Abstract
Iron is a vital element for plant and microbial growth; yet, the major portion of iron in soils is in the form of (oxi-)hydroxides with limited bioavailability, resulting in decreased crop yield quality. In response to iron deficiency, soil microorganisms produce siderophores that transform insoluble iron into a soluble form that plants and microorganisms can use. The abundance and activity of siderophore-producing bacteria (SPB) might be used as a biological assessment index for the fertility status of cultivated land. In order to achieve this goal, it is critical to investigate the influences of SPB on plant growth and soil quality. In this study, we performed a mixed-effect model meta-analysis on 342 research studies that compared plant growth with and without SPB. The findings revealed that SPB increased plant growth significantly (up to 30%). The stimulating effects on plants followed the sequences of pant weight, plant height, and germination rate.
1. Introduction
Iron, a vital element for almost all life, is essential for numerous biological activities, including electron transport, oxygen metabolism, nitrogen fixation, DNA and RNA synthesis, etc. [1,2]. Although it is the fourth most abundant metal in the earth’s crust, the bioavailability of iron is very low [3]. The content of soluble iron in the soil is insufficient for the proper development of plants (10−9–10−4 mol/L) [4] and microorganisms (10−7–10−5 mol/L). The soluble iron content in the majority of soils (particularly alkaline soils) is only picomolar (10−9–10−18 mol/L) [5,6,7]. In a highly aerobic alkaline environment, Fe2+ is readily oxidized in soils and forms iron oxides or hydroxides varied in compositions, degrees of crystallization, and solubility [1]. The solubility of Fe3+ (oxi-)hydroxides in soil decreases by a factor of 1000 for every unit increase in pH. Soluble iron is at its lowest concentration when the pH is between 7.5 and 8.5 [8]. Because soluble iron is scarce and largely found in poorly soluble Fe compounds in neutral or alkaline soils, crops suffer from severe iron deficiency [9]. In China, the soil is alkaline in the north, where iron deficiency is more severe, and acid in the southern region. Iron is an essential trace element for plant growth, and the amount of soluble iron has an impact on the health of plants. Plant leaves turn yellow from the veins due to a drop in chlorophyll content brought on by an iron deficit. Severe iron deficiency may cause plant death at the seedling stage, which would reduce agricultural productivity [10]. High iron intake, however, may also result in the release of reactive oxygen species and iron poisoning. Iron concentration needs to be maintained at a steady level for plants to grow properly. In recent years, iron deficiency in plants has been highlighted as one of the greatest agricultural challenges internationally [11].
To provide iron nutrition for themselves or their hosts, TonB-dependent transporters help transfer iron into cells [12]. The siderophore cell membrane’s transport mechanism is shown in Figure 1.
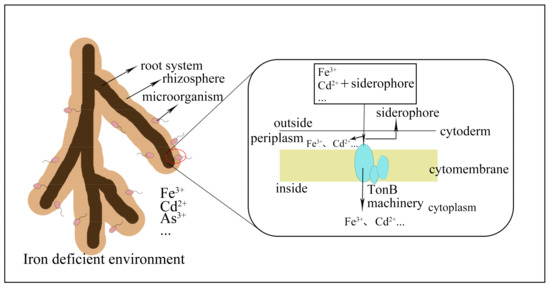
Figure 1.
Process of transferring metal ions into cells by siderophores in soil. Metal ions combine with the siderophore produced by bacteria near a plant’s root in dirt. Some complex compounds enter the cell membrane, whereas metal ions remain in the periplasm, and siderophores are released for cyclic usage. Others enter the cytoplasm via the cell membrane with the aid of TonB machinery.
For siderophores, there are more than 500 different chemical structures [13]. Siderophores can be divided into three broad categories [14] based on the functional groups that bind to Fe3+ and the varied topologies of siderophores: hydroxamate siderophores, catecholate siderophores, and carboxylate siderophores. Hydroxamate siderophores are the most frequently produced siderophores by both bacteria and fungi. The bacterial hydroxamic type is composed of hydroxylated and acylated alkylamines, as opposed to the fungal hydroxamic type, which is composed of hydroxylated and acylated ornithine groups [15]. Catechol is made by bacteria. This siderophore is lipophilic, has a strong affinity for iron, and can withstand environmental pH changes. In addition, catecholate siderophores may be utilized as a potent reducing agent to compete with pathogenic fungi for iron more efficiently. Few bacteria can create carboxylate siderophores. Different siderophores chelate Fe3+ in different ways. The most efficient chelators of Fe3+ are the carboxylate, hydroxamate, and catecholate types [13]. The iron-chelating ability of fungal siderophores is slightly less than that of bacterial siderophores among the siderophores of the same functional group [16]. The synthesis of siderophores is greatly influenced by metal ions in the soil. The main determinant of siderophores’ formation is the concentration of soluble iron. For example, the ability of Acinetobacter calcoaceticus to secrete is inhibited when the amount of soluble iron in the environment approaches 20 uM [17]. In the presence of iron, the cytoplasmic Fur protein of Pseudomonas aeruginosa inhibits siderophore production [18]. Fur regulates siderophore secretion by controlling gene expression in response to changes in iron concentration [19]. Additionally, research has demonstrated that some plants may produce chemicals such as furanone in the absence of iron to specifically encourage or hinder the development of siderophores [20]. Other metal ions, such as Cd2+, Hg2+, and Co2+, can similarly stimulate or inhibit the production of siderophores by bacteria [21,22]. For instance, Trichoderma produces siderophores when iron is scarce, and its production is boosted by high Zn2+ concentrations. The synthesis of siderophores also heavily depends on sources of carbon and nitrogen [23]. It has been shown that the synthesis of siderophores can be greatly increased by cultivating SPB in the lab using mannitol and sucrose as carbon sources and yeast extract and urea as nitrogen sources [20]. With a 13.3% increase in iron carrier synthesis in the presence of 5 g/L glucose in a PDA medium, strain RL1 had the highest rate [24]. The production of siderophores is also influenced by temperature, pH, C/N ratio, and medium type [15,16,17,18,19,20,21,22,23,24,25].
