1. Introduction
The major challenges for agriculture scientists and experts are to improve crop quality and yield with minimal inputs, focusing on environmental sustainability. To fulfill this aim, various breeding programs have been introduced, but they are time-consuming and species-specific methods. The use of an organic substance can stimulate healthy plant metabolism and improve their growth and development functions [
1].
Organic farming is generally characterized by lower crop yield as compared with conventional production systems, mainly because of the limitations imposed on fertilization (no use of chemical fertilizers) and on plant defense (no use of pesticides) [
2,
3,
4,
5].
Initially, plant biostimulants were used for organic production [
6], but as its benefits have been explored, it is now being adopted in sustainable agricultural practices and integrated cropping systems [
7].
Biostimulants are considered to be one of the most innovative and promising solutions for increasing the sustainability and profitability of agriculture [
8]. Biostimulants are defined as “any substance or microorganism applied to plants in order to increase the efficiency of nutrition, resistance to abiotic stress and quality characteristics of the crop, regardless of the content of nutrients in it” [
9]. Biostimulants are the extracts derived from organic raw substances containing bioactive compounds. Some common components of biostimulants are humic substances, mineral elements, amino acids, chitin, chitosan, vitamins, and poly- and oligosaccharides [
1]. The main categories of plant biostimulants include natural substances such as humic and fulvic acids, protein hydrolysates, seaweed extracts [
10,
11,
12], useful fungi (such as arbuscular mycorrhizal fungi and
Trichoderma spp.) [
13] and rhizobacteria that promote plant growth [
14]. Growth regulators affect the course of physiological processes and thereby allow changes to the metabolism of the plant organism and in the soil [
15,
16,
17,
18]. Modern biological products are indispensable for increasing germination of plant seeds, as they are able to increase resistance to plant diseases [
19,
20,
21,
22], abiotic stresses [
23,
24] and other stressful situations [
25]; accelerate flowering and fruiting; increase yield; and ensure the ecological purity of the crop [
26,
27,
28]. Biostimulants could also be considered for their implementation in the post-harvest management of fruits. Biostimulants containing mineral nutrients such as zinc and silicon might contribute with calcium to the strengthening of cell wall structure [
29], thereby allowing the preservation of fruit quality attributes for longer period. This is of particular interest for the organic apple production system, which is presently lacking any useful means to manage apple physiological disorders during storage. Biostimulants have been found active in promoting final crop quality and, more in detail, studies have highlighted the relevance of biostimulant applications for selected functional quality traits [
30].
Despite the large and increasing number of publications dealing with biostimulants [
16], science-based information on their optimal use, crop specificity, and interaction with growing conditions is many ways still incomplete. Studies on the effect of biostimulants on the growth and yield potential of plants have been conducted primarily on vegetable crops.
To further sustain the growth and profitability of the apple sector, the implementation of new agroecological means, such as biopreparations, in the management of horticultural systems is highly requested by growers. The use of these tools must nevertheless follow information derived from scientifically sound research about their effects on plant physiological and biochemical responses.
The priority in the application of adaptogenic preparations is a significant increase in the adaptive properties of plants and, as a result of their use, an increase in productivity and crop quality.
With this goal, this work aimed to investigate the effects of adaptogenic preparations of the Natural Plant Complex “White Pearl” line applications on the yield and fruit quality of apple trees belonging to the “Sinap Orlovsky” cultivar. The biopreparations were tested on apple trees, and, as far as we know, this was the first study in which the effectiveness of two adaptogenic preparations was evaluated simultaneously and during two consecutive growing seasons. In addition, their effect was also considered during the storage period of fruits by measuring the physiological disorder of “Sinap Orlovsky” apples.
2. Materials and Methods
2.1. Study Area and Research Conditions
The studies were carried out on the grounds of the laboratory of physiology of resistance of fruit plants and experimental plots of the Russian Research Institute of Fruit Crop Breeding (VNIISPK) from 2021–2022.
The institute is located in the Orel Region (53°00′ north, 36°00′ east), which is part of the Central Federal District of Russia. VNIISPK is located 368 km southwest of Moscow.
