Physiochemical Changes of European Pear cv. Conference and Asian Pear cv. Yali during Cold Storage
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials and Experimental Details
2.2. Measurements of Physiological Indexes
2.3. Saccharides Content
2.4. Ethylene Formation and Respiration Rate
2.5. Antioxidant Capacity
2.6. Determination of Organic Acids by HPLC
2.7. Statistical Analysis
3. Results and Discussion
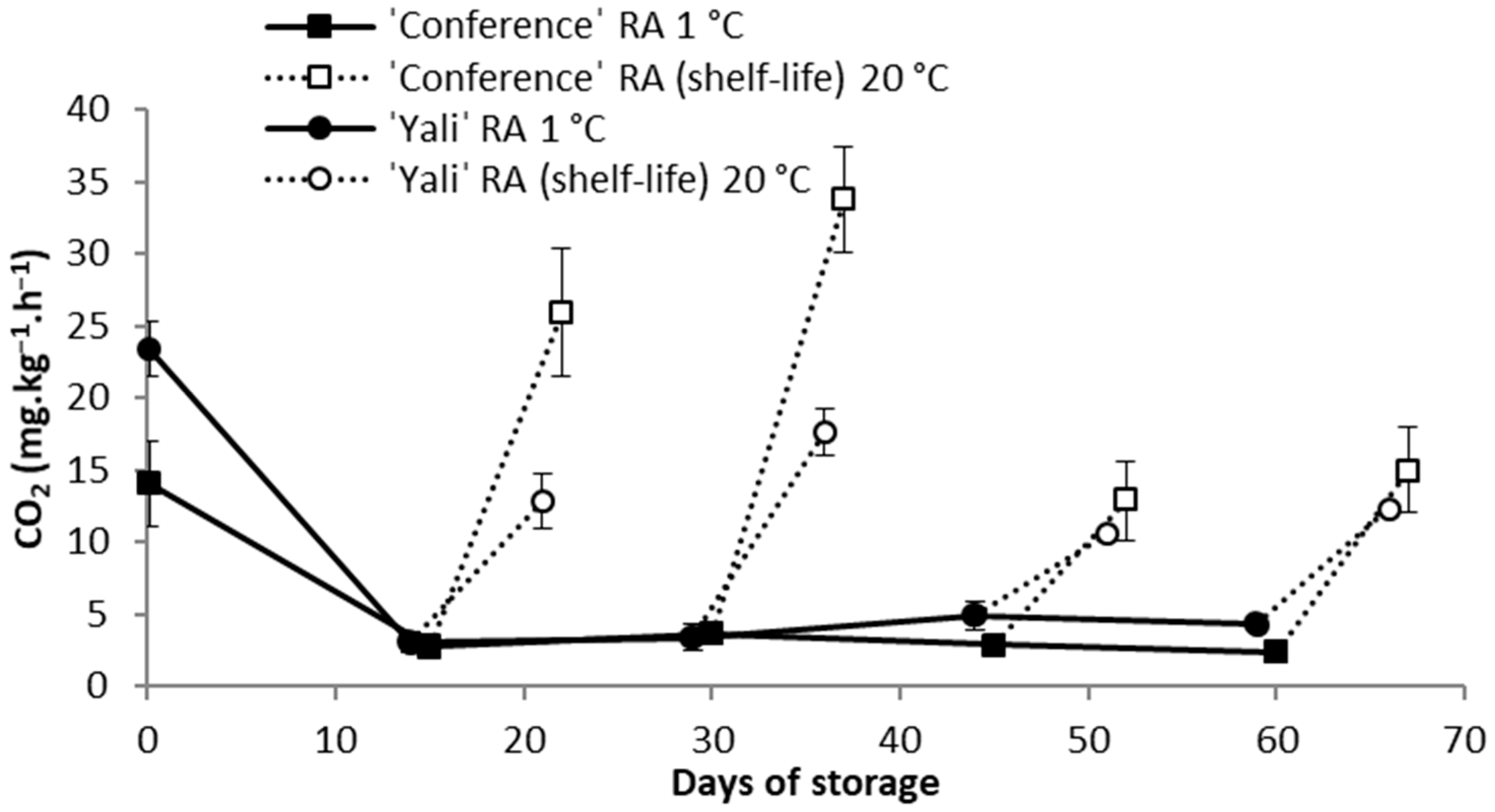
3.1. CO2 and Ethylene Formation at Refrigeration Temperature as a Function of Storage Temperature Change
3.2. Soluble Solids and Titratable Acidity during Storage at 1 °C and at 20 °C
3.3. Comparison of the Physicochemical Parameters of Asian and European Pear Fruit Varieties at the Beginning and End of Storage
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- FAO. Food and Agriculture Organization of the United Nations. 2022. Available online: www.fao.org (accessed on 21 March 2022).
