Abstract
In order to provide early selection indicators for the breeding of plants used for producing tea seed oil or harvesting tea, we investigated the relationships between flower morphology and fruit yields in tea plants. We analyzed 106 tea varieties to determine the relationships between flower morphological traits and fruit yields. Notably, the homogeneity of flower traits within the same tea plant variety was found to be very high. The average length and width measurements of certain phenotypic traits of tea plants, including pistil length, stamen length, stamen bundle inner width, stamen bundle outer width, and stigma width, were 11.8, 10.9, 2.5, 15.0, 3.7 mm, respectively. In this study, the flower traits that affect fruit yield appear to be related to the difficulty of pollination by insects (e.g., bees), in terms of their contacting the stigma. In 2013, three phenotypic trait variables showed significant effects on yield; namely, the stamen bundle outer width (negative), stigma width (positive), and stigma width minus the stamen bundle inner width (positive). In 2015, only the stamen bundle outer width had a significant negative effect on yield. Regarding pollen viability, in the TTC (2,3,5-triphenyl tetrazolium chloride) staining test, about 84% of the considered tea varieties presented pollen viability exceeding 70%. This indicates that most tea pollen has the ability to germinate normally after contact with the cross-pollinated stigma. The yields of all of the tea varieties exhibited a positively skewed distribution in 2013 and 2015. Although our results indicate that flowers in the anther superior group tend to produce fewer fruits than flowers in the stigma superior group in 2013, in the analysis of the effect of traits on yield, there were no significant differences in the relative positions of stigmas and anthers. In conclusion, we determined that the main trait affecting fruit yield is stamen bundle outer width, while the secondary trait affecting fruit yield is stigma width. However, the efficacy of the stigma width may also be affected by the position of the stigma relative to the anther and the stamen bundle inner width. These two traits have the potential to be used as reference indicators for early selection in future breeding programs.
1. Background
Tea is among the most widely consumed beverages globally, making it a vital cash crop in Taiwan. Taiwan has high tea genetic resources and diversity [1], and different varieties of tea plants have been grown in Taiwan for more than 200 years [2,3]. The tea cultivation area has reached 12,266 hectares and 14,341 metric tons of tea are produced annually [4]. Many studies have focused on the detection of tea genetic resources in Taiwan [5,6,7,8,9,10,11]. Tea plants are usually maintained in a state of vegetative growth due to commercial picking [12]; however, tea can also be used as an oil crop. The three species of Camellia plants used for oil extraction in Taiwan are C. oleifera, C. brevistyla (or C. tenuifolia), and tea (C. sinensis) [13,14]. The former two were previously used for seedling production to produce seeds, but most of the seed yield per unit area comes from a few individual plants. Therefore, in this line of study, the characteristics of the flowers that cause this phenomenon were first explored, and it was found that several of them present significant correlations. Due to the fact that some of Taiwan’s tea trees have excellent fruiting abilities and abundant yields, two lines of thought have developed: one is to develop varieties for the production of tea oil, while the other is to develop varieties with low fruiting capacity for harvesting tea. Therefore, in this study, we further examine the correlation between flower traits and fruiting ability with the goal of identifying early selection indicators for these two goals, thus serving to shorten the breeding period of tea plants.
When tea flowers bloom, four progressive development stages (S1 to S4) have been identified, based on the morphology of the tea flower: S1, balloon stage; S2, petals half open; S3, flowers soon after anthesis, petals open, stamens colored bright yellow; and S4, day after anthesis, stamens colored brown [15]. In different tea genotypes, anthers may be at the same position as the stigma, or higher or lower [15,16]. There have been few studies on the relationship between flower morphology and pollination in tea plants. In almond (Prunus amygdalus), also classified as a self-incompatible plant along with tea, some scholars have studied the influence of flower morphology on pollination. In some almond varieties, the effect of the style length on pollen tube growth and the stigma versus anther position have been found to determine the possibility of natural selfing [17]. The fruit yields of almond varieties differ considerably after self-pollination, where this difference may be caused by partial self-incompatibility [18]. In almond breeding, self-compatible germplasms are mainly screened. The relative position of the stigma and anthers determines the possibility of natural selfing in some almond varieties, and pollinators are not required due to the natural selfing of plants in some self-compatible almond varieties [19].
Most tea plants are self-incompatible [20]. Tea plants, including plants from the genus Camellia, typically exhibit late-acting self-incompatibility (LSI) [21,22,23,24,25,26]. Fluorescence microscopy has revealed blockages of the pollen tubes in self-pollinated tea plants [27]. Due to LSI, the pollen tubes of self-pollinated plants fail to fertilize and have trouble entering the ovule [21]. LSI hinders the pollen tube, which is present at the base of the style, ovary, or ovule [24,25]. Transcriptome analysis of the style after selfing and out-crossing has indicated that the LSI of tea plants may be controlled by gametophyte genes [26,28].
Self-incompatibility is the key to preventing inbreeding-induced decline and maintaining genetic diversity; however, the tendency to undergo selfing may itself be a driver of speciation, and this hypothesis should be investigated in future studies on diversity and speciation [29]. In the case of the manual pollination of tea plants, the success rate of artificial cross-hybridization ranges from 4.6 to 26% [16,30]. Some tea varieties exhibit a self-pollination rate of up to 20% [31]. A previous study has used SSR markers to confirm selfing, indicating that the variety “Ziyan” is self-compatible [32].
Pollen viability is very important for successful pollination, where in vitro pollen germination testing can be used to determine the germination rate of pollen [33]. Pollen germination ability has been correlated with temperature [34] and the concentration of certain ions, such as Ca2+, H+, and so on [35,36]. In practical agricultural applications, chemical staining can be used to distinguish between viable pollen grains [37]. Triphenyl tetrazolium chloride (TTC) can stain the cells of many plants [38], and a TTC staining test can be conducted to determine the viability of plant pollen, as the results of the TTC test are close to the actual germination rate [39,40].
In a natural environment, many types of insects visit and pollinate flowers in tea gardens [41]; however, other research has rarely considered whether insects are affected by flower shape traits when they come into contact with tea flowers. In southern China, wild bees and flies aid in the pollination of Camellia plants. The activity of visiting insects peaks between 10:00 and 14:00. In a previous study, insect cross-pollination significantly improved the fruit yields of three Camellia species; namely, C. osmantha, C. vietnamensis, and C. oleifera [42].
Tea fruit contains two seeds on average and matures between eight and nine months after pollination. Periods of more than one month and four months are required for the development of the zygote and embryo of tea plants, respectively [15]. Tea seeds have different sizes [43]. Oil is extracted from tea seeds in many parts of the world, and tea seed oil is primarily composed of unsaturated fatty acids—mainly oleic acid—and other fatty acids in small proportions [44]; as such, tea seed oil may be a potential source of natural antioxidants [45,46]. The oil content of tea seeds ranges from 16 to 25% [45,47].
The tea fruit yield directly affects the yield of the seeds, and the yield varies greatly between tea varieties. Furthermore, the shape of the tea flowers may vary, depending on the variety. To address whether flower morphology affects the reproductive growth of tea plants, we examined the relationship between flower morphology and fruit yields to identify early breeding selection indicators.
2. Methods
2.1. Plant Materials
A total of 106 tea varieties, with 20 individual plants for each variety, were examined for this study. These plants were planted with a spacing of 50 and 180 cm between plants and rows, respectively, in 1994, in the germplasm garden (at an altitude, longitude, and latitude of 200 m, 121.186, and 24.909, respectively) established by the Tea Research and Extension Station in Taiwan, with the exception of TTES Nos. 19–20 (which were planted in 2004) and TTES No. 21 (which was planted in 2008). The plants were planted following the same cultivation method, which involved watering once a week and fertilizing every March with 3000 kg/ha of organic fertilizer (produced by Lv Lin Biotechnology Co., Ltd., Changhua, Taiwan) with an N:P:K ratio of 5:2:2. The location of pollen collection for tea varieties was the Taitung branch of TRES (at an altitude, longitude, and latitude of 175 m, 121.130, and 22.908, respectively).