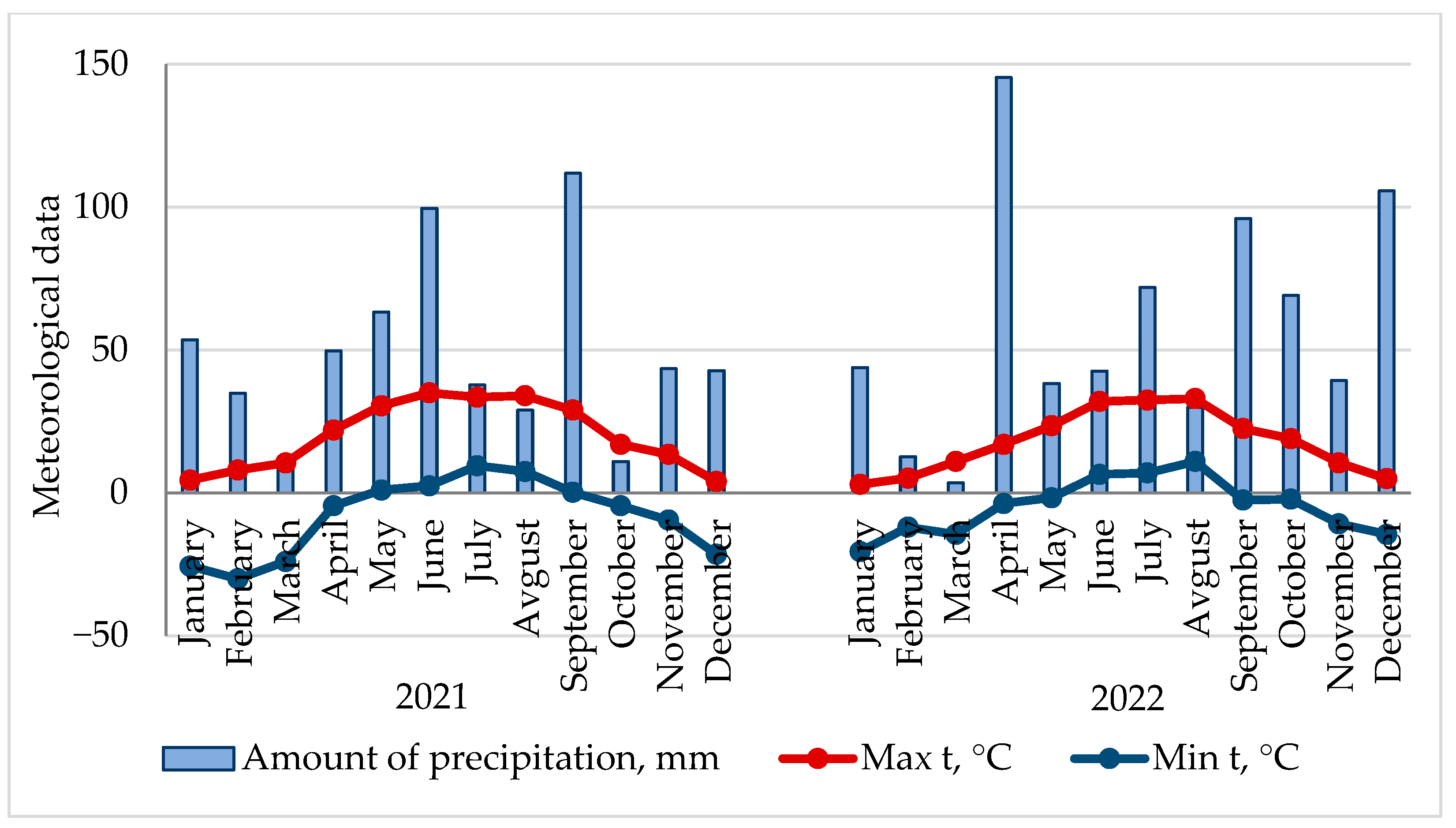
According to the data of the VNIISPK meteorological station, the winter period of 2021 in the Orel region was characterized by sharp drops in positive and negative temperatures. In spring, during the flowering period of garden crops, the minimum air temperature did not fall below 0 °C. The summer and autumn months were characterized by an uneven distribution of precipitation and temperature. Both low and high conditions of plant moisture were noted (
Figure 1).
The winter period of 2022 was characterized by moderate frosts without sudden temperature changes. In spring, a prolonged cold snap was observed in May, which led to a later flowering of fruit crops. Summer and autumn were also marked by an uneven distribution of precipitation and temperature. December was abnormally warm and rainy. The average daily air temperature of this winter month exceeded the norm by 6.4 °C, and the amount of precipitation exceeded the average annual value by 70.7 mm (
Figure 1).
The experiment was performed on agro-gray soils. The agro-gray soils are formed on loess-like loams underlain by dolomitic limestone. Agrochemical indicators of the soil in the studied orchard are presented in
Table 1.
2.2. Research Objects
The object of the study was apple cultivar “Sinap Orlovsky” was used from the bioresource collection of Russian Research Institute of Fruit Crop Breeding, growing on a medium–sized rootstock 54–118. “Sinap Orlovsky”, a cultivar having fruit of late winter maturation, has been developed from the crossing of the “Severny Sinap” and “Pamyat Michurina”. The cultivar is zoned for the Central and Central Chernozem regions of Russia. “Sinap Orlovsky” is precocious and winter-hardy. The fruits are of high quality and in terms of consumer qualities are characterized by a long shelf life. The disadvantage of the cultivar is that its fruits are predisposed to scald and bitter pitting [
31].
The cultivar was planted in the experimental plot with a spacing of 6 m × 3 m in 2013. Natural tinning was used in the aisles; herbicides were used in the trunk strips. The crown shape was spindle. Agronomical practices generally accepted for apple trees were used. The soil-forming rock was loess-like loam of medium mechanical composition.
The following products from AgroPlus Group of Companies LLC (Krasnodar, Russia) were tested: phytomodulator “White Pearl Universal Antifreeze” (“WPU Antifreeze”) and phytocorrector “White Pearl Drip Ca + Mg” (“WP Drip Ca + Mg”). The tested preparations are the Natural Plant Complex (NPC) “White Pearl” (“WP”).
