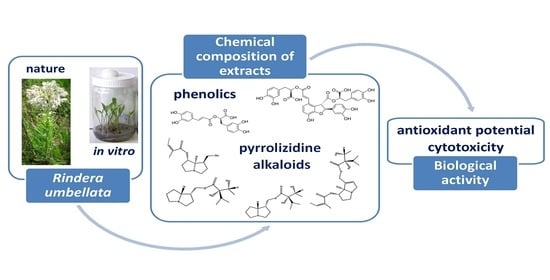
Chemical Characterization, Antioxidant Activity, and Cytotoxity of Wild-Growing and In Vitro Cultivated Rindera umbellata (Waldst. and Kit.) Bunge
Abstract
:1. Introduction
2. Materials and Methods
2.1. Wild-Growing Plant Material
2.2. In Vitro Culture Establishment
2.3. Preparation of Plant Extracts
2.4. Total Phenolic and Flavonoid Content Determination
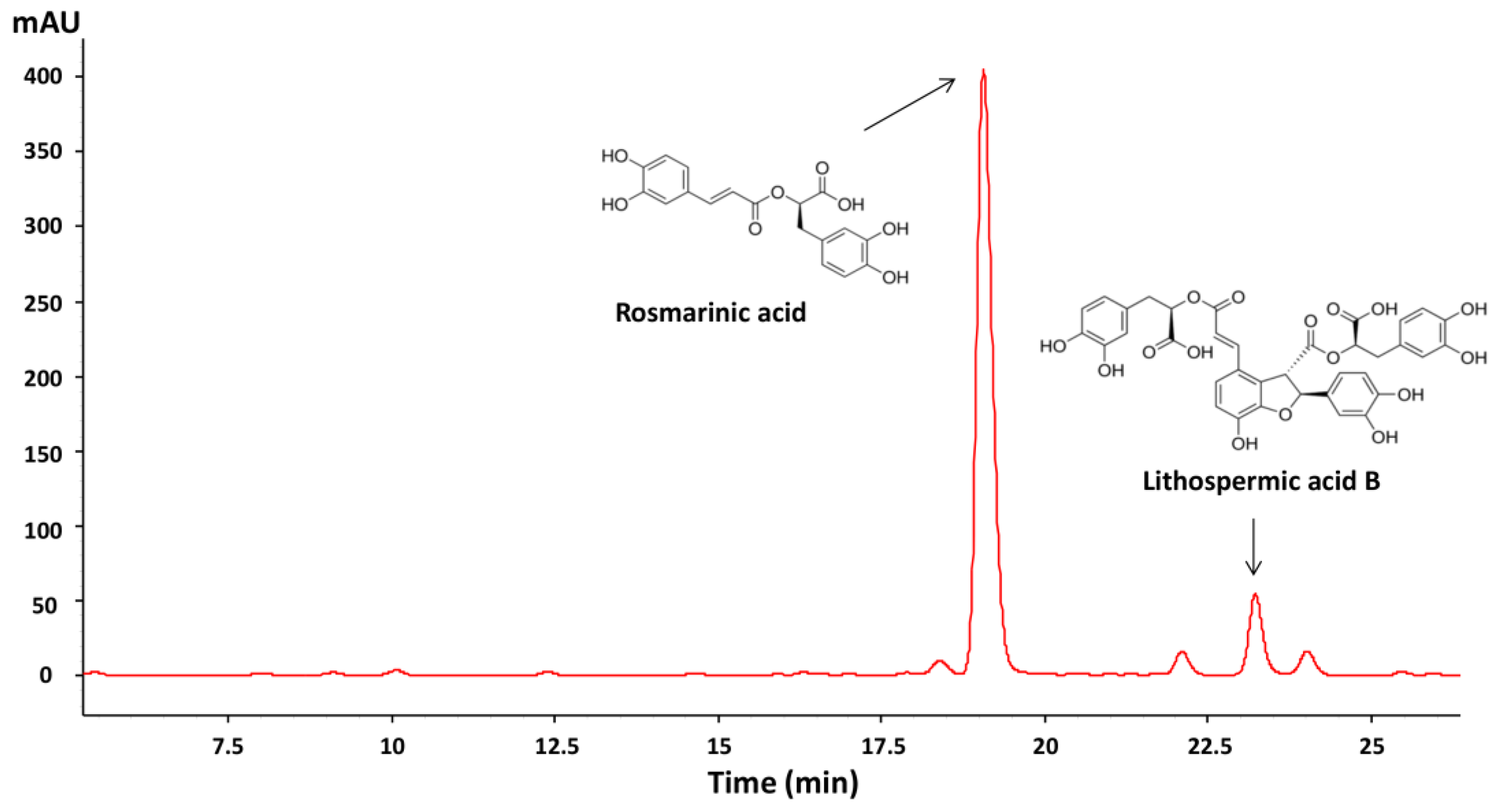
2.5. HPLC-DAD Analysis of Phenolic Compounds
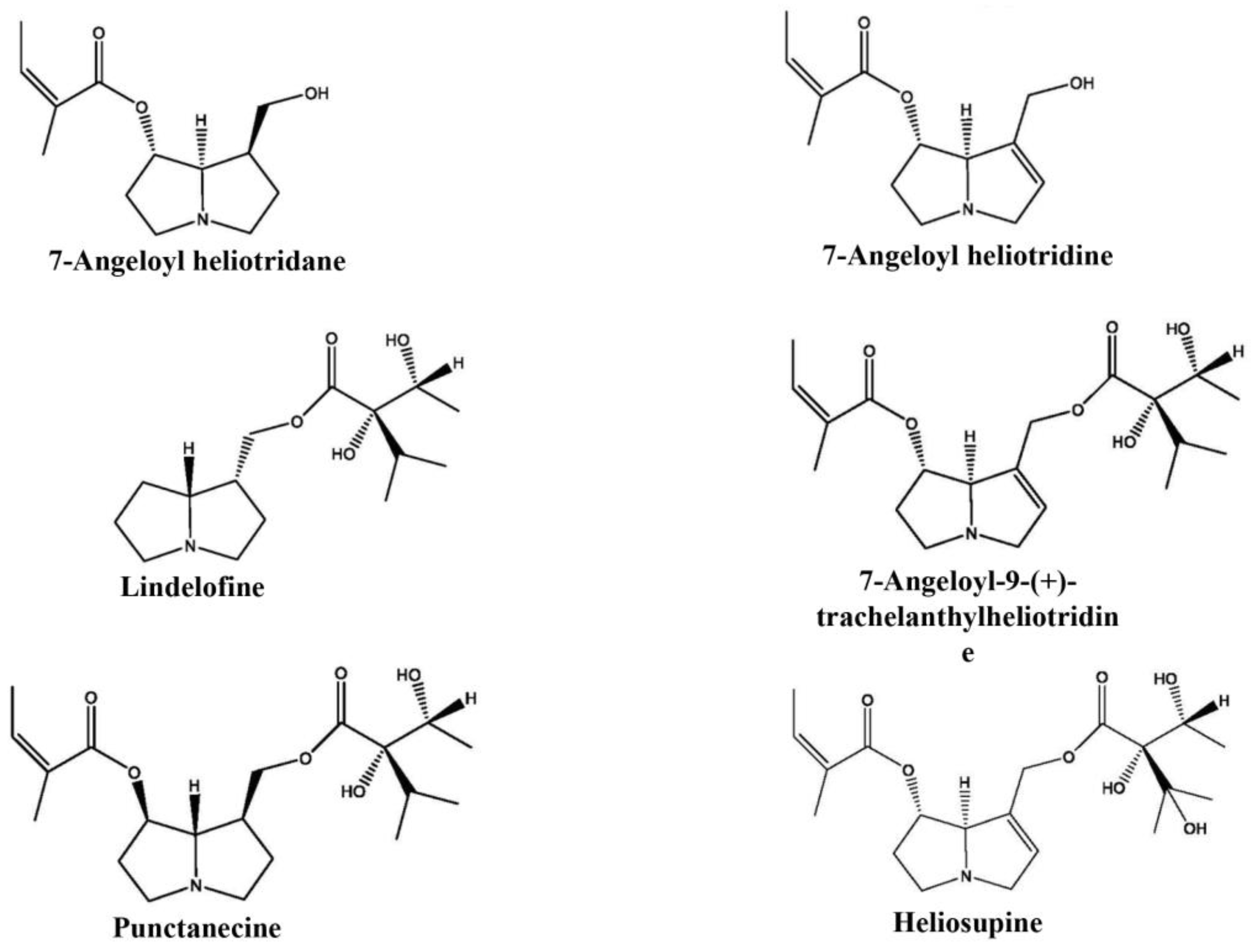
2.6. GC-MS/FID Analysis of Pyrrolizidine Alkaloids
2.7. Determination of Antioxidant Activity
2.8. Cytotoxicity Assay
2.9. Statistical Analysis
3. Results
3.1. Phenolic Compounds Content
3.2. Pyrrolizidine Alkaloids Presence in Wild and In Vitro Growing Plants
3.3. Antioxidant Activity
3.4. Cytotoxic Activity
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Diklić, N.; Niketić, M.; Tomović, G. Iščezli i krajnje ugroženi taksoni. In Crvena Knjiga Flore Srbije I; Ministry of Environment of the Republic of Serbia & Faculty of Biology, University of Belgrade & Institution for Protection of Nature of the Republic of Serbia: Belgrade, Serbia, 1999; pp. 251–479. [Google Scholar]
- Ljaljević Grbić, M.; Vukojević, J.G.; Janošević, D.; Grubišić, D.; Mijović, A. Gljive na ugroženim i retkim biljkama. In Proceedings of the 8th Symposium on the Flora of Southeastern Serbia and Neighboring Regions; Randjelovic, V., Ed.; Faculty of Sciences and Mathematics, Department of Biology and Ecology: Niš, Serbia, 2005; p. 23. [Google Scholar]
- Murthy, H.N.; Lee, E.-J.; Paek, K.-Y. Production of secondary metabolites from cell and organ cultures: Strategies and approaches for biomass improvement and metabolite accumulation. Plant Cell Tissue Organ Cult. (PCTOC) 2014, 118, 1–16. [Google Scholar] [CrossRef]