2.2. Flower Morphology
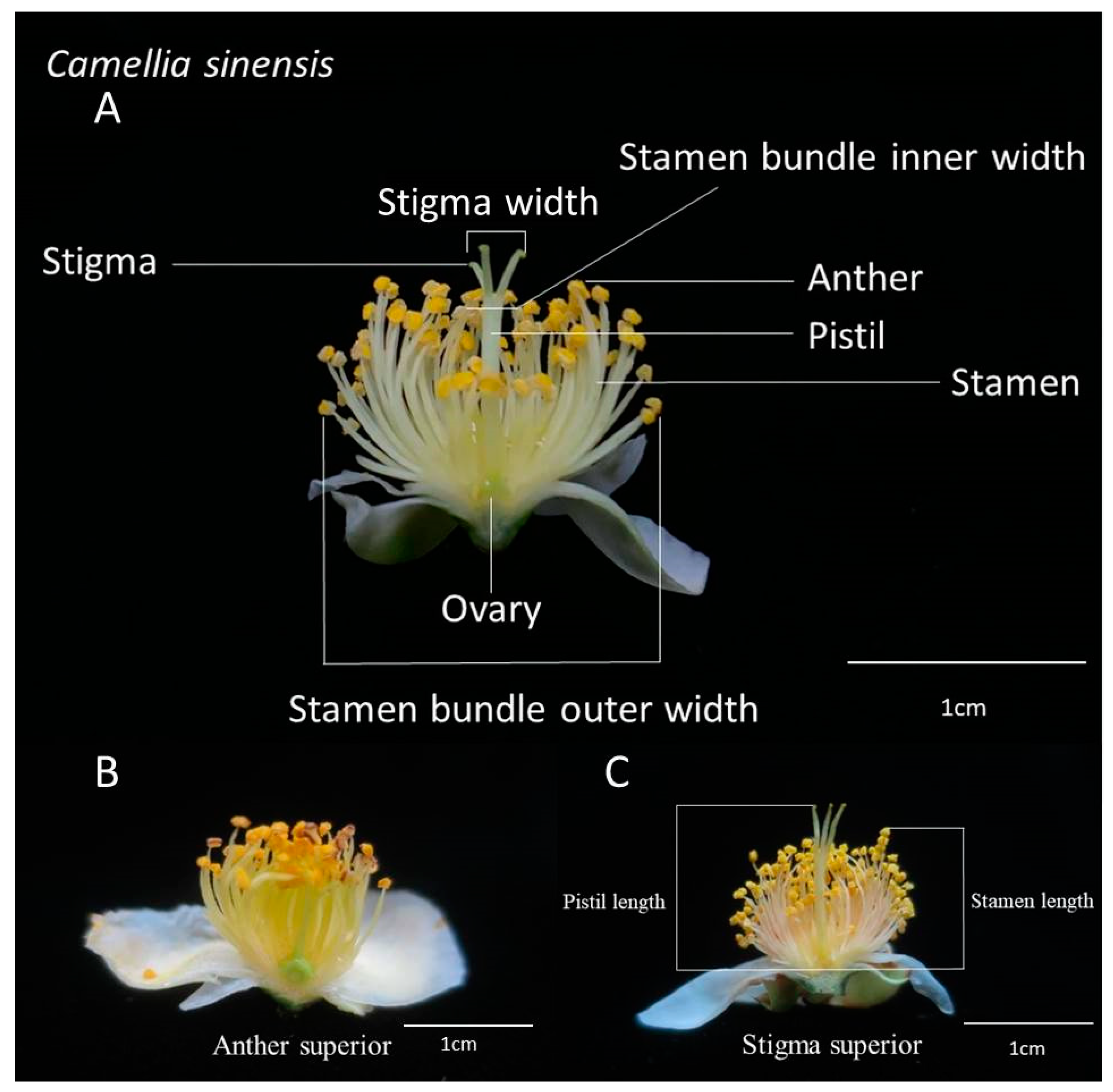
In November 2019, a total of 10 fresh and fully open flowers of each tea variety were collected every morning. The flower picking period covered when flowers had just fully opened and they were picked at the opportune moment. According to the study of Ariyarathna et al. [17], the flowers we picked belonged to stage S3. Within 2 weeks, 106 varieties of flowers were collected every morning, sealed in plastic bags, and taken to be measured indoors immediately. After collection, the characteristics of the tea flowers were determined indoors using a cursor ruler. These characteristics included the length of the pistil (the highest point), the length of the stamen (the highest point), the inner and outer widths of the stamen bundle, and the width of the style. We classified the varieties in which the length of the pistil was shorter than that of the stamen into the anther superior group, and those in which the length of the pistil was longer than or equal to that of the stamen into the stigma superior group (Table 1). Figure 1 presents a morphological diagram of the floral apparatus of the tea plants used in this study.

Table 1.
Intraclass correlation coefficients (ICCs) of flowers and the anther versus stigma position of the tea varieties.
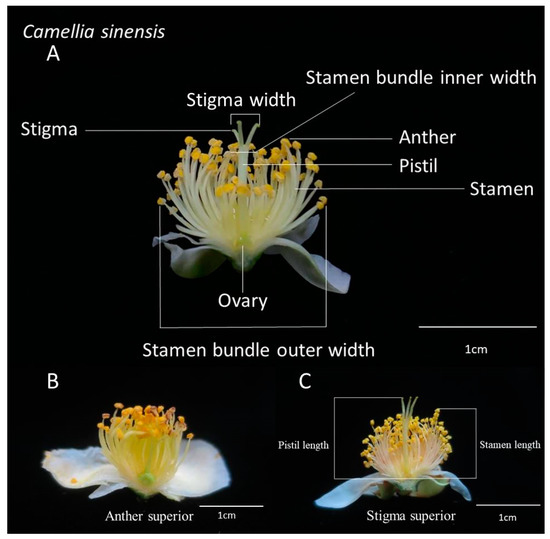
Figure 1.
Morphological diagram of the tea (C. sinensis) plant’s floral apparatus. (A) The measured flower organ; (B) the anther superior morphology; and (C) the stigma superior morphology. The positions for measurement of pistil length and stamen length are shown.
2.3. Pollen Viability Test of Tea Varieties
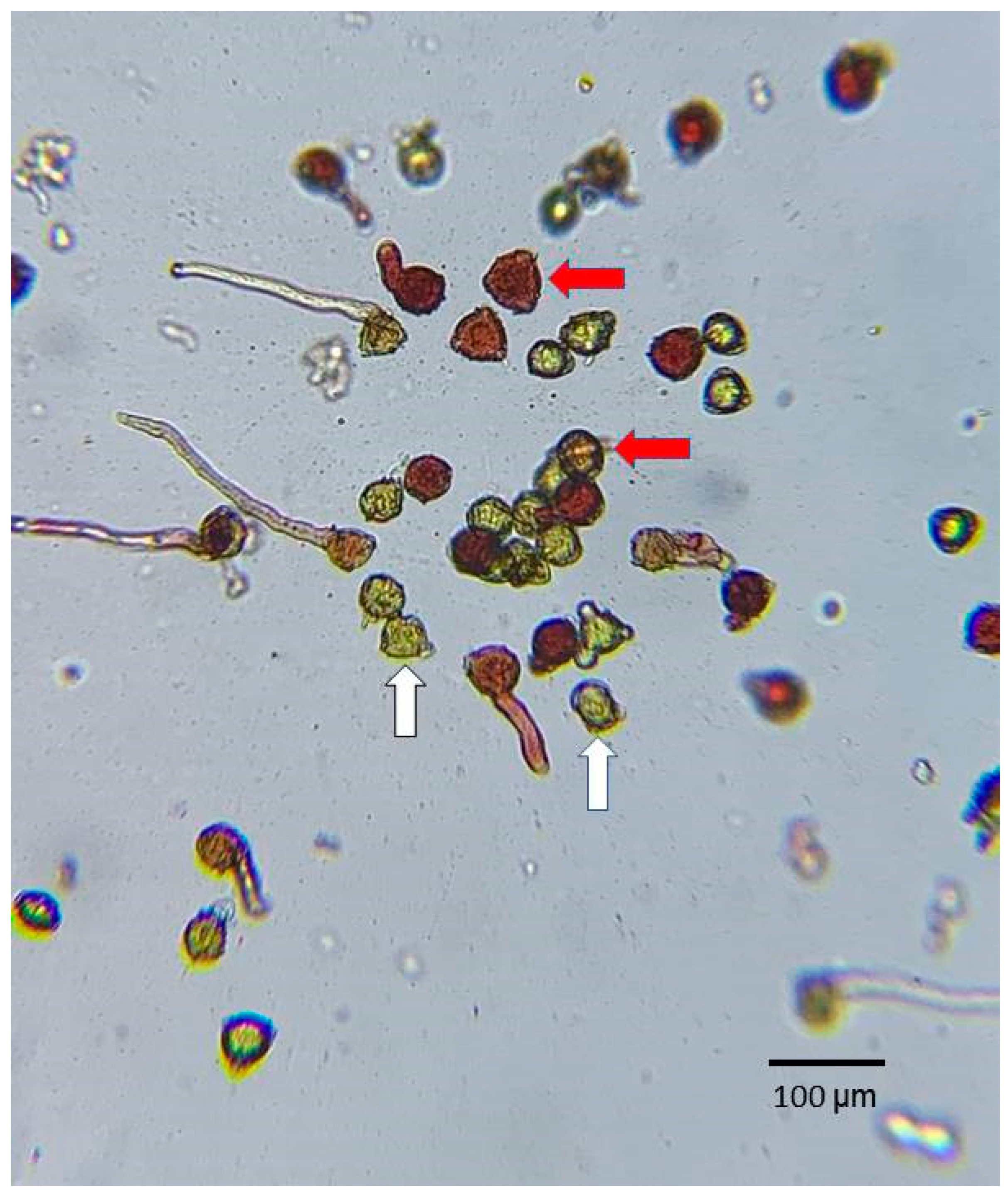
Pollen viability is highly important for fruiting. When plants are pollinated by insects or self-pollinated, the viability of the pollen affects the outcome of the pollination. To understand the viability of tea pollens in different varieties, pollen was obtained from the 75 tea varieties at the Taitung branch of TRES. The flowers used for the test were obtained between 8:00 and 10:00 a.m. On the same day, the pollen from three opened flowers was mixed and shaken onto a microscope slide for observation. The staining solution used in this experiment was 100 g L−1 sucrose + 5 g L−1 2,3,5-triphenyl tetrazolium chloride (TTC), and the prepared solution was secondary water with an adjusted pH of 5.5 (secondary water is filtered once more than deionized water; as this water is close to neutral, we adjusted the pH to 5.5 using citric acid). The TTC staining solution was pipetted onto the microscope slide containing the tea pollen, then covered with a cover slip. After 4 h, the pollen staining was observed (Microtech D1500) at a magnification of 100× and photographed with camera equipment through the eyepiece by randomly moving the slide to obtain five different views of the pollen. After the test, the image was displayed on a computer screen, and the total number of pollen grains and the number of stained pollen grains were counted manually. In the TTC staining test, pollen grains that turn red or pink are considered viable. The pollen viability for each view of the pollen was calculated according to the count of (stained pollens/total pollens) × 100% for each of the five views.
2.4. Fruit Yield
In mid-October of 2013 and 2015, we collected and weighed fresh fruits immediately from 20 trees of a single variety, and then calculated the average fruit yield per tree. In 2012, the plants were pruned (medium) to a height of 70 cm in late January, and the shoots were kept in cultivation to ensure that the flower buds in 2012 fully bloomed, retaining the fruit yield for harvest in 2013. In order to ensure consistency over the two years of investigations, in 2014, the plants were pruned again to the same height as in 2012. Therefore, we do not have data for the 2014 yield, instead retaining the fruit yield for harvest in 2015.
