Diagnostics and Description of a New Subspecies of Calluna vulgaris (L.) Hull from Western Siberia
Abstract
1. Introduction
2. Materials and Methods
2.1. Objects of Study
2.2. Genetic Analysis
2.3. Morphological and Anatomical Analysis
2.4. Chemophenotypic Analysis
2.5. Ecological Features
2.6. Statistical Analysis
3. Results and Discussion
3.1. Genetic Features
3.2. Morphological Features
3.3. Morphological and Anatomical Features
3.4. Chemotypic Analysis
3.5. Ecologic Range
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- POWO. Plants of the World Online. Facilitated by the Royal Botanic Gardens, Kew. 2023. Available online: http://www.plantsoftheworldonline.org/ (accessed on 13 May 2018).
- Seregin, A.P. (Ed.) Moscow Digital Herbarium: Electronic Resource; Moscow State University: Moscow, Russia, 2023; Available online: https://plant.depo.msu.ru/ (accessed on 2 February 2023).
- Mahy, G.; Nève, G. The application of spatial autocorrelation methods to the study of Calluna vulgaris population genetics. Belg. J. Bot. 1997, 129, 131–139. Available online: https://www.jstor.org/stable/20794390 (accessed on 13 May 2018).
- Sannikov, S.N.; Petrova, I.V.; Paule, L.; Egorov, E.V.; Cherepanova, O.E. Origin of the Atlantic Azorean insular population of Calluna vulgaris (L.) Hull. Cur. Plant Biol. 2019, 18, 100–108. [Google Scholar] [CrossRef]
- Effah, E.; Barrett, D.P.; Peterson, P.G.; Godfrey, A.J.R.; Potter, M.A.; Holopainen, J.K.; Clavijo McCormick, A. Natural Variation in Volatile Emissions of the Invasive Weed Calluna vulgaris in New Zealand. Plants 2020, 9, 283. [Google Scholar] [CrossRef] [PubMed]
- The Editorial Committee of the Flora of North America Ericaceae. Calluna vulgaris. In Flora of North America, Volume 8, Magnoliophyta: Paeoniaceae to Ericaceae; Kiger, R.W., Ed.; Oxford University Press: Oxford, UK, 2009; Volume 8, pp. 491–492. Available online: http://www.efloras.org/florataxon.aspx?flora_id=1&taxon_id=220002166 (accessed on 13 May 2018).
- Chapman, H.M.; Bannister, P. The spread of heather, Calluna vulgaris (L.) Hull, into indigenous plant communities of tongariro national park. N. Z. J. Ecol. 1990, 14, 7–16. Available online: https://www.jstor.org/stable/24053306 (accessed on 13 May 2018).
- Rogers, G.M.; Leathwick, J.R. North Island seral tussock grasslands. 3. The influence of heather (Calluna vulgaris) on rates of change from tussock grassland to shrubland. N. Z. J. Bot. 1996, 34, 473–487. [Google Scholar] [CrossRef]
- Chapman, H.M.; Bannister, P. Vegetative Production and Performance of Calluna vulgaris in New Zealand, with Particular Reference to Tongariro National-Park. N. Z. J. Ecol. 1994, 18, 109–121. Available online: https://newzealandecology.org/nzje/1954 (accessed on 13 May 2018).
- Petrova, I.V.; Cherepanova, O.E.; Kochubey, A.A.; Sannikova, N.S. Peculiarities of seed production and germination of Calluna vulgaris (L.) hull seeds in the Tobol region of Western Siberia//Successes of Modern Natural Science. Adv. Curr. Nat. Sci. 2016, 6, 104–109. [Google Scholar]
- Cherepanova, O.E.; Petrova, I.V.; Mishchikhina, Y.D. Leaf morphology and anatomy in marginal populations of common heather, Calluna vulgaris (L.) Hull from West Siberia and Atlantic Europe. Scvorcovie 2015, 2, 35–44. Available online: http://skvortsovia.uran.ru/2015/2104.pdf (accessed on 13 May 2018).
