Abstract
Drought stress severely limits crop growth and yield. With the atmospheric CO2 constantly increasing, plants will be affected by multiple effects of drought and increased CO2 in the future. Abscisic acid (ABA) plays vital roles in plant stress tolerance, especially drought stress. However, little is known about the effects of elevated CO2 concentration (e[CO2]) and exogenous ABA in cucumber (Cucumis sativus L.) response to drought stress. In the present study, we investigated the effects of e[CO2] and exogenous ABA on the drought tolerance of cucumber seedlings under the simulated drought stress induced by 5% polyethylene glycol 6000. The experiment was a split-plot design, in which the main factor was CO2 concentrations; atmospheric and elevated CO2 concentrations (~400 and 800 ± 40 μmol mol−1, respectively). The subplot factor was the combinations of exogenous ABA and its synthesis inhibitor sodium tungstate (Na2WO4); deionized water (control), 20 μM ABA, 2 mM Na2WO4, and 2 mM Na2WO4 + 20 μM ABA, which were applied to plant leaves. The results showed that compared with exogenous ABA application only, e[CO2] combined with exogenous ABA significantly increased the biomass, chlorophyll content, and net photosynthetic rate (Pn) of cucumber seedlings under drought stress. Meanwhile, e[CO2] and exogenous ABA were more efficient in reducing the contents of reactive oxygen species and malondialdehyde, promoting the accumulation of proline, soluble sugar, soluble protein, free amino acid, ascorbic acid, and glutathione. The ratios of ascorbic acid/dehydroascorbic acid (ASA/DHA), glutathione/oxidized glutathione (GSH/GSSG), as well as the activities of antioxidant enzymes were increased. In conclusion, e[CO2] and exogenous ABA synergistically alleviated oxidative damage of drought stress on cucumber seedlings by increasing antioxidant enzyme activities and accelerating the ASA–GSH cycle in cucumber seedlings, which in turn improved the drought tolerance of cucumber seedlings, and provided theoretical and practical support for further studies on the alleviation of drought stress in protected horticulture.
1. Introduction
Atmospheric CO2 concentrations have increased dramatically from the industrial revolution to the present and are expected to reach 600 μmol mol−1 by mid-century [1]. As atmospheric CO2 concentrations have increased, the frequency of droughts and high temperatures have increased worldwide, which in turn can lead to significant impacts on crop quality and yields [2,3]. This is because drought reduces the water potential of soil solution, induces oxidative stress which hampers many biological processes, causes osmotic stress, imbalance of free radical metabolism, membrane lipid peroxidation, and other damage to plant cells [4,5]. Meanwhile, when plants are subjected to drought stress, plant growth is limited and leads to reduced chlorophyll content, inhibited photosynthesis, reduced dry matter accumulation, and causes a reduction in crop growth and productivity [6].
Oxidative stress of plants was induced by the generation of reactive oxygen species (ROS) which include hydrogen peroxide (H2O2), superoxide anion (O2−), and oxygen-containing free radicals. The production and clearance of ROS in healthy plants maintains a dynamic balance. However, drought stress could destroy this dynamic balance, which leads to rising levels of ROS and thus hampers the growth of plants [7,8]. Plants have evolved a set of effective antioxidant defense systems to eliminate ROS [9], involving enzymatic and non-enzymatic antioxidant systems [10]. The enzymatic antioxidant system consists of superoxide peroxidase (POD), dismutase (SOD), and catalase (CAT). Furthermore, the abnormal accumulation of H2O2 can damage the membrane systems of tissues and organs, and subsequently the metabolic activities [11]. The ascorbate–glutathione (ASA–GSH) cycle is also an important way to remove H2O2 in plants [12,13], mainly in the cytoplasm and chloroplasts. ASA and GSH are two important antioxidants, which can scavenge reactive oxygen species and maintain the redox state of plants under various stresses [14,15,16] and their existing forms also affect their functions. When subjected to a certain degree of oxidative damage, plants can convert oxidized ascorbic acid and oxidized glutathione into their corresponding reduced state to reduce the damage [12,17]. Moreover, accumulation of osmotic proteins is a non-enzymatic antioxidant defense system to improve tissue water status by osmoregulation and to quench plant ROS under stress conditions.
For crops, CO2 enrichment can promote plant growth by improving the photosynthetic efficiency of plants [18]. Recent studies showed that elevated CO2 concentration (e[CO2]) could mitigate some deleterious effects in coffee plants at the physiological and metabolic level [19], and increase sugar accumulation of Populus leaves and upregulation of genes determining anthocyanin biosynthesis to prolong leaf longevity during natural autumnal senescence [20]. Moreover, e[CO2] plays an important role in alleviating the damage caused by abiotic stresses such as drought, salt, high temperature, etc. [21,22]. It can increase the activity of antioxidant enzymes like SOD, POD, CAT, ascorbate peroxidase (APX), and glutathione reductase (GR), and reduce the accumulation of reactive ROS and the degree of membrane lipid peroxidation [23], thereby improving plant resistance and alleviating oxidative damage caused by drought stress [24,25].
Abscisic acid (ABA) is known as the “stress hormone” of plants, and can alleviate the damage to plants in drought and other adverse events and improve the resistance of plants [26]. Plants release ABA in the roots, and then transfer it to the leaves through xylem fluid movement, resulting in a large accumulation of ABA content in the leaves [27]. The abundance of ABA reduces transpiration and improves water use efficiency by promoting stomatal closure [25,28]. Meanwhile, ABA works as the transmitter to trigger the response of plants to adversity stress, which improves the activities of related antioxidant enzymes to some extent in plants, thereby reducing the accumulation of reactive ROS in plants [29] and inducing plant tolerance to stress [30,31,32]. It was reported that the ASA–GSH cycle under ABA could reduce growth inhibition through larger antioxidant capacity, lower levels of ROS, and stronger tolerance to drought stress [11]. Thus, many drought-resistant varieties are more sensitive to ABA, while drought-susceptible varieties are the opposite [33]. Previous studies proved that the application of exogenous ABA could alleviate the oxidative damage to kiwifruit and soybean under drought stress, and enhance their drought resistance [34,35].
Recently, the effects of e[CO2] in the regulation of plant drought response to ABA were reported [36,37]. Chater et al. [38] showed that ABA could regulate the decrease in stomatal density caused by the increase in CO2 concentration. In addition, ABA could induce stomata closure under e[CO2] [39,40], which means that ABA could interact with CO2 to regulate the stomatal opening and closing and consequently facilitate plants to adapt to drought conditions. At present, there are many studies that have focused on the roles of CO2 and ABA in drought stress, but most of them have focused on stomatal movement and less on active oxygen metabolism and the ASA–GSH cycle which are important reducing substances in plants.
This study aims to explore the effects of e[CO2] and ABA in relieving oxidative damage of cucumber seedlings under drought stress. We hypothesized that e[CO2] and exogenous ABA would enhance the reactive oxygen metabolism and ASA cycling in cucumber seedlings under drought conditions, which would improve the drought tolerance of cucumber seedlings. The results of this research could not only provide useful information for enhancing crop drought tolerance but also could give guidance on high-yield production in the greenhouse.
2. Materials and Methods
2.1. Plant Materials and Treatments
This experiment was conducted in four self-designed open-top tunnels (6 m length, 6 m span, and 2.6 m ridge height) at the Horticulture Experimental Station of Shandong Agricultural University, China. Cucumber (Cucumis sativus L. cv. Jinyou No. 35; from Tianjin Kerun Cucumber Research Institute, Tianjin, China) seeds were washed with distilled water and then soaked in warm water for 6–8 h, and later incubated for 1.5 d in a dark environment with a relative humidity of 60–70% and temperature of 28 °C. Then, cucumber seeds were sown in 50-plug black plastic trays (one seed per plug containing a mixture of peat, perlite, and vermiculite with a 3:1:1 volume ratio). After the first true leaves appeared and the roots were carefully washed, all the seedlings were transplanted into the solution culture system and planted in 7 L containers filled with Yamazaki cucumber nutrient solution. Each container had six seedlings, and there were 16 such containers in each self-designed open-top tunnel. The nutrient solution was replaced every three days and aerated with an air pump for 30 s every three minutes. The pH of the nutrient solution was maintained at 6.8–7.0 and the contents of the solution included 0.5 mM NH4H2PO4, 2.0 mM Ca(NO3)2·4H2O, 3.2 mM KNO3, 1.0 mM MgSO4·7H2O, and full-strength trace elements.
The experiment was a split-plot design, in which the main factor was CO2 concentration: atmospheric CO2 concentration (a[CO2]; ~400 μmol mol−1) and elevated CO2 concentration (e[CO2]; 800 ± 40 μmol mol−1), and the subplot factor was the combinations of exogenous ABA and its synthesis inhibitor sodium tungstate (Na2WO4): deionized water (control), 20 μM ABA, 2 mM Na2WO4, and 20 μM ABA + 2 mM Na2WO4. The experiment was started when the second true leaf was fully expanded. For the first two days of the experiment, leaves of cucumber seedlings were, respectively, sprayed with deionized water (control), 20 μM ABA, 2 mM Na2WO4, and 20 μM ABA + 2 mM Na2WO4 twice a day (8:00 and 18:00), which inferred that plants in every four containers were sprayed with the same solution in the same tunnel. On the third day of the experiment, all the nutrient solutions were replaced with a nutrient solution containing 5% polyethylene glycol 6000 (PEG 6000) for simulating soil drought stress, which lasted for 5 days. According to the equation proposed by Michel and Kaufmann (1973) [41], the level of osmotic pressure in the hydroponic solution was −0.05 MPa when the PEG 6000 stress was 5%. On the third day of the experiment, a[CO2] and e[CO2] treatments were performed in different tunnels for 5 days, and CO2 was automatically released into the tunnels to maintain the target concentration. Both open-top tunnels were equipped the environmental control system (Auto 2000; Beijing, China) to provide CO2 via compressed CO2 cylinders under solenoid valve control. The experiment with eight treatments was repeated three times, in four containers with six seedlings each in one treatment. After the seventh day of treatment, leaves of cucumber seedlings in each treatment were sampled, then immediately frozen in liquid nitrogen, and stored at −80 °C fridge for physiological analyses.
2.2. Determination of Growth Parameters
The morphological parameters of seedlings were recorded immediately after 5 days of drought treatment, including plant height, diameter thickness, leaf area, fresh weight and dry weight of the whole plant. Plant height was measured using a ruler. The stem diameter was measured one centimeter below the cotyledons using a digital caliper. Leaf area was calculated using the formula SL = LL2, with SL representing the leaf area and LL representing the leaf length of the true leaves. The fresh and dry weights were determined by the weighing method by first weighing the shoot and root fresh weights of the plants separately, then drying them in an oven at 80 °C to a constant weight and weighing the dry weights.
