Effect of Chitosan and Micro-Carbon-Based Phosphorus Fertilizer on Strawberry Growth and Productivity
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Layout
2.2. Recorded Data
2.2.1. Vegetative Growth Parameters
2.2.2. Photosynthetic Pigments (Chlorophylla, Chlorophyllb, Carotenoid)
2.2.3. Fruit Yield and Its Components
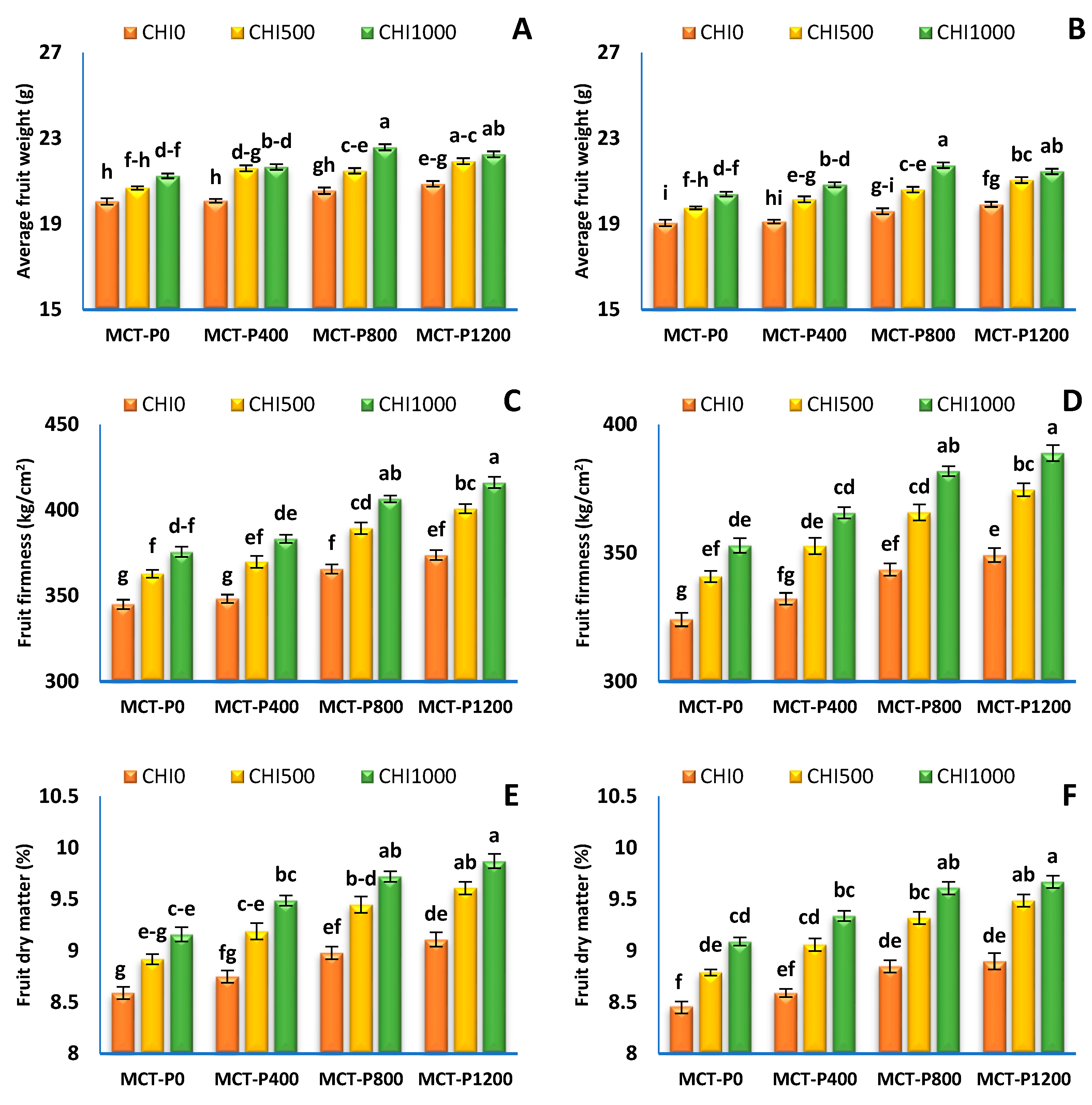
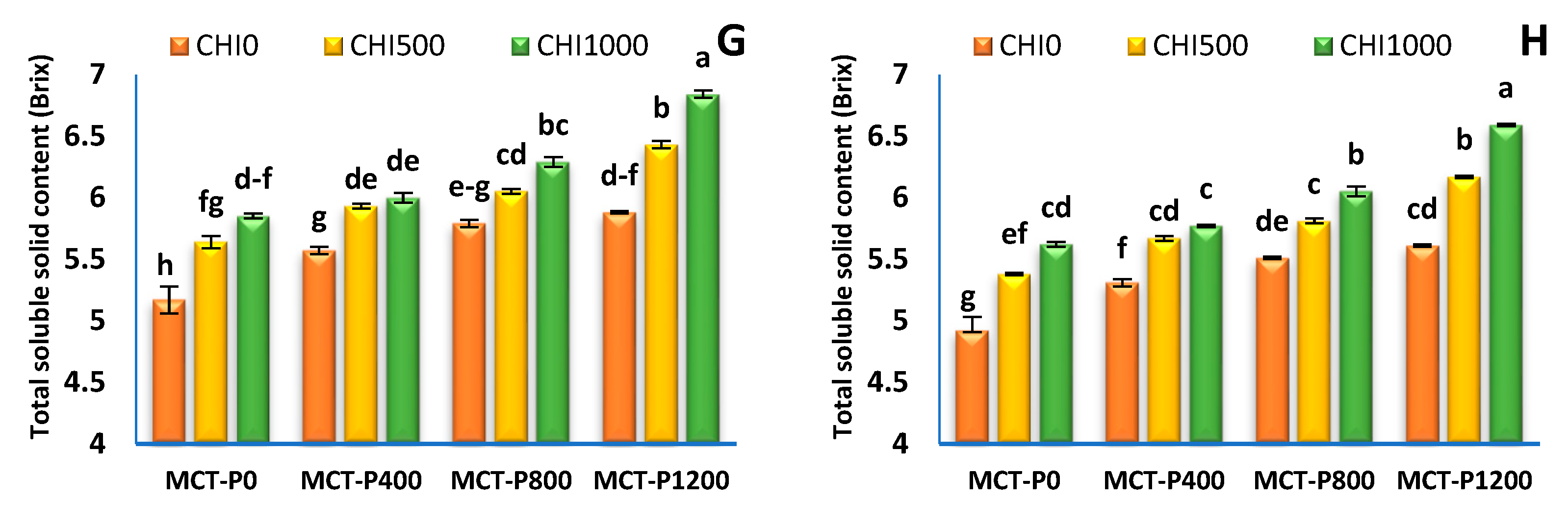
2.2.4. Fruit Physical Quality
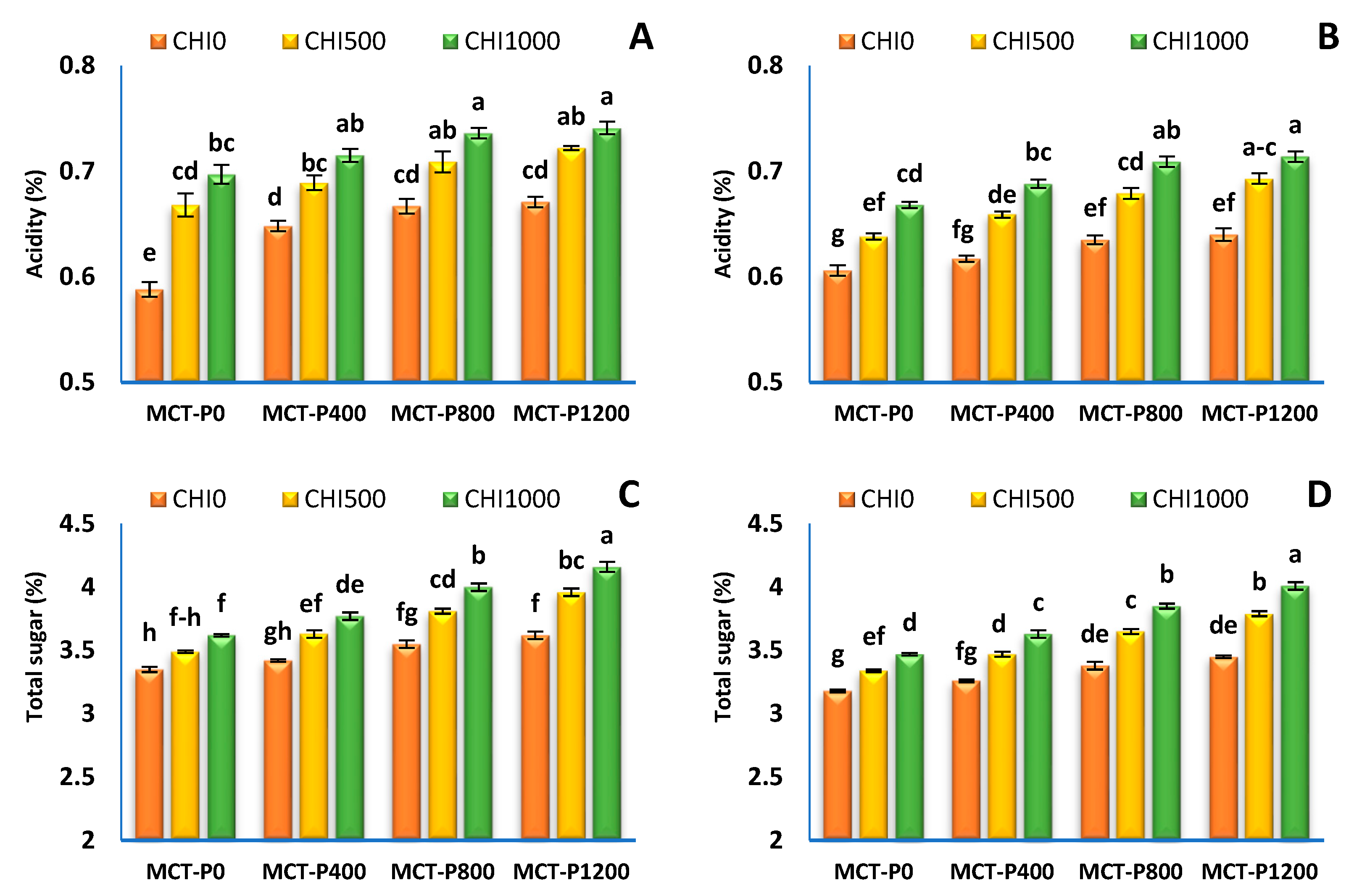
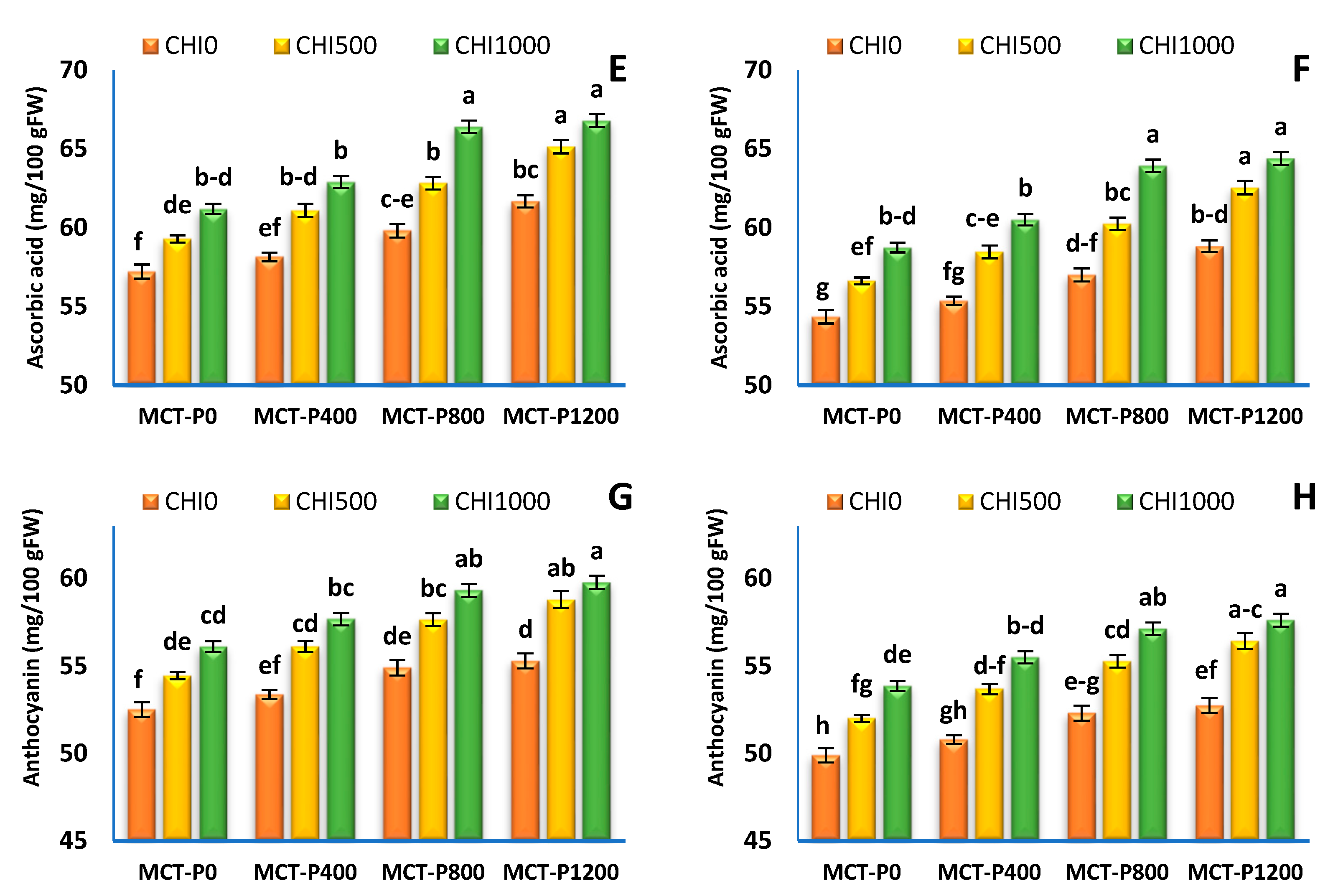
2.2.5. Fruit Chemical Quality
2.3. Statistical Analysis
3. Results
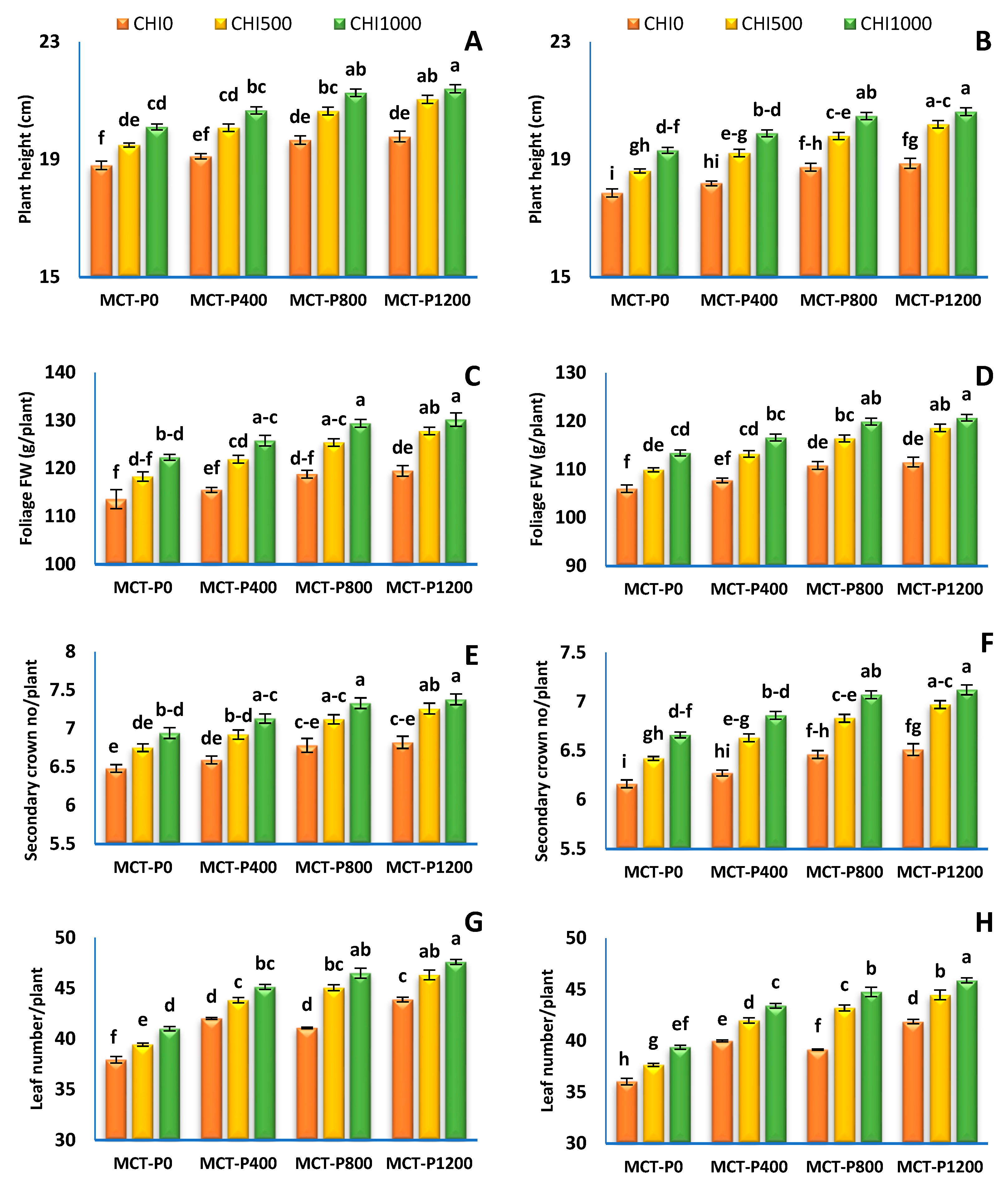
3.1. Vegetative Growth Characters
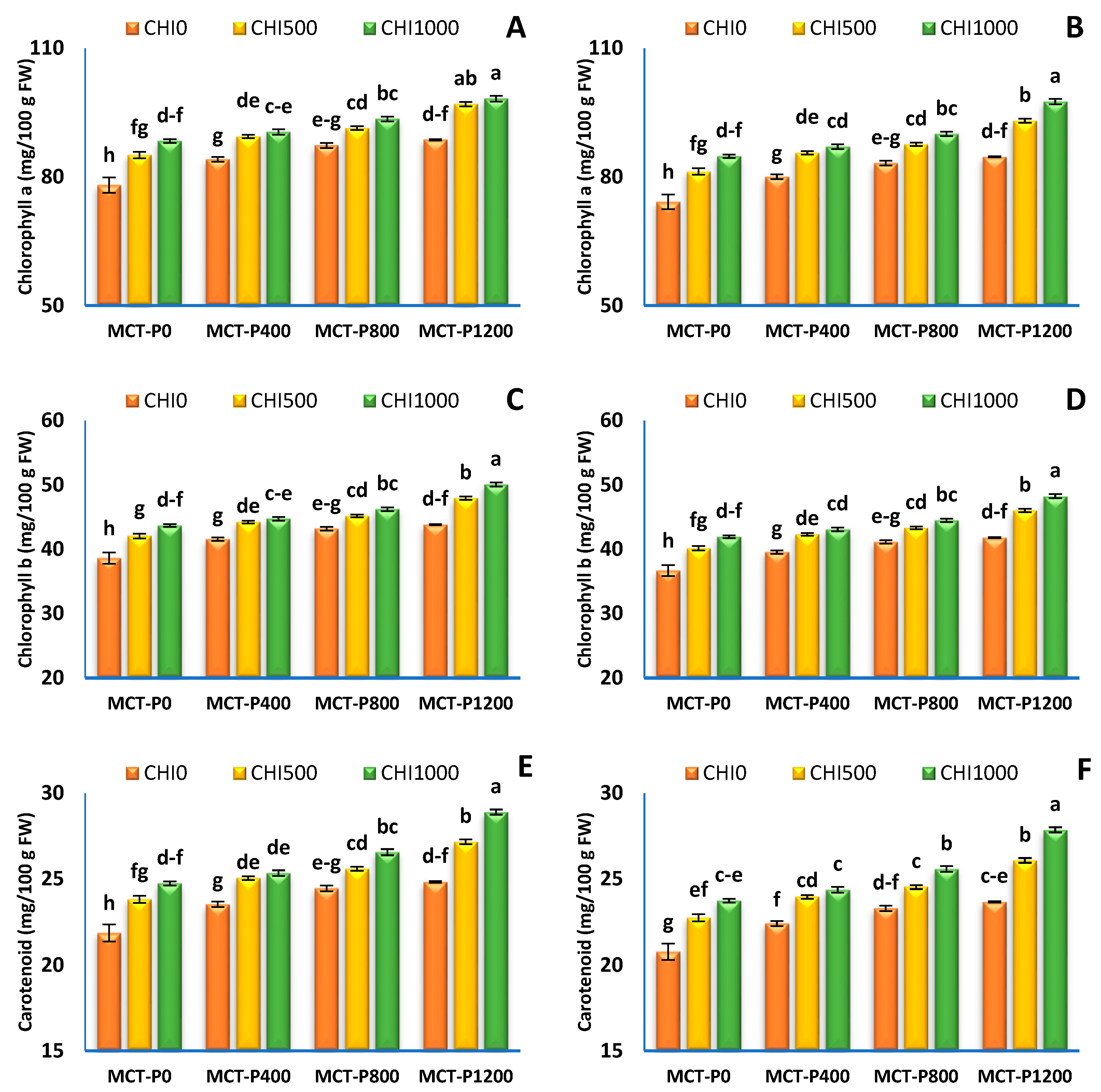
3.2. Photosynthetic Pigment Concentration
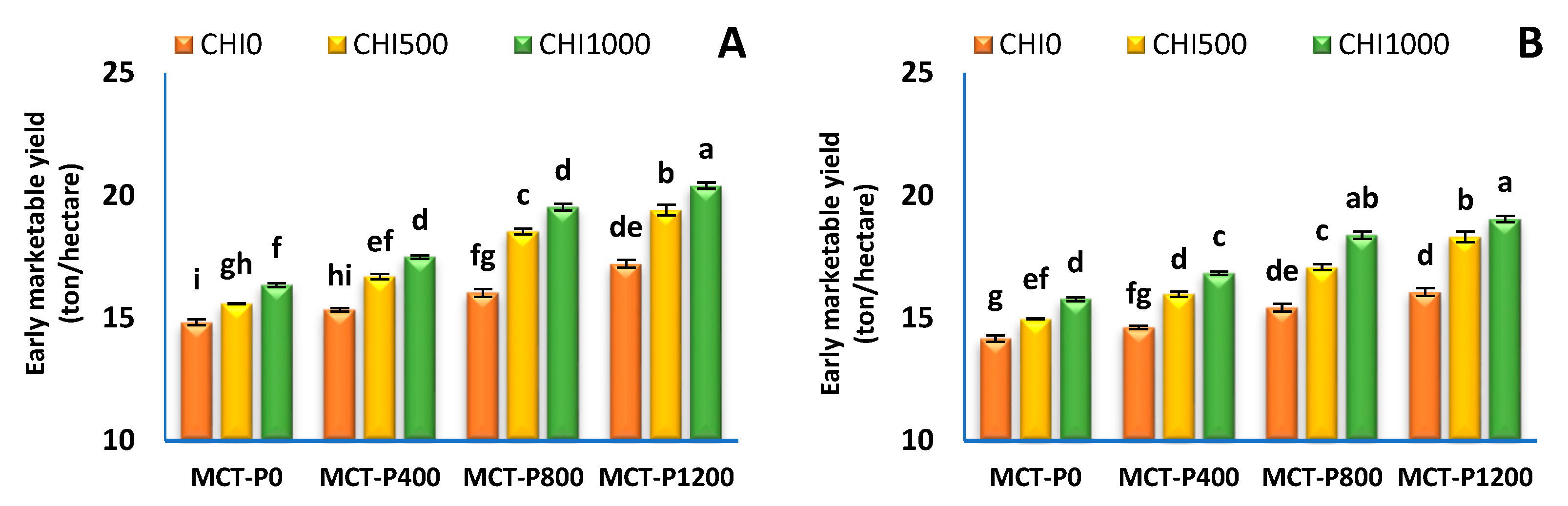
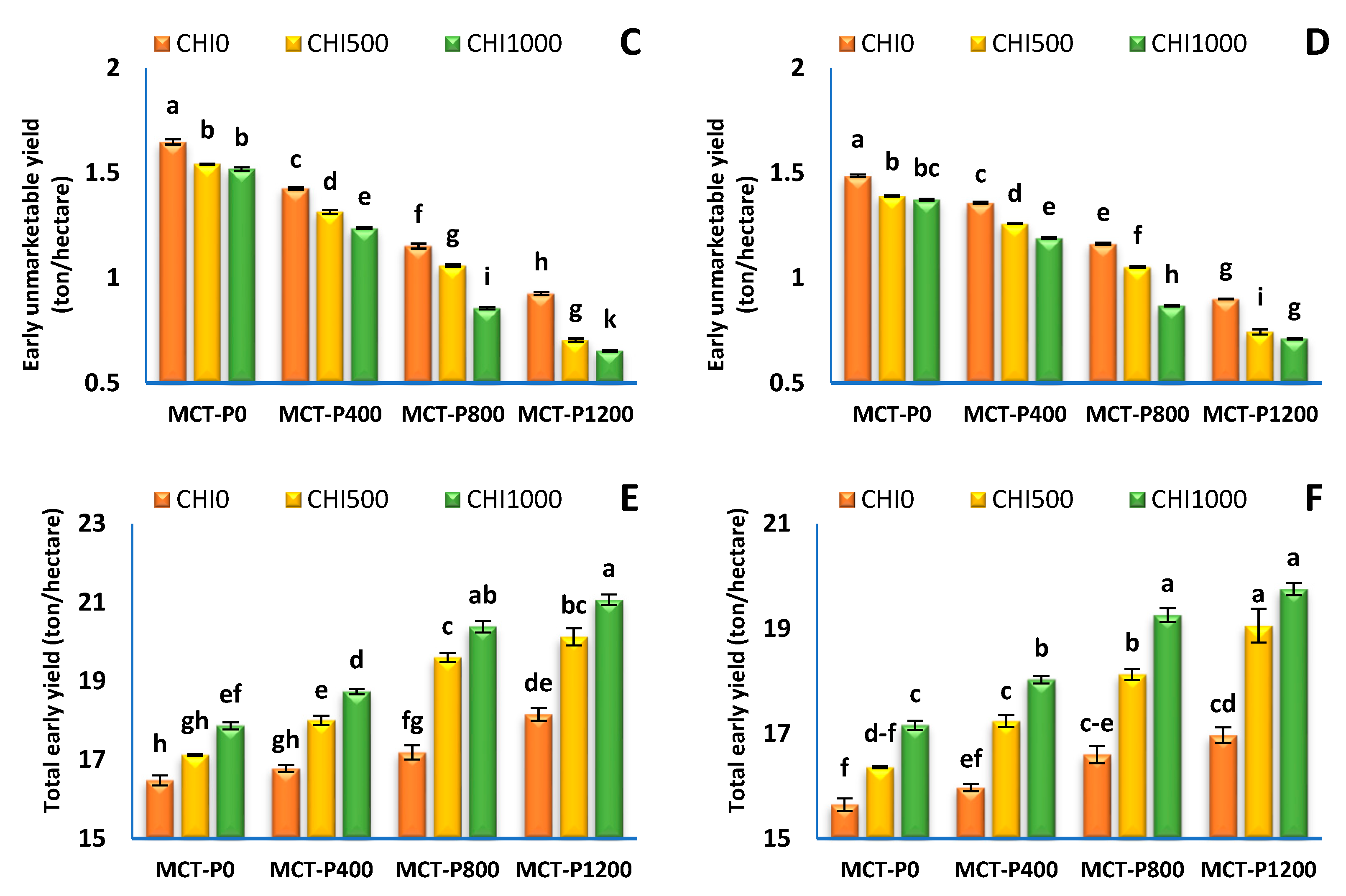
3.3. Fruit Yield and Its Components
3.4. Fruit Quality
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bach-Faig, A.; Berry, E.M.; Lairon, D.; Reguant, J.; Trichopoulou, A.; Dernini, S.; Medina, F.X.; Battino, M.; Belahsen, R.; Miranda, G.; et al. Mediterranean diet foundation expert group. Mediterranean diet pyramid today. Science and cultural updates. Public Health Nutr. 2011, 14, 2274–2284. [Google Scholar] [CrossRef] [PubMed]