- Gorchakovsky, P.L. Heather. Red Book of the Sverdlovsk Region; Korytin, N.S., Ed.; Basco: Yekaterinburg, Russia, 2008; 137p, Available online: https://natural-sciences.ru/ru/article/view?id=35975 (accessed on 2 February 2023). (In Russian)
- Sannikov, S.N.; Petrova, I.V.; Mishchikhina, Y.D.; Cherepanova, O.E.; Polezhaeva, M.A.; Dymshakova, O.S. Genetic divergence of Eastern European and Tobol populations of Calluna vulgaris (L.) Hull. Rus. J. Ecol. 2013, 44, 118–122. [Google Scholar] [CrossRef]
- Sannikov, S.N.; Petrova, I.V.; Cherepanova, O.E.; Dymshakova, O.S. Genetic and phenotypic differentiation of Calluna vulgaris (L.) Hull in Pritobolie and Europe. Rus. J. Gen. 2014, 50, 925–933. [Google Scholar] [CrossRef]
- Sannikov, S.N.; Petrova, I.V.; Paule, L.; Egorov, E.V.; Cherepanova, O.E.; Dymshakova, O.S. Pleistocene Refugia for Calluna vulgaris (L.) Hull Populations in the European Atlantic Region. Russ. J. Ecol. 2018, 49, 286–295. [Google Scholar] [CrossRef]
- Cherepanova, O.E.; Petrova, I.V.; Sannikova, N.S. Morpho-anatomical differentiation within populations of Calluna vulgaris L. (Hull) on the submeridional transect Murmansk-Batumi. Forest. Ideas 2018, 24, 163–170. Available online: https://forestry-ideas.info/issues/issues_Index.php?journalFilter=62 (accessed on 11 April 2021).
- Sannikov, S.N.; Petrova, I.V.; Cherepanova, O.E. Paleogeographic sketch of the history of the formation of the heather area in the Tobol region. News Orenb. Agr. Univ. 2013, 6, 185–187. Available online: https://elar.usfeu.ru/bitstream/123456789/5677/1/4.pdf (accessed on 11 April 2021).
- Devey, M.E.; Bell, J.C.; Smith, D.N. A genetic linkage map for Pinus radiate based on RFLP, RAPD and microsatellite markers. Theor. Appl. Genet. 1996, 92, 673–679. [Google Scholar] [CrossRef] [PubMed]
- Dowling, T.E.; Childs, M.R. Impact of hybridization on a threatened trout of the southwestern United States. Conserv. Biol. 1992, 6, 355–364. Available online: https://www.jstor.org/stable/2386036 (accessed on 11 April 2021). [CrossRef]
- Rendell, S.; Ennos, R.A. Chloroplast DNA diversity in Calluna vulgaris (heather) populations in Europe. Mol. Ecol. 2002, 11, 69–78. [Google Scholar] [CrossRef]
- Hall, T.A. BIOEDIT: A user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp. Ser. 1999, 41, 95–98. [Google Scholar] [CrossRef]
- Excoffier, L.; Laval, G.; Schneider, S. Arlequin Ver. 3.5.1.2.: An Integrated Software Package for Population Genetics Data Analysis. Computational and Molecular Population Genetics Lab (CMPG) Bern Inst. Zoology. 2006. Available online: https://pubmed.ncbi.nlm.nih.gov/19325852/ (accessed on 11 April 2021).
- Sannikov, S.N. Ekologiya i Geografiya Estestvennogo Vozobnovleniya Sosny Obyknovennoy, 1st ed.; Nauka: Yekaterinburg, Russia, 1992; 264p. (In Russian) [Google Scholar]
- Jalal, M.A.F.; Read, D.J.; Haslam, E. Phenolic composition and its seasonal variation in Calluna vulgaris II. Phytochemistry 1982, 21, 1397–1401. Available online: https://agris.fao.org/agris-search/search.do?recordID=US201301987396 (accessed on 11 April 2021). [CrossRef]
- Kanunnikova, Y.S.; Dzhavakhyan, M.A. Determination of Flavonoids in the Golden Volodushka Herb (Herba Bupleuri aurei) by HPLC. In Proceedings of the II International Conference New Tasks of Modern Medicine, St. Petersburg, Russia, 22–27 May 2013; pp. 88–90. (In Russian). [Google Scholar]
- Schellenberg, J.; Bergmeier, E. The Calluna life cycle concept revisited: Implications for heathland management. Biol. Conserv. 2022, 31, 119–141. [Google Scholar] [CrossRef]
- Sannikova, N.S.; Sannikov, S.N.; Petrova, I.V.; Mishchikhina, Y.D.; Cherepanova, O.E. Competition factors of edificator tree stand: Quantitative analysis and synthesis. Russ. J. Ecol. 2012, 43, 426–432. [Google Scholar] [CrossRef]
- StatSoft, Inc. STATISTICA (Data Analysis Software System), Version 10. 2011. Available online: http://www.statsoft.com (accessed on 13 May 2018).