- Espinosa-Leal, C.A.; Puente-Garza, C.A.; García-Lara, S. In vitro plant tissue culture: Means for production of biological active compounds. Planta 2018, 248, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Yaseen, M.; Ahmad, T.; Sablok, G.; Standardi, A.; Hafiz, I.A. Review: Role of carbon sources for in vitro plant growth and development. Mol. Biol. Rep. 2013, 40, 2837–2849. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Kim, J.; Kim, N.; Lee, D.; Lee, H.; Lee, D.Y.; Kim, K.H. Metabolomic Elucidation of the Effect of Sucrose on the Secondary Metabolite Profiles in Melissa officinalis by Ultraperformance Liquid Chromatography-Mass Spectrometry. ACS Omega 2020, 5, 33186–33195. [Google Scholar] [CrossRef]
- George, E.F.; Hall, M.A.; Klerk, G.-J.D. Plant Tissue Culture Procedure—Background. In Plant Propagation by Tissue Culture: Volume 1. The Background; George, E.F., Hall, M.A., Klerk, G.-J.D., Eds.; Springer: Dordrecht, The Netherlands, 2008; pp. 1–28. [Google Scholar]
- Arnold, T.; Appel, H.; Patel, V.; Stocum, E.; Kavalier, A.; Schultz, J. Carbohydrate translocation determines the phenolic content of Populus foliage: A test of the sink-source model of plant defense. New Phytol. 2004, 164, 157–164. [Google Scholar] [CrossRef] [PubMed]
- Babenko, L.; Smirnov, O.; Romanenko, K.; Trunova, O.; Kosakivska, I. Phenolic compounds in plants: Biogenesis and functions. Ukr. Biochem. J. 2019, 91, 5–18. [Google Scholar] [CrossRef]
- Soobrattee, M.A.; Neergheen, V.S.; Luximon-Ramma, A.; Aruoma, O.I.; Bahorun, T. Phenolics as potential antioxidant therapeutic agents: Mechanism and actions. Mutat. Res. 2005, 579, 200–213. [Google Scholar] [CrossRef]
- Bhambhani, S.; Kondhare, K.R.; Giri, A.P. Diversity in Chemical Structures and Biological Properties of Plant Alkaloids. Molecules 2021, 26, 3374. [Google Scholar] [CrossRef]
- Dai, J.; Mumper, R.J. Plant phenolics: Extraction, analysis and their antioxidant and anticancer properties. Molecules 2010, 15, 7313–7352. [Google Scholar] [CrossRef]
- Debnath, B.; Singh, W.S.; Das, M.; Goswami, S.; Singh, M.K.; Maiti, D.; Manna, K. Role of plant alkaloids on human health: A review of biological activities. Mater. Today Chem. 2018, 9, 56–72. [Google Scholar] [CrossRef]