- Giampieri, L.; Alvarez-Suarez, J.M.; Mazzoni, L.; Romandini, S.; Bompadre, S.; Diamanti, J.; Capocasa, F.; Mezzetti, B.; Quiles, J.L.; Ferreiro, M.S.; et al. The potential impact of strawberry on human health. Nat. Prod. Res. 2013, 27, 448–455. [Google Scholar] [CrossRef]
- Romandini, S.; Mazzoni, L.; Giampieri, F.; Tulipani, S.; Gasparrini, M.; Forbes-Hernandez, T.Y.; Locorotondo, N.; D’Alessandro, M.; Mezzetti, B.; Bompadre, S.; et al. Effects of an acute strawberry (Fragaria × ananassa) consumption on the plasma antioxidant status of healthy subjects. J. Berry Res. 2013, 3, 169–179. [Google Scholar] [CrossRef]
- Basu, A.; Nguyen, A.; Betts, N.M.; Lyons, T.J. Strawberry as a functional food: An evidence-based review. Crit. Rev. Food Sci. Nutr. 2014, 54, 790–806. [Google Scholar] [CrossRef] [PubMed]
- Nishu, S.M.; Bandral, J.D. Nutritional benefits and value added products of strawberry. Indian Farmer 2021, 8, 67–73. [Google Scholar]
- Hannum, S.M. Potential impact of strawberries on human health: A review of the science. Crit. Rev. Food Sci. Nutr. 2004, 44, 1–17. [Google Scholar] [CrossRef] [PubMed]
- FAOSTAT—Food and Agriculture Organization Corporate Statistical Database 2022. FAO Online Database. Available online: http://www.fao.org/faostat/en/#data (accessed on 1 March 2023).
- Sharma, K.; Negi, M. Effect of organic manures and inorganic fertilizers on plant growth of strawberry (Fragaria x ananassa) cv. Shimla delicious under mid-hill conditions of Uttarakhand. J. Pharmacogn. Phytochem. 2019, 8, 1440–1444. [Google Scholar]