2.3. Measurement of Photosynthetic Pigment Content and Gas Exchange
Chlorophyll a, chlorophyll b, and carotenoid contents of cucumber leaves were extracted according to the 95% ethanol extraction method (1 sheet) by grinding 0.5 g of fresh leaf samples in a mortar and adding 10 mL of 95% ethanol. The samples were left in the dark at 4 °C for 48 h and then centrifuged at 8000× g for 15 min. The absorbance values of the supernatants were measured at 665 nm, 649 nm and 470 nm using a spectrophotometer. The contents of chlorophyll a, chlorophyll b, and carotenoids were calculated according to the formulae of Lichtenthaler and Wellburn (1983) [42].
Photosynthetic gas exchange parameters were measured using a CIRAS-3 (PP Systems, US) portable plant photosynthesis meter for net photosynthetic rate (Pn), stomatal conductance (Gs), transpiration rate (Tr), and water use efficiency (WUE = Pn/Tr). Measurement conditions: leaf chamber temperature was 25 °C, light intensity was 1000 μmol m−2 s−1, and CO2 was measured using buffer bottles taking a relatively stable 3~4 m high air at a concentration of about 400 ± 10 μmol mol−1.
2.4. Measurement of MDA and ROS Contents
The content of malondialdehyde (MDA) was determined using the thiobarbituric acid (TBA) colorimetric method [43]. The O2− generation rate was determined by the method of Elstner and Heupel [44]. The content of H2O2 was determined using reagent kits (Comin Biotechnology Co., Ltd., Suzhou, China) according to the instructions of the manufacturer (http://www.cominbio.com/a/shijihe/shenghuashiji/yanghuayukangyanghuaxilie/2016/0422/877.html) accessed on 14 February 2021.
2.5. Measurement of Osmolytes’ Contents
The content of proline was determined according to the ninhydrin coloration method [45]. Determination of free amino acid content was by the ninhydrin method [46]. The contents of soluble sugars were determined by the anthrone method, and the contents of soluble proteins were determined by the Coomassie Brilliant Blue G-250 method [47].
2.6. Determination of Leaf ASA/DHA and GSH/GSSG
The content of ascorbate (ASA) was estimated according to the method proposed by [48]. The content of reduced glutathione (GSH) was determined by colorimetry of 5, 5-dithiobis (2-nitrobenzoic acid) (DTNB) [49]. Dehydroascorbate (DHA) and oxidized glutathione (GSSG) contents were determined using reagent kits (Comin Biotechnology Co., Ltd., Suzhou, China) according to the manufacturer’s instructions. The content of DHA was calculated by measuring the rate of ASA generation in the system. The content of GSSG was appraised by abolishing GSH with a derivatizing agent 2-vinyl pyridine (http://www.cominbio.com/a/shijihe/shenghuashiji/guguanggantaixilie/2014/0226/212.html) accessed on 14 February 2021.
2.7. Enzyme Assays
Superoxidase dismutase (SOD) enzyme activity was assessed by measuring the inhibition of the photochemical reduction of nitroblue tetrazolium (NBT) at 560 nm [50]; peroxidase (POD) enzyme activity was measured by the guaiacol method [51]; catalase (CAT) enzyme activity was analyzed by measuring the decline in absorbance at 240 nm.
Ascorbate peroxidase (APX) activity was measured according to the method of [52]. Glutathione reductase (GR), glutathione peroxidase (GPX), and dehydroascorbate reductase (DHAR) activities were determined using reagent kits (Comin Biotechnology Co., Ltd., Suzhou, China) according to the instructions of the manufacturer (http://www.cominbio.com/a/shijihe/shenghuashiji/guguanggantaixilie/) accessed on 14 February 2020.
2.8. Statistical Analyses
Statistical analyses were carried out by Microsoft Excel 2019 (Microsoft Corporation, Redmond, WA, USA) and DPS 15.10 (Zhejiang University, Hangzhou, China). Data were presented as the means of six seedlings in each treatment with three repetitions. Split-plot analyses of variances were applied to evaluate the interactions between CO2 and the combinations of ABA and Na2WO4. Duncan’s multiple polar difference test (α = 0.05) was used for all data to test for the presence of significant differences between treatments. Graphics were plotted with SigmaPlot 12.5 (Systat Software Inc, San Jose, CA, USA).
3. Results
3.1. Effects of Exogenous ABA and Elevated CO2 on Growth Parameters of Cucumber Seedlings under Drought Stress
The results showed that e[CO2] significantly promoted the growth of cucumber seedlings under drought stress compared with a[CO2] (Figure 1), which specifically increased plant height, stem thickness, leaf area, and dry as well as fresh weights to different degrees, except for the Na2WO4 treatment (Table 1). Likewise, under a[CO2], the plant fresh as well as dry weights and stem thickness of cucumbers in the ABA treatment were significantly higher than those of the control treatment, increasing by 7%, 14%, and 22%, respectively. The plant growth under the Na2WO4 treatment was the lowest compared to other treatments. Additionally, all growth parameters of the Na2WO4 + ABA treatment were significantly higher than those in the Na2WO4 treatment, while ABA spraying under e[CO2] was more beneficial to plant growth under drought stress compared with other treatments (Table 1, Figure 1).

Figure 1.
Side view of cucumber seedlings on day 5 under different treatments.

Table 1.
Effects of elevated CO2 and exogenous ABA on the growth of cucumber seedlings under drought stress.
3.2. Effects of Exogenous ABA and Elevated CO2 on Photosynthetic Properties of Cucumber Seedling Leaves under Drought Stress
Under e[CO2], exogenous ABA significantly increases the contents of chlorophyll a (Chl a), chlorophyll b (Chl b), and total chlorophyll (Total Chl), by 15%, 16%, and 15%, respectively, compared with the control treatment, but did not significantly affect the contents of chlorophyll a/b (Chl a/Chl b) and carotenoids (Table 2). On the other hand, under a[CO2], Na2WO4 treatment showed the lowest content of all pigments compared with control treatment, and the differences between the levels of Chl b and carotenoid content were significant compared with the control group, but exogenous ABA spraying also did not significantly promote the accumulation of photosynthetic pigment content in leaves under drought stress.

Table 2.
Effects of elevated CO2 and exogenous ABA on photosynthetic pigment content of cucumber seedlings under drought stress.
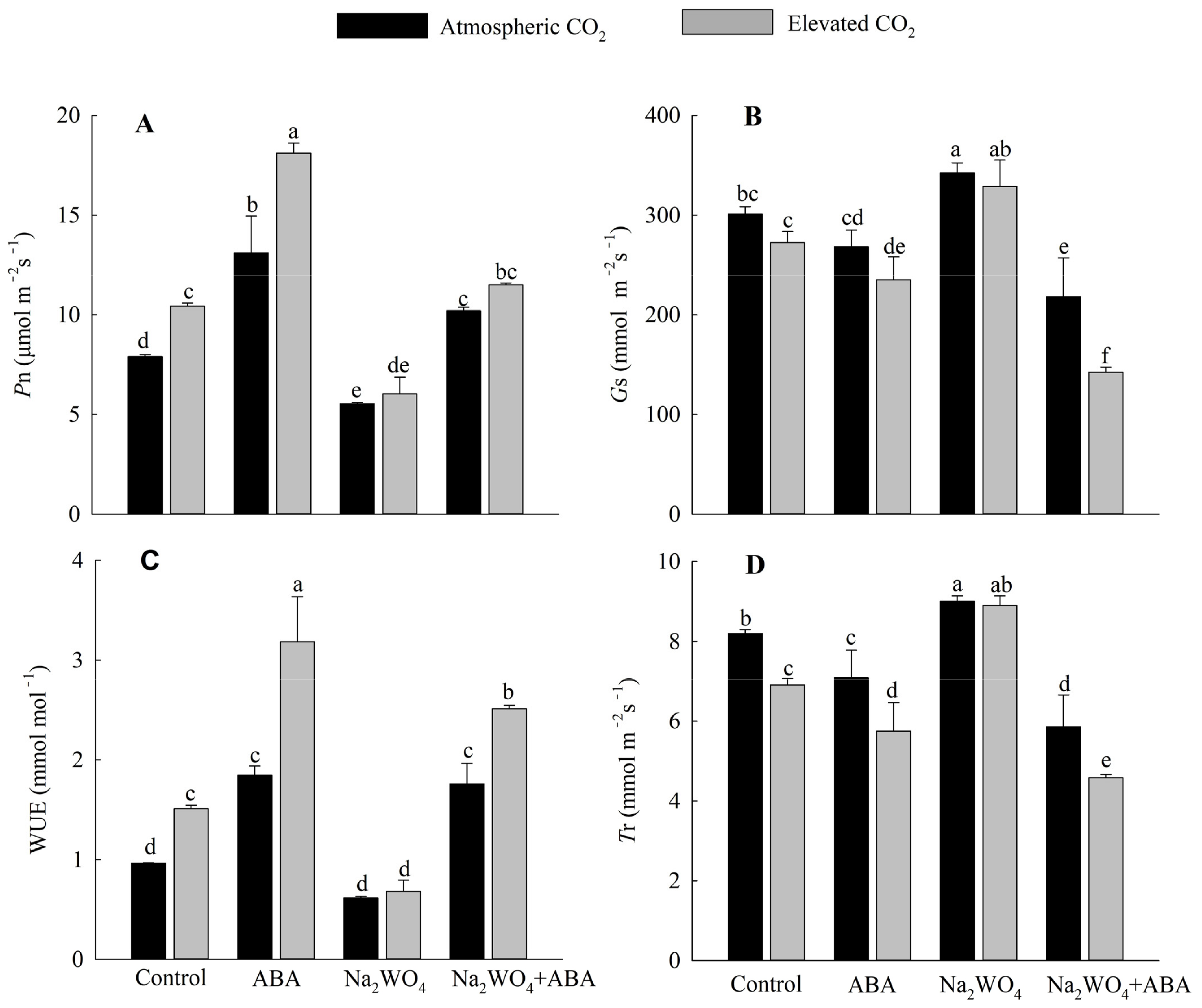
In control and ABA treatments, e[CO2] significantly increased Pn and WUE in drought stressed cucumber plants compared with a[CO2] (Figure 2A,C). Under e[CO2] and a[CO2], Na2WO4 treatment resulted in the lowest Pn and WUE compared with the control treatment. However, exogenous ABA spraying significantly enhanced the Pn and WUE in leaves under e[CO2]. Under a[CO2], Pn was significantly increased by 66% with ABA treatment and decreased by 30% with Na2WO4 treatment compared with control treatment, and increased by 84% with Na2WO4 + ABA treatment compared with Na2WO4 treatment. e[CO2] treatment reduced plant Gs and Tr compared to those under a[CO2], but this reduction was not significant under Na2WO4 treatment (Figure 2B,D).