At present, SPB has been found to promote the growth of many plants. For example, it has been found that Bacillus subtilis Bs-15 has a good effect on the prevention and control of pepper Fusarium wilt and growth promotion [26]. Moreover, SPB can raise the amount of nutrients in the soil to the point where wheat plant growth has been seen even under drought-like situations. [27]. The inoculation of SPB in lettuce increased nitrogen, phosphorus, and potassium nutrients under drought stress and promoted its growth [28]. Therefore, the use of SPB to promote crop growth to produce microbial iron fertilizer will be expected to replace some chemical fertilizers. At the same time, the antibacterial effect of SPB can also be applied in biological control.
However, there is little research on the variables that affect SPB from the perspective of a plant. We conducted a global study involving 342 comparative data points from 30 studies in the literature over the course of the preceding five years in order to carefully assess the effects of adding SPB to soil on plant development and the factors influencing the growth-promoting influence of SPB on plants. Our goals are to (1) quantify the changes in plant growth caused by the addition of SPB and (2) examine the effects of different plant family and genus classifications and measurement techniques on these changes. As a result, we will be able to use SPB as rhizobacteria that promote growth more successfully.
2. Materials and Methods
2.1. Date Sources
To locate the relevant literature for our meta-analysis, we searched the Web of Science and China National Knowledge Infrastructure (CNKI) for articles investigating the growth-promoting impact of siderophore-producing bacteria published between 2018 and 2022. The literature search was performed following guidelines from Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) [29] (Table S1). The search terms were “siderophore and growth-promoting” or “siderophore and plant”. In addition, the following criteria were used to evaluate the publications’ integrity, relevance, and scientific merit: (1) We ensured that each research was independent and based on real measurement data; (2) each study was required to disclose the effects of SPB on plant development with or without the substance; (3) the research must include the kinds and development of plants; (4) the statistical information, including mean values of root length and root dry weight, standard deviation (SD), and sample size, was collected directly from the tables of the published articles or from the graphs using GetData 2.20.
The selected studies provided information on (1) the names and families of tested plants; and (2) germination rate, plant height, root length, leaf fresh weight, leaf dry weight, root fresh weight, root dry weight, total fresh weight, and total dry weight. As a consequence, we narrowed down the findings to 30 scientific journal papers containing 342 observations (Table S1).
2.2. Date Analysis
We employed the natural logarithmic response ratio () to evaluate the effect size of SPB on plant growth.
where and are the mean value of the variable with (treatment) or without (control) the addition of SPB.
The variance of each observation was calculated as follows:
where and are the SD of each observation, while and are the sample size of each variable in treatment and control groups. If we can only extract the standard error (SE) from a study, the SD should be recalculated.
This meta-analysis was performed using the cumulative effect size. We used the random-effect model to perform the following calculation: For the within-case variance (τ2), we use the restricted maximum likelihood (REML). The weight () of each observation and the total variance between observations in the model () were calculated as follows:
where k is the sample size of the study.
The statistical tests were deemed significant at a p-value of less than 0.05. All meta-analysis processes were performed using Openmee, and all figures were created with GraphPad Prism.
2.3. Model Diagnostic and Influencing Factor Analysis
Using a linear mixed-effect model, we looked into how the plant family and measurement mode affected these impacts. The funnel plot analysis and fail-safe number test were used with the Openmee software and a 95% confidence interval to examine the model’s diagnostics.