- Wang, S.Y. Antioxidant and health benefits of Strawberry. Acta Hortic. 2014, 1049, 49–62. [Google Scholar] [CrossRef]
- Giampieri, F.; Forbes-Hernandez, T.Y.; Gasparrini, M.; Alvarez-Suarez, J.M.; Afrin, S.; Bompadre, S.; Quiles, J.L.; Mezzettia, B.; Battino, M. Strawberry as a health promoter: An evidence-based review. Food Funct. 2015, 6, 1386–1398. [Google Scholar] [CrossRef]
- Mohamed, F.A.; Gaber, S.M. Effect of organic manure and chemical fertilization on the growth, yield and quality characteristics of strawberry. J. Agric. Sci. Mansoura Univ. 2002, 27, 561–572. [Google Scholar] [CrossRef]
- Ahmad, H.; Sajjid, M.; Hayat, S.; Ullah, R.; Ali, M.; Jamal, A.; Rahman, A.; Aman, Z.; Ali, J. Growth, yield and fruit quality of strawberry (Frageria ananasa Dutch) under different phosphorus levels. Res. Agric. 2017, 2, 19–28. [Google Scholar] [CrossRef]
- Muhaba, S.; Dakora, F. Symbiotic performance, shoot biomass and water-use efficiency of three groundnut (Arachis hypogaea L.) genotypes in response to phosphorus supply under field conditions in Ethiopia. Front. Agric. Sci. Eng. 2020, 7, 455–466. [Google Scholar] [CrossRef]
- Dikr, W.; Abayechaw, D. Effects of phosphorus fertilizer on agronomic, grain yield and other physiological traits of some selected legume crops. J. Biol. Agric. Healthc. 2022, 12, 1–13. [Google Scholar]
