Quality and Yield of Edible Vegetables from Landscape Design
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Site
2.2. Experimental Design
- V1—40-centimetre-high raised beds;
- V2—20-centimetre-high raised beds;
- V3—ground-level beds, which represent the control version.
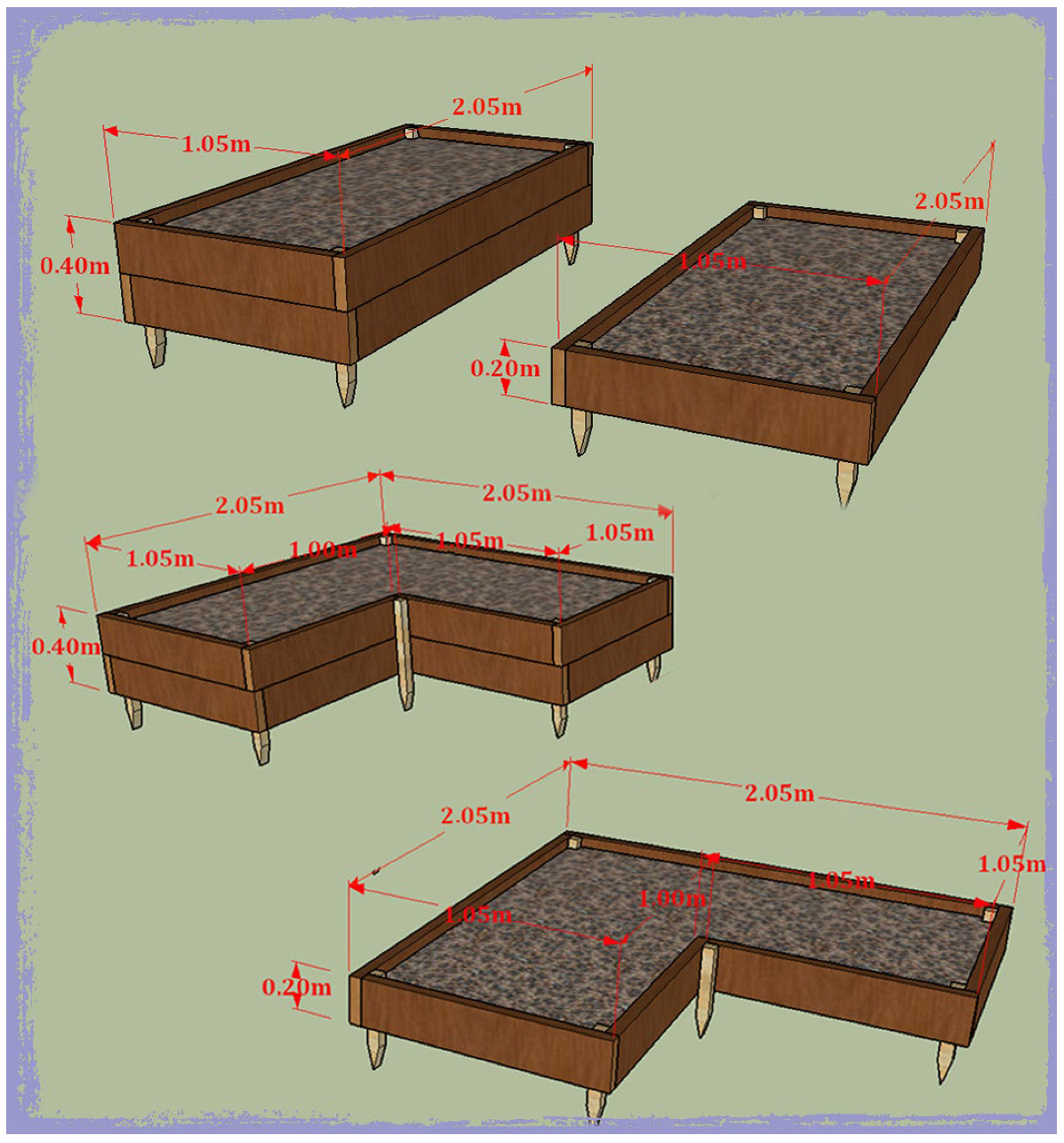
2.3. Biotechnical Materials
2.3.1. Raised Bed Design and Construction
2.3.2. Substrate and Irrigation
2.3.3. Soil Analysis
2.3.4. Climatic Conditions
2.4. Biological Material
- Seedlings—kale ‘Kadet’, ‘Scarlet’, and ‘Nero di Toscana’; chard ‘Bright Lights’; leek ‘Blue de Solaise’; white onion ‘Di Parma’; cucumber ‘Ekol’; eggplant ‘Black Beauty’; tomato ‘Tigerella’; tomato ‘Black Cherry’; sweet pepper ‘Barbara’; parsley ‘Triple Moss Curled’; celeriac ’Giant Prague’; carrot ’Cosmic Purple’; carrot ’Royal Chantenay’; basil ‘Italiano Classico Genovese’; basil ‘Serafim’; oregano ‘Kreta’; medicinal sage ‘Chrestensen’; mint ‘Cinderella’, thyme ‘Di Provenza’; French marigold ‘Nana’; silver ragwort ‘Silverdust’;
- Direct sowing in the field—patty pan squash ‘Óvári Fehér’; dwarf bean ‘Nano Supernano Giallo’; common bean ’Violeta de Iasi’;
- Potted plants—fountain grass ‘Cassian’; butterfly bush ‘Gaudi Red’; Russian sage ‘Little Spire’; New York aster ‘Starshine’; lavender ‘Munstead’; rosemary ‘Green Ginger’; lamb’s ear ‘Silver Carpet’; woodland sage ‘Caradonna’.