- Teng, Y.; Tanabe, K. Reconsideration on the origin of cultivated pears native to East Asia. Acta Hortic. 2004, 634, 175–182. [Google Scholar] [CrossRef]
- Villalobos-Acuña, M.; Mitcham, E.J. Ripening of European pears: The chilling dilemma. Postharvest Biol. Technol. 2008, 49, 187–200. [Google Scholar] [CrossRef]
- Pham, Q.T.; Liou, N.S. Investigating texture and mechanical properties of Asian pear flesh by compression tests. J. Mech. Sci. Technol. 2017, 31, 3671–3674. [Google Scholar] [CrossRef]
- Dong, Y.; Zhang, S.; Wang, Y. Compositional changes in cell wall polyuronides and enzyme activities associated with melting/mealy textural property during ripening following long-term storage of ‘Comice’ and ‘d’Anjou’ pears. Postharvest Biol. Technol. 2018, 135, 131–140. [Google Scholar] [CrossRef]
- Saquet, A.A. Storability of ‘Conference’ Pear Under Various Controlled Atmospheres. Erwerbs-Obstbau 2018, 60, 275–280. [Google Scholar] [CrossRef]
- Saquet, A.A.; Streif, J.; Bangerth, F. Energy metabolism and membrane lipid alterations in relation to brown heart development in ‘Conference’ pears during delayed controlled atmosphere storage. Postharvest Biol. Technol. 2003, 30, 123–132. [Google Scholar] [CrossRef]
- Pedreschi, R.; Franck, C.; Lammertyn, J.; Erban, A.; Kopka, J.; Hertog, M.; Verlinden, B.; Nicolai, B. Metabolic profiling of ‘Conference’ pears under low oxygen stress. Postharvest Biol. Technol. 2009, 51, 123–130. [Google Scholar] [CrossRef]
- Streif, J. Effects of AVG on harvest date, storage and economic return of ‘Conference’ pear. Acta Hortic. 2008, 796, 161–165. [Google Scholar] [CrossRef]
- Rizzolo, A.; Bucheri, M.; Bianchi, G.; Grassi, M.; Vanoli, M. Quality of ‘Conference’ pears as affected by low oxygen stress, dynamically controlled atmosphere and 1-MCP treatment. Acta Hortic. 2014, 1079, 343–350. [Google Scholar] [CrossRef]
- Saquet, A.A.; Streif, J. Fermentative metabolism in ‘Conference’ pears under various storage conditions. J. Hortic. Sci. Biotechnol. 2006, 81, 910–914. [Google Scholar] [CrossRef]
- Saquet, A.A.; Almeida, D.P.F. Ripening physiology and biochemistry of ‘Rocha’ pear as affected by ethylene inhibition. Postharvest Biol. Technol. 2017, 125, 161–167. [Google Scholar] [CrossRef]
- Lindo-García, V.; Larrigaudière, C.H.; Echeverría, G.; Murayama, H.; Soria, Y.; Giné-Bordonaba, J. New insights on the ripening pattern of ‘Blanquilla’ pears: A comparison between on- and off-tree ripened fruit. Postharvest Biol. Technol. 2019, 150, 112–121. [Google Scholar] [CrossRef]
- Predieri, S.E.; Gatti, E. Effects of cold storage and shelf-life on sensory quality and consumer acceptance of ‘Abate Fetel’ pears. Postharvest Biol. Technol. 2009, 51, 342–348. [Google Scholar] [CrossRef]
- Sugar, D.; Basile, S.R. Low-temperature induction of ripening capacity in ‘Comice’ and ‘Bosc’ pears as influenced by fruit maturity. Postharvest Biol. Technol. 2009, 51, 278–280. [Google Scholar] [CrossRef]