- Gong, H.; Xiang, Y.; Wako, B.K.; Jiao, X. Complementary effects of phosphorus supply and planting density on maize growth and phosphorus use efficiency. Front. Plant Sci. 2022, 13, 983788. [Google Scholar] [CrossRef] [PubMed]
- Taiz, L.; Zeiger, E. Fisiologia Vegetal, 5th ed.; Artmed: Porto Alegre, Brazil, 2013; 918p. [Google Scholar]
- Johan, P.D.; Ahmed, O.H.; Omar, L.; Hasbullah, N.A. Phosphorus transformation in soils following co-application of charcoal and wood ash. Agronomy 2021, 11, 2010. [Google Scholar] [CrossRef]
- Malhi, S.S.; Haderlein, L.K.; Pauly, D.G.; Johnston, A.M. Improving fertilizer phosphorus use efficiency. Better Crops 2022, 86, 8–9. [Google Scholar]
- Antón-Herrero, R.; García-Delgado, C.; Mayans, B.; Camacho-Arévalo, R.; Eymar, E. Impact of new micro carbon technology-based fertilizers on growth, nutrient efficiency and root cell morphology of Capsicum annuum L. Agronomy 2020, 10, 1165. [Google Scholar] [CrossRef]
- Antón-Herrero, R.; García-Delgado, C.; Mayans, B.; Camacho-Arévalo, R.; Delgado-Moreno, L.; Eymar, E. Biostimulant effects of micro carbon technology (MCT®)-based fertilizers on soil and Capsicum annuum culture in growth chamber and field. Agronomy 2022, 12, 70. [Google Scholar] [CrossRef]
- Crawford, T. Use of Micro Carbon Technology to Improve Phosphorus Availability. SW Ag Summit. Available online: https://sbsoliplant.nl/en/mct/ (accessed on 17 November 2022).