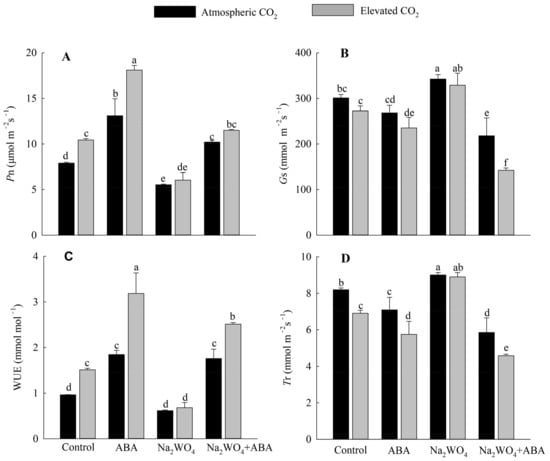
Figure 2.
Effects of elevated CO2 and exogenous ABA on net photosynthetic rate (A), stomatal conductance (B), water use efficiency (C), and transpiration rate (D) in leaves of cucumber seedlings under drought stress. Different lowercase letters represent significant differences between treatments at the 0.05 level. Values were the means ± SD (n = 3).
3.3. ROS and MDA Contents
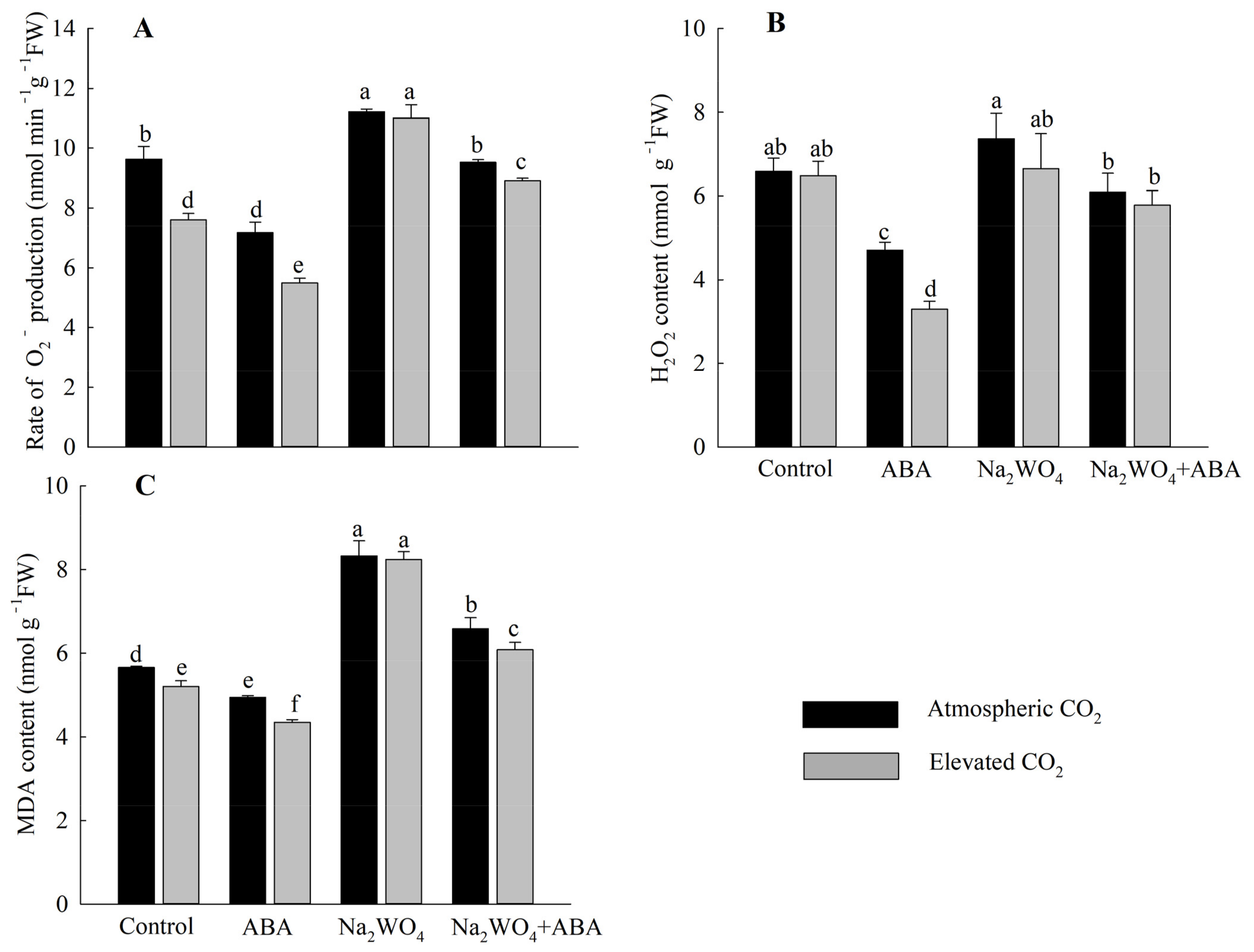
Except for the Na2WO4 treatment, e[CO2] reduced superoxide O2− production rate and MDA contents compared with a[CO2] treatment (Figure 3A,C). Under the a[CO2], compared with the control treatment, ABA treatment significantly reduced the production rate of O2−, the content of H2O2 and MDA by 26%, 29%, and 13%, respectively. O2− production rate, H2O2, and MDA content of Na2WO4 + ABA treatment were lower than those of Na2WO4 treatment. In contrast, plants with the Na2WO4 treatment had the highest rate of O2− production, H2O2, and MDA contents compared to other treatments, both in a[CO2] and e[CO2]. Compared with the control treatment, Na2WO4 significantly increased the O2 − production rate, H2O2, and MDA contents by 16%, 12%, and 47%, respectively (Figure 3).
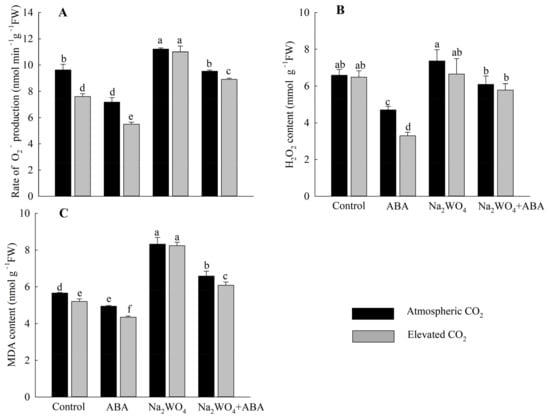
Figure 3.
Effects of elevated CO2 and exogenous ABA on O2− production rate (A), H2O2 content (B), and MDA content (C) in leaves of cucumber seedlings under drought stress. Different lowercase letters represent significant differences between treatments at the 0.05 level. Values were the means ± SD (n = 3).
3.4. Osmoregulation Substances’ Contents
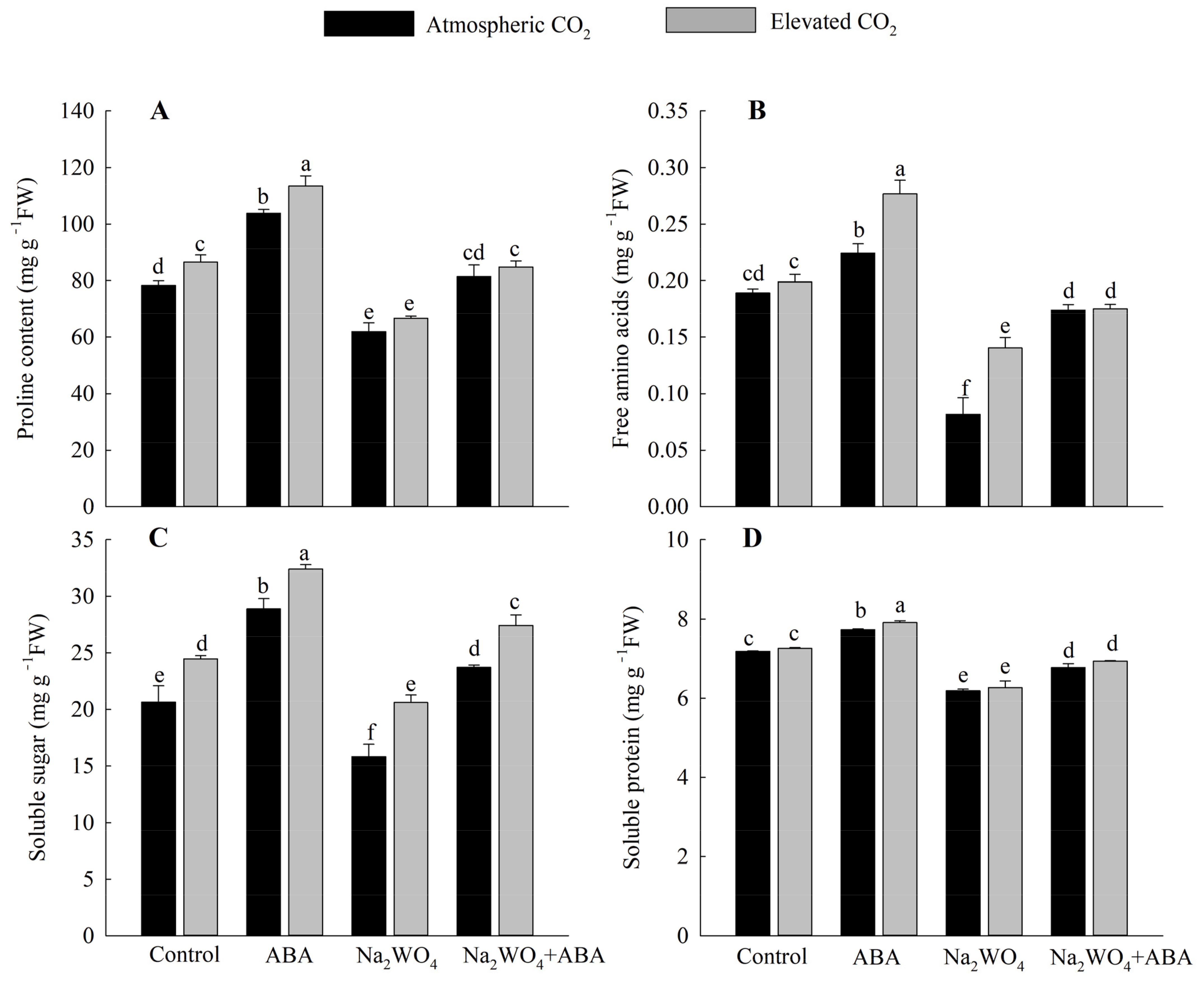
As shown in Figure 4, at the a[CO2] level, the contents of proline, free amino acids, soluble sugar, and soluble protein in the leaves of ABA-treated seedlings under drought stress were significantly higher than those of other treatments. The contents of proline, free amino acids, soluble sugar, and soluble protein in the ABA treatment significantly increased by 32%, 19%, 40%, and 8%, respectively, compared with the control treatment. Na2WO4 treatment had the lowest contents of each substance, which decreased by 21%, 57%, 23%, and 14% compared with the control treatment. Furthermore, e[CO2] further promoted the accumulation of osmoregulation substances in seedlings, and the differences were significant under ABA treatment. Compared with a[CO2], the contents of proline, free amino acid, and soluble sugar increased by 9%, 23%, and 12%, respectively.