- Torregrosa, L.; Echeverria, G.; Illa, J.; Giné-Bordonaba, J. Ripening behaviour and consumer acceptance of ‘Conference’ pears during shelf life after long term DCA-storage. Postharvest Biol. Technol. 2019, 155, 94–101. [Google Scholar] [CrossRef]
- Fonseca, S.; Monteiro, L.; Barreiro, M.G.; Pais, M.S. Expression of genes encoding cell wall modifying enzymes is induced by cold storage and reflects changes in pear fruit texture. J. Exp. Bot. 2005, 56, 2029–2036. [Google Scholar] [CrossRef] [Green Version]
- Chiriboga, M.A.; Shotsmans, W.C.; Larrigaudière, C.; Dupille, E.; Recasens, I. How to prevent ripening blockage in 1-MCP-treated ‘Conference’pears. J. Sci. Food Agric. 2011, 91, 1781–1788. [Google Scholar] [CrossRef]
- Makkumrai, W.; Anthon, G.E.; Sivertsen, H.; Ebeler, S.E.; Negre-Zakharov, F.; Barrett, D.M.; Mitcham, J.E. Effect of ethylene and temperature conditioning on sensory attributes and chemical composition of ‘Bartlett’ pears. Postharvest Biol. Technol. 2014, 97, 44–61. [Google Scholar] [CrossRef]
- Torregrosa, L.; Echeverria, G.; Illa, J.; Torres, R.; Jordi Giné-Bordonaba, J. Spatial distribution of flavor components and antioxidants in the flesh of ‘Conference’ pears and its relationship with postharvest pathogens susceptibility. Postharvest Biol. Technol. 2020, 159, 111004. [Google Scholar] [CrossRef]
- Yan, S.; Li, L.; He, L.; Liang, L.; Li, X. Maturity and cooling rate affects browning, polyphenol oxidase activity and gene expression of ‘Yali’ pears during storage. Postharvest Biol. Technol. 2013, 85, 39–44. [Google Scholar] [CrossRef]
- Li, M.; Zhi, H.; Dong, Y. Textural property and cell wall metabolism of ‘Golden Bosc’ and ‘d’Anjou’ pears as influenced by oxygen regimes after long-term controlled atmosphere storage. Postharvest Biol. Technol. 2019, 151, 26–35. [Google Scholar] [CrossRef]
- Li, L.; Zhang, Y.; Fan, X.; Wang, J.; Liang, L.; Yan, S.; Xiao, L. Relationships between activated oxygen metabolism and browning of ‘Yali’ pears during storage. J. Food Process. Preserv. 2020, 44, 14392. [Google Scholar] [CrossRef]
- Wu, G.X.; Zhou, H.W.; Wang, J.M. Biochemical mechanism and substances determination of enzymic browning of ‘Yali’ pear (Pyrus bretschneideri Rehd.). Acta Hortic. Sin. 1992, 19, 198–202. [Google Scholar]
- Cao, J.K.; Jiang, W.B. Induction of resistance in ‘Yali’ pear (Pyrus bretschneideri Rehd.) fruit against postharvest diseases by acibenzolar-S-methyl sprays on trees during fruit growth. Sci. Hortic. 2006, 110, 181–186. [Google Scholar] [CrossRef]
- Wang, Z.H.; Wang, W.H.; Tong, W. Effects of different cooling methods on physiology and core browning of ‘Yali’ pear treated with 1-MCP. J. Fruit Sci. 2011, 28, 513–517. [Google Scholar]
- Liang, L.Y.; Jin, Z.; Hao, L.P.; Yan, S.J. Effects of Different Cooling Methods on Postharvest Physiology of Yali Pears during Ice Temperature Storage. Adv. Mater. Res. 2012, 554, 1072–1075. [Google Scholar] [CrossRef]
- Itai, A.; Kotaki, T.; Tanabe, K.; Tamura, F.; Kawaguchi, D.; Fukuda, M. Rapid identification of 1-aminocyclopropane-1-carboxylate (ACC) synthase genotypes in cultivars of Japanese pear (Pyrus pyrifolia Nakai) using CAPS markers. Theor. Appl. Genet. 2003, 106, 1266–1272. [Google Scholar] [CrossRef]