- Bibi, A.; Ibrar, M.; Shalmani, A.; Rehan, T.; Quratulain. A review on recent advances in chitosan applications. Pure Appl. Biol. 2021, 10, 1217–1229. [Google Scholar] [CrossRef]
- Yu, J.; Wang, D.; Geetha, N.; Khawar, K.M.; Jogaiah, S.; Mujtaba, M. Current trends and challenges in the synthesis and applications of chitosan-based nanocomposites for plants: A review. Carbohydr. Polym. 2021, 261, 117904. [Google Scholar] [CrossRef] [PubMed]
- Bakhoum, G.S.; Sadak, M.S.; Tawfik, M.M. Chitosan and chitosan nanoparticle effect on growth, productivity and some biochemical aspects of Lupinus termis L. pant under drought conditions. Egypt. J. Chem. 2022, 65, 537–549. [Google Scholar]
- El Hadrami, A.; Adam, L.; El Hadrami, I.; Daayf, F. Chitosan in plant protection. Mar. Drugs 2010, 8, 968–987. [Google Scholar] [CrossRef] [PubMed]
- Algam, S.; Xie, G.; Li, B.; Yu, S.; Su, T.; Larsen, J. Effects of Paenibacillus strains and chitosan on plant growth promotion and control of Ralstonia wilt in tomato. J. Plant Pathol. 2010, 92, 593–600. [Google Scholar]
- Solgi, M. The application of new environmentally friendly compounds on postharvest characteristics of cut carnation (Dianthus caryophyllus L.). Braz. J. Bot. 2018, 41, 515–522. [Google Scholar] [CrossRef]
- Nguyen, T.; Thi, T.; Nguyen, T.T.; Le, T.; Vo, D.; Nguyen, D.; Nguyen, C.; Nguyen, D.; Nguyen, T.; Bach, L. Investigation of chitosan nanoparticles loaded with protocatechuic acid (Pca) for the resistance of Pyricularia oryzae fungus against rice blast. Polymers 2019, 11, 177. [Google Scholar]
- Hidangmayum, A.; Dwivedi, P.; Katiyar, D.; Hemantaranjan, A. Application of chitosan on plant responses with special reference to abiotic stress. Physiol. Mol. Biol. Plants 2019, 25, 313–326. [Google Scholar] [CrossRef]
- Farouk, S.; Ghoneem, K.M.; Ali, A.A. Induction and expression of systemic resistance to downy mildew disease in cucumber by elicitors. Egypt. J. Phytopathol. 2008, 36, 95–111. [Google Scholar]
- Farouk, S.; Mosa, A.A.; Taha, A.A.; Ibrahim, H.M.; El-Gahmery, A.M. Protective effect of humic acid and chitosan on radish (Raphanus sativus L. var. sativus) plants subjected to cadmium stress. J. Stress Physiol. Biochem. 2011, 7, 99–116. [Google Scholar]
- Farouk, S.; Ramadan, A.A. Improving growth and yield of cowpea by foliar application of chitosan under water stress. Egypt. J. Biol. 2012, 14, 14–26. [Google Scholar] [CrossRef]
- Helaly, M.; Farouk, S.; Arafa, S.A.; Amhimmid, N.B. Inducing salinity tolerance of rosemary (Rosmarinus Officinalis, L.) plants by chitosan or zeolite application. Asian J. Adv. Agric. Res. 2018, 5, 34. [Google Scholar] [CrossRef]
- Farouk, S.; Al-Sanoussi, A.J. The role of biostimulants in increasing barley plant growth and yield under newly cultivated sany soil. Cercet. Agron. În Mold. 2019, 2, 114–125. [Google Scholar]
- Faqir, Y.; Ma, J.; Chai, Y. Chitosan in modern agriculture production. Plant Soil Environ. 2021, 67, 679–699. [Google Scholar] [CrossRef]
- Khalil, H.A.; Badr Eldin, R.M. Chitosan improves morphological and physiological attributes of grapevines under deficit irrigation conditions. J. Hortic. Res. 2021, 29, 9–22. [Google Scholar] [CrossRef]
- Bittelli, M.; Flury, M.; Campbell, G.S.; Nichols, E.J. Reduction of transpiration through foliar application of chitosan. Agric. For. Meteorol. 2001, 107, 167–175. [Google Scholar] [CrossRef]
- Rahman, M.; Mukta, J.A.; Sabir, A.A.; Gupta, D.R.; Mohi-Ud-Din, M.; Hasanuzzaman, M.; Miah, M.G.; Rahman, M.; Islam, M.T. Chitosan biopolymer promotes yield and stimulates accumulation of antioxidants in strawberry fruit. PLoS ONE 2018, 13, e0203769. [Google Scholar] [CrossRef]
- Khordadi Varamin, J.; Fanoodi, F.; Masoud Sinaki, J.; Rezvan, S.; Damavandi, A. Foliar application of chitosan and nano-magnesium fertilizers influence on seed yield, oil content, photosynthetic pigments, antioxidant enzyme activities of sesame (Sesamum indicum L.) under water- limited conditions. Not. Bot. Horti Agrobot. Cluj-Napoca 2020, 48, 2228–2243. [Google Scholar] [CrossRef]
- Akhtar, G.; Faried, H.N.; Razzaq, K.; Ullah, S.; Wattoo, F.M.; Shehzad, M.A.; Sajjad, Y.; Ahsan, M.; Javed, T.; Dessoky, E.S.; et al. Chitosan-Induced Physiological and Biochemical Regulations Confer Drought Tolerance in Pot Marigold (Calendula officinalis L.). Agronomy 2022, 12, 474. [Google Scholar] [CrossRef]
- Page, A.L.; Miller, R.H.; Keeney, D.R. Methods of Soil Analysis—Chemical and Microbiology Properties; SSSA Inc.: Madison, WI, USA, 1982. [Google Scholar]
- Lichtenthaler, H.K.; Wellburn, A.R. Determinations of total carotenoids and chlorophylls a and b of leaf extracts in different solvents. Biochem. Soc. Trans. 1983, 11, 591–592. [Google Scholar] [CrossRef]
- Kumar, S.; Kumar, R.; Nambi, V.E.; Gupta, R.K. Postharvest changes in antioxidant capacity, enzymatic activity, and microbial profle of strawberry fruits treated with enzymatic and divalent ions. Food Bioprocess. Technol. 2014, 7, 2060–2070. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis, 20th ed.; Association of Official Analytical Chemists: Arlington, VA, USA, 1990. [Google Scholar]
- Mazumdar, B.C.; Majumder, K. Methods on Physico-Chemical Analysis of Fruits; Daya Publishing House: New Delhi, India, 2003. [Google Scholar]
- Sadasivam, S.; Manickam, A. Biochemical Methods, 3rd ed.; New Age International (P) Ltd. Publishers: New Delhi, India, 2008. [Google Scholar]