- Marchev, A.S.; Vasileva, L.V.; Amirova, K.M.; Savova, M.S.; Koycheva, I.K.; Balcheva-Sivenova, Z.P.; Vasileva, S.M.; Georgiev, M.I. Rosmarinic acid—From bench to valuable applications in food industry. Trends Food Sci. Technol. 2021, 117, 182–193. [Google Scholar] [CrossRef]
- Qin, T.; Rasul, A.; Sarfraz, A.; Sarfraz, I.; Hussain, G.; Anwar, H.; Riaz, A.; Liu, S.; Wei, W.; Li, J.; et al. Salvianolic acid A & B: Potential cytotoxic polyphenols in battle against cancer via targeting multiple signaling pathways. Int. J. Biol. Sci. 2019, 15, 2256–2264. [Google Scholar] [CrossRef] [PubMed]
- Bulgakov, V.P.; Inyushkina, Y.V.; Fedoreyev, S.A. Rosmarinic acid and its derivatives: Biotechnology and applications. Crit. Rev. Biotechnol. 2012, 32, 203–217. [Google Scholar] [CrossRef] [PubMed]
- Perić, M.; Dmitrović, S.; Živković, S.; Filipović, B.; Skorić, M.; Simonović, A.; Todorović, S. In vitro growth, morphogenesis, and acclimatization of endangered Rindera umbellata (Waldst. & Kit.) Bunge. HortScience 2012, 47, 1123–1128. [Google Scholar]
- Mandić, B.M.; Simić, M.R.; Vučković, I.M.; Vujisić, L.V.; Novaković, M.M.; Trifunović, S.S.; Nikolić-Mandić, S.D.; Tešević, V.V.; Vajs, V.V.; Milosavljević, S.M. Pyrrolizidine Alkaloids and Fatty Acids from the Endemic Plant Species Rindera umbellata and the Effect of Lindelofine-N-oxide on Tubulin Polymerization. Molecules 2013, 18, 10694–10706. [Google Scholar] [CrossRef] [PubMed]
- Mandić, B.M.; Vlajić, M.D.; Trifunović, S.S.; Simić, M.R.; Vujisić, L.V.; Vučković, I.M.; Novaković, M.M.; Nikolić-Mandić, S.D.; Tešević, V.V.; Vajs, V.V.; et al. Optimisation of isolation procedure for pyrrolizidine alkaloids from Rindera umbellata Bunge. Nat. Prod. Res. 2015, 29, 887–890. [Google Scholar] [CrossRef] [PubMed]