3. Results
3.1. Variation in Plant Family Growth under SPB
Plant growth increased by 30.00% on SPB based on 342 observations. Our paired observations reported 10 plant families: Pailionaceae (30 studies), Rosaceae (12 studies), Liliaceae (2 studies), Leguminosae (28 studies), Compositae (14 studies), Musaceae (3 studies), Solanaceae (49 studies), Cucurbitaceae (21 studies), Cruciferae (52 studies), and Gramineae (131 studies). The way SPB affected plant growth was different for each plant family (Figure 2, Table S2). Compared with the control, our results showed that adding SPB had a significant effect on plants from different families, especially the Compositae and Cucurbitaceae, which were helped by 49.1% and 50.8%, respectively. Gramineae, Rosaceae, and Musaceae, on the other hand, were only helped by 15.60%, 17.20%, and 22.30%, respectively. At the same time, the different numbers of paired observations showed that the results of the meta-analysis did not change much when the number of observations was decreased. This showed that our analysis was accurate.
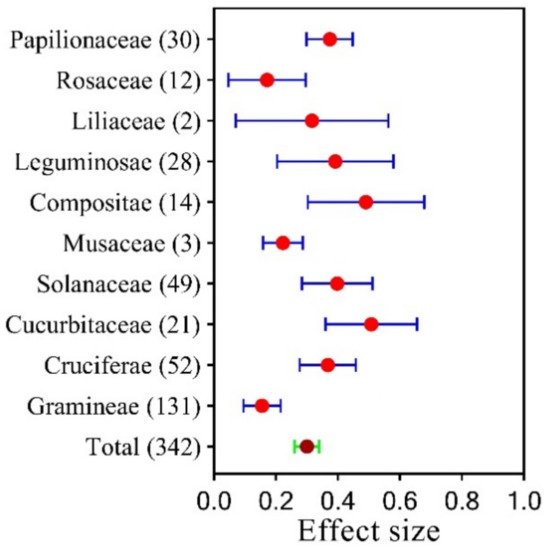
Figure 2.
The size of the effect of SPB on different plant families. The error bars in the circles display the 95% confidence intervals (CIs) for the overall mean. The answer is deemed significant if the CI does not overlap with zero. The number in parentheses indicates the number of observations.
SPB had different encouraging effects on plants belonging to different families. The majority of current research is focused on graminaceous plants, with little attention paid to other plant families. Although SPB had the least effect on graminaceous plants, it had the lowest error rate (Figure 2).
3.2. Growth Changes under SPB for Various Measurement Modes
Different measurement methods could produce different results (Figure 3, Table S3). When compared to the control group, the plant’s overall dry weight increased by 83.00%. Additionally, there was a 23.70% rise in root length and a 26.80% increase in plant height, while the germination rate has just increased by 5.60%.
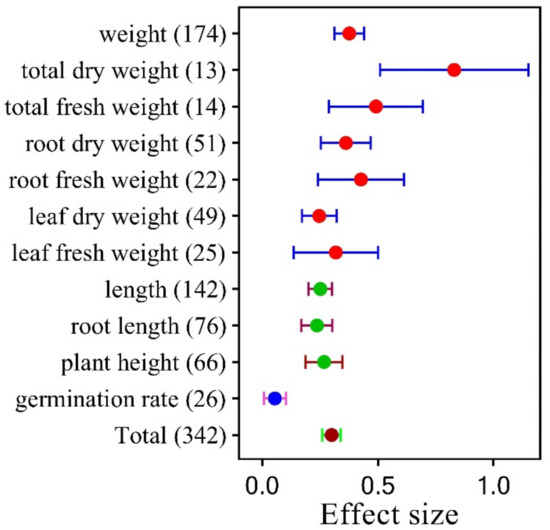
Figure 3.
The effect size of measurement methods under SPB. The error bars in the circles display the 95% confidence intervals (CIs) for the overall mean. The answer is deemed significant if the CI does not overlap with zero. The number in parentheses indicates the number of observations.
The effects of SPB were primarily reported in terms of changes in biomass, with weight increase preferred over plant length increase (Figure 3). This might be because iron supplementation promotes plant photosynthesis and the accumulation of organic materials.
3.3. Potential Bias in Publications
The funnel plot analysis proved that our research was less susceptible to publication bias and that our finding was trustworthy (Figure 4), and the fail-safe number test showed that 22,332,149 trials with opposing results would be required to overturn our conclusions.
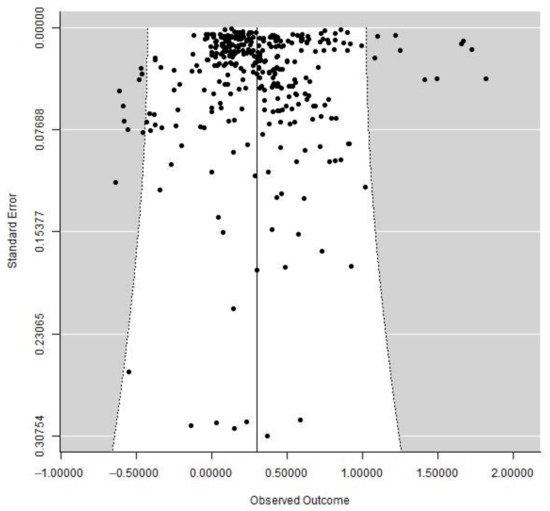
Figure 4.