- Norman, G.R.; Streiner, D.L. PDQ Statistics, 3rd ed.; BC Deckker Inc.: London, UK, 2003. [Google Scholar]
- Marschner, H. Mineral Nutrition of Higher Plants, 2nd ed.; Academic Press Inc.: San Diego, CA, USA, 1990. [Google Scholar]
- Ibrahim, M.; Iqbal, M.; Tang, Y.-T.; Khan, S.; Guan, D.-X.; Li, G. Phosphorus mobilization in plant–soil environments and inspired strategies for managing phosphorus: A review. Agronomy 2022, 12, 2539. [Google Scholar] [CrossRef]
- Sultana, S.; Islam, M.; Khatun, M.A.; Hassain, M.A.; Huque, R. Effect of foliar application of oligo-chitosan on growth, yield and quality of tomato and eggplant. Asian J. Agric. Res. 2017, 11, 36–42. [Google Scholar] [CrossRef]
- Khan, W.M.; Prithiviraj, B.; Smith, D.L. Effect of foliar application of chitin oligosaccharides on photosynthesis of maize and soybean. Photosynthetica 2002, 40, 621–624. [Google Scholar] [CrossRef]
- Hameed, A.; Sheikh, M.; Hameed, A.; Farooq, T.; Basra, S.; Jamil, A. Chitosan priming enhances the seed germination, antioxidants, hydrolytic enzymes, soluble proteins and sugars in wheat seeds. Agrochimica 2013, 67, 32–46. [Google Scholar]
- Dzung, N.A.; Khanh, V.T.P.; Dzung, T.T. Research on impact of chitosan oligomers on biophysical characteristics, growth, development and drought resistance of coffee. Carbohydr. Polym. 2011, 84, 751–755. [Google Scholar] [CrossRef]
- Farouk, S.; Belal, B.E.A.; El-Sharkawy, H.H.A. The role of some elicitors on the management of Roumy Ahmar grapevines downy mildew disease and it’s related to inducing growth and yield characters. Sci. Hort. 2017, 225, 646–658. [Google Scholar] [CrossRef]
- Chibu, H.; Shibayama, H. Effects of Chitosan Applications on the Growth of Several Crops. In Proceedings of the Eighth International Chitin and Chitosan Conference and Fourth Asia Pacific Chitin and Chitosan Symposium, Yamaguchi, Japan, 21–23 September 2000; pp. 235–239. [Google Scholar]
- Bistgani, Z.E.; Hashemi, M.; Hamid, R. Fertilizer source and chitosan effect on productivity, nutrient accumulation, and phenolic compounds of Thymus daenensis Celak. Agron. J. 2021, 113, 5499–5515. [Google Scholar] [CrossRef]
- Mohamed, R.A.; Abd El-Aal, H.; Abd El-Aziz, M.G. Effects of phosphorus, zinc and their interactions on vegetative growth characters, yield and fruit quality of strawberry. J. Hort. Sci. 2011, 3, 106–114. [Google Scholar]
- Wondimkun, D.; Hailu, G. Evaluation of the agronomic traits and grain yield of mung bean (Vigna radiata L.) by different levels of phosphorus fertilizer with row spacings at abine germama kebele in Adamitulu Jido kombolcha Wereda. J. Biol. Agric. Healthc. 2022, 12, 25–36. [Google Scholar]
- Landi, L.; De MiccolisAngelini, R.M.; Pollastro, E.; Feliziani, S.; Faretra, F.; Romanazzi, G. Global transcriptome analysis and identification of differentially expressed genes in strawberry after preharvest application of Benzothiadiazole and chitosan. Front. Plant Sci. 2017, 8, 235. [Google Scholar] [CrossRef] [PubMed]
- Arega, A.; Zenebe, M. Common bean (Phaseolus vulgaris L.) varieties response to rates of blended NPKSB fertilizer at Arba Minch, Southern Ethiopia. In Proceedings of the Advances in Crop Science and Technology, Workshop, Addis Ababa, Ethiopia, 1–3 October 1990; 114p. [Google Scholar]
- Abdel-Mawgoud, A.M.R.; Tantawy, A.S.; El-Nemr, M.A.; Sassine, Y.N. Growth and yield responses of strawberry plants to chitosan application. Eur. J. Sci. Res. 2010, 39, 161–168. [Google Scholar]
- Balibrea, M.A.; Andujar, C.M.; Cuartero, J.; Bolarin, M.C.; Alfocea, F.P. The high fruit soluble sugar content in wild Lycopersicon species and their hybrids with cultivars depends on sucrose import during ripening rather than on sucrose metabolism. Funct. Plant Biol. 2006, 33, 279–288. [Google Scholar] [CrossRef]
- El-Miniawy, S.M.; Ragab, M.E.; Youssef, S.M.; Metwally, A.A. Response of strawberry plants to foliar spraying of chitosan. Res. J. Agric. Biol. Sci. 2013, 9, 366–372. [Google Scholar]
- Mozos, I.; Flangea, C.; Vlad, D.C.; Gug, C.; Mozos, C.; Stoian, D.; Luca, C.T.; Horbańczuk, J.O.; Horbańczuk, O.K.; Atanasov, A.G. Effects of anthocyanins on vascular health. Biomolecules 2021, 11, 811. [Google Scholar] [CrossRef] [PubMed]
- Aaby, K.; Skrede, G.; Wrolstad, R.E. Phenolic composition and antioxidant activities in flesh and achenes of strawberries (Fragaria ananassa). J. Agric. Food Chem. 2005, 53, 4032–4040. [Google Scholar] [CrossRef] [PubMed]
- Stoner, G.D.; Huang, C. Molecular mechanisms involved in chemoprevention of black raspberry extracts: From transcription factors to their target genes. Nutr. Cancer 2006, 54, 69–78. [Google Scholar]
- Zhang, D.; Quantick, P.C. Effects of chitosan coating on enzymatic browning and decay during postharvest storage of litchi (Litchi chinensis Son.) fruit. Postharv. Biol. Technol. 1997, 12, 195–202. [Google Scholar] [CrossRef]
- Ekinci, M.; Turan, M.; Yildirim, E.; Güneş, A.; Kotan, R.; Dursun, A. Effect of plant growth promoting rhizobac_teria on growth, nutrient, organic acid, amino acid and hormone content of cauliflower (Brassica oleracea L. var. botrytis) transplants. Acta Sci. Pol. Hortorum Cultus 2014, 13, 71–85. [Google Scholar]
- Scalzo, J.; Politi, A.; Pellegrini, N.; Mezzetti, B.; Battino, M. Plant genotype affects total antioxidant capacity and phenolic contents in fruit. Nutrition 2005, 21, 207–213. [Google Scholar] [CrossRef] [PubMed]