- Murashige, T.; Skoog, F. A Revised Medium for Rapid Growth and Bio Assays with Tobacco Tissue Cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Mandić, B.; Gođevac, D.; Beškoski, V.; Simić, M.R.; Trifunović, S.S.; Tešević, V.; Vajs, V.; Milosavljević, S.M. Pyrrolizidine alkaloids from seven wild-growing Senecio species in Serbia and Montenegro. J. Serb. Chem. Soc. 2009, 74, 27–34. [Google Scholar] [CrossRef]
- Šiler, B.; Živković, S.; Banjanac, T.; Cvetković, J.; Nestorović Živković, J.; Ćirić, A.; Soković, M.; Mišić, D. Centauries as underestimated food additives: Antioxidant and antimicrobial potential. Food Chem. 2014, 147, 367–376. [Google Scholar] [CrossRef]
- Hansen, M.B.; Nielsen, S.E.; Berg, K. Re-examination and further development of a precise and rapid dye method for measuring cell growth/cell kill. J. Immunol. Methods 1989, 119, 203–210. [Google Scholar] [CrossRef] [PubMed]
- Badisa, R.B.; Darling-Reed, S.F.; Joseph, P.; Cooperwood, J.S.; Latinwo, L.M.; Goodman, C.B. Selective cytotoxic activities of two novel synthetic drugs on human breast carcinoma MCF-7 cells. Anticancer Res. 2009, 29, 2993–2996. [Google Scholar]
- Petersen, M.; Simmonds, M.S. Rosmarinic acid. Phytochemistry 2003, 62, 121–125. [Google Scholar] [CrossRef]
- Rao, S.R.; Ravishankar, G.A. Plant cell cultures: Chemical factories of secondary metabolites. Biotechnol. Adv. 2002, 20, 101–153. [Google Scholar] [CrossRef]
- Ganos, C.; Aligiannis, N.; Chinou, I.; Naziris, N.; Chountoulesi, M.; Mroczek, T.; Graikou, K. Rindera graeca (Boraginaceae) Phytochemical Profile and Biological Activities. Molecules 2020, 25, 3625. [Google Scholar] [CrossRef]
- Schramm, S.; Köhler, N.; Rozhon, W. Pyrrolizidine Alkaloids: Biosynthesis, Biological Activities and Occurrence in Crop Plants. Molecules 2019, 24, 498. [Google Scholar] [CrossRef]
- Hartmann, T. From waste products to ecochemicals: Fifty years research of plant secondary metabolism. Phytochemistry 2007, 68, 2831–2846. [Google Scholar] [CrossRef]
