An Optimized Protocol for In Vitro Regeneration of Ocimum basilicum cv. FT Italiko
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material, In Vitro and In Vivo Germination
2.2. Establishment of Cultures for Direct Regeneration
2.3. Data Collection and Statistical Analysis
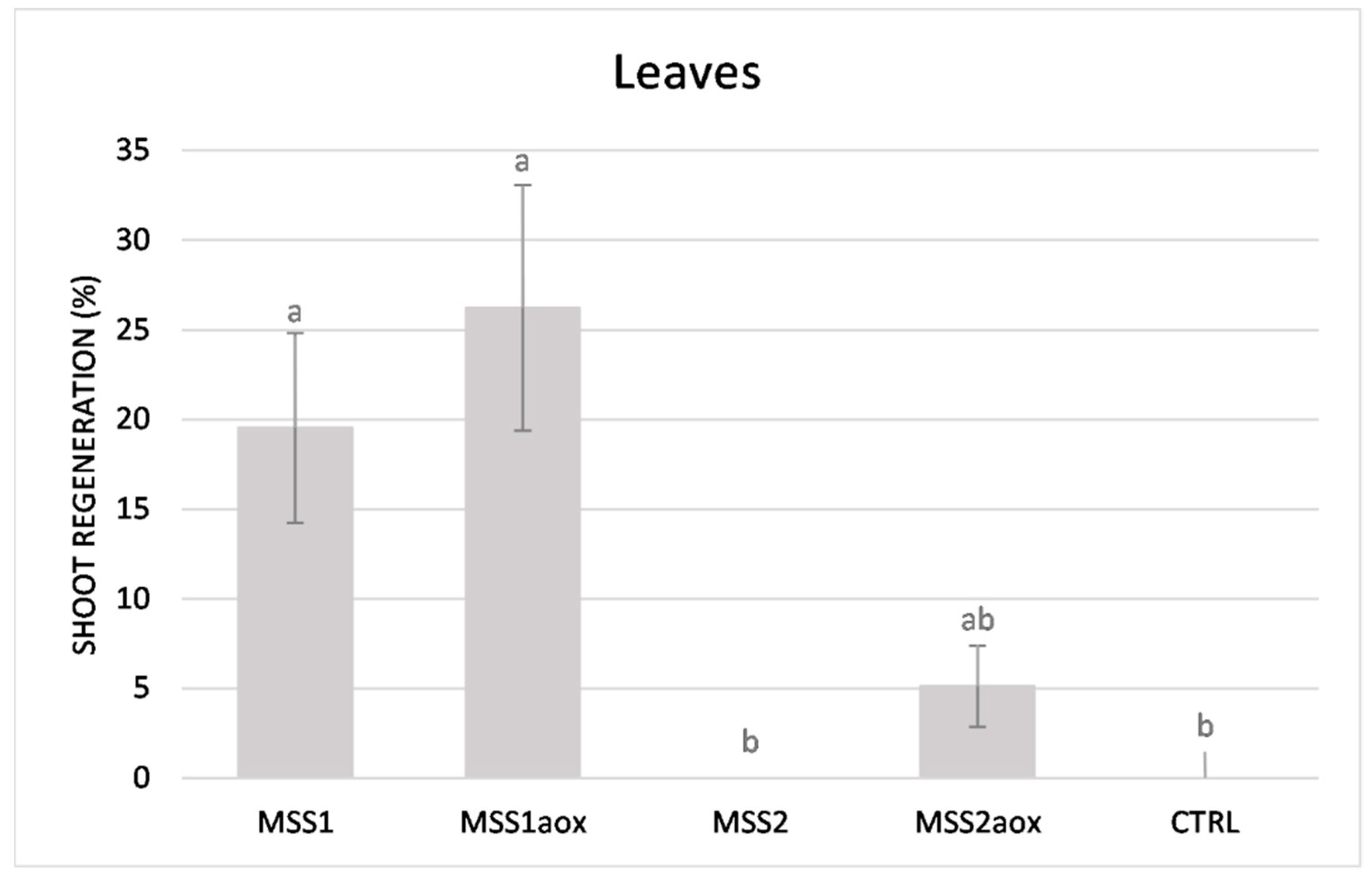
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| BA | 6-benzyladenine |
| IAA | indole-3-acetic acid |
| DOP | Denominazione di Origine Protetta (Protected Designation of Origin) |
| MS | medium Murashige and Skoog (1962) medium |
| MSS | Murashige and Miller shoot multiplication medium B |
| PGR | plant growth regulator |
| TDZ | thidiazuron |
References
- Filip, S. Basil (Ocimum basilicum L.) a source of valuable phytonutrients. Int. J. Clin. Nutr. Diet. 2017, 3, 118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sestili, P.; Ismail, T.; Calcabrini, C.; Guescini, M.; Catanzaro, E.; Turrini, E.; Anam, L.; Saeed, A.; Fimognari, C. The potential effects of Ocimum basilicum on health: A review of pharmacological and toxicological studies. Expert Opin. Drug Metab. Toxicol. 2018, 14, 679–692. [Google Scholar] [CrossRef] [PubMed]
- Petersen, M.; Simmonds, M.S. Rosmarinic acid. Phytochemistry 2003, 62, 121–125. [Google Scholar] [CrossRef] [PubMed]
- Salvadeo, P.; Boggia, R.; Evangelisti, F.; Zunin, P. Analysis of the volatile fraction of “Pesto Genovese” by headspace sorptive extraction (HSSE). Food Chem. 2007, 105, 1228–1235. [Google Scholar] [CrossRef]
- Belbahri, L.; Calmin, G.; Pawlowski, J.; Lefort, F. Phylogenetic analysis and real time PCR detection of a presumbably undescribed Peronospora species on sweet basil and sage. Mycol. Res. 2005, 109, 1276–1287. [Google Scholar] [CrossRef] [PubMed]
- Minuto, G.; Minuto, A.; Gullino, M.L.; Garibaldi, A. Lotta chimica alla peronospora del basilico: Primi risultati. Inf. Fitopatol.-Dif. Delle Piante 2004, 54, 54–57. [Google Scholar]
- Minuto, A.; Bogliolo, A.; Vinotti, P.; Formisano, G.; Benza, G.; Lanteri, A.; Minuto, G. Sensibilità varietale di basilico a Peronospora belbahrii. Atti Giornate Fitopatol. 2014, 2, 543–546. [Google Scholar]
- Cohen, Y.; Ben Naim, Y.; Falach, L.; Rubin, A.E. Epidemiology of basil downy mildew. Phytopathology 2017, 107, 1149–1160. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cardi, T. Cisgenesis and genome editing: Combining concepts and efforts for a smarter use of genetic resources in crop breeding. Plant Breed. 2016, 135, 139–147. [Google Scholar] [CrossRef]