- Sójka, M.; Miszczak, A.; Sikorski, P.; Zagibajło, K.; Karlińska, E.; Kosmala, M. Pesticide residue levels in strawberry processing by-products that are rich in ellagitannins and an assessment of their dietary risk to consumers. NFS J. 2015, 1, 31–37. [Google Scholar] [CrossRef]
- Xoca-Orozco, L.Á.; Cuellar-Torres, E.A.; González-Morales, S.; Gutiérrez-Martínez, P.; López-García, U.; Herrera-Estrella, L.; Vega-Arreguín, J.; Chacón-López, A. Transcriptomic analysis of avocado hass (Persea americana Mill) in the interaction system fruit-chitosan-Colletotrichum. Front. Plant Sci. 2017, 8, 956. [Google Scholar] [CrossRef] [PubMed]
- Rouached, H.; Arpat, A.B.; Poirier, Y. Regulation of phosphate starvation responses in plants signaling players and cross-talks. Mol. Plant 2010, 3, 288–299. [Google Scholar] [CrossRef] [PubMed]
- Niu, Y.; Chai, R.; Dong, H. Effect of elevated CO2 on phosphorus nutrition of phosphate-deficient Arabidopsis thaliana (L.) Heynh under different nitrogen formsm. J. Exp. Bot. 2012, 64, 355–367. [Google Scholar] [CrossRef] [PubMed]
- Johnson, C.R. Phosphorus nutrition on mycorrhizal colonization, photosynthesis, growth and nutrient composition of Citrus aurantium. Plant Soil 1984, 80, 35–42. [Google Scholar] [CrossRef]
- Fredeen, A.L.; Rao, I.M.; Terry, N. Influence of phosphorus nutrition on growth and carbon partitioning in Glycine max. Plant Physiol. 1989, 89, 225–230. [Google Scholar] [CrossRef] [PubMed]



| Seasons | Silt (%) | Clay (%) | Sand (%) | Texture Soil | pH | E.C (dSm−1) | Organic Matter (%) | CaCO3 (%) | N (mg/Kg Soil) | P (mg/Kg Soil) | K (meq/100 g Soil) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 2020/2021 | 39.5 | 35.2 | 25.3 | Clay loamy | 8.12 | 1.63 | 1.78 | 3.88 | 276 | 32 | 6.79 |
| 2021/2022 | 39.7 | 35.5 | 24.8 | Clay loamy | 8.19 | 1.69 | 1.61 | 3.61 | 266 | 30 | 6.66 |
| Treatments | Plant Height (cm). | Foliage FW (g/Plant) | Secondary Crown No/Plant | Leaf No/Plant | Leaf Area (cm2)/Plant | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| S1 | S2 | S1 | S2 | S1 | S2 | S1 | S2 | S1 | S2 | |
| Micro-carbon phosphorus (MCT-P, mgL−1) | ||||||||||
| MCT-P 0 | 19.47 ± 0.19 c | 18.59 ± 0.16 c | 118.1 ± 1.43 c | 109.8 ± 1.1 c | 6.72 ± 0.07 c | 6.41 ± 0.07 c | 39.46 ± 0.46 c | 37.69 ± 0.48 c | 1053 ± 11 c | 989 ± 10 c |
| MCT-P 400 | 19.95 ± 0.23 b | 19.10 ± 0.28 b | 121.1 ± 1.55 b | 112.5 ± 1.3 a | 6.88 ± 0.08 b | 6.59 ± 0.08 b | 43.68 ± 0.46 b | 41.80 ± 0.50 b | 1080 ± 13 b | 1014 ± 11 b |
| MCT-P 800 | 20.53 ± 0.24 a | 19.67 b ± 0.26 a | 124.5 ± 1.59 a | 115.7 ± 1.3 a | 7.08 ± 0.08 a | 6.79 ± 0.09 a | 44.21 ± 0.82 b | 42.38 ± 0.85 b | 1111 ± 13 a | 1043 ± 12 a |
| MCT-P 1200 | 20.74 ± 0.25 a | 19.90 ± 0.26 a | 125.8 ± 1.71 a | 116.9 ± 1.4 a | 7.15 ± 0.09 a | 6.86 ± 0.09 a | 45.94 ± 0.57 a | 44.08 ± 0.61 a | 1122 ± 14 a | 1054 ± 13 a |
| ANOVA p values | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** |
| Chitosan (CHI mgL−1) | ||||||||||
| CHI 0 | 19.34 ± 0.13 c | 18.41 ± 0.15 c | 116.9 ± 0.9 c | 109.1 ± 0.7 c | 6.67 ± 0.05 c | 6.35 ± 0.04 c | 41.23 ± 0.65 c | 39.26 ± 0.64 c | 1044 ± 7 c | 983 ± 6 c |
| CHI 500 | 20.32 ± 0.18 b | 19.46 ± 0.15 b | 123.4 ± 1.1 b | 114.5 ± 1.0 b | 7.01 ± 0.06 b | 6.71 ± 0.06 b | 43.67 ± 0.79 b | 41.83 ± 0.78 b | 1100 ± 10 b | 1032 ± 9 b |
| CHI 1000 | 20.86 ± 0.16 a | 20.08 ± 0.22 a | 126.9 ± 1.0 a | 117.6 ± 0.9 a | 7.19 ± 0.06 a | 6.92 ± 0.05 a | 45.06 ± 0.76 a | 43.36 ± 0.75 a | 1131 ± 9 a | 1060 ± 8 a |
| ANOVA p values | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** |
| Treatments | Chlorophylla (mg/100 g FW) | Chlorophyllb (mg/100 g FW) | Carotenoids (mg/100 g g FW) | |||
|---|---|---|---|---|---|---|
| S1 | S2 | S1 | S2 | S1 | S2 | |
| Micro-carbon phosphorus (MCT-P, mgL−1) | ||||||
| MCT-P 0 | 83.83 ± 1.61 d | 80.07 ± 1.65 d | 41.45 ± 0.79 d | 39.60 ± 0.81 d | 23.48 ± 0.45 d | 22.43 ± 0.46 d |
| MCT-P 400 | 87.99 ± 1.02 c | 84.22 ± 1.09 c | 43.51 ± 0.50 c | 41.65 ± 0.54 c | 24.65 ± 0.28 c | 23.59 ± 0.30 c |
| MCT-P 800 | 90.72 ± 0.93 b | 86.95 ± 1.02 b | 44.86 ± 0.46 b | 43.00 ± 0.50 b | 25.54 ± 0.31 b | 24.48 ± 0.33 b |
| MCT-P 1200 | 94.61 ± 1.52 a | 91.74 ± 1.92 a | 47.28 ± 0.92 a | 45.37 ± 0.95 a | 26.96 ± 059 a | 25.87 ± 0.60 a |
| ANOVA p values | *** | *** | *** | *** | *** | *** |
| Chitosan (CHI mgL−1) | ||||||
| CHI 0 | 84.53 ± 1.30 c | 80.50 ± 1.27 c | 41.80 ± 0.64 c | 39.81 ± 0.62 c | 23.68 ± 0.36 c | 22.55 ± 0.35 c |
| CHI 500 | 90.71 ± 1.31 b | 86.88 ± 1.30 b | 44.86 ± 0.64 b | 42.97 ± 0.64 b | 25.41 ± 0.36 b | 24.34 ± 0.36 b |
| CHI 1000 | 92.62 ± 1.14 a | 89.85 ± 1.47 a | 46.17 ± 0.73 a | 44.43 ± 0.72 a | 26.39 ± 0.47 a | 25.39 ± 0.47 a |
| ANOVA p values | *** | *** | *** | *** | *** | *** |
| Treatments | Early Yield (Ton/Hectare) | Total Yield (Ton/Hectare) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Marketable | Unmarketable | Total | Marketable | Unmarketable | Total | |||||||
| S1 | S2 | S1 | S2 | S1 | S2 | S1 | S2 | S1 | S2 | S1 | S2 | |
| Micro-carbon phosphorus (MCT-P, mgL−1) | ||||||||||||
| MCT-P 0 | 15.59 ± 0.22 d | 14.97 ± 0.23 d | 1.570 ± 0.020 a | 1.416 ± 0.018 a | 17.16 ± 0.20 d | 16.39 ± 0.22 d | 67.92 ± 0.54 d | 64.60 ± 0.62 d | 3.544 ± 0.083 a | 3.656 ± 0.092 a | 71.46 ± 0.47 d | 68.25 ± 0.54 d |