2.5. Determinations and Analyses Performed
2.6. Statistical Analysis
3. Results and Discussions
3.1. Results on Dry Weight and Moisture Content
3.2. Results on Yield Quality
3.3. Average Yield Results
4. Conclusions and Recommendations
- Further research is needed to explore the compositional attributes and potential benefits of sweet pepper ‘Barbara’ in dry weight, moisture content, calcium content, and anti-nutritive compounds.
- Conduct additional studies to investigate the dietary fibre benefits of version V3 of tomato ‘Tigerella’ and its potential implications for nutrition and health.
- Investigate the effects of different raised bed heights (40 cm and 20 cm) on various plant species’ growth, yield, and nutrient content to optimise the benefits of raised bed gardening.
- Consider using raised beds (at a height of 40 cm or 20 cm) for cultivating plants, as they do not significantly affect most plants’ dry weight and moisture content compared to ground-level beds.
- Select plant varieties based on their average yield and specific growth requirements. For example, consider cucumber ‘Ekol’ for higher average yields and common bean ‘Violeta de Iasi’ for better yields in V1 or V2.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hangan, A.-M.-R. Possibilities of Using Some Vegetable Species with Multiple Uses in the Concept of Urban and Periurban Gardens. Ph.D Thesis, ”Ion Ionescu de la Brad” Iasi University of Life Sciences, Iasi, Romania, 28 January 2022. [Google Scholar]
- Xie, Q.; Yue, Y.; Hu, D. Residents’ Attention and Awareness of Urban Edible Landscapes: A Case Study of Wuhan, China. Forests 2019, 10, 1142. [Google Scholar] [CrossRef]
- Alloway, B.J. Contamination of Soils in Domestic Gardens and Allotments: A Brief Overview. Land Contam. Reclam. 2004, 12, 179–187. [Google Scholar] [CrossRef]
- Stoleru, V.; Sellitto, V.M. Pest Control in Organic Systems. In Integrated Pest Management (IPM): Environmentally Sound Pest Management; Intech: Rijeka, Croatia, 2016. [Google Scholar]
- Stoleru, V.; Munteanu, N.; Hura, C. Organophosphorus Pesticide Residues in Soil and Vegetable, through Different Growing Systems. Environ. Eng. Manag. J. 2015, 14, 1465–1473. [Google Scholar] [CrossRef]
- Gregory, M.M.; Leslie, T.W.; Drinkwater, L.E. Agroecological and Social Characteristics of New York City Community Gardens: Contributions to Urban Food Security, Ecosystem Services, and Environmental Education. Urban Ecosyst. 2016, 19, 763–794. [Google Scholar] [CrossRef]
- Vittori Antisari, L.; Ventura, F.; Simoni, A.; Piana, S.; Rossi Pisa, P.; Vianelloz, G. Assessment of Pollutants in Wet and Dry Deposition in a Suburban Area around a Wast-to-Energy Plants (WEP) in Norther Italy. J. Environ. Prot. 2013, 4, 16–25. [Google Scholar] [CrossRef]
- Leake, J.R.; Adam-Bradford, A.; Rigby, J.E. Health Benefits of ‘Grow Your Own’ Food in Urban Areas: Implications for Contaminated Land Risk Assessment and Risk Management? Environ. Health 2009, 8, S6. [Google Scholar] [CrossRef]
- Timofeev, I.; Kosheleva, N.; Kasimov, N. Health Risk Assessment Based on the Contents of Potentially Toxic Elements in Urban Soils of Darkhan, Mongolia. J. Environ. Manag. 2019, 242, 279–289. [Google Scholar] [CrossRef]