The NPC phytomodulator “White Pearl Universal Antifreeze” is a suspension of a group of minerals of natural origin containing a concentrate of extracts of spruce needles, pine and Siberian fir. The composition includes the following: mineral elements SiO2 5.6%, N (common) 2–6%, CaO 5000 ppm, MgO 7000 ppm, K2O B 130 ppm, Zn 150 ppm, Mo 200 ppm, Al2O3 1600 ppm and other micro elements; vitamins A (carotene, lutein), D (phytosterols), E, K, B1, B2, B6, PP, H; and phytoncides (volatile oils), chlorophyll, flavonoids, sugars, proteins and amino acids.
The NPC phytocorrector “White Pearl Drip Ca + Mg” is an extract of vegetative mass of oceanic bioflora on an organo-mineral basis. The composition includes the following: bioelements Ca 3490.0 ppm, Mg 2829.0 ppm, P 42.9 ppm, K 38.8 ppm, S 0.3 ppm, Fe 68.7 ppm, Mn 3.65 ppm, B 3.37 ppm, Cu 0.85 ppm, Zn 0.05 ppm, Si 0.1 ppm, Se 0.003 ppm, J 2.1 ppm and Mo 0.01 ppm; mineral elements SiO2 5.6%, CaO 0.4%, MgO 0.4%, K2O 0.2%, Fe2O3 0.4% and Al2O3 0.16%; vitamins A (carotene, lutein), D (phytosterols), E, K, B1, B2, B6, PP and H; and chlorophyll, sulfonic acids, humic acids, sugars, proteins and amino acids.
2.3. Regulations for the Use of Adaptogenic Products in the Experiment
The experiment was performed with two variations: 1—control (foliar sprays with water); and 2—foliar sprays with preparations: 1% solution of “White Pearl Universal Antifreeze” + 1% solution of “White Pearl Drip Ca + Mg”. There were 3 repetitions of each experiment. In each repetition, there were 5 accounting trees.
The tested biological products are intended specifically for foliar sprays.
Foliar sprays were carried out with the RT-16LI knapsack sprayer (Patriot, Beijing, China), with a solution of the tested biological products directly on the trees of the experimental apple cultivar. This was done so that the nutrients that fall in the form of small drops on the bark and foliage of the apple tree were absorbed faster by the plants. Foliar sprays of apple trees were carried out in the morning, in calm weather. The consumption rate of the 1% solution of the tested biological products was 100 mL per 10 L of water.
In the early spring period, to prevent damage to generative organs from recurrent spring frosts, “Sinap Orlovsky” plants were treated twice with a 1% NPC “WPU Antifreeze” solution.
The first foliar sprays of the experimental cultivar trees were performed in the first ten days of April, and the second was performed at 20 days.
The next treatment was carried out in early May with a 1% solution of NPC “WPU Antifreeze” + 1% solution of NPC “WP Drip Ca + Mg”.
In summer, a four-fold leaf treatment with preparations of a 1% solution of NPC “WPU Antifreeze” + 1% solution of NPC “WP Drip Ca + Mg” was carried out in order to preserve the fruits from shedding and increase yield and fruit quality. Foliar sprays were performed at specific dates: 14 days after flowering; “fruit-hazel” (
Figure 2a), “fruit-walnut” (
Figure 2b) and 25 days before harvesting.
2.4. Determination of the Fractional Composition of Water
The fractional composition of water in the bark of annual shoots and fruit buds of apple trees was determined by the Okuntsov–Marinchik method [
32]. This method is based on the changing concentration of sucrose solution while immersing plant tissue therein. Prepared samples weighing 0.4 g were immersed in a 30% sucrose solution (in three replicates). Some of the water from the plant tissue passed into the solution, reducing its concentration. Based on the initial volume of the solution and its initial and final concentration, the amount of water that entered the solution from tissues was determined. The content of bound water was calculated from the difference in the total water content and the water that passed into the solution. The concentration of the sucrose solution was determined using a PAL-1 digital refractometer (Atago, Tokyo, Japan). The total water content in the bark of annual shoots and fruit buds was determined by the formula:
where
W shows the total hydration from the wet mass, %;
m1 denotes mass of crude weighed bark of annual shoots and fruit buds, g;
m2 denotes mass of absolutely dry weighed bark of annual shoots and fruit buds, g.
To determine the absolutely dry weight, the mass of absolutely dry weighed bark of annual shoots and fruit buds was laid out in aluminum containers and dried in an oven at a temperature of 105 °C until constant weight was reached.
2.5. Determination of Low Molecular Weight Carbohydrates and Proline Amino Acid
The amount of low molecular weight carbohydrates (sucrose, glucose) in the bark of annual shoots and fruit buds was determined in three replicates based on a resorcinol re-agent reaction at a wavelength of 520 nm. For this, 0.5 g of the material was rubbed in 10 mL of ethanol heated to 80 °C, and the tubes were heated in a UT-4301 E (Ulab, Shanghai, China) water bath (at t = 100 °C) for 10 min. The contents of the tubes were centrifuged for 10 min at 7000×
g rpm in centrifuge B4i (Jouan, Morlaas Zone Industrielle Berlanne, Morlaàs, France). Next, 50 μL of 5 N NaOH was added to 0.5 mL of the supernatant. It was heated in a water bath UT-4301 E (Ulab, Shanghai, China) (at t = 100 °C) for 10 min. After cooling, 0.5 mL of resorcinol reagent (100 mg of resorcinol +250 mg of thiou-rea in 100 mL of ice-cold CH
3COOH) and 3.5 mL of 30% HCl were added. Test tubes were heated in the bath for 10 min. After cooling, the optical density was determined on a BioRad SmartSpec Plus spectrophotometer (BioRad, Hercules, CA, USA) at a wavelength of 520 nm. The disaccharide content was calculated using a calibration curve constructed for pure sucrose and glucose [
33].