- Saquet, A.A. Storage of Pears. Sci. Hortic. 2019, 246, 1009–1016. [Google Scholar] [CrossRef]
- Łysiak, G.P.; Rutkowski, K.; Walkowiak-Tomczak, D. Effect of Storage Conditions on Storability and Antioxidant Potential of Pears cv. ‘Conference’. Agriculture 2021, 11, 545. [Google Scholar] [CrossRef]
- Valero, D.; Serrano, M. Postharvest Biology and Technology for Preserving Fruit Quality; CRC Press: Boca Raton, FL, USA, 2010; pp. 69–88. ISBN 1-4398-0267-X. [Google Scholar] [CrossRef]
- Yi, X.; Zhao, B.; Tang, Y.; Xu, Z. Transcriptome analysis reveals the regulation of metabolic processes during the post-harvest cold storage of pear. Genomics 2020, 112, 3933–3942. [Google Scholar] [CrossRef]
- Streif, J. Ernte, Lagerung und Aufbereitung. In Lucas’ Anleitung zum Obstbau; Winter, F., Lucas, E., Eds.; Eugen Ulmer: Stuttgart, Germany, 1992; Volume 31, pp. 304–337. [Google Scholar]
- Kolniak-Ostek, J.; Kłopotowska, D.; Rutkowski, K.P.; Skorupi’nska, A.; Kruczy´nska, D.E. Bioactive Compounds and HealthPromoting Properties of Pear (Pyrus communis L.) Fruits. Molecules 2020, 25, 4444. [Google Scholar] [CrossRef] [PubMed]
- Sinha, A.; Gill, P.P.S.; Jawandha, S.K.; Kaur, P.; Grewal, S.K. Chitosan-enriched salicylic acid coatings preserves antioxidant properties and alleviates internal browning of pear fruit under cold storage and supermarket conditions. Postharvest Biol. Technol. 2021, 182, 111721. [Google Scholar] [CrossRef]
- Brahem, M.; Renard, C.M.G.C.; Eder, S.; Loonis, M.; Ouni, R.; Mars, M.; Le Bourvellec, C. Characterization and quantification of fruit phenolic compounds of European and Tunisian pear cultivars. Food Res. Int. 2017, 95, 125–133. [Google Scholar] [CrossRef] [PubMed]
- Verzhuk, V.G.; Murashev, S.V.; Belova, A.Y. Determination of tissue elasticity of apple, pear, and quince fruits for predicting losses during cold storage. Russ. Agric. Sci. 2012, 38, 272–274. [Google Scholar] [CrossRef]
- Konopacka, D.; Rutkowski, K.P.; Kruczýnska, D.E.; Skorupínska, A.; Płocharski, W. Quality potential of some new pear cultivars–hoe to obtain fruit of the best sensory characteristics? J. Hortic. Res. 2014, 22, 71–84. [Google Scholar] [CrossRef] [Green Version]
- Fonseca, S.C.; Oliveira, F.A.; Brecht, J.K. Modelling respiration rate of fresh fruits and vegetables for modified atmosphere packages: A review. J. Food Eng. 2002, 52, 99–119. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT-Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Pellegrini, N.; Serafini, M.; Colombi, B.; Del Rio, D.; Salvatore, S.; Bianchi, M.; Brighenti, F. Total antioxidant capacity of plant foods, beverages and oils consumed in Italy assessed by three different in vitro assays. J. Nutr. 2003, 133, 2812–2819. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Benzie, I.F.; Szeto, Y.T. Total antioxidant capacity of teas by the ferric reducing/antioxidant power assay. J. Agric. Food Chem. 1999, 47, 633–636. [Google Scholar] [CrossRef]