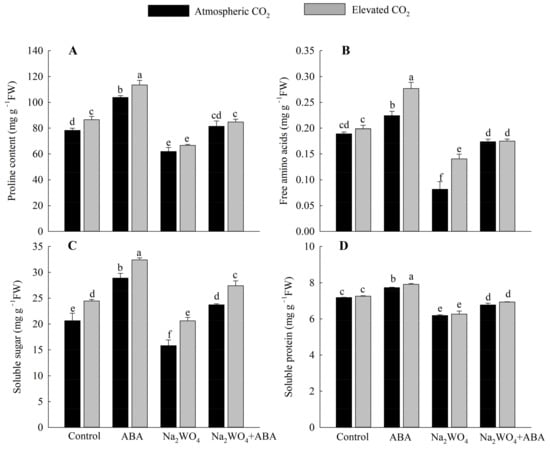
Figure 4.
Effects of elevated CO2 and exogenous ABA on proline content (A), free amino acids (B), soluble sugar (C), and soluble protein (D) in leaves of cucumber seedlings under drought stress. Different lowercase letters represent significant differences between treatments at the 0.05 level. Values were the means ± SD (n = 3).
3.5. Antioxidant Enzyme Activities, ASA, DHA Contents and ASA/DHA Ratio
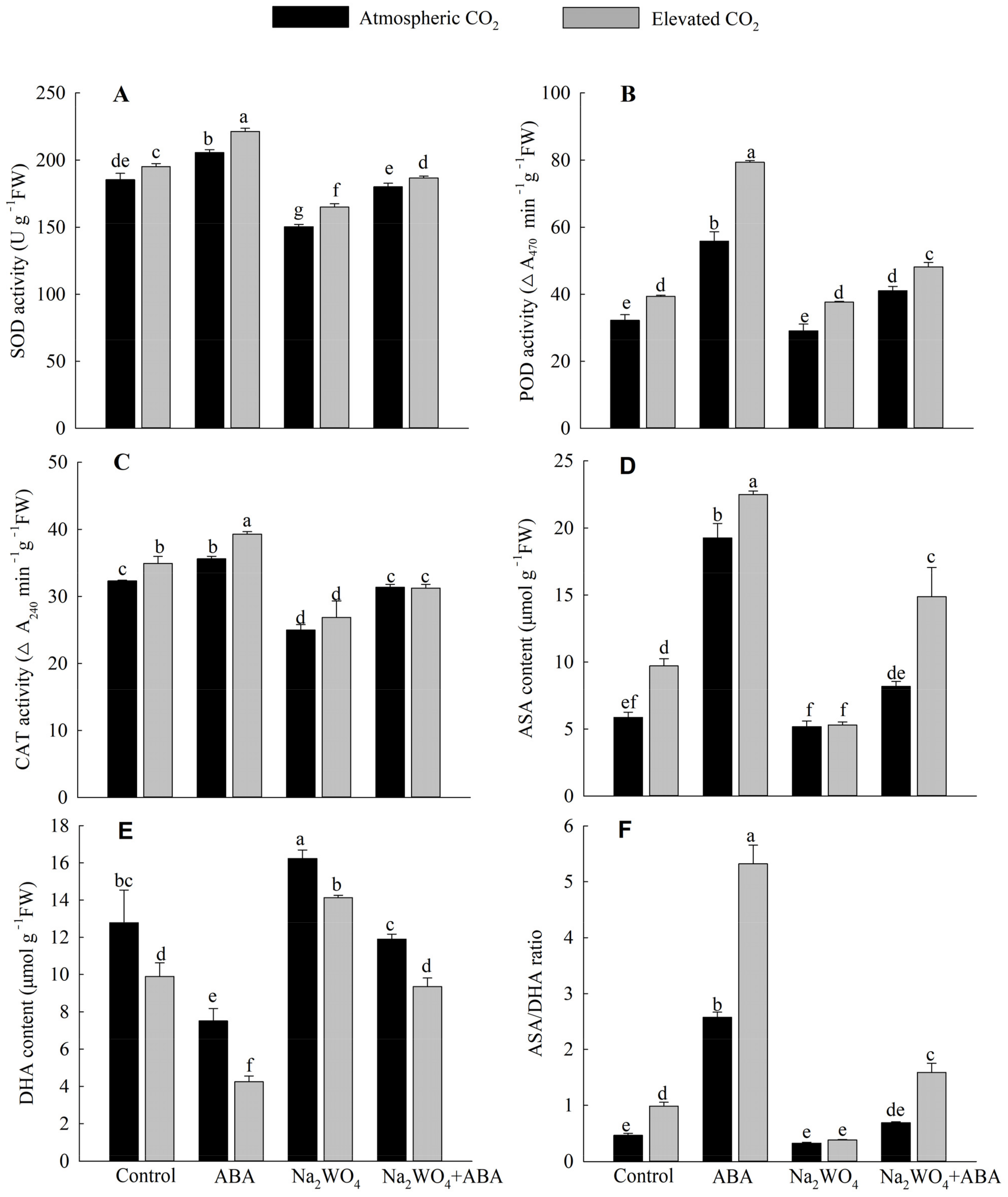
It can be observed from Figure 5 that under a[CO2], the activities of SOD, POD, and CAT in leaves of cucumber seedlings treated with ABA were the highest, significantly increased by 11%, 73%, and 10%, respectively, compared with the control treatment. Compared with the control treatment, three enzyme activities of Na2WO4 treatment were decreased by 18.88%, 9.8%, and 22.72%. The enzyme activities under Na2WO4 + ABA treatment were higher than those under Na2WO4 treatment, which indicated that exogenous ABA could weaken the inhibition of sodium tungstate on antioxidant enzymes. Compared with a[CO2], e[CO2] further significantly increased the activities of SOD and POD in all treatments. Under e[CO2], compared with the control treatment, ABA treatment significantly increased the activities of SOD, POD, and CAT (Figure 5A–C).
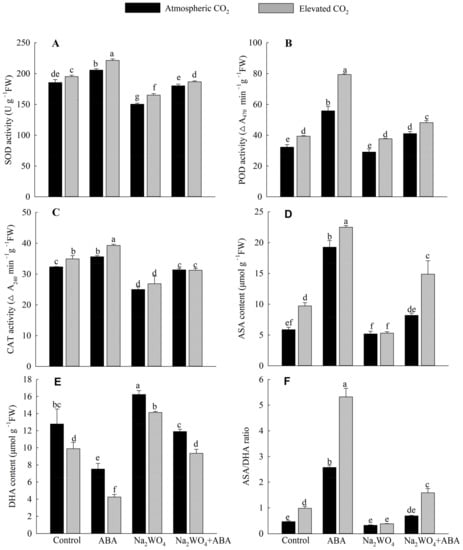
Figure 5.
Effects of elevated CO2 and exogenous ABA on SOD activity (A), POD activity (B), CAT activity (C), ASA content (D), DHA content (E), and ASA/DHA ratio (F) in leaves of cucumber seedlings under drought stress. Different lowercase letters represent significant differences between treatments at the 0.05 level. Values were the means ± SD (n = 3).
It is noticeable that at a[CO2], the content of ASA and the ratio of ASA/DHA were the highest and the content of DHA was the lowest in ABA treatment while being opposite with Na2WO4 treatment (Figure 5D,F). The content of ASA and the ratio of ASA/DHA under Na2WO4 + ABA treatment were increased by 58% and 115%, respectively, and the content of DHA was decreased by 23% compared with Na2WO4 treatment. Compared with a[CO2], e[CO2] could further increase the content of ASA and the ratio of ASA/DHA in cucumber seedling leaves and the differences were significant except for Na2WO4 treatment, and the content of DHA decreased significantly under e[CO2] (Figure 5D–F).
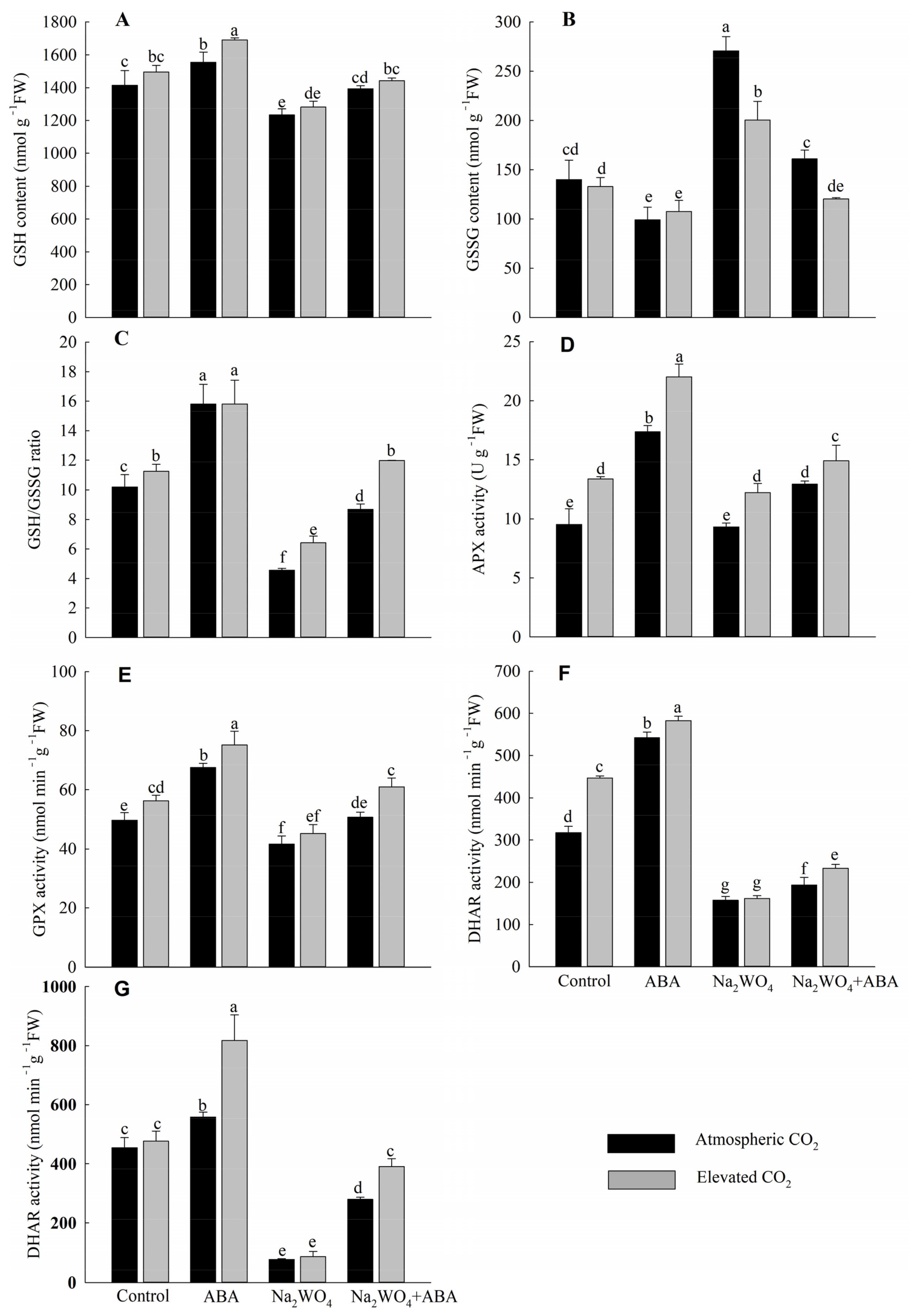
3.6. GSH, GSSG Contents, GSH/GSSG Ratio and Ascorbate–Glutathione Cycle Enzyme Activities
Under a[CO2], the ABA treatment had the highest GSH content and GSH/GSSH ratio, and the lowest GSSG content compared to the other treatments, while the opposite was observed with the Na2WO4 treatment (Figure 6A–C). The content of GSH and the ratio of GSH/GSSG of Na2WO4 + ABA treatment were increased by 13% and 90%, respectively, and the content of GSSG was decreased by 41% compared with Na2WO4 treatment. Compared with a[CO2], e[CO2] increased the ratio of GSH/GSSG in cucumber seedling leaves and the differences were significant except for ABA treatment (Figure 6C).