- Graikou, K.; Damianakos, H.; Ganos, C.; Sykłowska-Baranek, K.; Jeziorek, M.; Pietrosiuk, A.; Roussakis, C.; Chinou, I. Chemical Profile and Screening of Bioactive Metabolites of Rindera graeca (A. DC.) Bois. & Heldr. (Boraginaceae) In Vitro Cultures. Plants 2021, 10, 834. [Google Scholar] [CrossRef]
- Seremet, O.C.; Olaru, O.T.; Gutu, C.M.; Nitulescu, G.M.; Ilie, M.; Negres, S.; Zbarcea, C.E.; Purdel, C.N.; Spandidos, D.A.; Tsatsakis, A.M.; et al. Toxicity of plant extracts containing pyrrolizidine alkaloids using alternative invertebrate models. Mol. Med. Rep. 2018, 17, 7757–7763. [Google Scholar] [CrossRef]
- Petersen, M. Rosmarinic acid: New aspects. Phytochem. Rev. 2013, 12, 207–227. [Google Scholar] [CrossRef]
- Kikowska, M.; Budzianowski, J.; Krawczyk, A.; Thiem, B. Accumulation of rosmarinic, chlorogenic and caffeic acids in in vitro cultures of Eryngium planum L. Acta Physiol. Plant. 2012, 34, 2425–2433. [Google Scholar] [CrossRef]
- DiCosmo, F.; Misawa, M. Plant cell and tissue culture: Alternatives for metabolite production. Biotechnol. Adv. 1995, 13, 425–453. [Google Scholar] [CrossRef] [PubMed]
- Petersen, M.; Szabo, E.; Meinhard, J.; Karwatzki, B.; Gertlowski, C.; Kempin, B.; Fuß, E. Biosynthesis and accumulation of rosmarinic acid in suspension cultures ofColeus blumei. Plant Cell Tissue Organ Cult. 1995, 43, 89–92. [Google Scholar] [CrossRef]
- Lu, N.; Bernardo, E.L.; Tippayadarapanich, C.; Takagaki, M.; Kagawa, N.; Yamori, W. Growth and Accumulation of Secondary Metabolites in Perilla as Affected by Photosynthetic Photon Flux Density and Electrical Conductivity of the Nutrient Solution. Front. Plant Sci. 2017, 8, 708. [Google Scholar] [CrossRef]
- Santos-Gomes, P.C.; Seabra, R.M.; Andrade, P.B.; Fernandes-Ferreira, M. Phenolic antioxidant compounds produced by in vitro shoots of sage (Salvia officinalis L.). Plant Sci. 2002, 162, 981–987. [Google Scholar] [CrossRef]
- D’Amelia, V.; Aversano, R.; Chiaiese, P.; Carputo, D. The antioxidant properties of plant flavonoids: Their exploitation by molecular plant breeding. Phytochem. Rev. 2018, 17, 611–625. [Google Scholar] [CrossRef]