The proline content was determined in three replicates by reaction with ninhydrin reagent [
34]. To do this, a 500-mg sample of bark of annual shoots and fruit buds was ground in distilled water and boiled in a UT-4301E (Ulab, Shanghai, China) water bath (at t = 100 °C) for 10 min. After this, the homogenate was centrifuged at 7000×
g rpm in a B4i (Jouan, Morlaas Zone Industrielle Berlanne, France) centrifuge, 2 mL of the extract was taken and 2 mL of glacial acetic acid and 2 mL of ninhydrin reagent (30 mL of ice-cold CH
3COOH + 20 mL of 6 M H
3PO
4 + 1.25 g of ninhydrin) were added, followed by boiling in a UT-4301 E (Ulab, Shanghai, China) water bath (at t = 100 °C) for 1 h. The amino acid content was calculated using a calibration curve on a BioRad SmartSpec Plus spectrophotometer (BioRad, Hercules, CA, USA) constructed for pure proline at a wavelength of 520 nm. Proline content was expressed in mg per 1 kg of wet weight [
35].
2.6. Modeling of Spring Frosts
Artificial freezing of apple buds and flowers was carried out in a PSL-2KPH (Espec, Osaka, Japan) climatic chamber. Spring frosts were simulated (−3°, −3.5° and −4 °C). The exposure time of the freezing was 3 h. The rate of temperature decrease was 1 °C per hour. Before evaluation, the experimental material was kept at a temperature of +22 °C until signs of damage to the buds and flowers appeared. For artificial freezing, branches of the tested cultivar were cut off in the orchard so that there were 100 flowers and 100 buds in total. After freezing, a visual assessment of damage to flowers and buds of the cultivar was carried out. Damage to the pistils and stamens was assessed by the darkening of the tissues. The number of damaged flowers and buds was calculated from the total number of flowers and buds. The degree of damage to flowers and buds was expressed as a percentage.
2.7. Yield, Average Weight, Biochemical Analysis and Keeping Quality of Apple Fruits
The weight accounting of the yield was carried out per tree in kg by weighing during the period of removable fruit maturity in accordance with the methodology [
36]. The average yield from 1 accounting tree for each repetition of the experiment was calculated by dividing the total weight of the crop (harvested fruit crop + economically usable wind fallen fruits) by the number of accounting plants in the repetition.
The yield as a whole for the cultivar in the center from 1 ha was calculated by the formula:
where
Y—yield, c/ha;
A—average yield per 1 tree, kg;
B—nutrition area of 1 tree, m2;
100—the coefficient of conversion of weight in kilograms to weight in hundredweight and m2 area to hectares.
The commercial qualities, biochemical composition and keeping quality of apple fruits were studied according to the methodology [
36]. The “Sinap Orlovsky” fruits were selected based on typicality of shape, color and degree of maturity.
To characterize the weight of the fruits, 100 apples were selected from each repetition of the experiment. The average weight of the fruits was determined by weighing 100 fruits and dividing the resulting weight by their number.
For biochemical analysis of fruits during harvesting, 10 fruits were selected in 3 repetitions of each variant of the experiment.
Determination of sugars (sum, monosaccharide, sucrose) was carried out according to Bertrand’s method, which is based on the reducing action of sugar on alkaline solution of tartarate complex with cupric ions; the cuprous oxide formed is dissolved in a warm acid solution of ferric alum. The ferric alum is reduced to FeSO
4 which is titrated against standardized KMnO
4; Cu equivalence is correlated with the table to obtain the amount of reducing sugar. This is based on the alkaline solution of tartarate complex of cupric ion [
37].
Ascorbic acid was determined by titration of oxalic acid extracts with Tilman’s paint (2,6-dichlorophenolindophenol). All determinations, starting from taking the sample and ending with titration, were carried out within 1 h. The titer of the Tilman’s paint was determined by the method of S.M. Prokoshev.
In total, 40 apples in 3 repetitions of each experiment variant were selected for fruit storage. The fruits were stored in a CV114-S (Polair, Volzhsk, Russia) refrigerator at a temperature of +2 °C. After storage, the degree of damage by scald, bitter pitting, wilting and rotting of fruits was determined.
2.8. Statistical Analysis
The obtained data were evaluated using mathematical statistics using single-factor analysis of variance ANOVA (Version 22, SPSS Statistics). The critical significance level between control and treatment was assumed to be 5%. The results were presented in the form of M ± m.
3. Results
3.1. The Effect of Adaptogenic Preparations on the Fractional Composition of Water in Annual Shoots and Fruit Buds of Apple Trees in Spring
The degree of hydration of plants is one of the essential indicators of the water regime. The concentration of cell juice and the water potential of individual plant organs are associated with the water content [
38]. It is known that the mobility and activity of water directly depends on its state in the plant cell. From this point of view, it is customary in plant physiology to distinguish between free and bound water. Free water moves easily, enters into various biochemical reactions, evaporates during transpiration and freezes at low temperatures. Bound water, which plays a structure-forming role, is not a solvent and it has a reduced freezing point, which will significantly affect the resistance to low-temperature stress of the protoplast of the cell and the plant as a whole [
39].