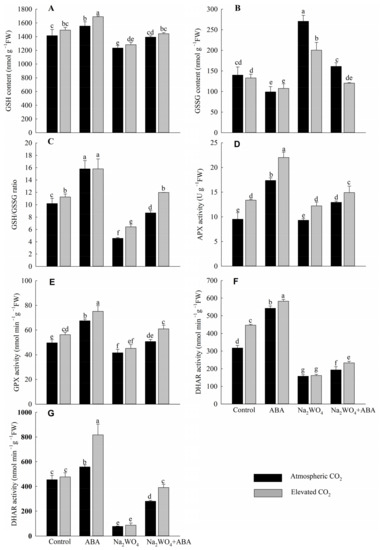
Figure 6.
Effects of elevated CO2 and exogenous ABA on GSH content (A), GSSG content (B), GSH/GSSG ratio (C), APX activity (D), GPX activity (E), DHAR activity (F), and GR activity (G) in leaves of cucumber seedlings under drought stress. Different lowercase letters represent significant differences between treatments at the 0.05 level. Values were the means ± SD (n = 3).
Compared with the control treatment, ABA significantly increased the activities of APX, GPX, DHAR, and GR in the leaves of cucumber seedlings under a[CO2] by 82%, 36%, 71%, and 23%, respectively (Figure 6D–G). While Na2WO4 treatment decreased them by 2%, 16%, 50%, and 83%, respectively, compared with the control treatment, the differences between DHAR and GR were significant (Figure 6F,G). The enzyme activities under Na2WO4 + ABA treatment were significantly higher than those under Na2WO4 treatment. Except for Na2WO4 treatment, e[CO2] enhanced the activities of APX, GPX, and DHAR significantly, while e[CO2] significantly enhanced GR activity only under ABA and Na2WO4 + ABA treatments (Figure 6D–G).
4. Discussion
Drought stress is one of the largest and most persistent constraints in agricultural development [53]. The cucumber is a major horticultural species, which is frequently used as a model plant to explore plant processes in response to drought stress [54]. At present, other evidence has already indicated that e[CO2] and exogenous ABA can alleviate drought stress in cucumbers [53,55]. However, most studies are aimed at a single effect of either e[CO2] or exogenous ABA spraying for the alleviation of drought stress. CO2 and ABA signals have a variety of interactions on the drought response in plants, which can work orchestrally to allow plants adapt to adverse environments [56]. In this study, we investigated the growth, photosynthetic capacity, active osmoregulation, and antioxidant enzyme activities of cucumber seedlings under e[CO2] and/or exogenous ABA to assess the effects of interaction between e[CO2] and/or exogenous ABA on the drought resistance of cucumber seedlings.
There is a common consensus that drought stress has a significant inhibitory effect on plant growth, in particular on biomass. e[CO2] provides the opportunity to promote the growth of drought-stressed plants and increase plant biomass accumulation [57]. Additionally, ABA can also act as an emergency stress hormone and play a pivotal role in signal transduction under drought stress, thus slowing down the damage to plants [58]. The results of this experiment showed that e[CO2] significantly enhanced the growth of cucumber seedlings under drought stress (Figure 1). On top of that, the application of ABA was more beneficial to plant growth (Table 1). These discoveries suggest that e[CO2] and exogenous ABA positively enhanced the tolerance of cucumber seedlings to drought stress. Moreover, its enhanced drought tolerance might be due to e[CO2] induced stomatal closure and reduced Gs, and consequently a decreased water dissipation [59], and this effect is simultaneously enhanced by exogenous ABA [60].
The photosynthetic pigment content is one of the most important physiological traits to determine the photosynthetic capacity and plant growth level of the plant [61]. The results of this experiment showed that e[CO2] and exogenous application of ABA significantly increased the photosynthetic pigment content in leaves of cucumber exposed to drought stress (Table 2), probably because e[CO2] and ABA can reduce the content of ROS in leaves (Figure 3), thus reducing the degradation of chlorophyll by ROS [62]. The increase in chlorophyll content is also a crucial factor that enables the increase in photosynthetic rate in cucumber. Photosynthesis is the most fundamental physiological activity of plants, involving light energy transfer, carbon fixation, CO2 uptake, and O2 release and other related processes, and also plays a key role in balancing the carbon cycle of land ecosystems and is responsible for climate change [63]. In addition, CO2, as a raw material for photosynthesis, has a significant contribution to the effects of photosynthesis, especially for C3 plants [64]. In our study, e[CO2] significantly improved the photosynthetic gas exchange parameters of cucumbers under drought stress (Figure 2). The stomatal conductance and transpiration rate of cucumber leaves were reduced under the e[CO2], but the net photosynthetic rate of cucumber leaves did not decrease (Figure 2). This is probably because the increase in CO2 concentration enhanced the difference in CO2 concentration between the external environment and the leaf pulp cells, which improved the CO2 diffusion dynamics, and the CO2 from the environment could still enter the leaves; therefore, even though there is no significant change in the intercellular CO2 concentration, current results are still consistent with the findings of Zheng et al. [63]. Similarly, abiotic stresses such as drought can cause damage to the photosynthetic organs of plants, and ABA can alleviate this damage while increasing the content of photosynthetic pigments under stress (Table 2), enhancing the stability of the cystoid membrane, improving the light energy capture ability, strengthening the photochemical reactions of leaves, and protecting the photosynthetic machinery, thus ultimately increasing the photosynthetic rate [65], which is consistent with the results of this experiment.
Drought stress is an important factor limiting the growth and development of plants [66], which can induce the generation of ROS such as O2− and H2O2. Excess ROS can damage cell membranes, proteins, RNA and DNA molecules, reduce photosynthetic efficiency and lead to leaf wilting [67]. To alleviate the damage from ROS, plants evolved a sophisticated antioxidant system to maintain a dynamic balance between the production and clearance of ROS in stressful environments [68]. Previous studies have shown that exogenous spraying of ABA can improve the activities of antioxidant enzymes SOD, POD, and CAT, which reduces the content of MDA and the accumulation of ROS in maize and kiwifruit under drought stress [35,69]. Similar results were observed in our experiment; ABA treatment reduced O2− production rate, MDA and H2O2 content and increased POD SOD, and CAT activities when compared to the control treatment under drought stress (Figure 3 and Figure 5). Nevertheless, after spraying the ABA inhibitor Na2WO4, both O2− production rate, H2O2, and MDA content were significantly increased compared with the control treatment. These results inferred that exogenous ABA can increase the activities of antioxidant enzymes to eliminate the accumulation of ROS, reduce the degree of membrane lipid peroxidation and protect the integrity of membrane structure, reduce the damage of drought stress on the cucumber seedling biofilm system, and enhance its antioxidant capacity, which was inconsistent with previous studies of cotton [70]. e[CO2] further increased the activities of antioxidant enzymes and decreased the contents of ROS and MDA under drought stress [71,72], which were observed in our results (Figure 3 and Figure 5). The above observation could be explained by the e[CO2] promoting photosynthetic carbon assimilation and inhibiting photorespiration, thus reducing ROS accumulation and H2O2 production, limiting the Mehler reaction by increasing the utilization rate of NADP+ in photosystem I (PSI) and producing more reducing coenzyme II (NADPH) for the ASA–GSH cycle, which finally induces antioxidant enzyme activity to reduce the damage of drought stress to cucumber seedlings [73,74]. Under the treatment of Na2WO4, the mitigation ability of CO2 on cucumber seedlings under drought stress was weakened. For example, there were no significant differences between O2− production rate, H2O2, and MDA contents under e[CO2] and a[CO2] (Figure 3), which might be related to the ABA level, implying that exogenous ABA signaling may be involved in regulating plant growth and oxidative stress response to e[CO2]. However, the specific mechanism is not clear yet and needs further study.
Osmoregulation is an important way for plants to resist drought stress. It means that plants actively accumulate organic solutes or inorganic ions to reduce osmotic potential under water stress, to promote plant cells to absorb water from the outside, and to guarantee their physiological processes function normally [75,76]. ABA is a stress hormone and studies have shown that exogenous spraying of ABA can improve the drought tolerance of plants by maintaining water balance, inducing the antioxidant system, maintaining membrane stability, and improving the level of osmotic adjustment substances such as soluble sugar, soluble protein, and amino acids [77,78]. It was reported that exogenous spraying of ABA increased the content of soluble sugar and soluble protein and reduced the damage degree of Axonopus compressus under drought stress [78], which is consistent with our results (Figure 4C,D). Proline would accumulate first during drought stress to maintain the osmotic balance between the cell matrix and the environment in wheat and papaya [79,80,81]. Our results showed that exogenous ABA could increase the content of proline and free amino acids in cucumber seedling leaves (Figure 4A,B). Moreover, e[CO2] can promote the accumulation of osmotic adjustment substances such as soluble sugar, soluble protein, proline, and free amino acids in cucumber seedlings (Figure 4). When cucumber seedlings were subjected to drought stress, e[CO2] and exogenous ABA could synergistically increase the accumulation of proline and other osmolytes, the osmotic adjustment ability of seedlings, and then their drought resistance (Figure 4). These results indicated that exogenous ABA interacts with e[CO2] on the regulation of plant growth in drought conditions.