2.5. Statistical Analysis
Continuous data, including yield and phenotypic traits, are presented as the median and interquartile range (IQR). Yield and phenotypic traits between the two groups of “pistil length shorter than stamen length” and “pistil length longer than stamen length” were compared using the non-parametric Mann–Whitney test. The non-parametric statistical hypothesis test was considered preferable due to the skewed yield distribution and the small sample size in the anther superior group, having pistil length < stamen length (N = 16).
Correlations between yield and phenotypic traits were evaluated by determining Spearman’s correlation coefficients (Spearman’s ρ, a non-parametric measure of rank correlation). Logistic linear regression models were used to determine the effects of phenotypic traits on yield (likelihood of high yield greater than the median). Additionally, the phenotypic trait variables with p-values of <0.2 in the univariate models were included in the model selection process. Then, the final multivariate model was determined by the conditional backward method. The corresponding odds ratios for the likelihood of higher yield in logistic regression models and weighting coefficients in general linear models with 95% confidence intervals were summarized and tabulated. A two-sided p-value less than 0.05 was considered statistically significant. All statistical analyses were performed using the IBM SPSS Statistics 25.0 software (IBM Corporation, Armonk, New York, NY, USA).
3. Results
3.1. Evaluation for the Homogeneity of Phenotypic Traits within Species
The homogeneity of phenotypic traits within the varieties was evaluated by calculating the intraclass correlation coefficient (ICC). The Cronbach’s alpha model of internal consistency was used, based on the average inter-item correlation. The interpretation of the ICC was based on the guidelines reported by Koo and Li (2016) [48]; namely, ICC values of <0.5, 0.5–0.75, 0.75–0.9, and >0.9 indicate poor, moderate, good, and excellent homogeneity, respectively.
Table 1 presents the homogeneity of phenotypic traits within the varieties, based on the ICC values. The ICCs of all varieties were >0.9, indicating the presence of excellent homogeneity within each set of 10 flowers for the 106 varieties of C. sinensis. Thus, the averaged phenotypic traits were directly used in subsequent statistical analyses.
3.2. Differences in Phenotypic Traits between Groups
In this study, the average length and width measurements of certain phenotypic traits of tea plants, including pistil length, stamen length, stamen bundle inner width, stamen bundle outer width, and stigma width, were 11.8, 10.9, 2.5, 15.0, 3.7 mm, respectively. The stamen length in the anther superior group was comparable to that in the stigma superior group (median value: 10.7 vs. 10.9 mm). Furthermore, the pistil length and stigma width in the anther superior group were significantly shorter and lower than those in the stigma superior group, respectively (median value: 10.1 vs. 12.2 mm and 3.0 vs. 4.0 mm, respectively; p < 0.001). In addition, the anther superior group had a significantly lower stigma width minus stamen bundle inner width than the stigma superior group (median values: 0.6 vs. 1.4 mm; p = 0.023); see Table 2.

Table 2.
Comparison of phenotypic traits between the anther and stigma superior groups.