Thus, in early April, the foliar sprays of “Sinap Orlovsky” trees with a 1% solution of NPC “WPU Antifreeze” increased the hydration of the bark annual shoots by 1.5% compared to the control. The content of free water in annual apple shoots was significantly reduced, by 4.5% in the variant with the treatment. The level of colloidal water at the same time significantly increased by 7.0% in treated trees against control ones (
Figure 3). This section may be divided by subheadings. It should provide a concise and precise description of the experimental results and their interpretation, as well as the experimental conclusions that can be drawn.
After 20 days, a second set of foliar sprays was carried out. After the second foliar sprays of “Sinap Orlovsky” with a 1% solution of NPC “WPU Antifreeze”, an insignificant decrease in the free water content in annual shoots was noted compared to the control. The amount of bound water in the bark annual shoots was at the same level in both variants. In the fruit buds of treated trees, compared with the control ones, the level of bound water significantly increased by 8.4% against the background of a significant decrease of 4.2% of free water (
Figure 4).
The subsequent foliar sprays of the experimental plants in early May with a tank mixture (1% solution of NPC “WPU Antifreeze” + 1% solution of NPC “WP Drip Ca + Mg”) also significantly affected the proportion of bound water in fruit buds. In the treated of “Sinap Orlovsky” trees, fruit buds contained 5.7% more colloidally bound water than the controls. The content of free water in the fruit buds was significantly reduced, by 4.5% in the variant with treatments (
Figure 5). The resistance of plants to adverse environmental conditions is determined by the state of intracellular water. It is bound water that affects the resistance to low-temperature stress of plants, since it has a reduced freezing point.
Thus, the 3-repetition foliar sprays with adaptogenic preparations carried out in spring increased the resistance to dehydration of the bark annual shoots and fruit buds of “Sinap Orlovsky” trees against the background of an increase in bound water, which would help reduce the freezing temperature of water inside the plant cells in spring frosts.
3.2. The Effect of Adaptogenic Preparations on the Level of Low-Molecular Osmoprotectors in the Bark of Annual Shoots and Fruit Buds of Apple Trees in Spring
The spring foliar sprays of apple trees with a 1% solution of NPC “WPU Antifreeze” increased the content of free proline both in the bark of annual shoots and in the fruit buds of the plants (
Table 2). The maximum effect of an increase in amino acid compared to the control in the bark of annual shoots (67.0% more than in the control) and in fruit buds (12.7% more than in the control) was noted in early April. At the same time, the maximum content of free proline was noted in the fruit buds of the plants in the variant with treatments. The proline amino acid, in addition to its building function in protein biosynthesis, performs an antioxidant and osmoregulatory role. Therefore, as an antioxidant, this amino acid is able to “extinguish” an excessive amount of the active oxygen form during the development of oxidative stress against the background of adverse environmental factors. As an osmoprotector, proline increases the concentration of cell juice, which prevents the formation of intracellular ice and prevents damage to cell membranes. By increasing the content of free proline in the cells and tissues of apple plants, NPC “WPU Antifreeze” prevents the development of not only oxidative stress, but also the formation of intracellular ice, which increases the resistance of cells and tissues to both negative and low positive temperatures.
As in the case with proline and bound water, foliar sprays with NPC “WPU Anti-freeze” contributed to an increased level of sugars, both in the bark of annual apple shoots and in the fruit buds (
Table 3). At the same time, at the beginning of April, the amount of sugars in the experimental version did not significantly differ from the control, which may be due to the onset of vegetation and intensification of physiological and biochemical processes. This assumption is supported by a further decrease in the level of sugars at the end of April. It is known that sugars are a substrate for respiration, as a result of which both energy and plastic equivalents are formed for the growth and development of plants. However, it should be noted that in the variant with treatment, the intensity of sugar re-duction was significantly lower compared to the control. Therefore, during the interval of the first ten days of April till the end of the third ten days of April, the amount of sugars in the bark of annual shoots in control plants decreased by a factor of 1.76, in experimental plants by a factor of 1.74; in the fruit buds by a factor of 4.1 versus 3.3 in experimental plants, respectively (
Table 3). The lower intensity of the reduction in the amount of sugars under the influence of treatment with a phytomodulator is probably associated with some inhibition of the expenditure of sugars on life support processes, and above all with the inhibition of respiration processes when plants exit the state of forced dormancy.
An increase in the amino acid proline in the bark of annual shoots by 16.5% and in fruit buds by 22.7% was noted in the experiment with the treatment with biological preparations in comparison with the control. Along with this, there was an in-crease in the amount of sugars both in the bark of annual shoots by 1.2 times, and in fruit buds by 1.6 times in “Sinap Orlovsky” under the action of adaptogenic preparations (
Table 4). The experimental plants treated with a tank mixture were generally characterized by an increased background of the amount of sugars compared to the control trees, which is of importance for the protective effect of low-molecular carbohydrates in conditions of sudden spring frosts.
Along with this, there was an increase in the amount of sugars both in the bark of annual shoots by 1.2 times, and in fruit buds by 1.6 times in “Sinap Orlovsky” under the action of adaptogenic preparations (
Table 4). The experimental plants treated with a tank mixture were generally characterized by an increased background of the amount of sugars compared to the control trees, which is of importance for the protective effect of low molecular carbohydrates in conditions of sudden spring frosts.