- Windows Excel. Available online: https://www.techtarget.com/searchenterprisedesktop/definition/Excel (accessed on 13 May 2018).
- Arlequin (Version 3.5.1.2): An Integrated Software Package for Population. Arlequin Suite Ver 3.5. Available online: http://cmpg.unibe.ch/software/arlequin3512/man/Arlequin35.pdf (accessed on 13 May 2018).
- AxioVision Imaging System. (Based on Release 4.8 June 2009). Available online: https://premium.fm.usp.br/download/Tutorial_Completo_AxioVision_48_Zeiss.pdf (accessed on 11 April 2021).
- Mohamed, B.F.; Gimingham, C.H. The Morphology of Vegetative Regeneration in Calluna vulgaris. New Phytol. 1970, 69, 743–750. Available online: http://www.jstor.org/stable/2430528 (accessed on 11 April 2021). [CrossRef]
- Stevens, P.F. Calluna, Cassiope and Harrimanella: A taxonomic and evolutionary problem. New Photol. 1970, 69, 1131–1148. Available online: https://nph.onlinelibrary.wiley.com/doi/pdfdirect/10.1111/j.1469-8137.1970.tb02494.x (accessed on 13 May 2018). [CrossRef]
- Bartoli, G.; Bottega, S.; Forino, L.M.C.; Castiglione, M.R.; Tagliasacchi, A.M.; Grilli, I.; Spano, C. Morpho-physiological plasticity contributes to tolerance of Calluna vulgaris in an active geothermal field. Aust. J. Bot. 2013, 61, 107–118. [Google Scholar] [CrossRef]
- Gimingham, C.H. Biological flora of British Isles: Calluna Salisb. A monotypic genus. J. Ecol. 1960, 48, 455–483. Available online: http://www.jstor.org/stable/20145563 (accessed on 13 May 2018). [CrossRef]