3.3. Pollen Viability
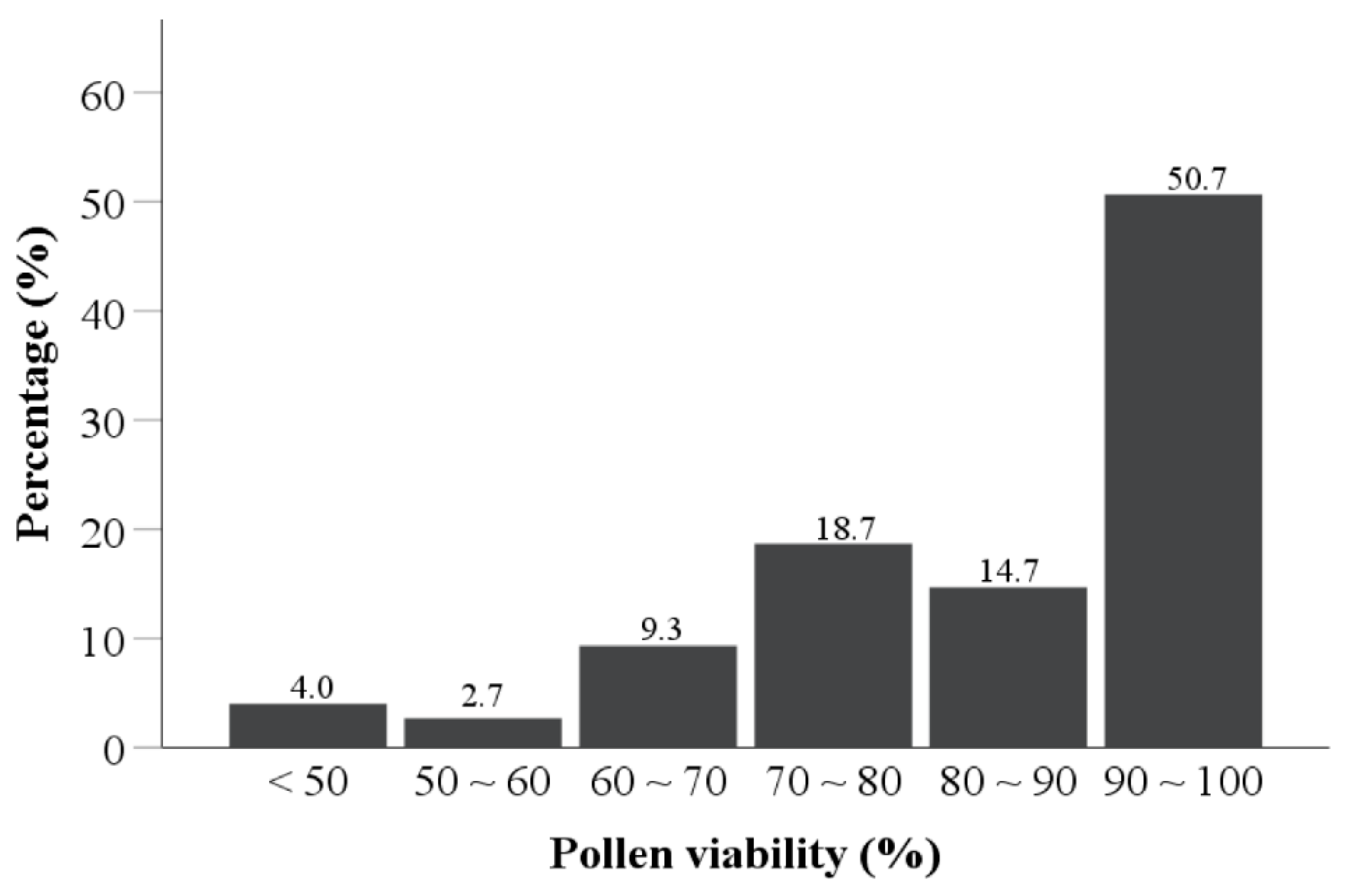
The pollen viability of 75 tea varieties was evaluated according to the average count of (stained pollens/total pollens) × 100% for the five views in the TTC staining test, where pollen grains that turned red or pink in the test were considered to be viable (Figure 2). About half of the tea cultivars had pollen viability over 90% (50.7%), one-third (33.3%) of the tea cultivars had pollen viability in the range of 70–90%, and 12% of the tea cultivars had pollen viability in the range of 50–70%. Additionally, only three (4.0%) of the tea cultivars had pollen viability less than 50% (Figure 3). This indicates that most of the tested tea pollen possessed the ability to germinate normally after contact with a cross-pollinated stigma.
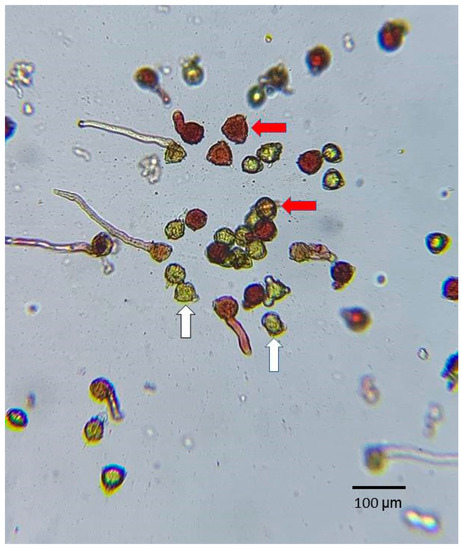
Figure 2.
TTC (2,3,5-triphenyl tetrazolium chloride) staining test of tea pollen. Pollen grains that turned red or pink (indicated by red arrows) were considered viable. Pollen grains lacking red color (indicated by white arrows) were classified as inactive. Magnification, 100×.
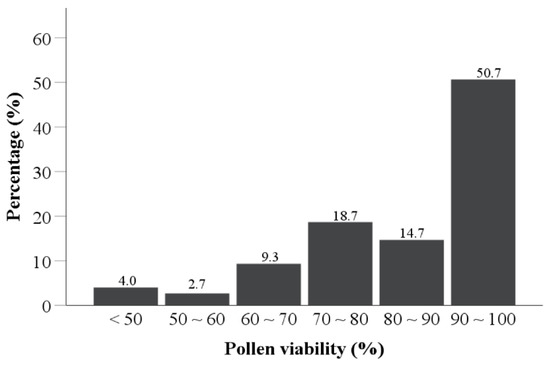
Figure 3.
Pollen viability distribution for 75 tea varieties.
3.4. Yield Distribution of Test Tea Varieties within Two Years
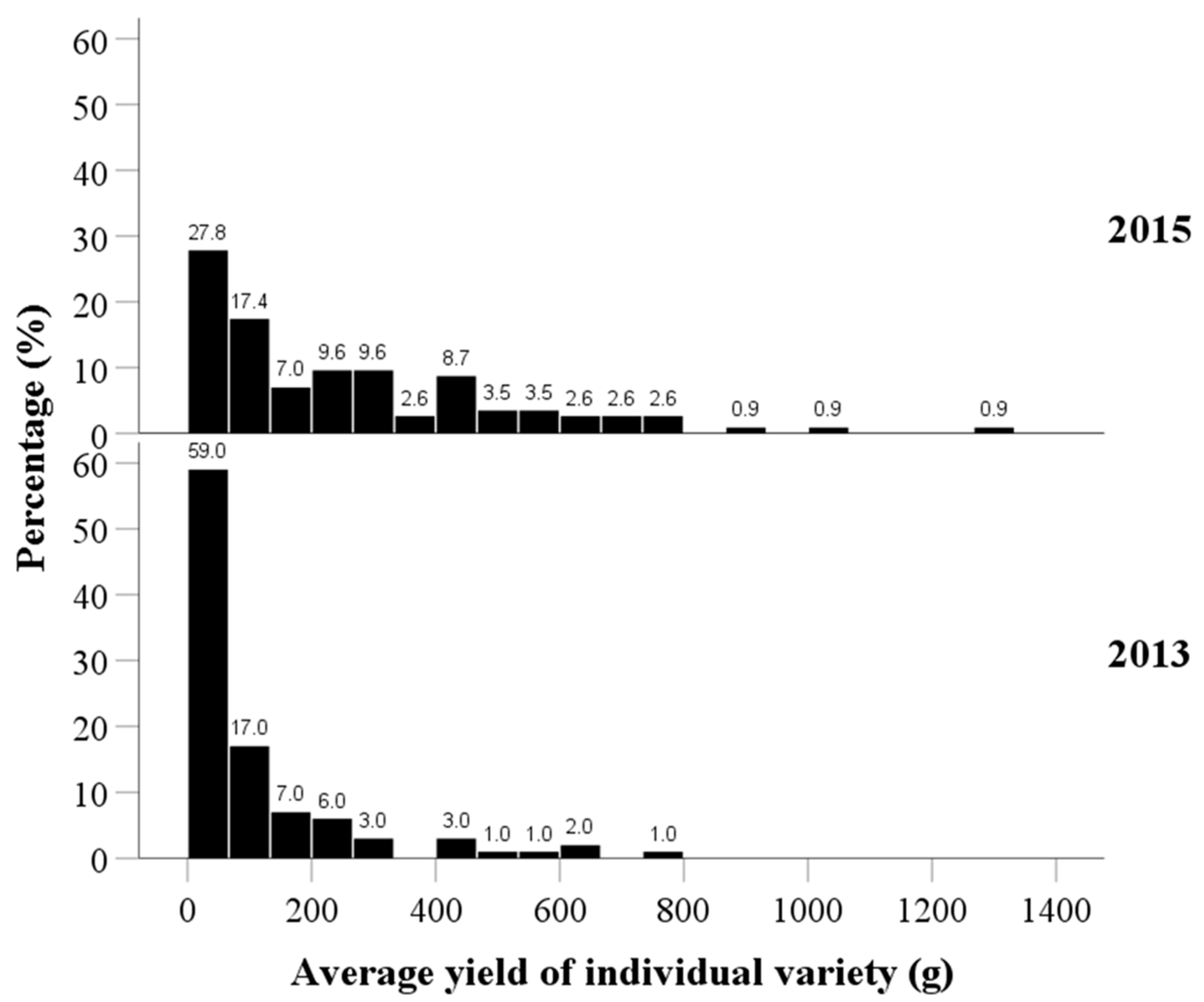
The average fruit yield per plant for each tea variety exhibited a positively skewed distribution (Figure 4). The findings indicated that over half of the plants had low yields (of 0–200 g), accounting for 83.0% (59.0 + 17.0 + 7.0 = 83.0%) and 52.2% (27.8 + 17.4 + 7.0 = 52.2%) in 2013 and 2015, respectively. The plants that had higher yields (over 400 g) accounted for 8.0% (3.0 + 1.0 + 1.0 + 2.0 + 1.0 = 8.0%) and 26.2% (8.7 + 3.5 + 3.5 + 2.6 + 2.6 + 2.6 + 0.9 + 0.9 + 0.9 = 26.2%) in 2013 and 2015, respectively. Additionally, the yields of individual plants in 2015 were significantly improved, when compared to those in 2013 (median of 39.4 g in 2013 vs. 175.0 g in 2015; p < 0.001).