The funnel plot analysis of all paired data. The 95% confidence interval (CI) is indicated by the white area in the center. Every single black dot stands for a distinct pair of observations. The result is reliable and less prone to publication bias if the black dot is symmetrical with regard to the solid line in the center.
4. Discussion
Siderophores are among the phytohormones produced by microbes. Insoluble Fe3+ may be used more easily in the environment when siderophores are present, enhancing the bioavailability of Fe3+ [30]. Under different iron concentrations, the growth-promoting effect of SPB was different [31]. Plants may directly absorb and utilize the complex produced by siderophores and Fe3+, enhancing their iron nutritional status. Previous studies have shown that siderophores during the germination and emergence phases [32] increase the germination rate and seedling activity of popular crops such as tomatoes [33,34], cucumbers [35], wheat [36,37,38], etc. In addition, siderophores may deliver nutrients to plants in heavy-metal-polluted soils to reduce stress toxicity and enhance plant development [39]. For instance, the addition of a suspension of the siderophore fungus led to a progressive increase in the fresh weight of Shanghaiqing and the amount of photosynthetic pigment in the leaves [25]. When the highest concentration of spore solution (3.2*108 CFU/mL) was used, the fresh weight of stems and leaves increased by 74.0%, while the fresh weight of the plants as a whole increased by 75.0% [25].
4.1. The Inhibitory Effect of SPB on Plant Pathogens
The production of siderophores is one of the most effective techniques for reducing plant diseases [40]. PGPR may restrict the amount of plant inter-root pathogens by secreting siderophores, which compete with ambient trace iron to deprive pathogenic bacteria of iron [32]. For instance, growth-promoting rhizosphere-produced siderophores effectively ward off fungal infections such as Phytophthora, Pythium, and Sclerotinia plant diseases [41]. Additionally, some research has indicated that siderophores have an impact on the growth of fungi. The siderophore produced by Pseudomonas aeruginosa FP6 may greatly reduce Colletotrichum gloeosporioides growth during the growth of peppers [21]. Seven of the siderophore-producing strains tested by Vijay Karuppiah et al. against Fusarium oxysporum f. sp., lycopersici MTCC10270 (Fol), Fusarium equiseti MFol, and Sarocladium sp. SWL showed in vitro antifungal activity [42]. Neofusicoccum kwambonambiense XKD-1, the pathogen that causes strawberry root rot, was completely inhibited by Penicilium asturianum XK-12 siderophore fermentation broth when 20% (volume fraction) of Penicilium asturianum was added [43].
The majority of SPB are PGPR, which provides another explanation for the inhibitory effect of siderophores on harmful bacteria. Unlike pathogens, SPB is indigenous bacteria. When the chelating ability and generation of siderophores produced by pathogens and PGPR in soil are identical, SPB can prioritize controlling iron atoms in soil [44].
4.2. The Effect of SPB on Other Metal Ions
In the soil, siderophores might increase the bioavailability of heavy metals. The siderophore can chelate other metal ions in addition to Fe3+ ions, such as cadmium, lead, nickel, arsenic, aluminum, magnesium, zinc, copper, cobalt, strontium, and iron [45,46]. Rhodococcus strains that can produce siderophores have been found to be able to extract the metal ions V (III), Ga (III), and In (III) from mixed metal solutions [24]. The chelation of heavy metals and siderophores not only reduces the toxicity of heavy metal ions but also increases the activity of heavy metals in the rhizosphere of plants, increases the uptake and accumulation of heavy metals by plants, and improves phytoremediation effectiveness. Following inoculation with P. aeruginosa, Armelle Braud observed that the absorption of Cr and Pb in the above-ground region of maize increased by 5.4 and 3.8 times, respectively [47]. Peat moss and iron-producing bacteria combined, according to Hanna Virpiranta’s research, have a 96% nickel adsorption capability [48]. Free heavy metal ions can easily enter cells through the cell membrane of bacteria through active or passive absorption. However, due to the increase in molecular weight caused by the presence of siderophores, some heavy metal ions that have been chelated with them are unable to enter bacteria via porin. This may reduce the toxicity of heavy metals to bacteria. Several arsenic-tolerant bacteria will produce siderophores in an arsenic-contaminated environment. When siderophores chelate with ferrihydrite, realgar, etc., iron ore is dissolved. Furthermore, siderophores may indirectly affect the way microbes reduce arsenic by using other substances [49]. When heavy metals bind to free siderophores, they prevent iron ions from being chelated, which reduces the amount of accessible iron and encourages the synthesis of more siderophores, which can help bacteria or plants thrive and partially shield them from the toxicity of heavy metals [50]. The YQ9 is a high-siderophore of B. vietnamiensis, and investigations have shown that it can withstand high concentrations of Pb2+, Zn2+, Cu2+, and Cd2+ with minimal inhibitory values of 3000, 5000, 4500, and 1000 mol/L, respectively [51,52]. Additionally, it has been found that siderophore bacteria may increase their siderophore–pyoverdine production in response to Cd2+, enhancing the strains’ resistance to the metal [53]. Carmen Tamariz-Angeles et al. found that the survival rate of alfalfa seedlings rose after inoculation with the SPB BEP17-Dm, BEP18-Dm, or BRU16 when the environment’s Cd2+, Pb2+, or Al+ concentrations were 0.5–1.0 mM, 0.5–1.0 mM, and 2.5–5.0 mM, respectively [54]. When Cd2+ is given, SPB takes part in siderophore formation, whereas cell Cd2+ accumulation declines [55]. The influence of siderophores will, however, decrease as the level of heavy metals increases, as demonstrated by the fact that the effect of the cadmium-resistant siderophore bacteria LY02 was significantly reduced at a Cd2+ concentration of 1.3 mM [56].