- Nunes, S.; Madureira, A.R.; Campos, D.; Sarmento, B.; Gomes, A.M.; Pintado, M.; Reis, F. Therapeutic and nutraceutical potential of rosmarinic acid-Cytoprotective properties and pharmacokinetic profile. Crit. Rev. Food Sci. Nutr. 2017, 57, 1799–1806. [Google Scholar] [CrossRef]
- Hajam, Y.A.; Rani, R.; Ganie, S.Y.; Sheikh, T.A.; Javaid, D.; Qadri, S.S.; Pramodh, S.; Alsulimani, A.; Alkhanani, M.F.; Harakeh, S.; et al. Oxidative stress in human pathology and aging: Molecular mechanisms and perspectives. Cells 2022, 11, 552. [Google Scholar] [CrossRef]
- Järvinen, K.; Pietarinen-Runtti, P.; Linnainmaa, K.; Raivio, K.O.; Krejsa, C.M.; Kavanagh, T.; Kinnula, V.L. Antioxidant defense mechanisms of human mesothelioma and lung adenocarcinoma cells. Am. J. Physiol. Lung Cell. Mol. Physiol. 2000, 278, L696–L702. [Google Scholar] [CrossRef]
- Babich, H.; Schuck, A.G.; Weisburg, J.H.; Zuckerbraun, H.L. Research strategies in the study of the pro-oxidant nature of polyphenol nutraceuticals. J. Toxicol. 2011, 2011, 467305. [Google Scholar] [CrossRef]
- Martin-Cordero, C.; Leon-Gonzalez, A.J.; Calderon-Montano, J.M.; Burgos-Moron, E.; Lopez-Lazaro, M. Pro-oxidant natural products as anticancer agents. Curr. Drug Targets 2012, 13, 1006–1028. [Google Scholar] [CrossRef]
- Chen, L.; Huang, S.; Li, C.Y.; Gao, F.; Zhou, X.L. Pyrrolizidine alkaloids from Liparis nervosa with antitumor activity by modulation of autophagy and apoptosis. Phytochemistry 2018, 153, 147–155. [Google Scholar] [CrossRef]

| Plant Parts | TPC (mg GAE g−1 DW) | TFC (mg RE g−1 DW) | RA (mg g−1 DW) | LAB (mg RAE g−1 DW) |
|---|---|---|---|---|
| Leaves | 32.37 ± 0.15 c | 22.87 ± 2.12 c | 0.28 ± 0.04 b | 0.04 ± 0.02 a |
| Flowers | 25.31 ± 1.70 b | 13.41 ± 2.31 b | 0.24 ± 0.04 b | Nd |
| Roots | 26.93 ± 0.22 b | 19.10 ± 3.15 bc | 0.06 ± 0.01 a | 1.12 ± 0.21 b |
| Stems | 7.26 ± 1.17 a | 0.81 ±0.44 a | 0.02 ± 0.01 a | Nd |
| Carbohydrate Concentration (M) | TPC (mg GAE g−1 DW) | TFC (mg RE g−1 DW) | RA (mg g−1 DW) | LAB (mg RAE g−1 DW) | |