- Hangan, A.-M.-R.; Cojocaru, A.; Teliban, G.-C.; Amișculesei, P.; Stoleru, V. Preliminary Study Regarding the Use of Medicinal and Decorative Plants in the Concept of Peri-Urban Gardens with Role on Environmental Protection. Sci. Pap. Ser. B Hortic. 2020, 64, 389–396. [Google Scholar]
- Prime, H.; Wade, M.; Browne, D.T. Risk and Resilience in Family Well-Being during the COVID-19 Pandemic. Am. Phycol. 2020, 75, 631–643. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhang, Y.; Zhai, J. Home Garden with Eco-Healing Functions Benefiting Mental Health and Biodiversity During and after the COVID-19 Pandemic: A Scoping Review. Front. Public Health 2021, 9, 740187. [Google Scholar] [CrossRef] [PubMed]
- Palar, K.; Hufstedler, E.L.; Hernandez, K.; Chang, A.; Ferguson, L.; Lozano, R.; Weiser, S.D. Nutrition and Health Improvements after Participation in an Urban Home Garden Program. J. Nutr. Educ. Behav. 2019, 51, 1037–1046. [Google Scholar] [CrossRef]
- Abdulkadir, A.; Dossa, L.H.; Lompo, D.J.-P.; Abdu, N.; van Keulen, H. Characterization of Urban and Peri-Urban Agroecosystems in Three West African Cities. Int. J. Agric. Sustain. 2012, 10, 289–314. [Google Scholar] [CrossRef]
- Hamilton, A.J.; Burry, K.; Mok, H.-F.; Barker, S.F.; Grove, J.R.; Williamson, V.G. Give Peas a Chance? Urban Agriculture in Developing Countries. A Review. Agron. Sustain. Dev. 2014, 34, 45–73. [Google Scholar] [CrossRef]
- Specht, K.; Siebert, R.; Hartmann, I.; Freisinger, U.B.; Sawicka, M.; Werner, A.; Thomaier, S.; Henckel, D.; Walk, H.; Dierich, A. Urban Agriculture of the Future: An Overview of Sustainability Aspects of Food Production in and on Buildings. Agric. Hum. Values 2014, 31, 33–51. [Google Scholar] [CrossRef]
- Tei, F.; Benincasa, P.; Farneselli, M.; Caprai, M. Allotment Gardens for Senior Citizens in Italy: Current Status and Technical Proposals. Acta Hortic. 2010, 881, 91–96. [Google Scholar] [CrossRef]
- Olivier, D.W.; Heinecken, L. Beyond Food Security: Women’s Experiences of Urban Agriculture in Cape Town. Agric. Hum. Values 2017, 34, 743–755. [Google Scholar] [CrossRef]
- He, B.; Zhu, J. Constructing Community Gardens? Residents’ Attitude and Behaviour towards Edible Landscapes in Emerging Urban Communities of China. Urban For. Urban Green. 2018, 34, 154–165. [Google Scholar] [CrossRef]
- Fischer, L.; Brinkmeyer, D.; Karle-Bhat, S.J.; Cremer, K.; Huttner, E.; Seebauer, M.; Nowikow, U.; Schütze, B.; Voigt, P.; Völker, S.; et al. Biodiverse Edible Schools: Linking Healthy Food, School Gardens and Local Urban Biodiversity. Urban For. Urban Green. 2018, 40, 35–43. [Google Scholar] [CrossRef]
- Clarke, L.; Jenerette, D. Biodiversity and Direct Ecosystem Service Regulation in the Community Gardens of Los Angeles, CA. Landsc. Ecol. 2015, 30, 367–653. [Google Scholar] [CrossRef]
- Klepacki, P.; Kujawska, M. Urban Allotment Gardens in Poland: Implications for Botanical and Landscape Diversity. J. Ethnobiol. 2018, 38, 123–137. [Google Scholar] [CrossRef]
- Samtiya, M.; Aluko, R.E.; Dhewa, T.; Moreno-Rojas, J.M. Potential Health Benefits of Plant Food-Derived Bioactive Components: An Overview. Foods 2021, 10, 839. [Google Scholar] [CrossRef] [PubMed]