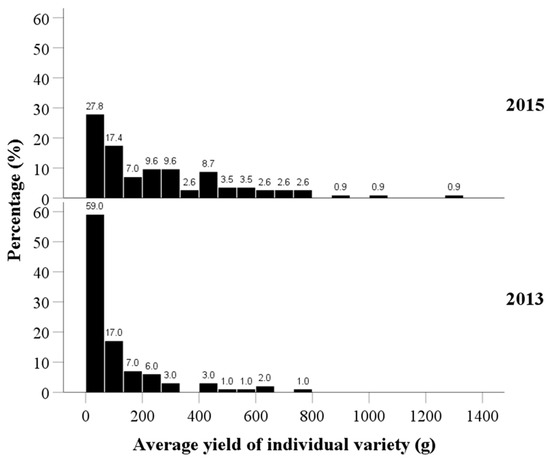
Figure 4.
Distribution of the average fruit yield per plant of tea varieties in 2013 and 2015.
3.5. Distribution of Fruit Yield between the Anther and Stigma Superior Groups
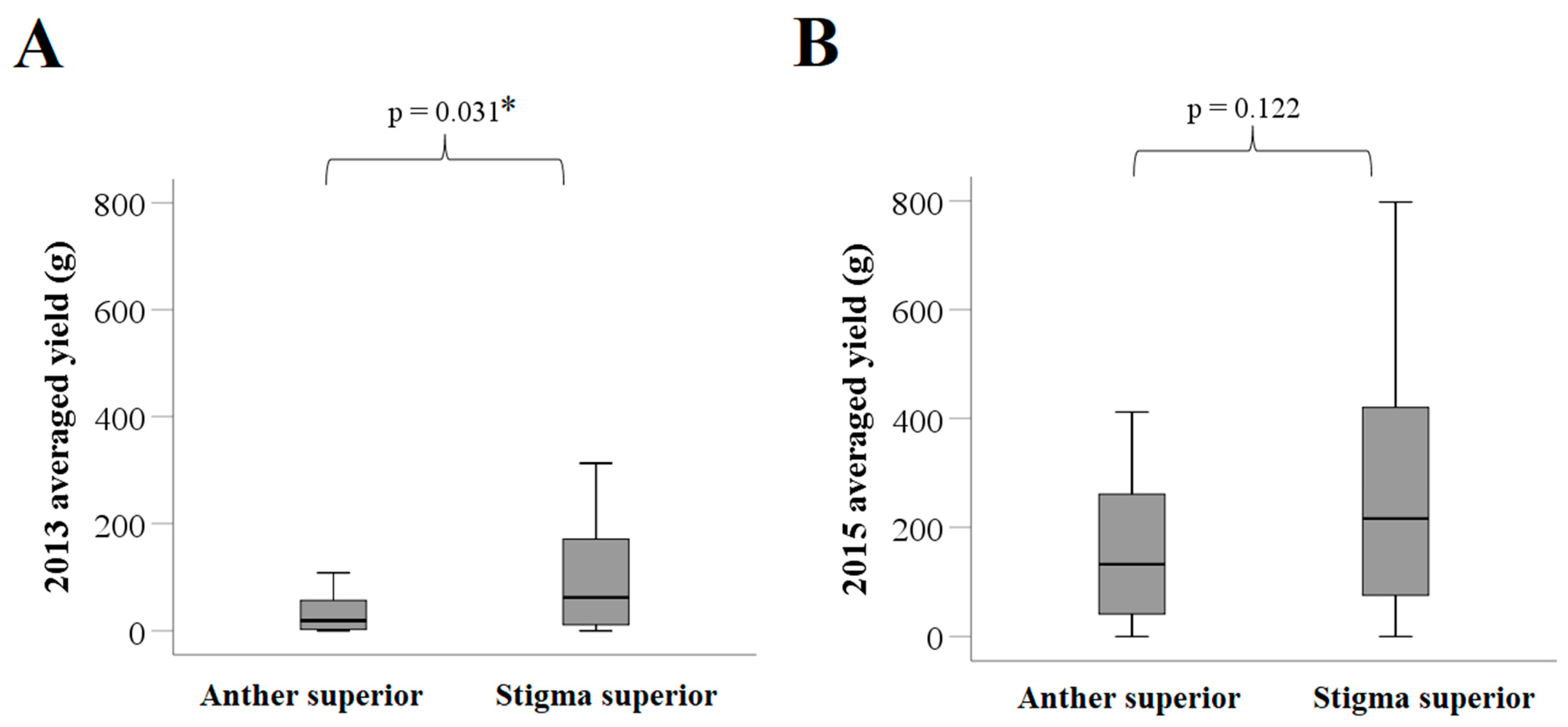
A total of 106 tea varieties (with 20 individual plants per variety) were examined in this study. Among the 106 varieties, the average length of the pistil was shorter than that of the stamen for 16 (15.1%) varieties, with a median difference of −0.3 mm. These 16 varieties formed the anther superior group. The remaining 90 (84.9%) varieties, with a median difference of 1.0 mm between the length of the pistil and that of the stamen, were included in the stigma superior group (Table 1). In 2013, the fruit yield of the anther superior group was significantly lower than that of the stigma superior group (median of 19.1 vs. 62.0 g; p = 0.031; Figure 5A). Notably, the same difference was not statistically significant in 2015 (median of 132.3 vs. 215.9 g; p = 0.122; Figure 5B).
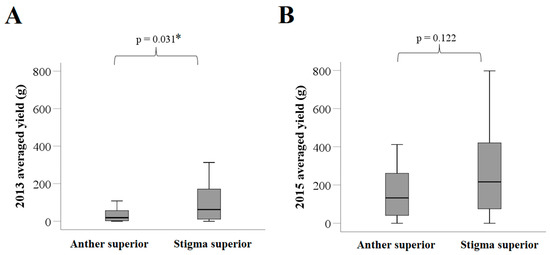
Figure 5.
Distribution of fruit yields between the anther and stigma superior groups of the tea varieties. (A) Fruit yield distribution in 2013; and (B) fruit yield distribution in 2015. The p-values in the figure were obtained based on the non-parametric Mann–Whitney test. * p < 0.05 indicates that the difference between the groups was statistically significant.
3.6. Monotonic Correlations between Phenotypic Traits and Yield
Next, we sought to understand the effect of tea plant flower traits on the average yield of tea plants at two years. As presented in Table 3, for the total group, yield was significantly negatively correlated with stamen length, stamen bundle outer width, and stamen bundle outer width minus stamen bundle inner width (Spearman’s ρ = −0.196, −0.243, and −0.254 and p = 0.044, 0.012, and 0.008, respectively). Meanwhile, yield was significantly positively correlated with the stigma width minus the stamen bundle inner width (Spearman’s ρ = 0.202; p = 0.038).

Table 3.
Correlations of yield versus phenotypic traits by group on the average yield of tea plants at two years.
Similar results were observed in the stigma superior group. Yield was negatively correlated with the stamen length, stamen bundle outer width, and stamen bundle outer width minus stamen bundle inner width (Spearman’s ρ = −0.228, −0.303, and −0.301 and p = 0.031, 0.004, and 0.004, respectively). Furthermore, a positive correlation was observed between yield and stigma width minus the stamen bundle inner width (Spearman’s ρ = 0.216, p = 0.041). In the anther superior group, yield was negatively correlated with the stigma width minus the stamen bundle inner width (Spearman’s ρ = −0.556, p = 0.025).