|---|---|---|---|---|---|
| Sucrose | 0.003 | 18.63 ± 1.78 a | 15.37 ± 2.86 b | 0.43 ± 0.04 ab | 0.05 ± 0.02 a |
| 0.01 | 29.37 ± 0.96 b | 8.37 ± 1.48 a | 0.22 ± 0.03 a | 0.01 ± 0.01 a | |
| 0.03 | 29.58 ± 1.11 b | 16.65 ± 0.38 bc | 0.42 ± 0.01 ab | 0.01 ± 0.01 a | |
| 0.06 | 26.08 ± 1.86 b | 17.76 ± 2.29 bc | 0.46 ± 0.04 ab | 0.07 ± 0.01 a | |
| 0.1 | 27.87 ± 1.33 b | 22.15 ± 1.33 c | 0.71 ± 0.11 b | 0.10 ± 0.02 a | |
| 0.3 | 27.95 ± 1.40 b | 61.97 ± 1.49 d | 2.52 ± 0.17 c | 0.11 ± 0.11 a | |
| Glucose | 0.003 | 13.92 ± 0.01 a | 10.83 ± 1.56 abc | 0.23 ± 0.06 a | 0.04 ± 0.02 a |
| 0.01 | 14.43 ± 0.21 a | 8.23 ± 0.98 ab | 0.18 ± 0.01 a | 0.01 ± 0.01 a | |
| 0.03 | 18.02 ± 0.46 b | 13.55 ± 2.61 bc | 0.30 ± 0.06 ab | 0.02 ± 0.02 a | |
| 0.06 | 21.45 ± 0.20 c | 14.40 ± 2.56 c | 0.40 ± 0.06 b | 0.02 ± 0.01 a | |
| 0.1 | 20.26 ± 0.01 c | 12.56 ± 0.66 bc | 0.28 ± 0.05 ab | 0.00 ± 0.00 a | |
| 0.3 | 26.74 ± 0.97 d | 6.60 ± 1.69 a | 0.23 ± 0.06 a | 0.00 ± 0.00 a | |
| Fructose | 0.003 | 17.95 ± 0.17 b | 14.10 ± 0.32 ab | 0.23 ± 0.01 a | 0.04 ± 0.15 a |
| 0.01 | 19.25 ± 0.86 b | 11.26 ± 1.46 a | 0.26 ± 0.04 a | 0.06 ± 0.02 ab | |
| 0.03 | 24.50 ± 0.97 c | 10.68 ± 0.32 a | 0.24 ± 0.04 a | 0.05 ± 0.01 a | |
| 0.06 | 20.63 ± 1.53 bc | 14.94 ± 3.08 ab | 0.34 ± 0.08 a | 0.07 ± 0.02 b | |
| 0.1 | 13.75 ± 1.42 a | 18.43 ± 1.99 b | 0.46 ± 0.10 a | 0.08 ± 0.05 b | |
| 0.3 | 11.24 ± 1.18 a | 31.21 ± 2.55 c | 1.51 ± 0.22 b | 0.05 ± 0.05 a |
| Pyrrolizidine Alkaloids | Retention Time (min) | Wild-Growing Plants | In Vitro Plants |
|---|---|---|---|
| 7-angeloyl heliotridane | 12.349 | flw, lv | lv |
| 7-angeloyl heliotridine | 13.171 | flw, lv | |
| lindelofine | 15.176 | flw, lv, rt | rt |
| 7-angeloyl-9-(+)-trachelanthylheliotridine | 19.571 | flw, lv | |
| punctanecine | 19.746 | flw, lv | |
| heliosupine | 20.288 | lv |
| IC50 (mM RAE g−1 DW) | ||
|---|---|---|
| Wild Growing Plants | DPPH● | ABTS•+ |
| Leaves | 2.31 ± 0.23 a | 0.77 ± 0.01 a |
