 ± 2.39 b | 17.81 ± 0.84 a | 2.18 ± 0.11 a | 0.27 ± 0.04 |
| 2.4(mM) | 24.66 ± 2.48 a | 18.77 ± 0.62 a | 2.31 ± 0.16 a | 0.21 ± 0.04 |
| LSD | 1.11 | 1.39 | 0.3 | 0.06 |
| Fresh Weight | Dry Weight | Chlorophyll a | Chlorophyll b | Total Chlorophyll | Carotenoid | SOD | Total Proline Content | Total Protein Content | MDA | GPX | APX | Essential Oil Content | Linalool | Dodecane | Linalool Acetate | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Fresh Weight | 1.00 | |||||||||||||||
| Dry Weight | 0.99 ** | 1.00 | ||||||||||||||
| Chlorophyll a | 0.61 * | 0.57 * | 1.00 | |||||||||||||
| Chlorophyll b | 0.94 ** | 0.92 ** | 0.67 ** | 1.00 | ||||||||||||
| Total Chlorophyll | 0.80 ** | 0.77 ** | 0.94 ** | 0.87 ** | 1.00 | |||||||||||
| Carotenoid | 0.86 ** | 0.85 ** | 0.71 ** | 0.78 ** | 0.80 ** | 1.00 | ||||||||||
| SOD | −0.76 ** | −0.79 ** | −0.19 | −0.70 ** | −0.43 | −0.58 * | 1.00 | |||||||||
| Total Proline Content | −0.56* | −0.61 * | −0.05 | −0.51 * | −0.25 | −0.39 | 0.89 ** | 1.00 | ||||||||
| Total Protein Content | 0.89 ** | 0.88 ** | 0.83 ** | 0.87 ** | 0.92 ** | 0.95 ** | −0.55* | −0.37 | 1.00 | |||||||
| MDA | −0.81 ** | −0.80 ** | −0.87 ** | −0.83 ** | −0.93 ** | −0.82 ** | 0.37 | 0.23 | −0.93 ** | 1.00 | ||||||
| GPX | −0.89 ** | −0.89 ** | −0.32 | −0.85 ** | −0.57* | −0.62 ** | 0.92 ** | 0.75 ** | −0.65 ** | 0.51* | 1.00 | |||||
| APX | −0.85 ** | −0.86 ** | −0.29 | −0.77 ** | −0.52 * | −0.68 ** | 0.95 ** | 0.85 ** | −0.63 ** | 0.48 | 0.931 ** | 1.00 | ||||
| Essential Oil Content | −0.15 | −0.19 | 0.20 | 0.01 | 0.13 | −0.24 | 0.48 | 0.59* | −0.09 | −0.03 | 0.20 | 0.43 | 1.00 | |||
| Linalool | 0.64 ** | 0.64 ** | 0.37 | 0.69 ** | 0.53 * | 0.57 * | −0.71 ** | −0.54* | 0.55* | −0.47 | −0.65 ** | −0.74 ** | −0.38 | 1.00 | ||
| Dodecane | 0.59 * | 0.63 ** | 0.23 | 0.48 | 0.36 | 0.73 ** | −0.62* | −0.55* | 0.63 ** | −0.45 | −0.48 | −0.62 ** | −0.64 ** | 0.54 * | 1.00 | |
| Linalool Acetate | −0.72 ** | −0.74 ** | −0.26 | −0.67 ** | −0.46 | −0.68 ** | 0.80 ** | 0.69 ** | −0.64 ** | 0.51* | 0.70 ** | 0.81 ** | 0.59* | −0.80 ** | −0.85 ** | 1.00 |
| Treatments (BABA and Water Deficit Stress) | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N. | Constituents | RI | RI * | 0 (mM) | 0.8 (mM) | 1.6 (mM) | 2.4 (mM) | ||||||||||||
| 35 | 55 | 75 | 100 | 35 | 55 | 75 | 100 | 35 | 55 | 75 | 100 | 35 | 55 | 75 | 100 | ||||
| 1 | n-Nonane | 900 | 898 | 0.10 | tr. | tr. | - | tr. | - | tr. | tr. | - | - | tr. | - | tr. | - | tr. | - |
| 2 | Citronellene | 930 | 929 | 0.06 | - | 0.05 | 0.10 | 0.06 | - | 0.07 | tr. | - | 0.06 | - | - | 0.05 | - | - | 0.11 |
| 3 | β-Pinene | 974 | 972 | 0.36 | 0.34 | 0.425 | 0.39 | 0.33 | 0.32 | 0.39 | 0.38 | 0.26 | 0.02 | 0.4 | 0.195 | 0.265 | 0.29 | 0.41 | 0.41 |