Thus, adaptogenic preparations also reduced the risk of damage to the fruit buds of apple trees by negative temperature by increasing low-molecular osmoprotectors at the beginning of the growing season.
3.3. The Effect of Adaptogenic Preparations on the Resistance of Apple Buds and Flowers to Spring Frosts
The evaluation of the results of artificial freezing showed a positive effect of foliar sprays with adaptogenic preparations on the resistance of apple flowers and buds to spring frosts. After exposure to negative temperatures of −3°, −3.5° and −4 °C, a decrease in the proportion of dead buds in the variant with foliar sprays was noted by 8.6%, 7.8% and 3.4%, respectively, although statistically the differences between the variants were not confirmed (
Figure 6a). Foliar sprays with a tank mixture with preparations of the “White Pearl” line significantly affected the resistance of “Sinap Orlovsky” flowers to spring frosts of −3.5 °C and −4 °C. The proportion of damaged flowers at temperatures of −3.5 °C and −4 °C significantly decreased by 6.3% and 8.2%, respectively, compared with the control (
Figure 6b).
Thus, the preparations of the NPC “White Pearl” line prevented the destruction of cell membranes and dehydration of plant cells by increasing bound water and the most powerful osmolytically active substance-free proline and sugars during low-temperature stress, thereby reducing the risk of damage to buds and flowers of garden crops by spring frosts.
3.4. The Effect of Adaptogenic Preparations on the Water Regime of Apple Leaves in Summer
All physiological processes in the plant normally proceed only with sufficient water supply to the plant. Water is a necessary component and an important factor in the structure of the cytoplasm of living cells. It participates in cell metabolism, in hydrolytic and synthetic processes, promotes the interaction of molecules [
38].
In the summer of 2021, foliar sprays with preparations of 1% NPC “White Pearl Antifreeze” + 1% NPC “White Pearl Drip Ca + Mg” contributed to an increase in free water in the “Sinap Orlovsky” leaf apparatus by 2.1% compared to the control (
Figure 7). At the same time, the proportion of bound water in the variant with foliar sprays was 2.3% lower. The overall hydration of the apple tree leaf apparatus was at the same level in both versions of the experiment. Probably, foliar sprays contributed to the strengthening of metabolic processes during the period of active growth and development of apple fruits against the background of an increase in the proportion of free water, which contributed to the intensive outflow of organic substances accumulated in the leaves during photosynthesis to the fruits.
In the summer period of 2022, as well as of 2021, after the foliar sprays with a tank mixture of 1% NPC “WPU Antifreeze” + 1% NPC “WP Drip Ca + Mg”, a 2.1% increase in free water was noted in the leaf apparatus of “Sinap Orlovsky” trees compared to the control (
Figure 8). At the same time, the proportion of bound water in the variant with foliar sprays was 1.1% lower than in the control. Free water, being a solvent and the main transporter of organic substances, will presumably contribute to their intensive outflow from leaves to fruits.
Thus, foliar sprays with adaptogenic preparations had a positive effect on the water regime of apple leaves, contributing to the intensive transition of bound water into a more mobile form, which was necessary for the normal functioning of plants during the formation and ripening of fruits, which subsequently affected the increase in the average fruit weight and yield of apple.
3.5. The Effect of Adaptogenic Preparations on the Proline Accumulation in Apple Leaves and Fruits in Summer
In the first ten days of June, the treatments reduced the content of free proline in leaf tissue by 43.7% and did not significantly affect its level in fruits (
Table 5). However, in the third ten days of June, the analysis showed an increase in the amino acid content in both leaves (by 62.8%) and fruits (by 12.4%) compared to the control. This increase in proline under the influence of treatments may be associated with the urgent need for a building material, i.e., protein, for more intensive fruit growth. This assumption is supported by the fact that in the second ten days of July, a decrease in the level of free proline was noted in the fruits of the experimental variant, while its growth was still continuing in the leaf apparatus. In the first ten days of August, when the need for building material had virtually completely disappeared, an even greater decrease in the amount of proline was noted both in the leaf apparatus and in fruits, and in the variant with treatment it was to a greater extent. Thus, in the treated plants, the proline content in the fruits was 23.01% less than in the control indicators. Apparently, this indicates that the applied drugs initially contributed to a more intensive growth of apple fruits.
At the same time, foliar sprays did not practically affect the content of free proline in the leaf apparatus and only during the “fruit-hazel” phenophase did it reduce its level by 32.7% compared to the control (
Table 6). The amount of free proline in fruits was affected by foliar sprays only by the middle of the growing season. Thus, during the “fruit-walnut” phenophase, the study showed that the level of proline in the experimental version was 23.0% higher than in the control. However, in general, in both variants, the amount of proline decreased by the middle of the growing season, which can be explained by the intensive growth of fruits and the expenditure of amino acids on the biosynthesis of protein substances necessary for growth processes.
Thus, foliar sprays of plants with adaptogenic preparations contributed to the regulation of donor–acceptor leaf–fruit relations against the background of the accumulation of the amino acid proline in summer, which subsequently affected the increase in the average fruit weight and yield of apples.