| Flowers | 4.14 ± 0.77 a | 1.15 ± 0.28 a |
| Roots | 1.71 ± 0.54 a | 1.03 ± 0.17 a |
| Stems | 52.56 ± 3.57 b | 10.06 ± 3.35 b |
| Plants grown in vitro | ||
| 0.3 M sucrose | 0.52 ± 0.04 a | 0.27 ± 0.01 a |
| 0.1 M sucrose | 2.07 ± 0.33 b | 1.06 ± 0.16 b |
| HT-29 | A549 | MRC-5 | |||
|---|---|---|---|---|---|
| IC50 (mg mL−1) | SI | IC50 (mg mL−1) | SI | IC50 (mg mL−1) | |
| Wild-growing leaves | 1.65 ± 0.18 | Na | Nd | Na | Nd |
| In vitro grown leaves | |||||
| 0.3 M sucrose | 1.25 ± 0.14 | 2.56 | 4.00 ± 0.17 | 0.80 | 3.20 ± 0.15 |
| 0.1 M sucrose | 3.19 ± 0.11 | 0.90 | 3.50 ± 0.22 | 0.80 | 2.90 ± 0.20 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Todorović, S.; Perić, M.; Nikolić, B.; Mandić, B.; Cvetković, S.; Bogdanović, M.; Živković, S. Chemical Characterization, Antioxidant Activity, and Cytotoxity of Wild-Growing and In Vitro Cultivated Rindera umbellata (Waldst. and Kit.) Bunge. Horticulturae 2023, 9, 381. https://doi.org/10.3390/horticulturae9030381
Todorović S, Perić M, Nikolić B, Mandić B, Cvetković S, Bogdanović M, Živković S. Chemical Characterization, Antioxidant Activity, and Cytotoxity of Wild-Growing and In Vitro Cultivated Rindera umbellata (Waldst. and Kit.) Bunge. Horticulturae. 2023; 9(3):381. https://doi.org/10.3390/horticulturae9030381
Chicago/Turabian StyleTodorović, Slađana, Marija Perić, Biljana Nikolić, Boris Mandić, Stefana Cvetković, Milica Bogdanović, and Suzana Živković. 2023. "Chemical Characterization, Antioxidant Activity, and Cytotoxity of Wild-Growing and In Vitro Cultivated Rindera umbellata (Waldst. and Kit.) Bunge" Horticulturae 9, no. 3: 381. https://doi.org/10.3390/horticulturae9030381
APA StyleTodorović, S., Perić, M., Nikolić, B., Mandić, B., Cvetković, S., Bogdanović, M., & Živković, S. (2023). Chemical Characterization, Antioxidant Activity, and Cytotoxity of Wild-Growing and In Vitro Cultivated Rindera umbellata (Waldst. and Kit.) Bunge. Horticulturae, 9(3), 381. https://doi.org/10.3390/horticulturae9030381