| 4 | 1 -Octen-3-ol | 977 | 975 | 0.87 ± 0.02 | 0.60 ± 0.08 | 0.71 ± 0.09 | 0.76 ± 0.04 | 1.16 ± 0.05 | 0.63 ± 0.00 | 0.83 ± 0.07 | 0.69 ± 0.08 | 0.8 ± 0.02 | 0.54 ± 0.1 | 0.80 ± 0.04 | 0.42 ± 0.06 | 1.08 ± 0.03 | 0.60 ± 0.04 | 0.83 ± 0.01 | 0.38 ± 0.22 |
| 5 | β-Myrcene | 988 | 988 | tr. | - | - | 2.57 ± 0.26 | 2.31 ± 0.02 | 1.33 ± 0.77 | 2.86 ± 0.12 | - | - | 2.51 ± 0.18 | - | 0.38 ± 0.16 | - | - | - | 1.18 ± 0.68 |
| 6 | n-Decane | 1000 | 998 | tr. | - | - | - | - | - | - | - | tr. | 0.08 | - | - | tr. | - | - | - |
| 7 | p-Cymene | 1024 | 1021 | - | tr. | - | - | - | tr. | - | tr. | - | - | - | - | tr. | - | - | - |
| 8 | Limonene | 1025 | 1025 | - | - | tr. | - | - | - | - | - | - | - | tr. | - | - | - | tr. | - |
| 9 | 1,8-Cineole | 1026 | 1027 | 1.42 ± 0.1 | 1.42 ± 0.06 | 1.71 ± 0.18 | 1.84 ± 0.01 | 1.68 ± 0.03 | 1.42 ± 0.01 | 1.51 ± 0.04 | 1.51 ± 0.09 | 1.43 ± 0.06 | 1.22 ± 0.07 | 1.65 ± 0.05 | 1.33 ± 0.04 | 1.71 ± 0.03 | 1.27 ± 0.09 | 1.65 ± 0.06 | 1.52 ± 0.00 |
| 10 | (Z)-β-Ocimene | 1032 | 1035 | 0.98 | 0.42 | 0.40 | 0.41 | 0.38 | 0.38 | 0.44 | 0.42 | 0.37 | 0.36 | 0.43 | 0.37 | 0.22 | 0.36 | 0.38 | 0.39 |
| 11 | (E)-β-Ocimene | 1044 | 1045 | 0.65 | 0.45 | 0.49 | 0.49 | 0.78 | 0.43 | 0.52 | 0.51 | 0.64 | 0.39 | 0.56 | 0.40 | 0.91 | 0.43 | 0.56 | 0.54 |
| 12 | γ-Terpinene | 1054 | 1055 | 0.11 | 0.11 | 0.12 | 0.17 | 0.15 | 0.12 | 0.15 | 011 | 0.09 | 0.14 | 0.1 | 0.09 | 0.13 | 0.13 | 0.11 | 0.12 |
| 13 | Terpinolene | 1086 | 1084 | 0.23 | 0.19 | 0.18 | 0.16 | 0.30 | 0.19 | 0.23 | 0.17 | 0.25 | 0.15 | 0.21 | 0.13 | 0.37 | 0.175 | 0.2 | 0.19 |
| 14 | Linalool | 1096 | 1102 | 42.57 ± 0.76 | 41.58 ± 1.05 | 40.81 ± 0.67 | 35.76 ± 0.23 | 47.30 ± 0.27 | 41.40 ± 1.19 | 38.56 ± 1.08 | 38.52 ± 0.71 | 46.12 ± 0.29 | 39.08 ± 0.57 | 42.18 ± 1.17 | 34.43 ± 1.89 | 39.84 ± 0.17 | 36.95 ± 1.22 | 41.15 ± 0.55 | 33.75 ± 0.08 |
| 15 | cis-Pinocamphone | 1172 | 1168 | 0.81 | tr. | - | - | - | - | - | 0.07 | - | - | tr. | tr. | - | - | - | 0.06 |
| 16 | α-Terpineol | 1186 | 1187 | 0.37 | 0.33 | 0.32 | 0.30 | 0.31 | 0.29 | 0.28 | 0.12 | 0.32 | 0.29 | 0.30 | 0.19 | 0.40 | 0.27 | 0.15 | 0.41 |
| 17 | n-Dodecane | 1200 | 1198 | 3.52 ± 0.3 | 2.54 ± 0.29 | 2.31 ± 0.22 | 2.47 ± 0.11 | 4.35 ± 0.36 | 2.38 ± 0.3 | 2.53 ± 0.28 | 2.32 ± 0.19 | 3.7 ± 0.03 | 1.87 ± 0.11 | 3.2 ± 0.27 | 2.19 ± 0.16 | 5.34 ± 0.52 | 2.35 ± 0.05 | 2.59 ± 0.05 | 1.37 ± 0.04 |
| 18 | Nerol | 1227 | 1225 | 0.65 | 0.43 | 0.41 | 0.29 | 0.81 | 0.39 | 0.43 | 0.48 | 0.62 | 0.39 | 0.55 | 0.21 | 1.07 | 0.41 | 0.46 | 1.52 |
| 19 | Carvone | 1239 | 1239 | - | tr. | - | 0.4 | - | - | - | - | - | - | tr. | - | - | - | tr. | 0.20 |
| 20 | Linalool acetate | 1254 | 1257 | 38.89 ± 0.71 | 45.58 ± 2 | 45.88 ± 0.45 | 46.32 ± 0.51 | 31.28 ± 1.55 | 44.46 ± 0.67 | 44.05 ± 2.17 | 48.58 ± 0.36 | 38.04 ± 0.18 | 46.18 ± 0.59 | 43.13 ± 1.86 | 52.06 ± 1.33 | 37.05 ± 1.88 | 45.49 ± 1.48 | 45.6 ± 0.48 | 48.66 ± 0.28 |