3.6. The Effect of Adaptogenic Preparations on the Accumulation of Glucose in the Leaves and Fruits of Apple Trees in Summer
An analysis of the glucose content during the growing season showed that in June, under the influence of treatments, lower carbohydrate values were noted, both in the leaf apparatus and in the fruits (
Table 7). However, starting from July, in the treated version, there was an increase in glucose biosynthesis compared to June: in the leaf apparatus, it was 4.2 times more intense in the control and 5.9 times more intense in the experiment; in the fruit, it was 2.5 times more intense in the control and 4.6 times more in the experiment. In this regard, in the second ten days of July, against the background of a decrease in the level of proline in fruits, the glucose content under the influence of treatments was 9.8% higher in leaves and 11.6% higher in fruits than in the control. In August, when the fruits were ripening, a decrease in glucose biosynthesis was noted in the leaf apparatus, with a continued increase of this carbohydrate in apples. It should be noted that in the leaves of the experimental plants, the glucose content was 20.0% higher than in the control, and in the fruits it was higher by 11.3%. The higher carbohydrate content under the action of treatments in experimental plants compared to the control is explained by the improvement of both photosynthetic activity and donor-acceptor relations between the leaf apparatus and the ripening fruit.
The analysis of the low molecular weight carbohydrate content during the growing season showed an ambiguous effect of the foliar sprays on glucose levels, both in the fruits and leaves of plants. Thus, in all the dates of the conducted studies, the amount of glucose in the leaf apparatus in the experimental version did not significantly differ from the control (
Table 8). Conversely, in fruits, the prevalence or decrease in the amount of the studied carbohydrate varied in the experimental variants compared to the control depending on the time of the growing season. Therefore, 14 days after flowering, under the influence of treatments, the amount of glucose exceeded the control values by 87.5%, in the “fruit-hazel” phenophase it did not significantly differ from the control, and in subsequent periods it was lower than in the control variant by 18.0–76.2%. Apparently, a gradual decrease in the amount of glucose in fruits by the middle of the growing season in the experimental variant is associated with an intense load on plants by the future yield and, as a consequence, a biological dilution of glucose concentration.
Thus, foliar sprays of apple plants with adaptogenic preparations during the summer period definitely did not affect the carbohydrate metabolism of the donor–acceptor leaf–fruit relationship, and therefore it is necessary to continue the studies to draw reliable conclusions.
3.7. The Effect of Adaptogenic Preparations on the Fruit Qualities and Apple Yield
Due to a biological feature of apple trees (the frequency of fruiting) in the reporting year of 2021, the low yield of the experimental cultivar “Sinap Orlovsky” was noted, but the effectiveness of the applied biological preparations was traced at the same level as in 2022.
With the onset of harvest maturity of “Sinap Orlovsky” fruits in 2021, a significant increase in the average yield from the tree by 1.8 times was recorded against the background of foliar sprays of plants with the preparations of the NPC “White Pearl” line (
Figure 9). The average weight of the fruit of the studied cultivar increased by 10.0 g compared to the control, which affected its productivity.
In 2022, a significant 1.7-fold increase in the average yield from a tree was also noted after foliar sprays of plants with biological preparations (
Figure 9). Based on the treatment with the tested drugs, the average weight of the fruit of the studied cultivar increased by 30.6 g compared to the control.
When taking into account the weight of the yield of “Sinap Orlovsky” apples (
Figure 10 and
Figure 11), a significant increase in the yield by 1.7 times was noted in the variant with treatment with preparations of the NPC “White Pearl” line. The increase in the yield amounted to 86.6 c/ha (
Figure 10). The adaptogenic preparations contributed to the improvement of fruit quality. The average weight of the fruit of the studied cultivar significantly increased by 20.3 g in the variant with treatments compared to the control.
The results of the biochemical analysis of apple fruits showed that foliar sprays with preparations of the NPC “White Pearl” line improved the taste qualities of the fruits of “Sinap Orlovsky” compared to the control by increasing the amount of sucrose by 25.6% and ascorbic acid by 20.2% (
Table 9).
Thus, foliar sprays with preparations of the NPC “White Pearl” line significantly increased the yield and quality of fruits, as well as favorably affecting the consumer and commodity qualities of “Sinap Orlovsky” fruits.
3.8. The Results of Fruits of Apple Cultivar “Sinap Orlovsky” Storage
After harvesting, the apples were laid in for storage on 13 September 2021 and 9 September 2022 at a temperature of +2 °C. The fruits were removed from storage in early April. The storage duration of the studied variants was 211 days. According to the yield of commercial fruits in the experiment, significant differences were revealed between the variants at the 5% significance level. “Sinap Orlovsky” apples treated with adaptogenic preparations of the NPC “White Pearl” line had a ratio of 92.8% marketable fruits to a 7.2% waste (
Figure 12b and
Figure 13b), and untreated control fruits had a marketable yield and waste of 78.0% and 22.0% (
Figure 12a and
Figure 13a), respectively.
The fruits of the studied cultivar predisposed to scald were least affected by this functional disorder after being treated with adaptogenic preparations. The degree of damage was 4.7% (
Figure 12b). The fruits of the control variant had a percentage of damage by scald of 11.7 (
Figure 12a), although statistically the differences between the variants were not confirmed, as well as for another physiological disorder, i.e., bitter pitting (this indicates an imbalance of mineral composition). In the control variant, 5% of fruits (
Figure 12b) were identified as having bitter pitting, and in the variant with treatments with preparations of the NPC “White Pearl” line only 2.5% (
Figure 12a) were identified.