- Zhang, X.; Low, Y.C.; Lawton, M.A.; Simon, J.E.; Di, R. CRISPR-editing of sweet basil (Ocimum basilicum L.) homoserine kinase gene for improved downy mildew disease resistance. Front. Genome Ed. 2021, 3, 629–769. [Google Scholar] [CrossRef] [PubMed]
- Navet, N.; Tian, M. Efficient targeted mutagenesis in allotetraploid sweet basil by CRISPR/Cas9. Plant Direct 2020, 4, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Forti, C.; Barberini, S.; Laura, M.; Ciorba, R.; Mascarello, C.; Giovannini, A.; Ruffoni, B.; Savona, M. Messa a punto di protocolli di rigenerazione in vitro in Ocimum basilicum cv FT Italiko, finalizzati al miglioramento genetico via genome editing. In Proceedings of the V Convegno Nazionale Sulla Micropropagazione: Un Incontro Tra Gli Operatori Di Settore E Della Ricerca, Bari, Italy, 12–14 October 2022; Atti Giornate Scientifiche SOI: Bari, Italy, 2022. submitted. [Google Scholar]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Phippen, W.B.; Simon, J.E. Shoot regeneration of young leaf explants from basil (Ocimum basilicum L.). Vitr. Cell. Dev. Biol.-Plant 2000, 36, 250–254. [Google Scholar] [CrossRef]
- Huang, L.C.; Murashige, T. Plant tissue culture media: Major constitutents, their preparation and some applications. Tca Man. 1977, 3, 539–548. [Google Scholar] [CrossRef]
- Verma, S.K.; Sahin, G.; Das, A.K.; Gurel, E. In vitro plant regeneration of Ocimum basilicum L. is accelerated by zinc sulfate. Vitr. Cell. Dev. Biol.-Plant 2016, 52, 20–27. [Google Scholar] [CrossRef]
- Dode, L.B.; Bobrowski, V.L.; Braga, E.J.B.; Seixas, F.K.; Schuch, M.W. In vitro propagation of Ocimum basilicum L. (Lamiaceae). Acta Sci. Biol. Sci. 2003, 25, 435–437. [Google Scholar] [CrossRef]
- Oliveira, R.C.D. Cultivo In Vitro e Ex Vitro de Cultivares de Manjericão (Ocimum basilicum L.). Ph.D. Thesis, Universidade Federal de Uberlândia, Uberlândia, Brazil, 2020. [Google Scholar] [CrossRef]
- Khan, S.; Fahim, N.; Singh, P.; Rahman, L.U. Agrobacterium tumefaciens mediated genetic transformation of Ocimum gratissimum: A medicinally important crop. Ind. Crops Prod. 2015, 71, 138–146. [Google Scholar] [CrossRef]
- Laura, M.; Forti, C.; Barberini, S.; Ciorba, R.; Mascarello, C.; Cassetti, A.; Giovannini, A.; Ruffoni, B.; Savona, M. Genome editing of Ocimum basilicum L. through CRISPR / Cas9 to induce resistance to the pathogen Peronospora belbahrii. In Proceedings of the 2nd PlantEd Conference on Plant Genome Editing—The Wide Range of Applications, Lecce, Italy, 20–22 September 2021. [Google Scholar]