- Butnariu, M.; Butu, A. Chemical Composition of Vegetables and Their Products. In Handbook of Food Chemistry; Cheung, P.C.K., Mehta, B.M., Eds.; Springer: Berlin/Heidelberg, Germany, 2015; pp. 627–692. ISBN 978-3-642-36605-5. [Google Scholar]
- Dhingra, D.; Michael, M.; Rajput, H.; Patil, R.T. Dietary Fibre in Foods: A Review. J. Food Sci. Technol. 2012, 49, 255–266. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Healthy Diet; World Health Organization: Geneva, Switzerland, 2020. [Google Scholar]
- Stan, N.; Stan, T. Legumicultură Generală; “Ion Ionescu de la Brad”: Iasi, Romania, 2010. [Google Scholar]
- Mihalache, G.; Peres, C.I.; Bodale, I.; Achitei, V.; Gheorghitoaie, M.V.; Teliban, G.C.; Cojocaru, A.; Butnariu, M.; Muraru, V.; Stoleru, V. Tomato Crop Performances under Chemical Nutrients Monitored by Electric Signal. Agronomy 2020, 10, 1915. [Google Scholar] [CrossRef]
- Hailu, A.A.; Addis, G. The Content and Bioavailability of Mineral Nutrients of Selected Wild and Traditional Edible Plants as Affected by Household Preparation Methods Practiced by Local Community in Benishangul Gumuz Regional State, Ethiopia. Int. J. Food Sci. 2016, 2016, 7615853. [Google Scholar] [CrossRef] [PubMed]
- Oyetayo, F.L.; Ibitoye, M.F. Phytochemical and Nutrient/Antinutrient Interactions in Cherry Tomato (Lycopersicon esculentum) Fruits. Nutr. Health 2012, 21, 187–192. [Google Scholar] [CrossRef] [PubMed]
- Abbaspour, N.; Hurrell, R.; Kelishadi, R. Review on Iron and Its Importance for Human Health. J. Res. Med. Sci. 2014, 19, 164. [Google Scholar]
- Gemede, H.F.; Ratta, N. Antinutritional Factors in Plant Foods: Potential Health Benefits and Adverse Effects. Int. J. Nutr. Food Sci. 2014, 3, 284–289. [Google Scholar] [CrossRef]
- Franceschi, V.R.; Nakata, P.A. Calcium Oxalate in Plants: Formation and Function. Annu. Rev. Plant Biol. 2005, 56, 41. [Google Scholar] [CrossRef]
- Popova, A.; Mihaylova, D. Antinutrients in Plant-Based Foods: A Review. Open Biotechnol. J. 2019, 13, 68–76. [Google Scholar] [CrossRef]
- Choi, W.C.; Parr, T.; Lim, Y.S. The Impact of Four Processing Methods on Trypsin-, Chymotrypsin-and Alpha-Amylase Inhibitors Present in Underutilised Legumes. J. Food Sci. Technol. 2019, 56, 281–289. [Google Scholar] [CrossRef]
- Dilip, S.; Thomas, A.; Singh Malik, J. Attitudes of Students on School Vegetable Garden and Gardening Activities in Kerala. Indian J. Ext. Educ. 2020, 56, 89–92. [Google Scholar]
- Schmitt, S.; Bryant, L.; Korucu, I.; Kirkham, L.; Katare, B.; Benjamin, T. The Effects of a Nutrition Education Curriculum on Improving Young Children’s Fruit and Vegetable Preferences and Nutrition and Health Knowledge. Public Health Nutr. 2018, 22, 28–34. [Google Scholar] [CrossRef] [PubMed]
- Sarti, A.; Dijkstra, C.; Nury, E.; Seidell, J.; Dedding, C. ‘I Eat the Vegetables Because I Have Grown Them with My Own Hands’: Children’s Perspectives on School Gardening and Vegetable Consumption. Child. Soc. 2017, 31, 429–440. [Google Scholar] [CrossRef]
- McAleese, J.; Rankin, L. Garden-Based Nutrition Education Affects Fruit and Vegetable Consumption in Sixth-Grade Adolescents. J. Am. Diet. Assoc. 2007, 107, 662–665. [Google Scholar] [CrossRef] [PubMed]
- Gavrilescu, M. Water, Soil, and Plants Interactions in a Threatened Environment. Water 2021, 13, 2746. [Google Scholar] [CrossRef]