3.7. Effects of Phenotypic Traits on Yield in 2013 and 2015
Logistic regression models were chosen to assess the effects of phenotypic traits on yield. As shown in Table 4, the plants were classified into two groups according to the 2013 yield data: higher yield (yield greater than the median yield of 39.4 g in 2013) and lower yield (yield less than the median yield of 39.4 g in 2013). Each phenotypic trait data point was included in a univariate logistic regression model, in order to test its effects on the likelihood of a higher yield. Additionally, three phenotypic trait variables showed significant effects on yield. Higher stamen bundle outer width was correlated with a lower likelihood of a higher yield, with the odds of a higher yield decreasing with each mm increase in the stamen bundle outer width, with an odds ratio of 0.82 (p = 0.024). Higher stigma width was correlated with a greater likelihood of higher yield: the odds of a higher yield increased with each mm increase in stigma width, with an odds ratio of 1.47 (p = 0.021). A higher stigma width minus stamen bundle inner width was correlated with a greater likelihood of a higher yield: the odds of a higher yield increased with each mm increase in the difference between the stigma width and the stamen bundle inner width, with an odds ratio of 1.53 (p = 0.007). After the model selection process, two variables (stigma width and stamen bundle outer width) were included in the final multivariate model. The results of the final multivariate model were consistent with those of the univariate models: when controlling the stamen bundle outer width under the same conditions, the odds of a higher yield increased with each mm increase in the stigma width, with an odds ratio of 1.72 (p = 0.003). Furthermore, when controlling the stigma width under the same conditions, the odds of a higher yield decreased with each mm increase in the stamen bundle outer width, with an odds ratio of 0.74 (p = 0.005); see Table 4.

Table 4.
Effects of phenotypic traits on the yield of tea varieties in 2013.
As shown in Table 5, the plants were also classified into two groups according to the 2015 yield data: higher yield (yield greater than the median yield of 175.0 g) and lower yield (yield less than the median yield of 175.0 g). Each phenotypic trait datapoint was included in a univariate logistic regression model to test its effects on yield. Only one of the phenotypic trait variables had a significant effect on the yield: a higher stamen bundle outer width was correlated with a lower likelihood of a higher yield, as the odds of a higher yield decreased with each mm increase in the stamen bundle outer width, with an odds ratio of 0.81 (p = 0.013). After the model selection process, the two variables of stamen bundle outer width and the difference between the pistil length and the stamen length were included in the final multivariable model. The results indicated that the odds of a high yield decreased with each mm increase in the stamen bundle outer width, with an odds ratio of 0.78 (p = 0.006), when fixing the difference between the pistil length and stamen length. However, the effect of the difference between pistil length and stamen length on the likelihood of a higher yield was not statistically significant (OR = 1.55; p = 0.053); see Table 5.

Table 5.
Effects of phenotypic traits on the yield of tea varieties in 2015.
4. Discussion
The fruiting ability of a tea plant is obviously determined by the success rates of pollination and fertilization, which are influenced by the success of pollination and self-incompatibility. Therefore, the difference in fruiting ability among tea populations, with their own anther epistatic flowers, should derive from the strength of their self-incompatibility. This phenomenon has obtained sufficient evidence in a bagging test of C. oleifera and C. brevistyla (or C. tenuifolia) (currently in the process of publishing). Therefore, self-compatible tea varieties should be explored in the future, in order to facilitate tea plant breeding for tea seed oil.
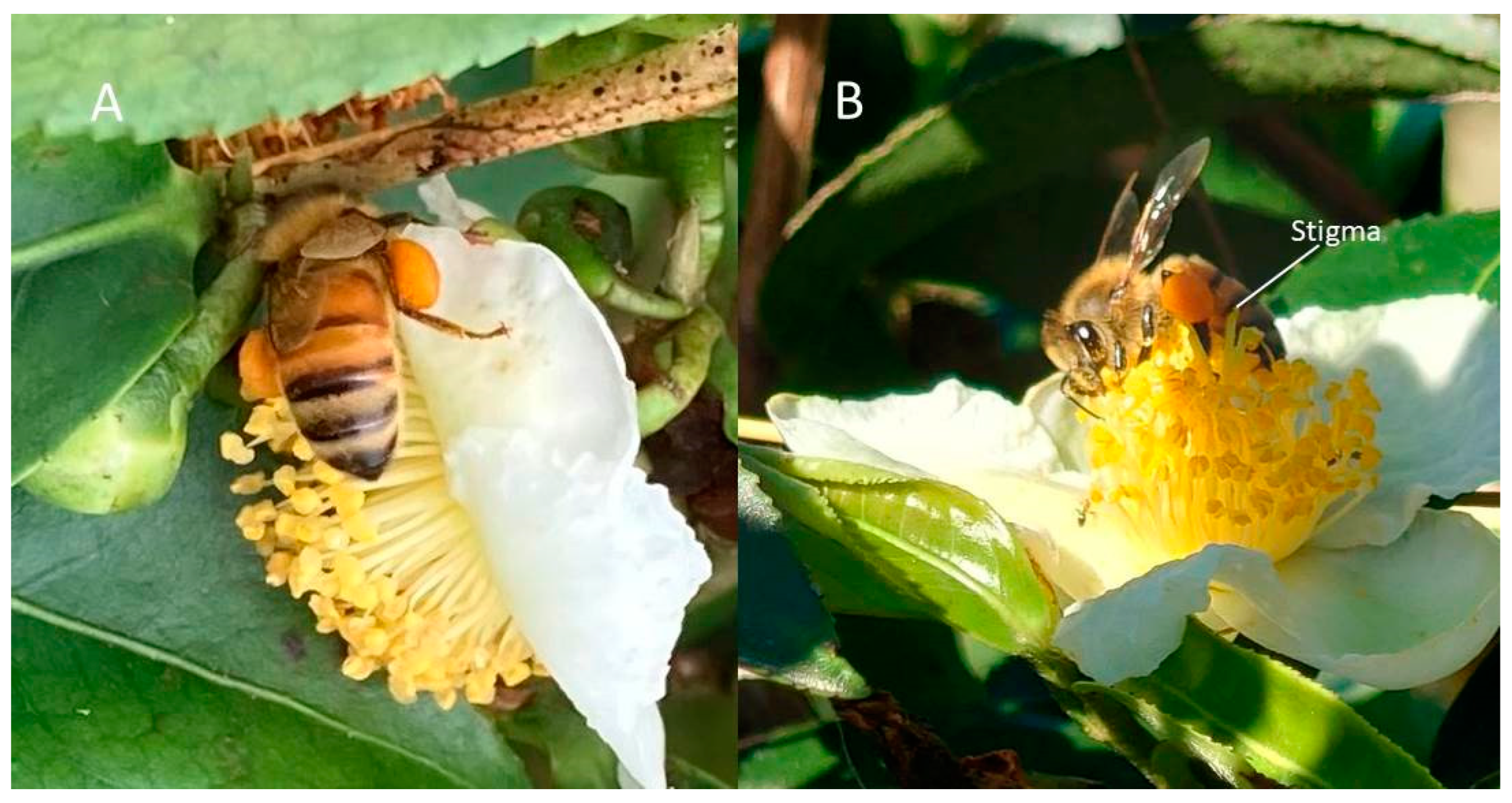
In our experiment, tea plant flower traits were assessed in order to explore their effects on fruit yields. We determined the intraclass correlation coefficients (ICC) of flowers, representing the consistency of tea flowers (Table 1). After determining the high homogeneity of flower traits in the study, many flower traits were related to the probability of pollination and, in fact, factors that are positively or negatively correlated seem to favor the stigma in obtaining pollen. From actual observations of the behavior of bees in the field, Figure 6A shows the case when the stigma is higher than the anther and opening, while Figure 6B shows the case when the stigma is lower than the anther. In Figure 6B, when the bees collect pollen, they are less likely to contact the stigma, as it is hidden under the anther. In Figure 6A, when the stigma is higher than the anther and has a larger width, the pollen carried by bees will more easily come into contact with the stigma. This point can be validated by looking at the obtained statistics: the fruit yield was higher with a larger width of stigma (Table 4). The results indicated that there exists a significant negative correlation between stamen bundle outer width and higher yield (Table 3, Table 4 and Table 5). It seems that bees collecting from flowers with a smaller stamen bundle outer width are more likely to touch the stigma in the middle of the flower, resulting in an increased pollination rate.