- Walter, H. Vegetation der Erde in Öko-Physiologischer Betrachtung, Bd. 2: Die Gemässigten und Arktischen Zonen; Gustav Fischer Verlag: Jena, Germany, 1968; pp. 334–336. Available online: https://www.persee.fr/doc/revec_0040-3865_1975_num_29_2_4892_t1_0334_0000_2 (accessed on 11 March 2023).
- Gaudio, N.; Balandier, P.; Dumas, Y.; Ginisty, C. Growth and morphology of three forest understorey species (Calluna vulgaris, Molinia coerulea and Pteridium aquilinum) according to light availability. For. Ecol. Manag. 2011, 261, 489–498. [Google Scholar] [CrossRef]
- Cucu, A.-A.; Baci, G.-M.; Cucu, A.-B.; Dezsi, Ş.; Lujerdean, C.; Hegeduş, I.C.; Bobiş, O.; Moise, A.R.; Dezmirean, D.S. Calluna vulgaris as a Valuable Source of Bioactive Compounds: Exploring Its Phytochemical Profile, Biological Activities and Apitherapeutic Potential. Plants 2022, 11, 1993. [Google Scholar] [CrossRef]
- Turland, N.J.; Wiersema, J.H.; Barrie, F.R.; Greuter, W.; Hawksworth, D.L.; Herendeen, P.S.; Knapp, S.; Kusber, W.-H.; Li, D.-Z.; Marhold, K.; et al. (Eds.) International Code of Nomenclature for Algae, Fungi, and Plants (Shenzhen Code) Adopted by the Nineteenth International Botanical Congress Shenzhen, China, July 2017; Koeltz Botanical Books: Oberreifenberg, Germany, 2018; 254p. [Google Scholar] [CrossRef]



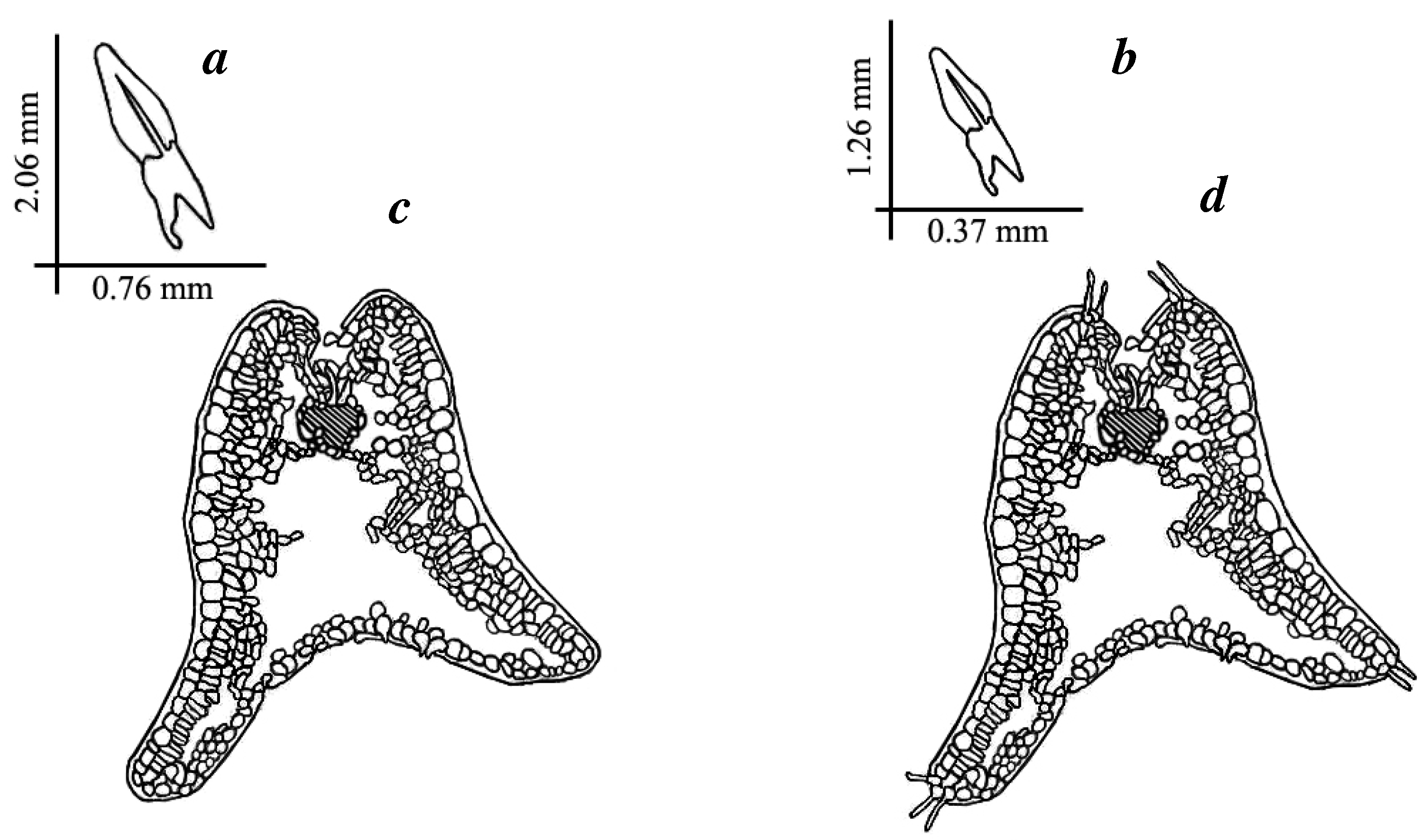

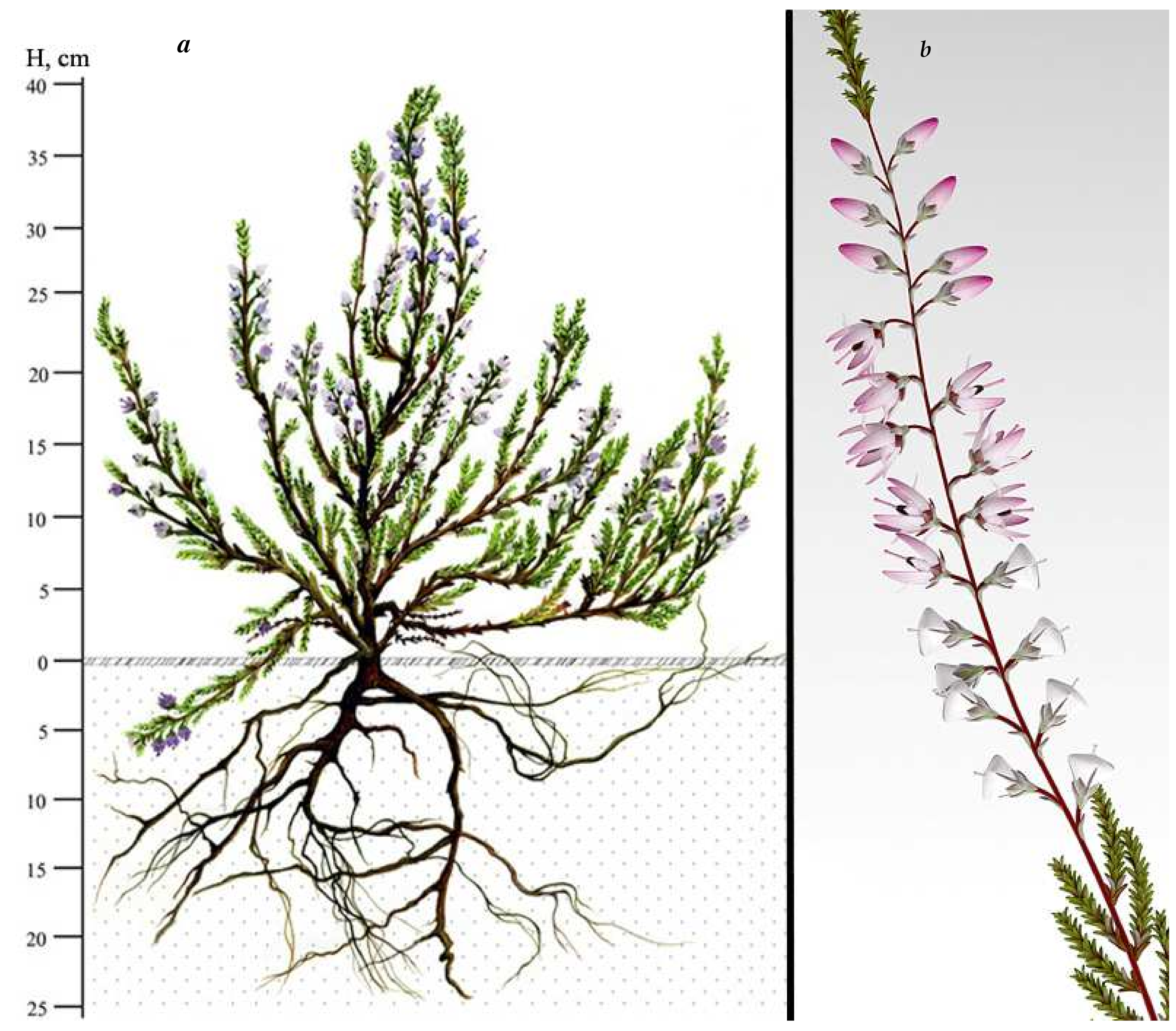
| Phylogenogeographic Groups of Populations | EEM | NES | WSP | SEE | WEA |
|---|---|---|---|---|---|
| Eastern-European-Mediterranean (EEM) | 0.00000 | ||||
| Northern-European-Scandinavian (NES) | 0.60004 | 0.00000 | |||
| Western-Siberian-Pritobolye (WSP) | 0.86929 | 0.63591 | 0.00000 | ||
| Southern-Eastern-European (SEE) | 0.43626 | 0.45641 | 0.90038 | 0.00000 | |