Therefore, due to their high tolerance for heavy metals, SPB may live and proliferate in large numbers in contaminated areas if they are introduced into the rhizomes of heavy metal hyperaccumulator plants. In areas with heavy metal pollution, it may chelate Fe3+ and other metal ions. It might promote the growth of super-enriched plants, enabling them to take up more metal ions. Moreover, studies have found that microorganisms with growth-promoting siderophores and growth-inhibiting siderophores have the opposite effect on pathogenic bacteria [57], which means an incorrect application of siderophores may have a negative impact on agricultural production. Therefore, the effects of different types of siderophores need to be further studied.
5. Conclusions
The addition of SPB to varied plant families may increase plant yield to a certain extent. The most significant effect of SPB on plant root, leaf, and germination rates was on root dry weight. Nevertheless, more attention should be paid to plant families and variations in biomass, as the economic value of diverse plant families differs, including ornamental and edible varieties. The appropriate use of SPB may increase agricultural yield, whereas a bad application may increase the heavy metal content of food.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/horticulturae9030370/s1, Table S1: The data extracted from the literature; Table S2: The size of the effect of SPB on different plant families; Table S3: The effect size of measurement methods under SPB.
Author Contributions
Conceptualization, J.W., T.B. and S.Z.; methodology, S.Z., T.B. and J.W.; software, S.Z., J.W. and J.H.; validation, J.W., S.Z. and Z.D.; formal analysis, S.Z., Y.W. and Z.D.; investigation, S.Z., A.B. and Z.D.; resources, S.Z., J.W. and Y.W.; data curation, S.Z., J.H. and Z.D.; writing—original draft preparation, S.Z., J.W., Y.M. and Y.W.; writing—review and editing, S.Z., A.B., J.W. and Y.W.; visualization, S.Z., J.W. and A.B.; supervision, J.W. and T.B.; project administration, J.W. and T.B.; funding acquisition, S.Z. and J.W. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Postgraduate Research and Practice Innovation Program of Jiangsu Province (Yangzhou University), grant number KYCX21_3254; and the Key research and development projects (social development) of Yangzhou, grant number YZ2022060.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Aguado-Santacruz, G.A.; Moreno-Gomez, B.; Jimenez-Francisco, B.; Garcia-Moya, E.; Preciado-Ortiz, R.E. Impact of the microbial siderophores and phytosiderophores on the iron assimilation by plants: A synthesis. Rev. Fitotec. Mex. 2012, 35, 9–21. [Google Scholar]
- Fardeau, S.; Demailly-Mullie, C.; Dassonville-Klimpt, A.; Audic, N.; Sonnet, P. Bacterial iron’s uptake: A promising solution against multidrug resistant bacteria. In Science against Microbial Pathogens: Communicating Current Research and Technological Advances; Formatex Research Center: Badajoz, España, 2011. [Google Scholar]
- Schalk, I.J. Metal trafficking via siderophores in Gram-negative bacteria: Specificities and characteristics of the pyoverdine pathway. J. Inorg. Biochem. 2008, 102, 1159–1169. [Google Scholar] [PubMed]
- Li, W.F.; Zhu, H.Y.; Lan, P. Research Progress of Iron Homeostasis Regulation in Strategy I Plants. Soil 2021, 53, 1101–1106. [Google Scholar]
- Dong, Z.Y.; Hu, J.J.; Hu, B.L. Regulation of microbial siderophore transport and its application in environmental remediation. Chin. J. biotechnol. 2019, 35, 2189–2200. [Google Scholar]
- Hu, B.H.; Zhao, C.G.; Yang, S.P. Influence of iron on siderophore and photosynthetic pigments biosynthesis by siderophore-producing Rhodopesudomonnas palustris. Acta Microbiol. Sin. 2014, 54, 408–416. [Google Scholar]
- Liu, J.T.; Yao, F.; Li, Z.Y.; Li, Q.M.; Huang, L.Y. Advances on the Mechanism of Iron Absorption in Plants. Chin. J. Trop. Agric. 2022, 42, 26–33. [Google Scholar]