| 21 | Thymol | 1289 | 1290 | 1.90 ± 0.22 | 1.18 ± 0.15 | 1.15 ± 0.08 | 1.45 ± 0.13 | 2.345 ± 0.23 | 1.12 ± 0.15 | 1.26 ± 0.14 | 1.25 ± 0.08 | 1.78 ± 0.00 | 0.97 ± 0.08 | 1.55 ± 0.13 | 0.64 ± 0.37 | 3.16 ± 0.38 | 1.22 ± 0.01 | 1.26 ± 0.02 | 1.04 ± 0.02 |
| 22 | Carvacrol | 1298 | 1299 | 0.125 ± 0.04 | 0.31 ± 0.02 | 0.29 ± 0.02 | 0.14 ± 0.08 | 0.05 ± 0.00 | 0.26 ± 0.01 | 0.23 ± 0.01 | - | tr. | 0.26 ± 0.02 | tr. | 0.06 ± 0.01 | 0.29 ± 0.02 | 2.58 ± 1.45 | 0.12 ± 0.07 | 0.41 ± 0.15 |
| 23 | Neryl acetate | 1361 | 1363 | 1.04 | 0.63 | 0.70 | 0.83 | 1.13 | 0.62 | 0.7 | 0.74 | 0.97 | 0.57 | 0.82 | 0.66 | 1.62 | 1.83 | 0.75 | 0.72 |
| 24 | Geranyl acetate | 1381 | 1382 | 2.04 ± 0.22 | 1.20 ± 0.15 | 1.32 ± 0.02 | 1.66 ± 0.13 | 2.19 ± 0.2 | 1.19 ± 0.14 | 1.34 ± 0.14 | 1.41 ± 0.03 | 1.85 ± 0.03 | 1.1 ± 0.03 | 1.57 ± 0.13 | 0.78 ± 0.36 | 3.16 ± 0.4 | 2.55 ± 0.69 | 1.42 ± 0.03 | 1.58 ± 0.12 |
| 25 | (E)-Caryophyllene | 1417 | 1413 | - | tr. | - | 0.21 | - | 0.06 | 0.12 | tr. | - | 0.18 | 0.02 | - | - | tr. | - | 0.11 |
| 26 | (E)-β-Farnesene | 1454 | 1454 | 0.32 | 0.47 | 0.43 | 0.48 | 0.27 | 0.46 | 0.42 | 0.46 | 0.34 | 0.48 | 0.4 | 0.53 | 0.47 | 1.23 | 0.39 | 0.46 |
| 27 | Bicyclogermacrene | 1500 | 1490 | 0.63 | 0.79 | 0.75 | 0.68 | 0.475 | 0.69 | 0.66 | 0.68 | 0.59 | 0.74 | 0.61 | 1.05 | 0.89 | 0.81 | 0.54 | 0.90 |
| 28 | Viridiflorol | 1592 | 1585 | 0.25 | 0.34 | 0.29 | 0.25 | 0.34 | 0.2 | 0.28 | 0.25 | 0.39 | 0.25 | 0.23 | 1.67 | 0.69 | 0.17 | 0.23 | 0.28 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Akbarzadeh, S.; Morshedloo, M.R.; Behtash, F.; Mumivand, H.; Maggi, F. Exogenous β-Aminobutyric Acid (BABA) Improves the Growth, Essential Oil Content, and Composition of Grapefruit Mint (Mentha suaveolens × piperita) under Water Deficit Stress Conditions. Horticulturae 2023, 9, 354. https://doi.org/10.3390/horticulturae9030354
Akbarzadeh S, Morshedloo MR, Behtash F, Mumivand H, Maggi F. Exogenous β-Aminobutyric Acid (BABA) Improves the Growth, Essential Oil Content, and Composition of Grapefruit Mint (Mentha suaveolens × piperita) under Water Deficit Stress Conditions. Horticulturae. 2023; 9(3):354. https://doi.org/10.3390/horticulturae9030354
Chicago/Turabian StyleAkbarzadeh, Soghra, Mohammad Reza Morshedloo, Farhad Behtash, Hasan Mumivand, and Filippo Maggi. 2023. "Exogenous β-Aminobutyric Acid (BABA) Improves the Growth, Essential Oil Content, and Composition of Grapefruit Mint (Mentha suaveolens × piperita) under Water Deficit Stress Conditions" Horticulturae 9, no. 3: 354. https://doi.org/10.3390/horticulturae9030354
APA StyleAkbarzadeh, S., Morshedloo, M. R., Behtash, F., Mumivand, H., & Maggi, F. (2023). Exogenous β-Aminobutyric Acid (BABA) Improves the Growth, Essential Oil Content, and Composition of Grapefruit Mint (Mentha suaveolens × piperita) under Water Deficit Stress Conditions. Horticulturae, 9(3), 354. https://doi.org/10.3390/horticulturae9030354