- Lin, Y.; Li, N.; Lin, H.; Lin, M.; Chen, Y.; Wang, H.; Ritenour, M.A.; Lin, Y. Effects of chitosan treatment on the storability and quality properties of longan fruit during storage. Food Chem. 2020, 306, 125627. [Google Scholar] [CrossRef]
- Brandes, N.; Zude-Sasse, M. Respiratory patterns of European pear (Pyrus communis L. ‘Conference’) throughout pre- and post-harvest fruit development. Heliyon 2019, 5, e01160. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, U.Y.; Choi, J.H.; Lee, J.H.; Oh, J.P.; Chun, J.P. Effect of 1-MCP Treatment on the Early-season Asian Pear Cultivar ‘Wonhwang’ in Response to Different Temperature Conditions during Simulated Exportation. Hortic. Sci. Technol. 2017, 35, 568–576. [Google Scholar] [CrossRef]
- Fagundes, C.; Carciofi, B.A.M.; Monteiro, A.R. Estimate of respiration rate and physicochemical changes of fresh-cut apples stored under different temperatures. Food Sci. Technol. 2013, 33, 60–67. [Google Scholar] [CrossRef] [Green Version]
- Liu, M.; Pirrello, J.; Chervin, C.; Roustan, J.P.; Bouzayen, M. Ethylene control of fruit ripening: Revisiting the complex network of transcriptional regulation. Plant Physiol. 2015, 169, 2380–2390. [Google Scholar] [CrossRef] [Green Version]
- Saquet, A.A.; Streif, J. Respiration rate and ethylene metabolism of ‘Jonagold’ apple and ‘Conference’ pear under regular air and controlled atmosphere. Bragantia 2017, 76, 335–344. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Zhao, L.; Fan, C.; Wang, P.; Cui, M.; Liu, L.; Yang, H.; Wang, J. Impact of methyl salicylate on storage quality, ethylene action, and protein profiling of ‘Zaosu’ pear (Pyrus bretschneideri). Sci. Hortic. 2020, 264, 109196. [Google Scholar] [CrossRef]
- Chiriboga, M.A.; Saladié, M.; Jordi Giné Bordonaba, J.G.; Recasens, I.; Jordi Garcia-Mas, J.; Christian Larrigaudière, C. Effect of cold storage and 1-MCP treatment on ethylene perception, signalling and synthesis: Influence on the development of the evergreen behaviour in ‘Conference’ pears. Postharvest Biol. Technol. 2013, 86, 212–220. [Google Scholar] [CrossRef]
- Busatto, N.; Farneti, B.; Tadiello, A.; Oberkofler, V.; Cellini, A.; Biasioli, F.; Delledonne, M.; Cestaro, A.; Noutsos, C.; Costa, F. Wide transcriptional investigation unravel novel insights of the on-tree maturation and postharvest ripening of ‘Abate Fetel’ pear fruit. Hortic. Res. 2019, 6, 32. [Google Scholar] [CrossRef] [Green Version]
- Chauvin, M.A.; Ross, C.F.; Pitts, M.; Kupferman, E.; Swanson, B. Relationship betweeninstrumental and sensory determination of apple and pear texture. J. Food Qual. 2010, 33, 181–198. [Google Scholar] [CrossRef]
- Wang, Y.; Sugar, D. 1-MCP efficacy in extending storage life of ’Bartlett’ pears is affected by harvest maturity, production elevation, and holding temperature during treatment delay. Postharvest Biol. Technol. 2015, 103, 1–8. [Google Scholar] [CrossRef]
- Chen, J.L.; Yan, S.; Feng, Z.; Xiao, L.; Hu, X.S. Changes in the volatile compounds and chemical and physical properties of Yali pear (Pyrus bertschneideri Reld) during storage. Food Chem. 2006, 97, 248–255. [Google Scholar] [CrossRef]