In addition to osmoregulation, the ratios of ascorbic acid/dehydroascorbic acid (ASA/DHA), and glutathione/oxidized glutathione (GSH/GSSG) could determine the stress level of plants and reflect the redox status of plants through the ASA–GSH cycle [12,17]. In the present study, we observed that the content of ASA, GSH, and the ratio of ASA/DHA as well as GSH/GSSG in cucumber seedlings were significantly increased after application of e[CO2] and exogenous ABA under drought stress (Figure 5D,F and Figure 6A,C), which is comparable to the studies of Jiang et al. [82] and Liu et al. [83]. These results implied that e[CO2] and ABA treatment are more favorable to provide a reduced state environment for plants, thus improving the antioxidant defense function of plants. The activity of the ASA–GSH cycle mainly depends on the activities of ascorbate peroxidase (APX), glutathione reductase (GR), glutathione peroxidase (GPX), and dehydroascorbate reductase (DHAR). Among them, APX directly reduces H2O2 to water using ascorbic acid as an electron donor [5]. The results from the present study showed that both ABA treatment and e[CO2] treatment can separately increase the activity of APX, and their interaction enlarged this effect (Figure 6D), indicating that CO2 and ABA can improve the ability to remove active oxygen in cucumber seedlings. DHAR can participate in the regeneration of ASA using GSH as substrate [84], and converting GSH to GSSG through GR [85]. In this pathway, ascorbate and GSH are not consumed in the cycling transfer of electrons but participate in the cycling transfer of reduction equivalent, thus eventually reducing H2O2 to H2O [7,85]. In this study, treatment with ABA and e[CO2] increased the activities of DHAR, GPX, and GR, the regeneration of ASA and GSH, and the activity as well as the operation of the whole ASA–GSH cycle system, thus it accelerated the activity clearance and alleviated the oxidative damage of cucumber seedlings under drought stress (Figure 5 and Figure 6). However, interestingly, after the exogenous application of the ABA synthesis inhibitor Na2WO4, the activities of DHAR, GPX, and GR were not significantly improved by e[CO2]. We speculated that exogenous ABA may be involved in the response of the plant ASA–GSH cycle to e[CO2] under drought stress as an important substance.
5. Conclusions
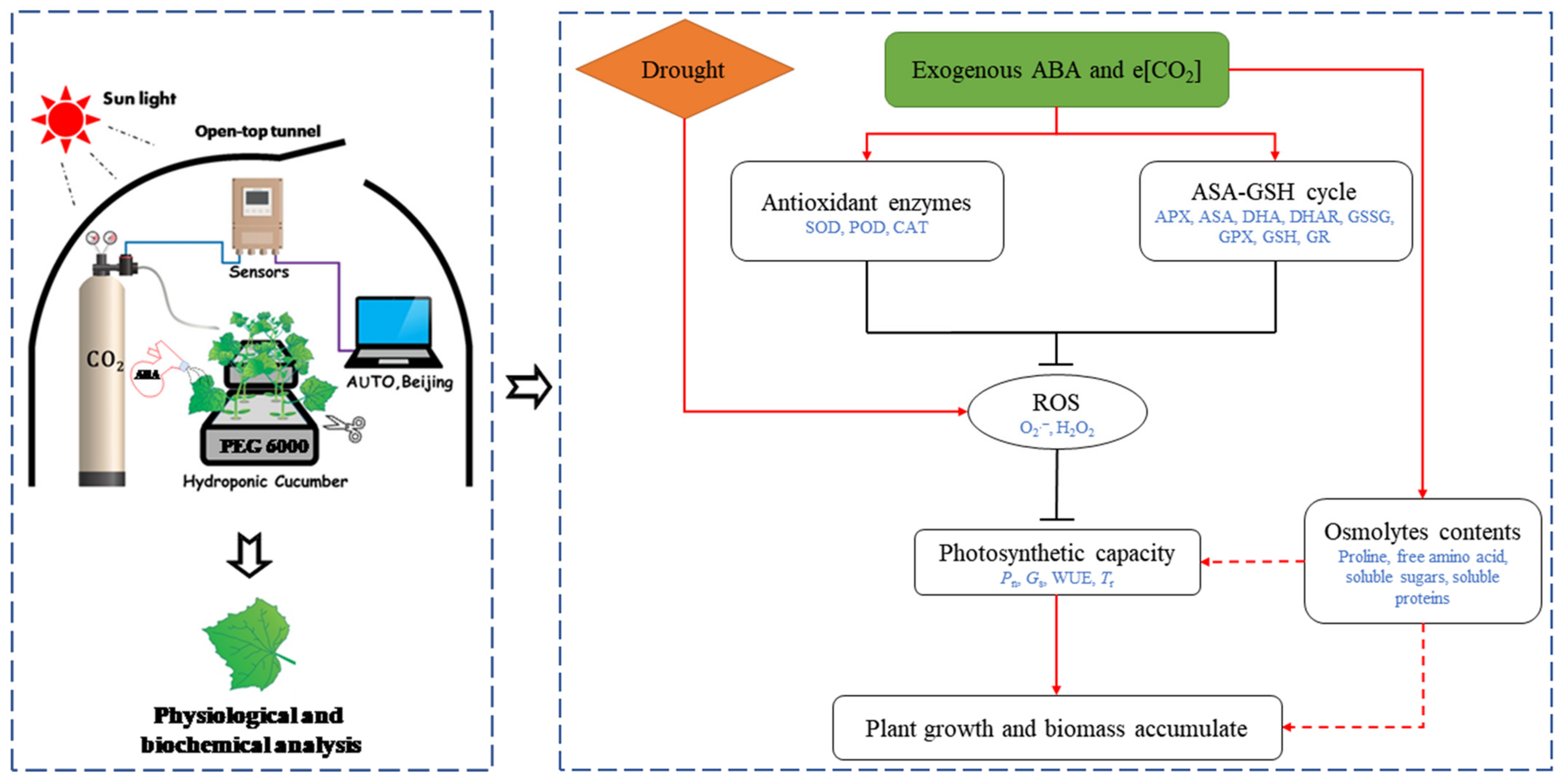
e[CO2] and exogenous ABA synergistically enhanced cucumber seedling drought tolerance by maintaining high photosynthetic capacity, increasing the activity of antioxidant enzymes, and accelerating the ASA–GSH cycle to alleviate the drought-induced oxidative damage in cucumber seedlings (Figure 7). Therefore, the combination of e[CO2] and exogenous ABA might be an effective way to increase crop yield, especially in arid and semi-arid regions. Furthermore, our results also indicated that ABA seemed to be involved in e[CO2]-induced tolerance to drought stress in cucumber seedlings. However, the mechanism of the ABA-mediated CO2 enhancement of drought resistance is still unclear. Future studies of cell membrane structure, intercellular and intracellular water transport-related proteins may shed light on the mechanisms of e[CO2]-induced drought tolerance.
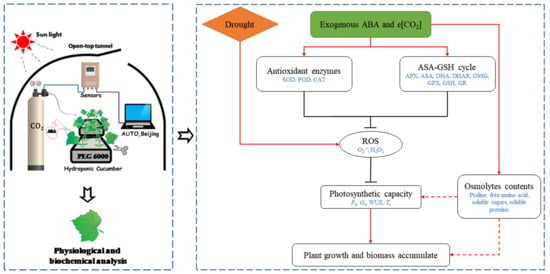
Figure 7.
Schematic diagram of elevated CO2 and exogenous ABA enhancement of drought resistance of cucumber seedlings. Arrow and bar-ended lines represent activation and inhibition, respectively. Dotted lines denote indirect regulation.
Author Contributions
Conceptualization, Z.B. and Q.L.; investigation, Q.S., X.H., T.W. and H.Q.; writing—original draft preparation, X.H., Q.S.; writing—review and editing, X.Y. and Y.C.; supervision, project administration, and funding acquisition, Z.B. and Q.L. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (31872154, 31902092); the Agricultural Science and Technology Innovation Project of the Chinese Academy of Agricultural Sciences (34-IUA-03).