- Parker, J.E.; Snyder, W.; Hamilton, G.C.; Rodriguez-Saona, C. Companion Planting and Insect Pest Control. In Weed and Pest Control—Conventional and New Challenges; InTech: Rijeka, Croatia, 2013; pp. 1–30. [Google Scholar]
- He, M.; Wang, Y.; Wang, W.J.; Xie, Z. Therapeutic Plant Landscape Design of Urban Forest Parks Based on the Five Senses Theory: A Case Study of Stanley Park in Canada. Int. J. Geoherit. Park. 2022, 10, 97–112. [Google Scholar] [CrossRef]
- Sofo, A.; Sofo, A. Converting Home Spaces into Food Gardens at the Time of COVID-19 Quarantine: All the Benefits of Plants in This Difficult and Unprecedented Period. Hum. Ecol. 2020, 48, 131–139. [Google Scholar] [CrossRef]
- Stoleru, V.; Munteanu, N.; Sellitto, V.M. New Approach of Organic Vegetable Systems. Aracne Editrice 2014, 126–147. [Google Scholar]
- Stoleru, V.V.; Munteanu, N.C.; Stoleru, C.M.V.; Rotaru, L.G. Cultivar Selection and Pest Control Techniques on Organic White Cabbage Yield. Not. Bot. Horti Agrobot. Cluj-Napoca 2012, 40, 190–196. [Google Scholar] [CrossRef]
- Rusu, O.-R.; Mangalagiu, I.; Amăriucăi-Mantu, D.; Teliban, G.-C.; Cojocaru, A.; Burducea, M.; Mihalache, G.; Roșca, M.; Caruso, G.; Sekara, A.; et al. Interaction Effects of Cultivars and Nutrition on Quality and Yield of Tomato. Horticulturae 2023, 9, 541. [Google Scholar] [CrossRef]
- Galea, F.-M. Increasing the Ornamental Value of Vegetable Crops by Optimizing the Design in the Intercropping System. from Iasi, Romania. Ph.D. Thesis, “Ion Ionescu de la Brad” University of Agricultural Sciences and Veterinary Medicine, Iasi, Romania, 29 March 2018. [Google Scholar]
- Roy, R.N.; Finck, A.; Blair, G.; Tandon, H. Plant Nutrition for Food Security. A guide for integrated nutrient management. FAO Fertil. Plant Nutr. Bull. 2006, 16, 368. [Google Scholar]
- Tandon, H.L.S. Fertilizers in Indian Agriculture from 20th to 21st Century; Fertiliser Development and Consultation Organisation: Delhi, India, 2004; ISBN 81-85116-52-0. [Google Scholar]
- AOAC International. Official Methods of Analysis of AOAC International; Association of Official Analytical Chemists: Gaithersburg, MD, USA, 2005. [Google Scholar]
- AOAC International. Official Methods of Analysis of AOAC International, 17th ed.; Association of Official Analytical Chemists: Gaithersburg, MD, USA, 2000. [Google Scholar]
- Davis, A.R.; Fish, W.W.; Perkins-Veazie, P. A Rapid Spectrophotometric Method for Analyzing Lycopene Content in Tomato and Tomato Products. Postharvest Biol. Technol. 2003, 28, 425–430. [Google Scholar] [CrossRef]
- Cadoni, E.; De Giorgi, M.R.; Medda, E.; Poma, G. Supercritical CO2 Extraction of Lycopene and β-Carotene from Ripe Tomatoes. Dye. Pigment. 1999, 44, 27–32. [Google Scholar] [CrossRef]
- Caruso, G.; De Pascale, S.; Cozzolino, E.; Cuciniello, A.; Cenvinzo, V.; Bonini, P.; Colla, G.; Rouphael, Y. Yield and Nutritional Quality of Vesuvian Piennolo Tomato PDO as Affected by Farming System and Biostimulant Application. Agronomy 2019, 9, 505. [Google Scholar] [CrossRef]
- Gheorghitoaie, M.-V.; Bodale, I.; Achitei, V.; Teliban, G.-C.; Cojocaru, A.; Caruso, G.; Mihalache, G.; Stoleru, V. Potential of Continuous Electric Current on Biometrical, Physiological and Quality Characteristics of Organic Tomato. Appl. Sci. 2022, 12, 4211. [Google Scholar] [CrossRef]