- Arzani, K.; Khoshghalb, H.; Malakouti, M.J.; Barzegar, M. Postharvest Physicochemical Changes and Properties of Asian (Pyrus serotina Rehd.) and European (Pyrus communis L.) Pear Cultivars. Hortic. Environ. Biotechnol. 2008, 49, 244–252. [Google Scholar]
- Gamrasni, D.; Ruth Ben-Arie, R.; Goldway, M. 1-Methylcyclopropene (1-MCP) application to Spadona pears at different stages of ripening to maximize fruit quality after storage. Postharvest Biol. Technol. 2010, 58, 104–112. [Google Scholar] [CrossRef]
- Feng, Y.X.; Cheng, Y.D.; He, J.G.; Li, L.M.; Guan, J.F. Effects of 1-methylcyclopropene and modified atmosphere packaging on fruit quality and superficial scald in Yali pears during storage. J. Integr. Agric. 2018, 17, 1667–1675. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.; Wang, Z.; Wu, J.; Wang, Q.; Hu, X. Chemical compositional characterization of eight pear cultivars grown in China. Food Chem. 2007, 104, 268–275. [Google Scholar] [CrossRef]
- Sha, S.; Li, J.; Wu, J.; Zhang, S. Characteristics of organic acids in the fruit of different pear species. Afr. J. Agric. Res. 2011, 6, 2403–2410. [Google Scholar] [CrossRef]
- Gao, H.Y.; Liao, X.J.; Hu, X.S. Study on determination and correlation of soluble sugars and organic acids in pear juice from different cultivars. Acta Agric. Bor-Sin. 2004, 19, 104–107. [Google Scholar]
- Gao, H.F.; Wang, Y.Z. Respiration and sugar-catabolism of Kuerle fragrant pear during storage. Acta Hortic. Sin. 1983, 10, 141–142. [Google Scholar]
- Kolniak-Ostek, J. Chemical composition and antioxidant capacity of different anatomical parts of pear (Pyrus communis L.). Food Chem. 2016, 203, 491–497. [Google Scholar] [CrossRef]
- Itai, A.; Hatanaka, R.; Irie, H. Effects of storage temperature on fruit quality and expression of sucrose phosphate synthase and acid invertase genes in Japanese pear. Hortic. J. 2015, 84, 227–232. [Google Scholar] [CrossRef] [Green Version]
- Yim, S.H.; Nam, S.H. Physiochemical, nutritional and functional characterization of 10 different pear cultivars (Pyrus spp.). J. Appl. Bot. Food Qual. 2016, 89, 73–81. [Google Scholar] [CrossRef]
- Hong, S.Y.; Lansky, E.; Kang, S.S.; Yang, M. A review of pears (Pyrus spp.), ancient functional food for modern times. BMC Complement. Med. Ther. 2021, 21, 219. [Google Scholar] [CrossRef] [PubMed]
- Silva, F.J.P.; Gomes, M.H.; Fidalgo, F.; Rodrigues, J.A.; Almeida, D.P.F. Antioxidant properties and fruit quality during long-term storage of ‘‘Rocha’’ pear: Effects of maturity and storage conditions. J. Food Qual. 2010, 33, 1–20. [Google Scholar] [CrossRef]
- Guan, J.; He, J.; Shen, C.; Li, L.; Wang, Y.; Cheng, Y. How Cultivars Influence Fruit Composition: Total Phenols, Flavonoids Contents, and Antioxidant Activity in the Pulp of Selected Asian Pears. In Processing and Impact on Active Components in Food; Preedy, V., Ed.; Academic Press: Cambridge, MA, USA, 2015; pp. 139–145. [Google Scholar] [CrossRef]