In addition to the above disorders, the control variant had overripe and browned fruits in the amounts of 2.7 and 1.2% (
Figure 12a), respectively. The treated fruits with adaptogenic preparations did not have similar damage.
Regarding microbiological diseases on the fruits of the control variant, minor damage (0.7% with partial and 0.7% with absolute rotting) to fruits was recorded, mainly by fruit rot moniliosis (
Monilia fruktigena Pers.). In the treated variant, the fruits had absolutely no microbiological damage (
Figure 12a). At the same time, an average withering of the fruits in the control variant was recorded, and in the variant with foliar sprays with adaptogenic preparations there was a slight withering of the apples.
Thus, foliar sprays with preparations of the NPC “White Pearl” line favorably affected the consumer and commodity qualities of “Sinap Orlovsky” fruits during storage.
4. Discussion
As a result of the conducted studies, the “Synap Orlovsky” apple trees treated with adaptogenic preparations of the NPC “White Pearl” line were generally characterized by an increased content of low molecular osmolytics and bound water compared to the control trees, which is of great importance in conditions of recurrent spring frosts. It is known that sugars, along with proline, increase the concentration of cell juice by increasing bound water, which has a protective effect under conditions of negative temperatures. In our experiment, the adaptogenic preparations of 1% solution of NPC “White Pearl Universal Antifreeze” + 1% solution of NPC “White Pearl Drip Ca + Mg” presumably prevented the destruction of cell membranes and dehydration of plant cells by increasing the most powerful osmolytically active substances—free proline and sugars—during low-temperature stress, thereby reducing the degree of freezing in the generative organs of apple trees. Thus, foliar sprays with a phytomodulator NPC “White Pearl Universal Antifreeze” and a phytocorrector NPC “White Pearl Drip Ca + Mg” significantly reduced the freezing of flowers by 6.3% and 10.4% at temperature conditions of −3.5 °C and −4 °C, respectively, in comparison with the control. Earlier, V.P. Popova and the authors [
40] reported the effectiveness of organomineral foliar sprays containing macro- and microelements, a complex of amino acids, with an increase in the adaptive properties of apple trees. Other studies showed that foliar sprays with the “Regalis” growth regulator enhanced resistance to adverse environmental factors in the Krasnodar Territory by increasing the intensity of metabolic processes [
41].
Summer foliar sprays with adaptogenic preparations of 1% solution of NPC “White Pearl Universal Antifreeze” + 1% solution of NPC “White Pearl Drip Ca + Mg” contributed to more intensive growth and ripening of apple fruits against the background of regulation of protein-carbohydrate metabolism, water regime and donor–acceptor leaf–fruit relations, which subsequently positively affected the increase in yield by 1.7 times and the average weight of the fruit by 20.3 g. Other researchers also report an increase in the yield of apple trees with the use of growth regulators. Thus, the silicon-containing growth regulator “Mival-Agro” increased the yield of Gala apples by 3.4 t/ha against the control [
42]. The use of the “Regalis” growth regulator also contributed to the active course of photosynthetic activity of apple plants and increased yield by 3.2–13.9 t/ha compared to the control variant [
41]. The use of “Albit” steadily increased the yield of apple cultivars, both in comparison with the control and with the production scheme of protection [
43]. Other authors, when using biostimulants based on alfalfa protein hydrolysate, seaweed extracts and B vitamins, noted an improvement in the quality and appearance of apple fruits. This study has also shown that biostimulants containing zinc are effective in reducing physiological disorders in apples during storage [
30]. In our experiment, foliar sprays with adaptogenic preparations increased the yield of commercial apple fruits by 14.8% compared to the control. Treatment with bioregulators containing progexadion-Ca and paclobutrazole increased the weight of fruits and the yield of pear cultivars. At the same time, a strong relationship was shown between the illumination of the pear crown and the yield [
44]. Biostimulants based on alfalfa protein hydrolysate, seaweed extracts and B vitamins also improved the taste and color of apple fruits [
30].
Physiological diseases of fruits that occur during storage can seriously affect the quality of apples and, consequently, lead to significant economic losses [
45]. At the same time, foliar sprays with adaptogenic preparations contributed to a decrease in fruits affected by scald by 2.5 times and bitter pitting by 2 times against the control. In addition, in the variant of the experiment with foliar sprays, there were 2.3 times fewer overripe and browned fruits. The combined use of calcium chloride with seaweed extract and with a Zn-containing product (Siliforce
®) was effective in reducing the spotting of Jonathan fruit during storage [
30,
46]. The combined application of Ca and Zn led to a higher concentration of these elements at the level of the fruit skin [
46], which may have strengthened the cell membranes [
47], while reducing the development of spotting during storage. Our results on apple storage are consistent with the results of other studies. Growth regulators “Buton” and “Mival-Agro” significantly increased the yield of standard products during the storage of apples. In addition, the authors recorded an increase in fruit weight and some acceleration of the ripening process of apples [
48].
Our tests of adaptogenic preparations of the NPC “White Pearl” line in apple plantations show the prospects of their use as additional techniques in traditional technologies of cultivation of this crop to regulate plant growth processes, increase resistance to spring frosts, improve fruit quality and yield, as well as to preserve consumer and commodity qualities of apples during storage.