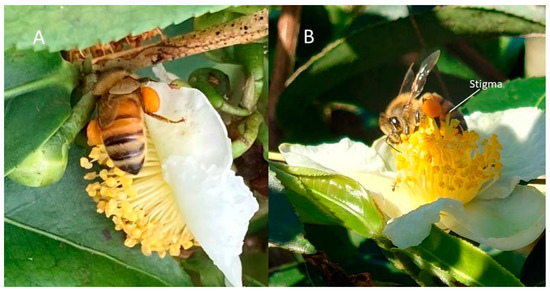
Figure 6.
Bee pollination. (A) Anther superior flower; and (B) stigma superior flower of C. sinensis.
It can be seen, from Table 3, that the effect of stigma width minus stamen bundle inner width on fruit yield reached a statistically significant level in all three groups; however, the anther superior group and stigma superior group presented completely opposite results. When the stigma was hidden under the anther, the wider the width of the stigma, the lower the yield; presumably due to the lower ease of contact for pollinating bees. When the stigma was above the anthers, the wider the stigma, the easier it is to contact the pollen carried by bees.
The difference in tea tree fruit yields between 2013 and 2015 is presumed to be due to the moderate pruning in 2012 and 2014 (the years before the yield survey), in order to homogenize the experiment. The difference may be due to spring pruning being conducted in early 2014, allowing for better shoot growth, which may have led to the yield difference between the two years. From Figure 4, it can be seen that the fruit yield of most tea tree varieties was not high, which may be due to the fact that tea tree varieties were selected to produce leaves rather than fruits in the past, resulting in a positively skewed yield distribution. The fruit yield measurements were taken in 2013 and 2015. If possible, in the future, using the flower traits from the previous year may lead to better results.
5. Conclusions
The results of this study indicated that the flowers collected from a single variety of tea plant typically present a high degree of homogeneity. In the described experiment, most tea varieties had higher stigmas than anthers, and most tea varieties presented pollen vitality exceeding 70%. In terms of the correlation between flower traits and yield, although we assumed that the relative position of the anthers and stigmas would affect fruit yield, the comparative results were not significant. From the results of the experiments on flower traits that significantly affect fruit yield, it can be speculated that different anther and stamen traits affect the difficulty of insects (e.g., bees) with respect to contacting the stigma, which affects the pollination results and, consequently, fruit yield. The main factor influencing flower traits is the stamen bundle outer width (negative), while the secondary influencing trait is the stigma width. However, the efficacy of the stigma width may be affected by the position of the stigma relative to the anther and the stamen bundle inner width as well. In the future, these flower traits can be used as early indicators for reference in breeding programs.
Author Contributions
S.-K.L., I.-Z.C. and S.-F.R. designed the study; S.-K.L. and C.-Y.H. performed the experiments; S.-K.L. wrote the manuscript; T.-C.S. provided the plant materials and provided recommendations for experiments. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported in part by Tea Research and Extension Station, Council of Agriculture, Executive Yuan in Taiwan. The project number was 111AS-4.1.7-MS-M1.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The plant materials were provided by the Tea Research and Extension Station, Council of Agriculture, Executive Yuan (TRES).
Acknowledgments
The authors thank the Tea Research and Extension Station (TRES) for providing the testing equipment.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| LSI | Late-acting self-incompatibility |
| ICC | Intraclass correlation coefficient |
| IQR | Interquartile range |
References
- Taniguchi, F.; Kimura, K.; Saba, T.; Ogino, A.; Yamaguchi, S.; Tanaka, J. Worldwide core collections of tea (Camellia sinensis) based on SSR markers. Tree Genet. Genomes 2014, 10, 1555–1565. [Google Scholar] [CrossRef]
- Chiu, T.F. Tea production and research in Taiwan. In Recent Development in Tea Production; Chiu, T.F., Wang, C.H., Eds.; Taiwan Tea Experiment Station: Taoyuan, Taiwan, 1988; pp. 121–129. [Google Scholar]
- Jun, I.M.; Lin, M.L. Present status of tea industry in Taiwan. Taiwan Tea Res. Bull. 1997, 16, 87–97. [Google Scholar]
- Council of Agriculture. Agricultural Statistics Yearbook in 2020; Council of Agriculture, Executive Yuan: Taipei, Taiwan, 2021; p. 40. [Google Scholar]
- Hu, C.Y. Studies on the Variations in Leaf Characters and DNA Sequences of Tea Germplasm in Taiwan. Master’s Thesis, National Taiwan University, Taipei, Taiwan, 2004. [Google Scholar]
- Hu, C.Y.; Tsai, Y.Z.; Lin, S.F. Using ISSR DNA markers to evaluate genetic diversity of tea germplasm in Taiwan. J. Agric. Assoc. China 2005, 6, 463–480. [Google Scholar]
- Hu, C.Y.; Tsai, Y.Z.; Lin, S.F. Evaluating the feasibility of molecular identification for made tea varieties. J. Agric. Assoc. China 2006, 7, 499–510. [Google Scholar]
- Hu, C.Y.; Lin, Y.C.; Hsieh, W.T.; Tseng, Y.H.; Lin, S.F.; Tsai, Y.Z. Using EST-SSR markers to identify tea (Camellia sinensis) cultivars in Taiwan. Taiwan Tea Res. Bull. 2011, 30, 9–22. [Google Scholar]
- Sanui, H. Tea breeding in Taiwan. In Tea Breeding in Taiwan during Japanese Occupation Period; Shyu, Y.S., Ed.; Tea Research and Extension Station: Taoyaun, Taiwan, 2011; pp. 4–51. [Google Scholar]
- Shyu, Y.S.; Juan, I.M. Retrospect of tea breeding in Taiwan. Taiwan Tea Res. Bull. 1993, 12, 1–17. [Google Scholar]
- Tsai, H.T.; Tsai, I.C.; Liaw, W.R.; Chang, C.K.; Wang, Y.W. Study on the genetic diversity among the selected Taiwan tea cultivars/lines using AFLP and RAPD markers. Taiwan Tea Res. Bull. 2003, 22, 17–32. [Google Scholar]
- Piyasundara, J.H.N.; Wickramasinghe, I.P.; Gunesekara, M.T.K.; Wijeratne, M.A.; Perera, S.A.C.N.; Ranathunga, M.A.B.; Mudalige, A.K. Reproductive phenology of tea (Camellia sinensis (L.) O. Kuntze) cultivars in Sri Lanka. Trop. Agric. Res. 2018, 29, 288–301. [Google Scholar] [CrossRef]
- Su, M.H.; Shih, M.C.; Lin, K.H. Chemical composition of seed oils in native Taiwanese Camellia species. Food Chem. 2014, 156, 369–373. [Google Scholar] [CrossRef]
- Hieh, W.M. The industry development process of Camellia oil in Taiwan. J. Agric. For. 2020, 67, 223–232. Available online: https://canr.nchu.edu.tw/upload/files/jaf/67-4-1%E8%87%BA%E7%81%A3%E8%8C%B6%E6%B2%B9%E7%94%A2%E6%A5%AD%E7%99%BC%E5%B1%95%E6%AD%B7%E7%A8%8B.pdf (accessed on 1 July 2022).
- Ariyarathna, H.A.C.K.; Gunasekare, M.T.K.; Kottawa-Arachchige, J.D.; Paskarathevan, R.; Ranaweera, K.K.; Ratnayake, M.; Kumara, J.B.D.A.P. Morpho-physiological and phenological attributes of reproductive biology of tea (Camellia sinensis (L.) O. Kuntze) in Sri Lanka. Euphytica 2011, 181, 203–215. [Google Scholar] [CrossRef]
- Ariyarathna, C.; Kottawa-Arachchi, J.; Gunasekare, M. Floral biology and breeding system of tea [Camellia sinensis L.]: Implication on the tea breeding programme. SLJ Tea Sci. 2007, 72, 31–43. Available online: https://www.researchgate.net/publication/268819279_Floral_Biology_and_Breeding_system_of_Tea_Camellia_sinensis_L_Implication_on_the_Tea_Breeding_Programme (accessed on 1 July 2022).