- Li, G.; Jia, H.; Wu, R.; Hussain, S.; Teng, Y. Characterization of aromatic volatile constituents in 11 Asian pear cultivars belonging to different species. Afr. J. Agric. Res. 2012, 7, 4761–4770. [Google Scholar] [CrossRef]
- Kumari, P.; Barman, K.; Patel, V.B.; Siddiqui, M.W.; Kole, B. Reducing postharvest pericarp browning and preserving health promoting compounds of litchi fruit by combination treatment of salicylic acid and chitosan. Sci. Hortic. 2015, 197, 555–563. [Google Scholar] [CrossRef]
- Aghdam, M.S.; Asghari, M.; Babalar, M.; Sarcheshmeh, M.A.A. Impact of salicylic acid on postharvest physiology of fruits and vegetables. In Eco-Friendly Technology for Postharvest Produce Quality; Siddiqui, M.W., Ed.; Academic Press: London, UK, 2016; pp. 243–268. [Google Scholar] [CrossRef]


| Variable | ‘Conference’ | ‘Yali’ | ‘Conference’ | ‘Yali’ |
|---|---|---|---|---|
| Before Storage | After Storage | |||
| Chromaticity L* | 55.08 ± 1.63 a | 61.47 ± 1.38 bc | 56.59 ± 2.04 ab | 62.82 ± 0.53 c |
| Chromaticity a* | −0.31 ± 2.16 bc | −7.71 ± 0.24 a | 2.43 ± 2.65 c | −6.75 ± 0.15 ab |
| Chromaticity b* | 31.54 ± 1.91 a | 41.37 ± 1.46 b | 34.00 ± 2.43 a | 42.96 ± 0.14 b |
| DPPH (mmol Trolox/kg FW) | 0.416 ± 0.007 ab | 0.443 ± 0.012 b | 0.377 ± 0.010 a | 0.429 ± 0.007 b |
| FRAP (mmol Trolox/kg FW) | 0.439 ± 0.015 a | 0.648 ± 0.023 c | 0.386 ± 0.012 a | 0.550 ± 0.014 b |
| Soluble solids (°Bx) | 11.9 ± 0.2 b | 9.4 ± 0.2 a | 13.1 ± 0.3 b | 9.3 ± 0.2 a |
| Saccharose (g 100 g−1) | 3.00 ± 0.06 c | 0.13 ± 0.05 a | 1.55 ± 0.38 b | 0.06 ± 0.03 a |
| Fructose (g 100 g−1) | 5.49 ± 0.07 b | 3.88 ± 0.09 a | 6.65 ± 0.14 c | 3.56 ± 0.21 a |
| Glucose (g 100 g−1) | 0.79 ± 0.04 a | 1.71 ± 0.04 c | 1.47 ± 0.02 bc | 1.29 ± 0.12 b |
| Titratable acidity (%) | 0.132 ± 0.011 a | 0.183 ± 0.006 b | 0.149 ± 0.006 a | 0.194 ± 0.002 b |
| Malic acid (mg·kg−1) | 2900 ± 54 c | 1700 ± 6 a | 2700 ± 43 b | 1800 ± 12 a |
| L-ascorbic acid (mg·kg−1) | 200 ± 17 a | 700 ± 12 b | 280 ± 24 a | 680 ± 23 b |
| Citric acid (mg·kg−1) | 140 ± 8 a | 1300 ± 46 b | 130 ± 14 a | 1300 ± 46 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Híc, P.; Kožíšková, J.; Omastová, P.; Balík, J.; Goliáš, J.; Horák, M. Physiochemical Changes of European Pear cv. Conference and Asian Pear cv. Yali during Cold Storage. Horticulturae 2023, 9, 378. https://doi.org/10.3390/horticulturae9030378
Híc P, Kožíšková J, Omastová P, Balík J, Goliáš J, Horák M. Physiochemical Changes of European Pear cv. Conference and Asian Pear cv. Yali during Cold Storage. Horticulturae. 2023; 9(3):378. https://doi.org/10.3390/horticulturae9030378
Chicago/Turabian StyleHíc, Pavel, Jarmila Kožíšková, Petra Omastová, Josef Balík, Jan Goliáš, and Miroslav Horák. 2023. "Physiochemical Changes of European Pear cv. Conference and Asian Pear cv. Yali during Cold Storage" Horticulturae 9, no. 3: 378. https://doi.org/10.3390/horticulturae9030378
APA StyleHíc, P., Kožíšková, J., Omastová, P., Balík, J., Goliáš, J., & Horák, M. (2023). Physiochemical Changes of European Pear cv. Conference and Asian Pear cv. Yali during Cold Storage. Horticulturae, 9(3), 378. https://doi.org/10.3390/horticulturae9030378