Data Availability Statement
The data presented in this study are available upon request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Intergovernmental Panel on Climate Change. Climate Change 2013: The Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2013; p. 1535. [Google Scholar]
- Cernusak, L.A.; Haverd, V.; Brendel, O.; Le Thiec, D.; Guehl, J.-M.; Cuntz, M. Robust Response of Terrestrial Plants to Rising CO2. Trends Plant Sci. 2019, 24, 578–586. [Google Scholar] [CrossRef] [PubMed]
- Chaves, M.M.; Maroco, J.P.; Pereira, J.S. Understanding plant responses to drought—From genes to the whole plant. Funct. Plant Biol. 2003, 30, 239–264. [Google Scholar] [CrossRef] [PubMed]
- Malik, M.A.; Wani, A.H.; Mir, S.H.; Rehman, I.U.; Tahir, I.; Ahmad, P.; Rashid, I. Elucidating the role of silicon in drought stress tolerance in plants. Plant Physiol. Biochem. 2021, 165, 187–195. [Google Scholar] [CrossRef] [PubMed]
- Bartels, D.; Sunkar, R. Drought and salt tolerance in plants. Crit. Rev. Plant Sci. 2005, 24, 23–58. [Google Scholar] [CrossRef]
- Dong, S.; Jiang, Y.; Dong, Y.; Wang, L.; Wang, W.; Ma, Z.; Yan, C.; Ma, C.; Liu, L. A study on soybean responses to drought stress and rehydration. Saudi J. Biol. Sci. 2019, 26, 2006–2017. [Google Scholar] [CrossRef]
- Foyer, C.H.; Noctor, G. Ascorbate and glutathione: The heart of the redox hub. Plant Physiol. 2011, 155, 2–18. [Google Scholar] [CrossRef]
- Møller, I.M.; Jensen, P.E.; Hansson, A. Oxidative modifications to cellular components in plants. Annu. Rev. Plant Biol. 2007, 58, 459–481. [Google Scholar] [CrossRef]
- Gill, S.S.; Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef]
- Ghassemi, S.; Ghassemi-Golezani, K.; Salmasi, S.Z. Changes in antioxidant enzymes activities and physiological traits of ajowan in response to water stress and hormonal application. Sci. Hortic. 2019, 246, 957–964. [Google Scholar] [CrossRef]
- Jiang, Z.; Zhu, H.; Zhu, H.; Tao, Y.; Liu, C.; Liu, J.; Yang, F.; Li, M. Exogenous ABA enhances the antioxidant defense system of maize by regulating the AsA-GSH cycle under drought stress. Sustainability 2022, 14, 3071. [Google Scholar] [CrossRef]
- Kaya, C.; Ashraf, M.; Alyemeni, M.N.; Ahmad, P. The role of endogenous nitric oxide in salicylic acid-induced up-regulation of ascorbate-glutathione cycle involved in salinity tolerance of pepper (Capsicum annuum L.) plants. Plant Physiol. Biochem. 2020, 147, 10–20. [Google Scholar] [CrossRef] [PubMed]
- Dipierro, N.; Mondelli, D.; Paciolla, C.; Brunetti, G.; Dipierro, S. Changes in the ascorbate system in the response of pumpkin (Cucurbita pepo L.) roots to aluminium stress. J. Plant Physiol. 2005, 162, 529–536. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Wang, X.; Hu, Y.; Hu, W.; Bi, Y. Glucose-6-phosphate dehydrogenase plays a pivotal role in tolerance to drought stress in soybean roots. Plant Cell Rep. 2013, 32, 415–429. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Liu, L.; Yu, Y.; Liu, W.; Lu, L.; Jin, C.; Lin, X. Nitric oxide alleviates aluminum-induced oxidative damage through regulating the ascorbate-glutathione cycle in roots of wheat. J. Integr. Plant Biol. 2015, 57, 550–561. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Ma, Y.; Huang, C.; Wan, Q.; Li, N.; Bi, Y. Glucose-6-phosphate dehydrogenase plays a central role in modulating reduced glutathione levels in reed callus under salt stress. Planta 2008, 227, 611–623. [Google Scholar] [CrossRef]
- Gallie, D.R. The role of l-ascorbic acid recycling in responding to environmental stress and in promoting plant growth. J. Exp. Bot. 2012, 64, 433–443. [Google Scholar] [CrossRef]
- Barton, C.V.M.; Duursma, R.A.; Medlyn, B.E.; Ellsworth, D.S.; Eamus, D.; Tissue, D.T.; Adams, M.A.; Conroy, J.; Crous, K.Y.; Liberloo, M.; et al. Effects of elevated atmospheric [CO2] on instantaneous transpiration efficiency at leaf and canopy scales in Eucalyptus saligna. Glob. Chang. Biol. 2012, 18, 585–595. [Google Scholar] [CrossRef]
- Marques, I.; Fernandes, I.; Paulo, O.S.; Lidon, F.C.; DaMatta, F.M.; Ramalho, J.C.; Ribeiro-Barros, A.I. A transcriptomic approach to understanding the combined impacts of supra-optimal temperatures and CO2 revealed different responses in the polyploid Coffea arabica and its diploid progenitor C. canephora. Int. J. Mol. Sci. 2021, 22, 3125. [Google Scholar] [CrossRef]
- Taylor, G.; Street, N.R.; Tricker, P.J.; Sjödin, A.; Graham, L.; Skogström, O.; Calfapietra, C.; Scarascia-Mugnozza, G.; Jansson, S. The transcriptome of Populus in elevated CO2. New Phytol. 2005, 167, 143–154. [Google Scholar] [CrossRef]
- Ksiksi, T.S.; Ppoyil, S.B.T.; Palakkott, A.R. CO2 enrichment affects eco-physiological growth of maize and alfalfa under different water stress regimes in the UAE. Physiol. Mol. Biol. Plants 2018, 24, 251–259. [Google Scholar] [CrossRef]
- Perry, L.G.; Shafroth, P.B.; Blumenthal, D.M.; Morgan, J.A.; LeCain, D.R. Elevated CO2 does not offset greater water stress predicted under climate change for native and exotic riparian plants. New Phytol. 2013, 197, 532–543. [Google Scholar] [CrossRef]
- Yan, F.; Li, X.; Liu, F. ABA signaling and stomatal control in tomato plants exposure to progressive soil drying under ambient and elevated atmospheric CO2 concentration. Environ. Exp. Bot. 2017, 139, 99–104. [Google Scholar] [CrossRef]
- Cui, Q.; Li, Y.; He, X.; Li, S.; Zhong, X.; Liu, B.; Zhang, D.; Li, Q. Physiological and iTRAQ based proteomics analyses reveal the mechanism of elevated CO2 concentration alleviating drought stress in cucumber (Cucumis sativus L.) seedlings. Plant Physiol. Biochem. 2019, 143, 142–153. [Google Scholar] [CrossRef]
- Li, J.; Wang, X.-Q.; Watson, M.B.; Assmann, S.M. Regulation of abscisic acid-induced stomatal closure and anion channels by guard cell AAPK kinase. Science 2000, 287, 300–303. [Google Scholar] [CrossRef]
- Vishwakarma, K.; Upadhyay, N.; Kumar, N.; Yadav, G.; Singh, J.; Mishra, R.K.; Kumar, V.; Verma, R.; Upadhyay, R.G.; Pandey, M. Abscisic Acid Signaling and Abiotic Stress Tolerance in Plants: A Review on Current Knowledge and Future Prospects. Front. Plant Sci. 2017, 8, 161. [Google Scholar] [CrossRef]
- Jiang, F.; Hartung, W. Long-distance signalling of abscisic acid (ABA): The factors regulating the intensity of the ABA signal. J. Exp. Bot. 2007, 59, 37–43. [Google Scholar] [CrossRef]
- Qaderi, M.M.; Kurepin, L.V.; Reid, D.M. Growth and physiological responses of canola (Brassica napus) to three components of global climate change: Temperature, carbon dioxide and drought. Physiol. Plant. 2006, 128, 710–721. [Google Scholar] [CrossRef]
- Du, Y.-L.; Wang, Z.-Y.; Fan, J.-W.; Turner, N.C.; He, J.; Wang, T.; Li, F.-M. Exogenous abscisic acid reduces water loss and improves antioxidant defence, desiccation tolerance and transpiration efficiency in two spring wheat cultivars subjected to a soil water deficit. Funct. Plant Biol. 2013, 40, 494–506. [Google Scholar] [CrossRef]
- Haisel, D.; Pospíšilová, J.; Synková, H.; Schnablová, R.; Baťková, P. Effects of abscisic acid or benzyladenine on pigment contents, chlorophyll fluorescence, and chloroplast ultrastructure during water stress and after rehydration. Photosynthetica 2006, 44, 606–614. [Google Scholar] [CrossRef]
- Parent, B.; Hachez, C.; Redondo, E.; Simonneau, T.; Chaumont, F.o.; Tardieu, F.o. Drought and abscisic acid effects on aquaporin content translate into changes in hydraulic conductivity and leaf growth rate: A trans-scale approach. Plant Physiol. 2009, 149, 2000–2012. [Google Scholar] [CrossRef]
- Wang, T.; Zhang, X.; Li, C. Growth, abscisic acid content, and carbon isotope composition in wheat cultivars grown under different soil moisture. Biol. Plant. 2007, 51, 181–184. [Google Scholar] [CrossRef]
- Kurahashi, Y.; Terashima, A.; Takumi, S. Variation in dehydration tolerance, ABA sensitivity and related gene expression patterns in D-genome progenitor and synthetic hexaploid wheat lines. Int. J. Mol. Sci. 2009, 10, 2733–2751. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Yang, L.; Li, Y.; Hou, J.; Huang, J.; Liang, W. Involvement of ABA- and H2O2-dependent cytosolic glucose-6-phosphate dehydrogenase in maintaining redox homeostasis in soybean roots under drought stress. Plant Physiol. Biochem. 2016, 107, 126–136. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Ma, F.; Li, M.; Liang, D.; Zou, J. Physiological responses of kiwifruit plants to exogenous ABA under drought conditions. Plant Growth Regul. 2011, 64, 63–74. [Google Scholar] [CrossRef]
- Fang, L.; Abdelhakim, L.O.A.; Hegelund, J.N.; Li, S.; Liu, J.; Peng, X.; Li, X.; Wei, Z.; Liu, F. ABA-mediated regulation of leaf and root hydraulic conductance in tomato grown at elevated CO2 is associated with altered gene expression of aquaporins. Hortic. Res. 2019, 6, 104. [Google Scholar] [CrossRef]
- Li, S.; Li, X.; Wei, Z.; Liu, F. ABA-mediated modulation of elevated CO2 on stomatal response to drought. Curr. Opin. Plant Biol. 2020, 56, 174–180. [Google Scholar] [CrossRef]
- Chater, C.; Peng, K.; Movahedi, M.; Dunn, J.A.; Walker, H.J.; Liang, Y.-K.; McLachlan, D.H.; Casson, S.; Isner, J.C.; Wilson, I.; et al. Elevated CO2-induced responses in stomata require ABA and ABA signaling. Curr. Biol. 2015, 25, 2709–2716. [Google Scholar] [CrossRef]
- Engineer, C.B.; Hashimoto-Sugimoto, M.; Negi, J.; Israelsson-Nordström, M.; Azoulay-Shemer, T.; Rappel, W.-J.; Iba, K.; Schroeder, J.I. CO2 sensing and CO2 regulation of stomatal conductance: Advances and open questions. Trends Plant Sci. 2016, 21, 16–30. [Google Scholar] [CrossRef]
- Tazoe, Y.; Santrucek, J. Superimposed behaviour of gm under ABA-induced stomata closing and low CO2. Plant Cell Environ. 2015, 38, 385–387. [Google Scholar] [CrossRef]
- Michel, B.E.; Kaufmann, M.R. The Osmotic Potential of Polyethylene Glycol 6000. Plant Physiol. 1973, 51, 914–916. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K.; Wellburn, A.R. Determinations of total carotenoids and chlorophylls a and b of leaf extracts in different solvents. Analysis 1983, 11, 591–592. [Google Scholar] [CrossRef]