- Kodad, O.; Socias i Company R. Floral characterization of some self-compatible almond selections. In XIII GREMPA Meeting on Almonds and Pistachios; Options Méditerranéennes: Série A. Séminaires Méditerranéens; Oliveira, M.M., Cordeiro, V., Eds.; CIHEAM: Zaragoza, Spain, 2005; pp. 161–166. [Google Scholar]
- MartÍNez-GarcÍAP, J.; Ortega, E.; Dicenta, F. Analysis of the expression of partial self-incompatibility in almond (Prunus dulcis). J. Hortic. Sci. Biotechnol. 2011, 86, 284–290. [Google Scholar] [CrossRef]
- Bernad, D.; R. Socias i Company. Characterization of some self-compatible almonds. II. Flower phenology and morphology. HortScience 1995, 30, 321–324. [Google Scholar] [CrossRef]
- Barua, P.K. Classification of the tea plant. Two Bud 1963, 10, 3–11. [Google Scholar]
- Chen, X.; Hao, S.; Wang, L.; Fang, W.; Wang, Y.; Li, X. Late-acting self-incompatibility in tea plant (Camellia sinensis). Biologia 2012, 67, 347–351. [Google Scholar] [CrossRef]
- Kumarihami, H.M.P.C.; Eun, U.O.; Atsushi, N.; Kwan, J.S. Comparative study on cross-compatibility between Camellia sinensis var. sinensis (China type) and C. sinensis var. assamica (Assam type) tea. Afr. J. Agric. Res. 2016, 11, 1092–1101. [Google Scholar] [CrossRef]
- Liao, T.; Yuan, D.Y.; Zou, F.; Gao, C.; Yang, Y.; Zhang, L.; Tan, X.F. Self-sterility in Camellia oleifera may be due to the prezygotic late-acting self-incompatibility. PLoS ONE 2014, 9, e99639. [Google Scholar] [CrossRef]
- Yang, M.J. Studies on the Self-Incompatibility of Tea. Master’s Thesis, National Taiwan University, Taipei, Taiwan, 1998. [Google Scholar]
- Yang, M.J.; Chen, Y.Z. Observation of the self-incompatibility phenomenon of tea. J. Chin. Soc. Hortic. Sci. 1998, 46, 83–92. [Google Scholar]
- Zhang, C.C.; Wang, L.Y.; Wei, K.; Wu, L.Y.; Li, H.L.; Zhang, F.; Cheng, H.; Ni, D.J. Transcriptome analysis reveals self-incompatibility in the tea plant (Camellia sinensis) might be under gametophytic control. BMC Genom. 2016, 17, 359. [Google Scholar] [CrossRef]
- Seth, R.; Bhandawat, A.; Parmar, R.; Singh, P.; Kumar, S.; Sharma, R.K. Global transcriptional insights of pollen-pistil interactions commencing self-incompatibility and fertilization in tea [Camellia sinensis (L.) O. Kuntze]. Int. J. Mol. Sci. 2019, 20, 539. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q.; Chen, C.; Zeng, Z.; Zou, Z.; Li, H.; Zhou, Q.; Chen, X.; Sun, K.; Li, X. Transcriptomic analysis between self- and cross-pollinated pistils of tea plants (Camellia sinensis). BMC Genom. 2018, 19, 289. [Google Scholar] [CrossRef] [PubMed]
- Wright, S.I.; Kalisz, S.; Slotte, T. Evolutionary consequences of self-fertilization in plants. Proc. R. Soc. B Biol. Sci. 2013, 280, 20130133. [Google Scholar] [CrossRef] [PubMed]
- Kottawa-Arachchi, J.D.; Ranatunga, M.A.B.; Ranaweera, K.K. Recent progress of intra-specific hybridization of tea (Camellia sinensis (L.) O. Kuntze) in Sri Lanka. SLJ Food Agric. 2019, 5, 19–26. [Google Scholar] [CrossRef][Green Version]
- Wachira, F.N.; Kamunya, S.K. Pseudo-self-incompatibility in some tea clones (Camellia sinensis (L.) O. Kuntze). J. Hortic. Sci. Biotechnol. 2005, 80, 716–720. [Google Scholar] [CrossRef]
- Tan, L.Q.; Liu, Q.L.; Zhou, B.; Yang, C.J.; Zou, X.; Yu, Y.Y.; Wang, Y.; Hu, J.H.; Zou, Y.; Chen, S.X.; et al. Paternity analysis using SSR markers reveals that the anthocyanin-rich tea cultivar ‘Ziyan’ is self-compatible. Sci. Hortic. 2019, 245, 258–262. [Google Scholar] [CrossRef]
- Shivanna, R.K.; Mohan Ram, Y.H. Pollination biology: Contributions to fundamental and applied aspects. Current Science. 1993, 65, 226–233. Available online: https://core.ac.uk/download/pdf/291544001.pdf (accessed on 1 July 2022).
- Muoki, C.; Wachira, F.; Pathak, R.; Kamunya, S. Potential male gametophyte competition among Camellia sinensis genotypes in isolated biclonal seed orchards. Afr. Crop Sci. J. 2010, 15, 59–66. [Google Scholar] [CrossRef][Green Version]
- Brewbaker, J.L.; Kwack, B.H. The essential role of calcium ion in pollen germination and pollen tube growth. Am. J. Bot. 1963, 50, 859–865. [Google Scholar] [CrossRef]
- Hepler, P.K.; Lovy-Wheeler, A.; McKenna, S.T.; Kunkel, J.G. Ions and pollen tube growth. In The Pollen Tube: A Cellular and Molecular Perspective; Malhó, R., Ed.; Springer: Berlin, Heidelberg, 2006; pp. 47–69. [Google Scholar]
- Peterson, R.; Slovin, J.P.; Chen, C. A simplified method for differential staining of aborted and non-aborted pollen grains. Int. J. Plant Biol. 2010, 1, 66–69. [Google Scholar] [CrossRef]
- Brown, W.V. A preliminary study of the staining of plant cells by tetrazolium chloride. Bull. Torrey Bot. Club 1954, 81, 127–136. [Google Scholar] [CrossRef]
- Lansac, A.R.; Sullivan, C.Y.; Johnson, B.E.; Lee, K.W. Viability and Germination of the Pollen of Sorghum [Sorghum bicolor (L.) Moench]. Ann. Bot. 1994, 74, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Sulusoglu, M.; Cavusoglu, A. In vitro pollen viability and pollen germination in cherry laurel (Prunus laurocerasus L.). Sci. World J. 2014, 2014, 657123. [Google Scholar] [CrossRef] [PubMed]
- Mitra, B.; Kumar Shah, S.; Mishra, P. Insect Fauna associated with the tea ecosystem of North Bengal, India. Rec. Zool. Surv. India 2018, 118, 178–193. [Google Scholar] [CrossRef]
- Wei, W.; Wu, H.; Li, X.; Wei, X.; Lu, W.; Zheng, X. Diversity, daily activity patterns, and pollination effectiveness of the insects visiting Camellia osmantha, C. vietnamensis, and C. oleifera in South China. Insects 2019, 10, 98. [Google Scholar] [CrossRef]
- Altuntas, E.; Yildiz, M. Some engineering properties of shelled and kernel tea (Camellia sinensis) seeds. Afr. J. Tradit. Complement. Altern. Med. 2017, 14, 39–45. [Google Scholar] [CrossRef]
- George, K.O.; Wanyoko, J.K.; Kinyanjui, T.; Moseti, K.O.; Wachira, F.N. Comparative assessment of the fatty acid profiles of crude oils extracted from seeds of selected tea (Camellia sinensis L.) cultivars. Food Nutr. Sci. 2016, 7, 1–7. [Google Scholar] [CrossRef]
- George, K.O.; Kinyanjui, T.; Wanyoko, J.; Moseti, O.K.; Wachira, F. Extraction and analysis of tea (Camellia sinensis) seed oil from different clones in Kenya. Afr. J. Biotechnol. 2013, 12, 841–846. [Google Scholar] [CrossRef]
- George, K.O.; Moseti, K.O.O.; Wanyoko, J.K.; Kinyanjui, T.; Wachira, F.N. Quantitation of the total catechin content in oils extracted from seeds of selected tea (Camellia sinensis (L.) O. Kuntze, Theaceae) Clones by RP-HPLC. Am. J. Plant Sci. 2015, 6, 1080–1089. [Google Scholar] [CrossRef]
- Barooah, A.K.; Singh, S.K.; Das, B.; Sarma, R.; Patel, P.K. Tea seed oil: Physicochemical profiling. J. Plant. Crops 2020, 48, 247–251. [Google Scholar] [CrossRef]
- Koo, T.K.; Li, M.Y. A guideline of selecting and reporting intraclass correlation coefficients for reliability research. J. Chiropr. Med. 2016, 15, 155–163. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).