- Lindsay, W.L. Iron oxide solubilization by organic matter and its effect on iron availability. Plant Soil 1991, 130, 27–34. [Google Scholar]
- Yu, X.M.; Zheng, F.C. The Development and Utilization of Siderophore on Plant Growth Promotion and Plant Disease Control. Chin. Agric. Sci. Bull. 2007, 23, 507–510. [Google Scholar]
- Sebastian, A.; Nangia, A.; Prasad, M.N.V. Carbon-Bound Iron Oxide Nanoparticles Prevent Calcium-Induced Iron Deficiency in Oryza sativa L. J. Agric. Food Chem. 2017, 65, 557–564. [Google Scholar]
- Ellermann, M.; Arthur, J.C. Siderophore-mediated iron acquisition and modulation of host-bacterial interactions. Free Radic. Bio. Med. 2017, 105, 68–78. [Google Scholar]
- Josts, I.; Veith, K.; Normant, V.; Schalk, I.J.; Tidow, H. Structural insights into a novel family of integral membrane siderophore reductases. PNAS 2021, 118, 34. [Google Scholar]
- Boukhalfa, H.; Crumbliss, A.L. Chemical aspects of siderophore mediated iron transport. Biometals 2002, 15, 325–339. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.L.; Lin, Q.Q.; Li, Y.; Yang, X.H.; Wang, S.Z.; Qiu, R.L. Application potential of siderophore-producing rhizobacteria in phytoremediation of heavy metals-contaminated soils: A review. J. Appl. Ecol. 2013, 24, 2081–2088. [Google Scholar]
- Chowdappa, S.; Jagannath, S.; Konappa, N.; Udayashankar, A.C.; Jogaiah, S. Detection and Characterization of Antibacterial Siderophores Secreted by Endophytic Fungi from Cymbidium aloifolium. Biomolecules 2020, 10, 1412. [Google Scholar]
- He, X.; Zhang, Q.; Li, C.; Li, M.G.; Xu, S.T.; Chen, S.Q.; Yang, P.W. Screening and Identification of Secretory Siderophore from Plant Pathogen. J. Yunnan Agric. Univ. 2020, 35, 235–242. [Google Scholar]
- Sarode, P.D.; Rane, M.R.; Chaudhari, B.L.; Chincholkar, S.B. Siderophoregenic Acinetobacter calcoaceticus Isolated from Wheat Rhizosphere with Strong PGPR Activity. Malays. J. Microbiol. 2009, 5, 6–12. [Google Scholar]
- Lee, J.W.; Helmann, J.D. Functional specialization within the Fur family of metalloregulators. Biometals 2007, 20, 485–499. [Google Scholar]
- Pi, H.; Helmann, J.D. Sequential induction of Fur-regulated genes in response to iron limitation in Bacillus subtilis. PNAS 2017, 114, 12785–12790. [Google Scholar]
- Sasirekha, B.; Srividya, S. Siderophore production by Pseudomonas aeruginosa FP6, a biocontrol strain for Rhizoctonia solani and Colletotrichum gloeosporioides causing diseases in chilli. Agric. Nat. Resour. 2016, 50, 250–256. [Google Scholar]
- Dimkpa, C.O.; Merten, D.; Svatoš, A.; Büchel, G.; Kothe, E. Metal-induced oxidative stress impacting plant growth in contaminated soil is alleviated by microbial siderophores. Soil Boil Biochem. 2009, 41, 154–162. [Google Scholar]
- Braud, A.; Geoffroy, V.; Hoegy, F.; Mislin, G.L.A.; Schalk, I.J. Presence of the siderophores pyoverdine and pyochelin in the extracellular medium reduces toxic metal accumulation in Pseudomonas aeruginosa and increases bacterial metal tolerance. Env. Microbiol. Rep. 2010, 2, 419–425. [Google Scholar]
- Hussein, K.A.; Joo, J.H. Zinc Ions Affect Siderophore Production by Fungi Isolated from the Panax ginseng Rhizosphere. J. Microbiol. Biotechn. 2019, 29, 105–113. [Google Scholar]
- Hofmann, M.; Heine, T.; Malik, L.; Hofmann, S.; Joffroy, K.; Senges, C.H.R.; Bandow, J.E.; Tischler, D. Screening for Microbial Metal-Chelating Siderophores for the Removal of Metal Ions from Solutions. Microorganisms 2021, 9, 1. [Google Scholar]
- Xu, J.L.; Zhang, P.; Li, M.F.; Liao, B.H.; Peng, P.Q.; Li, J.; Mei, J.X. Isolation, culture condition optimization, and preliminary application of siderophore-producing strains. Microbiology 2022, 49, 1004–1016. [Google Scholar]
- Yu, X.M.; Zhou, G.F.; Xin, L. Conditions for siderophore production by Bacillus subtilis Bs-15 and its effect on disease prevention and growth promotion of sweet pepper. Chin. J. Pesticide Sci. 2010, 12, 135–141. [Google Scholar]