| Western-European-Atlantic (WEA) | 0.53501 | 0.19462 | 0.64500 | 0.47764 | 0.00000 |
| Parameter | Typical C. vulgaris Populations | ||||
|---|---|---|---|---|---|
| Zavodouspenskoe | Luga | TST | p * | ||
| Morphological features | |||||
| Leaf length, mm | 2.06 ± 0.09 1 | 1.26 ± 0.03 | −18.2810 | 0.00001 | |
| Leaf thickness, mm | 0.76 ± 0.02 | 0.37 ± 0.03 | −10.9994 | 0.00001 | |
| Leaf elongation coefficient (LEC) | 0.39 ± 0.02 | 0.30 ± 0.02 | 2.0930 | 0.05 | |
| Anatomical features | |||||
| Stoma number, pc. | 13.60 ± 0.63 | 19.69 ± 1.14 | 4.3012 | 0.0002 | |
| Trichome number, pc. | 18.98 ± 0.56 | 15.65 ± 0.75 | −2.8235 | 0.008 | |
| Cuticle thickness, µm | 4.77 ± 0.33 | 3.97 ± 0.28 | 2.024 | 0.06 | |
| Upper epidermis cells | Height, µm | 38.08 ± 2.56 | 26.61 ± 1.44 | 2.069 | 0.05 |
| Length, µm | 56.54 ± 1.56 | 58.78 ± 2.4 | 2.035 | 0.03 | |
| Thickness, µm | 25.51 ± 1.22 | 29.39 ± 4.46 | 2.447 | 0.05 | |
| Area, µm2 | 1126.43 ± 61.40 | 1195.19 ± 56.37 | 1.991 | 0.06 | |
| Perimeter, µm | 166.37 ± 5.23 | 180.48 ± 5.02 | 2.035 | 0.03 | |
| LEC | 0.453 ± 0.01 | 0.490 ± 0.06 | 2.018 | 0.05 | |
| Palisade tissue cells | Length, µm | 32.02 ± 0.23 | 26.19 ± 1.14 | −4.9125 | 0.00004 |
| Thickness, µm | 13.82 ± 0.05 | 11.82 ± 0.55 | −3.5992 | 0.001 | |
| Area, µm2 | 402.25 ± 8.50 | 290.85 ± 19.87 | −3.9669 | 0.0005 | |
| Perimeter, µm | 112.23 ± 7.99 | 67.99 ± 4.98 | −2.7204 | 0.01 | |
| LEC | 0.432 ± 0.003 | 0.460 ± 0.03 | 2.101 | 0.05 | |
| Spongy parenchyma cells | Length, µm | 20.62 ± 0.44 | 16.76 ± 1.06 | −2.8758 | 0.007 |
| Thickness, µm | 15.12 ± 0.29 | 13.29 ± 1.48 | 1.973 | 0.05 | |