| MCT-P 400 | 16.52 ± 0.31 c | 15.81 ± 0.32 c | 1.326 ± 0.027 b | 1.269 ± 0.024 b | 17.84 ± 0.29 c | 17.08 ± 0.30 c | 69.02 ± 0.66 c | 66.04 ± 0.79 c | 3.096 ± 0.049 b | 2.980 ± 0.111 b | 72.11 ± 0.61 c | 69.02 ± 0.68 c |
| MCT-P 800 | 18.04 ± 0.52 b | 16.97 ± 0.43 b | 1.022 ± 0.043 c | 1.027 ± 0.043 bc | 19.06 ± 0.48 b | 17.99 ± 0.39 b | 71.37 ± 1.08 b | 68.38 ± 1.15 b | 2.395 ± 0.108 c | 2.318 ± 0.109 c | 73.77 ± 0.99 b | 70.70 ± 1.04 b |
| MCT-P 1200 | 19.02 ± 0.47 a | 17.81 ± 0.46 a | 0.761 ± 0.042 d | 0.785 ± 0.029 c | 19.78 ± 0.43 a | 18.60 ± 0.43 a | 73.08 ± 0.97 a | 70.00 ± 1.01 a | 2.092 ± 0.118 d | 2.129 ± 0.077 d | 75.18 ± 0.87 a | 72.13 ± 0.93 a |
| ANOVA p values | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** |
| Chitosan (CHI mgL−1) | ||||||||||||
| CHI 0 | 15.86 ± 0.27 c | 15.07 ± 0.22 c | 1.288 ± 0.08 a | 1.226 ± 0.066 a | 17.15 ± 0.20 c | 16.30 ± 0.16 c | 67.49 ± 0.44 c | 64.10 ± 0.47 c | 3.101 ± 0.15 a | 3.119 ± 0.188 a | 70.60 ± 0.35 c | 67.22 ± 0.35 c |
| CHI 500 | 17.56 ± 0.45 b | 16.59 ± 0.38 b | 1.155 ± 0.09 b | 1.111 ± 0.073 b | 18.72 ± 0.36 b | 17.70 ± 0.31 b | 70.68 ± 0.73 b | 67.59 ± 0.75 b | 2.757 ± 0.16 b | 2.744 ± 0.172 b | 73.44 ± 0.58 b | 70.34 ± 0.59 b |
| CHI 1000 | 18.45 ± 0.48 a | 17.52 ± 0.38 a | 1.066 ± 0.10 c | 1.035 ± 0.078 c | 19.52 ± 0.38 a | 18.55 ± 0.31 a | 72.87 ± 0.77 a | 70.07 ± 0.76 a | 2.488 ± 0.19 c | 2.448 ± 0.184 c | 75.36 ± 0.58 a | 72.51 ± 0.59 a |
| ANOVA p values | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** |
| Treatments | Average Fruit Weight (g) | Fruit Firmness (kg/ cm2) | Fruit Dry Matter (%) | Total Soluble Solid Content (Brix) | ||||
|---|---|---|---|---|---|---|---|---|
| S1 | S2 | S1 | S2 | S1 | S2 | S1 | S2 | |
| Micro-carbon phosphorus (MCT-P, mgL−1) | ||||||||
| MCT-P 0 | 20.66 ± 0.18 b | 19.73 ± 0.20 c | 361.1 ± 4.6 c | 339.2 ± 4.3 d | 8.89 ± 0.08 c | 8.78 ± 0.09 c | 5.56 ± 0.10 d | 5.31 ± 0.10 d |
| MCT-P 400 | 20.94 ± 0.23 b | 20.03 ± 0.25 b | 367.1 ± 5.2 c | 350.2 ± 5.0 c | 9.14 ± 0.11 b | 9.00 ± 0.11 b | 5.83 ± 0.06 c | 5.58 ± 0.07 c |
| MCT-P 800 | 21.54 ± 0.30 a | 20.64 ± 0.31 a | 387.2 ± 6.0 b | 363.7 ± 5.7 b | 9.39 ± 0.11 a | 9.26 ± 0.11 a | 6.04 ± 0.07 b | 5.79 ± 0.07 b |
| MCT-P 1200 | 21.68 ± 0.22 a | 20.80 ± 0.24 a | 396.9 ± 6.3 a | 370.9 ± 5.9 a | 9.53 ± 0.11 a | 9.36 ± 0.12 a | 6.38 ± 0.14 a | 6.12 ± 0.14 a |
| ANOVA p values | *** | *** | *** | *** | *** | *** | *** | *** |
| Chitosan (CHI mgL−1) | ||||||||
| CHI 0 | 20.39 ± 0.11 c | 19.41 ± 0.11 c | 358.1 ± 3.7 c | 337.2 ± 3.1 c | 8.86 ± 0.07 c | 8.70 ± 0.06 c | 5.60 ± 0.08 c | 5.33 ± 0.08 c |
| CHI 500 | 21.29 ± 0.15 b | 20.39 ± 0.15 b | 380.7 ± 4.7 b | 358.5 ± 4.0 b | 9.29 ± 0.08 b | 9.17 ± 0.08 b | 6.01 ± 0.09 b | 5.76 ± 0.08 b |
| CHI 1000 | 21.93 ± 0.17 a | 21.10 ± 0.16 a | 395.4 ± 5.1 a | 372.3 ± 4.3 a | 9.56 ± 0.08 a | 9.43 ± 0.07 a | 6.24 ± 0.11 a | 6.01 ± 0.11 a |
| ANOVA p values | *** | *** | *** | *** | *** | *** | *** | *** |
| Treatments | Acidity (%) | Total Sugar (%) | Ascorbic Acid (mg/100 g FW) | Anthocyanin (mg/100 g FW) | ||||
|---|---|---|---|---|---|---|---|---|
| S1 | S2 | S1 | S2 | S1 | S2 | S1 | S2 | |
| Micro-carbon phosphorus (MCT-P, mgL−1) | ||||||||
| MCT-P 0 | 0.651 ± 0.017 c | 0.637 ± 0.009 c | 3.49 ± 0.03 d | 3.33 ± 0.04 d | 59.23 ± 0.60 d | 56.57 ± 0.65 d | 54.36 ± 0.54 c | 51.92 ± 0.59 c |
| MCT-P 400 | 0.684 ± 0.010 b | 0.655 ± 0.010 b | 3.61 ± 0.05 c | 3.45 ± 0.05 c | 60.72 ± 0.71 c | 58.11 ± 0.77 c | 55.72 ± 0.65 b | 53.33 ± 0.70 b |
| MCT-P 800 | 0.704 ± 0.010 a | 0.647 ± 0.011 a | 3.79 ± 0.06 b | 3.63 ± 0.06 b | 63.02 ± 0.97 b | 60.40 ± 1.02 b | 57.29 ± 0.67 a | 54.91 ± 0.72 a |
| MCT-P 1200 | 0.711 ± 0.010 a | 0.682 ± 0.011 a | 3.91 ± 0.08 a | 3.75 ± 0.08 a | 64.55 ± 0.78 a | 61.94 ± 0.84 a | 57.96 ± 0.71 a | 55.61 ± 0.76 a |
| ANOVA p values | *** | *** | *** | *** | *** | *** | *** | *** |
| Chitosan (CHI mgL−1) | ||||||||
| CHI 0 | 0.643 ± 0.006 c | 0.624 ± 0.004 c | 3.49 ± 0.03 c | 3.32 ± 0.03 c | 59.21 ± 0.54 c | 56.39 ± 0.53 c | 54.02 ± 0.38 c | 51.44 ± 0.38 c |
| CHI 500 | 0.697 ± 0.007 b | 0.667 ± 0.006 b | 3.72 ± 0.05 b | 3.56 ± 0.05 b | 62.09 ± 0.67 b | 59.48 ± 0.67 b | 56.75 ± 0.51 b | 54.36 ± 0.52 b |
| CHI 1000 | 0.722 ± 0.006 a | 0.695 ± 0.005 a | 3.89 ± 0.06 a | 3.74 ± 0.06 a | 64.33 ± 0.73 a | 61.90 ± 0.73 a | 58.23 ± 0.46 a | 56.03 ± 0.46 a |
| ANOVA p values | *** | *** | *** | *** | *** | *** | *** | *** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Metwaly, E.-S.E.; AL-Huqail, A.A.; Farouk, S.; Omar, G.F. Effect of Chitosan and Micro-Carbon-Based Phosphorus Fertilizer on Strawberry Growth and Productivity. Horticulturae 2023, 9, 368. https://doi.org/10.3390/horticulturae9030368
Metwaly E-SE, AL-Huqail AA, Farouk S, Omar GF. Effect of Chitosan and Micro-Carbon-Based Phosphorus Fertilizer on Strawberry Growth and Productivity. Horticulturae. 2023; 9(3):368. https://doi.org/10.3390/horticulturae9030368
Chicago/Turabian StyleMetwaly, El-Saied E., Arwa Abdulkreem AL-Huqail, Saad Farouk, and Genesia F. Omar. 2023. "Effect of Chitosan and Micro-Carbon-Based Phosphorus Fertilizer on Strawberry Growth and Productivity" Horticulturae 9, no. 3: 368. https://doi.org/10.3390/horticulturae9030368
APA StyleMetwaly, E.-S. E., AL-Huqail, A. A., Farouk, S., & Omar, G. F. (2023). Effect of Chitosan and Micro-Carbon-Based Phosphorus Fertilizer on Strawberry Growth and Productivity. Horticulturae, 9(3), 368. https://doi.org/10.3390/horticulturae9030368