| Explant Type | PGR (Concentration) Added to MSS Medium | Compounds (Concentration) Added to MSS Medium | Medium Acronym |
|---|---|---|---|
| First true leaf (in vitro or in vivo) | BA (1 mg/L) | / | MSS1 |
| BA (1 mg/L) | +Citric acid (10 mg/L) + Ascorbic acid (100 mg/L) | MSS1aox | |
| TDZ (4 mg/L) | / | MSS2 | |
| TDZ (4 mg/L) | +Citric acid (10 mg/L) + Ascorbic acid (100 mg/L) | MSS2aox | |
| Hypocotyls (in vitro) | IAA (1 mg/L) | +ZnSO4 (12.9 mg/L) | MSS3 |
| TDZ (4 mg/L) | / | MSS2 | |
| TDZ (4 mg/L) | +Citric acid (10 mg/L) + Ascorbic acid (100 mg/L) | MSS2aox | |
| Cotyledons (in vitro) | IAA (1 mg/L) | +ZnSO4 (12.9 mg/L) | MSS3 |
| TDZ (4 mg/L) | / | MSS2 | |
| Cotyledonary nodes (CNs) (in vitro) | TDZ (4 mg/L) | / | MSS2 |
| Explant Type | Medium | Shoot Regeneration (%) | Mean n. of Shoots per Responsive Explants |
|---|---|---|---|
| Leaves (in vivo) | MSS1aox | 32.64 ± 7.79 | 1.52 ± 0.131 |
| Hypocotyls | MSS2aox | 9.18 ± 2.43 | 1.33 ± 0.333 |
| Cotyledons | MSS2aox | 19.7 ± 8.31 | 1.25 ± 0.214 |
| CNs | MSS2 | 93.5 ± 2.31 | 2.6 ± 0.254 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barberini, S.; Forti, C.; Laura, M.; Ciorba, R.; Mascarello, C.; Giovannini, A.; Ruffoni, B.; Savona, M. An Optimized Protocol for In Vitro Regeneration of Ocimum basilicum cv. FT Italiko. Horticulturae 2023, 9, 407. https://doi.org/10.3390/horticulturae9030407
Barberini S, Forti C, Laura M, Ciorba R, Mascarello C, Giovannini A, Ruffoni B, Savona M. An Optimized Protocol for In Vitro Regeneration of Ocimum basilicum cv. FT Italiko. Horticulturae. 2023; 9(3):407. https://doi.org/10.3390/horticulturae9030407
Chicago/Turabian StyleBarberini, Sara, Chiara Forti, Marina Laura, Roberto Ciorba, Carlo Mascarello, Annalisa Giovannini, Barbara Ruffoni, and Marco Savona. 2023. "An Optimized Protocol for In Vitro Regeneration of Ocimum basilicum cv. FT Italiko" Horticulturae 9, no. 3: 407. https://doi.org/10.3390/horticulturae9030407
APA StyleBarberini, S., Forti, C., Laura, M., Ciorba, R., Mascarello, C., Giovannini, A., Ruffoni, B., & Savona, M. (2023). An Optimized Protocol for In Vitro Regeneration of Ocimum basilicum cv. FT Italiko. Horticulturae, 9(3), 407. https://doi.org/10.3390/horticulturae9030407