| Area, µm2 | 252.68 ± 6.96 | 192.74 ± 17.48 | −3.1006 | 0.004 | |
| Perimeter, µm | 59.19 ± 0.89 | 54.99 ± 3.25 | 1.977 | 0.06 | |
| LEC | 0.738 ± 0.01 | 0.86 ± 0.15 | 2.011 | 0.05 | |
| Biologically Active Substances, mg g−1 | Typical C. vulgaris Populations | |
|---|---|---|
| Zavodouspenskoe | Luga | |
| Chlorogenic acid | 2.614 ± 0.005 1 | 1.911 ± 0.005 * |
| Oleic acid | 0.198 ± 0.005 | 0.208 ± 0.004 |
| Quercetin | 2.982 ± 0.004 | 1.194 ± 0.005 * |
| Myricetin | 2.361 ± 0.005 | 0.451 ± 0.005 * |
| Epicatechin | 2.004 ± 0.004 | 2.954 ± 0.004 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cherepanova, O.; Petrova, I.; Sannikov, S.; Mishchihina, Y. Diagnostics and Description of a New Subspecies of Calluna vulgaris (L.) Hull from Western Siberia. Horticulturae 2023, 9, 386. https://doi.org/10.3390/horticulturae9030386
Cherepanova O, Petrova I, Sannikov S, Mishchihina Y. Diagnostics and Description of a New Subspecies of Calluna vulgaris (L.) Hull from Western Siberia. Horticulturae. 2023; 9(3):386. https://doi.org/10.3390/horticulturae9030386
Chicago/Turabian StyleCherepanova, Olga, Irina Petrova, Stanislav Sannikov, and Yulia Mishchihina. 2023. "Diagnostics and Description of a New Subspecies of Calluna vulgaris (L.) Hull from Western Siberia" Horticulturae 9, no. 3: 386. https://doi.org/10.3390/horticulturae9030386
APA StyleCherepanova, O., Petrova, I., Sannikov, S., & Mishchihina, Y. (2023). Diagnostics and Description of a New Subspecies of Calluna vulgaris (L.) Hull from Western Siberia. Horticulturae, 9(3), 386. https://doi.org/10.3390/horticulturae9030386