- Lastochkina, O. Effect of endophytic Bacillus subtilis on drought stress tolerance of Triticum aestivum plants of steppe volga and forest-steppe west siberian agroecological groups. In Proceedings of the 2nd International Scientific Conference “Plants and Microbes: The Future of Biotechnology”, Saratov, Russia, 5–9 October 2020. [Google Scholar]
- Vivas, A.; Marulanda, A.; Ruiz-Lozano, J.M.; Barea, J.; Azcón, R. Influence of a Bacillus sp. on physiological activities of two arbuscular mycorrhizal fungi and on plant responses to PEG-induced drought stress. Mycorrhiza 2023, 13, 249–256. [Google Scholar]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulron, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. J. Clin. Epidemiol 2021, 134, 178–189. [Google Scholar]
- Gao, B.B.; Chai, X.F.; Huang, Y.M.; Wang, X.N.; Han, Z.H.; Xu, X.F.; Wu, T.; Zhang, X.Z.; Wang, Y. Siderophore production in pseudomonas SP. strain SP3 enhances iron acquisition in apple rootstock. J. Appl. Microbiol. 2022, 133, 720–732. [Google Scholar]
- Boiteau, R.M.; Markillie, L.M.; Hoyt, D.W.; Hu, D.H. Metabolic Interactions between Brachypodiumand Pseudomonas fluorescens under Controlled Iron-Limited Condition. mSystems 2021, 6, 1. [Google Scholar]
- Min, L.J.; Guo, L.; Ye, J.R. Mechanism of Burkholderia pyrrocinia JK-SH007 growth-promoting to plant via siderophore-mediation. J. Nanjing For. Univ. 2019, 43, 165–172. [Google Scholar]
- Radzki, W.; Gutierrez Mañero, F.J.; Algar, E.; Lucas García, J.A.; García-Villaraco, A.; Ramos Solano, B. Bacterial siderophores efficiently provide iron to iron-starved tomato plants in hydroponics culture. Antonie Van. Leeuwenhoek 2013, 104, 321–330. [Google Scholar] [PubMed]
- Zhou, C.; Zhu, L.; Guo, J.S.; Xiao, X.; Ma, Z.Y.; Wang, J.F. Bacillus subtilis STU6 Ameliorates Iron Deficiency in Tomato by Enhancement of Polyamine-Mediated Iron Remobilization. J. Agric. Food Chem. 2018, 67, 320–330. [Google Scholar] [PubMed]
- Al-Karablieh, N.; Al-Shomali, I.; Al-Elaumi, L.; Hasan, K. Pseudomonas fluorescens NK4 siderophore promotes plant growth and biocontrol in cucumber. J. Appl. Microbiol. 2022, 133, 1414–1421. [Google Scholar] [PubMed]
- Yang, K.Y.; Doxey, S.; McLean, J.E.; Britt, D.; Watson, A.; Al Qassy, D.; Jacobson, A.; Anderson, A.J. Remodeling of root morphology by CuO and ZnO nanoparticles: Effects on drought tolerance for plants colonized by a beneficial pseudomonad. Botany 2018, 96, 175–186. [Google Scholar]
- Yue, Z.H.; Chen, Y.J.; Hao, Y.W.; Wang, C.C.; Zhang, Z.F.; Chen, C.; Liu, H.Z.; Liu, Y.C.; Li, L.L.; Sun, Z.K. Bacillus sp. WR12 alleviates iron deficiency in wheat via enhancing siderophore- and phenol-mediated iron acquisition in roots. Plant Soil 2022, 471, 247–260. [Google Scholar]
- He, L.; Yue, Z.H.; Chen, C.; Li, C.Y.; Li, J.; Sun, Z.K. Enhancing Iron uptake and Alleviating Iron Toxicity in Wheat by Plant Growth-Promoting Bacteria: Theories and Practices. Int. J. Agric. Biol. 2020, 23, 190–196. [Google Scholar]
- Dimkpa, C.O.; Svatos, A.; Dabrowska, P.; Schmidt, A.; Boland, W.; Kothe, E. Involvement of siderophores in the reduction of metal-induced inhibition of auxin synthesis in Streptomyces spp. Chemosphere 2008, 74, 19–25. [Google Scholar]
- Etesami, H.; Maheshwari, D.K. Use of plant growth promoting rhizobacteria (PGPRs) with multiple plant growth promoting traits in stress agriculture: Action mechanisms and future prospects. Ecotoxicol. Environ. Saf. 2018, 156, 225–246. [Google Scholar]
- Yu, X.M.; Ai, C.X.; Xin, L.; Zhou, G.F. The siderophore-producing bacterium, Bacillus subtilis CAS15, has a biocontrol effect on Fusarium wilt and promotes the growth of pepper. Eur. J. Soil Biol. 2011, 47, 138–145. [Google Scholar]