- Dhindsa, R.S.; Plumb-Dhindsa, P.; Thorpe, T.A. Leaf senescence: Correlated with increased levels of membrane permeability and lipid peroxidation, and decreased levels of superoxide dismutase and catalase. J. Exp. Bot. 1981, 32, 93–101. [Google Scholar] [CrossRef]
- Elstner, E.F.; Heupel, A. Inhibition of nitrite formation from hydroxylammoniumchloride: A simple assay for superoxide dismutase. Anal. Biochem. 1976, 70, 616–620. [Google Scholar] [CrossRef]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid determination of free proline for water-stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Aurisano, N.; Bertani, A.; Reggiani, R. Involvement of calcium and calmodulin in protein and amino acid metabolism in rice roots under anoxia. Plant Cell Physiol. 1995, 36, 1525–1529. [Google Scholar] [CrossRef]
- Irigoyen, J.J.; Einerich, D.W.; Sánchez-Díaz, M. Water stress induced changes in concentrations of proline and total soluble sugars in nodulated alfalfa (Medicago sativd) plants. Physiol. Plant. 1992, 84, 55–60. [Google Scholar] [CrossRef]
- Kampfenkel, K.; Vanmontagu, M.; Inze, D. Extraction and determination of ascorbate and dehydroascorbate from plant tissue. Anal. Biochem. 1995, 225, 165–167. [Google Scholar] [CrossRef]
- Anderson, J.V.; Chevone, B.I.; Hess, J.L. Seasonal variation in the antioxidant system of eastern white pine needles 1: Evidence for thermal dependence. Plant Physiol. 1992, 98, 501–508. [Google Scholar] [CrossRef]
- Beyer, W.F.; Fridovich, I. Assaying for superoxide dismutase activity: Some large consequences of minor changes in conditions. Anal. Biochem. 1987, 161, 559–566. [Google Scholar] [CrossRef]
- Omran, R.G. Peroxide levels and the activities of catalase, peroxidase, and indoleacetic acid oxidase during and after chilling cucumber seedlings. Plant Physiol. 1980, 65, 407–408. [Google Scholar] [CrossRef]
- Nakano, Y.; Asada, K. Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol. 1981, 22, 867–880. [Google Scholar]
- Awan, S.A.; Khan, I.; Rizwan, M.; Zhang, X.; Brestic, M.; Khan, A.; El-Sheikh, M.A.; Alyemeni, M.N.; Ali, S.; Huang, L. Exogenous abscisic acid and jasmonic acid restrain polyethylene glycol-induced drought by improving the growth and antioxidative enzyme activities in pearl millet. Physiol. Plant. 2021, 172, 809–819. [Google Scholar] [CrossRef] [PubMed]
- Fan, H.-F.; Ding, L.; Du, C.-X.; Wu, X. Effect of short-term water deficit stress on antioxidative systems in cucumber seedling roots. Bot. Stud. 2014, 55, 46. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.B.; Li, M.; Li, Q.M.; Cui, Q.Q.; Zhang, W.D.; Ai, X.Z.; Bi, H.G. Combined effects of elevated CO2 concentration and drought stress on photosynthetic performance and leaf structure of cucumber (Cucumis sativus L.) seedlings. Photosynthetica 2018, 56, 942–952. [Google Scholar] [CrossRef]
- Li, Y.; Li, S.; He, X.; Jiang, W.; Zhang, D.; Liu, B.; Li, Q. CO2 enrichment enhanced drought resistance by regulating growth, hydraulic conductivity and phytohormone contents in the root of cucumber seedlings. Plant Physiol. Biochem. 2020, 152, 62–71. [Google Scholar] [CrossRef]
- Liang, F.; Yang, W.; Xu, L.; Ji, L.; He, Q.; Wu, L.; Ran, Y.; Yan, S. Closing extra CO2 into plants for simultaneous CO2 fixation, drought stress alleviation and nutrient absorption enhancement. J. CO2 Util. 2020, 42, 101319. [Google Scholar] [CrossRef]
- Kuromori, T.; Seo, M.; Shinozaki, K. ABA transport and plant water stress responses. Trends Plant Sci. 2018, 23, 513–522. [Google Scholar] [CrossRef]
- Hatfield, J.L.; Dold, C. Water-use efficiency: Advances and challenges in a changing climate. Front. Plant Sci. 2019, 10, 103. [Google Scholar] [CrossRef]
- Saradadevi, R.; Palta, J.A.; Siddique, K.H.M. ABA-mediated stomatal response in regulating water use during the development of terminal drought in wheat. Front. Plant Sci. 2017, 8, 1251. [Google Scholar] [CrossRef]
- Pan, T.; Wang, Y.; Wang, L.; Ding, J.; Cao, Y.; Qin, G.; Yan, L.; Xi, L.; Zhang, J.; Zou, Z. Increased CO2 and light intensity regulate growth and leaf gas exchange in tomato. Physiol. Plant. 2020, 168, 694–708. [Google Scholar] [CrossRef]
- Foreman, J.; Demidchik, V.; Bothwell, J.H.F.; Mylona, P.; Miedema, H.; Torres, M.A.; Linstead, P.; Costa, S.; Brownlee, C.; Jones, J.D.G.; et al. Reactive oxygen species produced by NADPH oxidase regulate plant cell growth. Nature 2003, 422, 442–446. [Google Scholar] [CrossRef]
- Zheng, Y.; Li, F.; Hao, L.; Yu, J.; Guo, L.; Zhou, H.; Ma, C.; Zhang, X.; Xu, M. Elevated CO2 concentration induces photosynthetic down-regulation with changes in leaf structure, non-structural carbohydrates and nitrogen content of soybean. BMC Plant Biol. 2019, 19, 255. [Google Scholar] [CrossRef]
- Ainsworth, E.A.; Ort, D.R. How do we improve crop production in a warming world? Plant Physiol. 2010, 154, 526–530. [Google Scholar] [CrossRef]
- Poór, P.; Borbély, P.; Czékus, Z.; Takács, Z.; Ördög, A.; Popović, B.; Tari, I. Comparison of changes in water status and photosynthetic parameters in wild type and abscisic acid-deficient sitiens mutant of tomato (Solanum lycopersicum cv. Rheinlands Ruhm) exposed to sublethal and lethal salt stress. J. Plant Physiol. 2019, 232, 130–140. [Google Scholar] [CrossRef]
- Farooq, M.; Wahid, A.; Kobayashi, N.; Fujita, D.; Basra, S.M.A. Plant drought stress: Effects, mechanisms and management. In Sustainable Agriculture; Springer: Dordrecht, The Netherlands, 2009; pp. 153–188. [Google Scholar]
- Mittler, R. Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci. 2002, 7, 405–410. [Google Scholar] [CrossRef]
- Xu, P.L.; Guo, Y.K.; Bai, J.G.; Shang, L.; Wang, X.J. Effects of long-term chilling on ultrastructure and antioxidant activity in leaves of two cucumber cultivars under low light. Physiol. Plant. 2008, 132, 467–478. [Google Scholar] [CrossRef]
- Ge, T.-d.; Sui, F.-g.; Bai, L.-p.; Lu, Y.-y.; Zhou, G.-s. Effects of water stress on the protective enzyme activities and lipid peroxidation in roots and leaves of summer maize. Agric. Sci. China 2006, 5, 291–298. [Google Scholar] [CrossRef]
- Hu, W.; Zhang, J.; Yan, K.; Zhou, Z.; Zhao, W.; Zhang, X.; Pu, Y.; Yu, R. Beneficial effects of abscisic acid and melatonin in overcoming drought stress in cotton (Gossypium hirsutum L.). Physiol. Plant. 2021, 173, 2041–2054. [Google Scholar] [CrossRef]
- Robredo, A.; Pérez-López, U.; de la Maza, H.S.; González-Moro, B.; Lacuesta, M.; Mena-Petite, A.; Muñoz-Rueda, A. Elevated CO2 alleviates the impact of drought on barley improving water status by lowering stomatal conductance and delaying its effects on photosynthesis. Environ. Exp. Bot. 2007, 59, 252–263. [Google Scholar] [CrossRef]
- Farfan-Vignolo, E.R.; Asard, H. Effect of elevated CO2 and temperature on the oxidative stress response to drought in Lolium perenne L. and Medicago sativa L. Plant Physiol. Biochem. 2012, 59, 55–62. [Google Scholar] [CrossRef]
- Miyake, C. Molecular mechanism of oxidation of P700 and suppression of ROS production in photosystem I in response to electron-sink limitations in C3 plants. Antioxidants 2020, 9, 230. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Jiang, Y.; Zhou, G. Response and adaptation of photosynthesis, respiration, and antioxidant systems to elevated CO2 with environmental stress in plants. Front. Plant Sci. 2015, 6, 701. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Zhang, Y.; Ji, X.; Downing, A.; Serpe, M. Combined effects of nitrogen deposition and water stress on growth and physiological responses of two annual desert plants in northwestern China. Environ. Exp. Bot. 2011, 74, 1–8. [Google Scholar] [CrossRef]
- Farouk, S.; AL-Huqail, A.A. Sustainable biochar and/or melatonin improve salinity tolerance in borage plants by modulating osmotic adjustment, antioxidants, and ion homeostasis. Plants 2022, 11, 765. [Google Scholar] [CrossRef]
- Chen, Z.; Wang, Z.; Yang, Y.; Li, M.; Xu, B. Abscisic acid and brassinolide combined application synergistically enhances drought tolerance and photosynthesis of tall fescue under water stress. Sci. Hortic. 2018, 228, 1–9. [Google Scholar] [CrossRef]
- Nawaz, M.; Wang, Z. Abscisic acid and glycine betaine mediated tolerance mechanisms under drought stress and recovery in axonopus compressus: A new insight. Sci. Rep. 2020, 10, 6942. [Google Scholar] [CrossRef]
- Kaur, G.; Asthir, B. Proline: A key player in plant abiotic stress tolerance. Biol. Plant. 2015, 59, 609–619. [Google Scholar] [CrossRef]
- Kaur, M.; Gupta, A.K.; Zhawar, V.K. Antioxidant response and lea genes expression under exogenous ABA and water deficit stress in wheat cultivars contrasting in drought tolerance. J. Plant Biochem. Biotechnol. 2014, 23, 18–30. [Google Scholar] [CrossRef]
- Mahouachi, J.; Argamasilla, R.; Gómez-Cadenas, A. Influence of exogenous glycine betaine and abscisic acid on papaya in responses to water-deficit stress. J. Plant Growth Regul. 2012, 31, 1–10. [Google Scholar] [CrossRef]
- Jiang, M.; Zhang, J. Role of abscisic acid in water stress-induced antioxidant defense in leaves of maize seedlings. Free Radic. Res. 2002, 36, 1001–1015. [Google Scholar] [CrossRef]
- Liu, Y.; Jiang, H.; Zhao, Z.; An, L. Abscisic acid is involved in brassinosteroids-induced chilling tolerance in the suspension cultured cells from Chorispora bungeana. J. Plant Physiol. 2011, 168, 853–862. [Google Scholar] [CrossRef]
- Kang, G.Z.; Li, G.Z.; Liu, G.Q.; Xu, W.; Peng, X.Q.; Wang, C.Y.; Zhu, Y.J.; Guo, T.C. Exogenous salicylic acid enhances wheat drought tolerance by influence on the expression of genes related to ascorbate-glutathione cycle. Biol. Plant. 2013, 57, 718–724. [Google Scholar] [CrossRef]
- Miller, G.; Suzuki, N.; Ciftci-Yilmaz, S.; Mittler, R. Reactive oxygen species homeostasis and signalling during drought and salinity stresses. Plant Cell Environ. 2010, 33, 453–467. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).