- Karuppiah, V.; Natarajan, S.; Gangatharan, M.; Aldayel, M.F.; Alsowayeh, N.; Thangavel, K. Development of siderophore-based rhizobacterial consortium for the mitigation of biotic and abiotic environmental stresses in tomatoes: An in vitro and in planta approach. J. Appl. Microbiol. 2022, 133, 3276–3287. [Google Scholar] [PubMed]
- Peng, W.J.; Zhan, Y.J.; Lei, P.; Sun, T.; Qian, J.Y.; Xu, H. Characteristics of siderophores production bu Penicilium astuiianum XK-12 and its effect on antibacterial activity. Jiangsu J. Agr. Sci. 2022, 38, 73–80. [Google Scholar]
- Kong, W.L.; Zhou, M.; Wu, X.Q. Characteristics of siderophores production by Rahnella aquatilis JZ-GX1 and its antagonism against forest pathogens. Microbiology 2019, 46, 3278–3285. [Google Scholar]
- Braud, A.; Hoegy, F.; Jezequel, K.; Lebeau, T.; Schalk, I.J. New insights into the metal specificity of the Pseudomonas aeruginosa pyoverdine-iron uptake pathway. Environ. Microbiol. 2009, 11, 1079–1091. [Google Scholar]
- Sayyed, R.Z.; Chincholkar, S.B. Growth and siderophores production in Alcaligenes faecalis is regulated by metal ions. Indian J. Microbiol. 2010, 50, 179–182. [Google Scholar]
- Braud, A.; Jezequel, K.; Bazot, S.; Lebeau, T. Enhanced phytoextraction of an agricultural Cr- and Pb-contaminated soil by bioaugmentation with siderophore-producing bacteria. Chemosphere 2009, 74, 280–286. [Google Scholar]
- Virpiranta, H.; Banasik, M.; Taskila, S.; Leiviska, T.; Halttu, M.; Sotaniemi, V.H.; Tanskanen, J. Isolation of Efficient Metal-Binding Bacteria from Boreal Peat Soils and Development of Microbial Biosorbents for Improved Nickel Scavenging. Water 2020, 12, 7. [Google Scholar]
- Liu, X.; Fu, J.W.; Da Silva, E.; Shi, X.X.; Cao, Y.; Rathinasabapathi, B.; Chen, Y.S.; Ma, L.Q. Microbial siderophores and root exudates enhanced goethite dissolution and Fe/As uptake by As-hyperaccumulator Pteris vittata. Environ. Pollut. 2017, 223, 230–237. [Google Scholar]
- Wu, W.W.; Xue, L.G.; Zhang, L.; Wang, S.M.; Chang, S.J.; Li, M.C.; Liu, Y.T.; He, Y.Y. Isolation of a siderophore-producing and cadmium-resistant bacteria and its effect on seed germination of ryegrass. Microbiology 2021, 48, 1895–1906. [Google Scholar]
- Wang, Y.J.; Huang, W.; Ali, S.W.; Li, Y.Q.; Yu, F.B.; Deng, H.H. Isolation, Identification, and Characterization of an Efficient Siderophore Producing Bacterium From Heavy Metal Contaminated Soil. Cur. Microbio. 2022, 79, 227. [Google Scholar]
- Wang, Y.J.; Feng, J.W.; Li, Y.Q.; Yu, F.B. Studies on growth-promoting properties of an efficient siderophore producing bacterium, Burkholderia vietnamiensis YQ9, and its effects on seed germination under heavy metal stress. Acta Sci. Circumst. 2022, 42, 430–437. [Google Scholar]
- Sinha, S.; Mukherjee, S.K. Cadmium-induced siderophore production by a high cd-resistant bacterial strain relieved cd toxicity in plants through root colonization. Curr. Microbiol. 2008, 56, 55–60. [Google Scholar] [CrossRef]
- Tamariz-Angeles, C.; Huaman, G.D.; Palacios-Robles, E.; Olivera-Gonzales, P.; Castaneda-Barreto, A. Characterization of siderophore-producing microorganisms associated to plants from high-Andean heavy metal polluted soil from Callejon de Huaylas (Ancash, Peru). Microbiol. Res. 2021, 250, 126811. [Google Scholar] [CrossRef] [PubMed]
- Dimkpa, C.O.; Merten, D.; Svatos, A.; Buechel, G.; Kothe, E. Siderophores mediate reduced and increased uptake of cadmium by Streptomyces tendae F4 and sunflower (Helianthus annuus), respectively. J. Appl. Microbiol. 2009, 107, 1687–1696. [Google Scholar] [CrossRef]
- Zhao, S.M.; Yu, F.B.; Ye, Z.Q.; Fang, X.B.; Lin, H.P. Isolation and identification of a cadmium-resistant and siderophores-producing strain. Environ. Poll. Control 2017, 39, 999–1002. [Google Scholar]
- Gu, S.H.; Wei, Z.; Shao, Z.Y.; Friman, V.P.; Cao, K.H.; Yang, T.J.; Kramer, J.; Wang, X.F.; Li, M.; Mei, X.L.; et al. Competition for iron drives phytopathogen control by natural rhizosphere microbiomes. Nat. Microbiol. 2020, 5, 1002. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).