- Shariff, A.H.M.; Wahab, P.N.Z.M.M.A.; Jahurul, A.H.; Huda, N.; Romes, N.B.; Zakaria, M.; Roslan, J.; Wahab, R.A.; Huyop, F. Nutrient Composition, Total Phenolic Content, and Antioxidant Activity of Tropical Kundasang-Grown Cucumber at Two Growth Stages. Chil. J. Agric. Res. 2021, 81, 220–227. [Google Scholar] [CrossRef]
- Ozgur, M.; Akpinar-Bayizit, A.; Ozcan, T.; Yilmaz-Ersan, L. Effect of Dehydration on Several Physico-Chemical Properties and the Antioxidant Activity of Leeks (Allium Porrum L.). Not. Bot. Horti Agrobot. Cluj-Napoca 2011, 39, 144–151. [Google Scholar] [CrossRef]
- Stoleru, V.; Inculet, S.-C.; Mihalache, G.; Cojocaru, A.; Teliban, G.-C.; Caruso, G. Yield and Nutritional Response of Greenhouse Grown Tomato Cultivars to Sustainable Fertilization and Irrigation Management. Plants 2020, 9, 1053. [Google Scholar] [CrossRef]
- Inculet, C.-S.; Mihalache, G.; Sellitto, V.M.; Hlihor, R.-M.; Stoleru, V. The Effects of a Microorganisms-Based Commercial Product on the Morphological, Biochemical and Yield of Tomato Plants under Two Different Water Regimes. Microorganisms 2019, 7, 706. [Google Scholar] [CrossRef]
- Goldbohm, R.; Brants, H.; Hulshof, K.; Van den Brandt, P.A. The Contribution of Various Foods to Intake of Vitamin A and Carotenoids in The Netherlands. Int. J. Vitam. Nutr. Res. 1998, 68, 378–383. [Google Scholar]
- Pelz, R.; Schmidt-Faber, B.; Heseker, H. Carotenoid Intake in the German National Food Consumption Survey. Z. Für Ernähr. 1998, 37, 319–327. [Google Scholar] [CrossRef] [PubMed]
- VandenLangenberg, G.M.; Brady, W.E.; Nebeling, L.C.; Block, G.; Forman, M.; Bowen, P.E.; Stacewicz-Sapuntzakis, M.; Mares-Perlman, J.A. Influence of Using Different Sources of Carotenoid Data in Epidemiologic Studies. J. Am. Diet Assoc. 1996, 96, 1271–1275. [Google Scholar] [CrossRef]
- Rao, A.; Waseem, Z.; Agarwal, S. Lycopene Content of Tomatoes and Tomato Products and Their Contribution to Dietary Lycopene. Food Res. Int. 1998, 31, 737–741. [Google Scholar] [CrossRef]
- Dzhos, E.; Golubkina, N.; Antoshkina, M.; Kondratyeva, I.; Koshevarov, A.; Shkaplerov, A.; Zavarykina, T.; Nechitailo, G.; Caruso, G. Effect of Spaceflight on Tomato Seed Quality and Biochemical Characteristics of Mature Plants. Horticulturae 2021, 7, 89. [Google Scholar] [CrossRef]
- Inthichack, P.; Nishimura, Y.; Fukumoto, Y. Diurnal Temperature Alternations on Plant Growth and Mineral Absorption in Eggplant, Sweet Pepper, and Tomato. Hortic. Environ. Biotechnol. 2013, 54, 37–43. [Google Scholar] [CrossRef]
- Noor, R.S.; Wang, Z.; Umair, M.; Yaseen, M.; Ameen, M.; Rehman, S.-U.; Khan, M.U.; Imran, M.; Ahmed, W.; Sun, Y. Interactive Effects of Grafting Techniques and Scion-Rootstocks Combinations on Vegetative Growth, Yield and Quality of Cucumber (Cucumis Sativus L.). Agronomy 2019, 9, 288. [Google Scholar] [CrossRef]
- Mi, S.; Zhang, X.; Wang, Y.; Ma, Y.; Sang, Y.; Wang, X. Effect of Different Fertilizers on the Physicochemical Properties, Chemical Element and Volatile Composition of Cucumbers. Food Chem. 2022, 367, 130667. [Google Scholar] [CrossRef] [PubMed]
- Schlering, C.; Schweiggert, R.; Dietrich, H.; Frisch, M.; Zinkernagel, J. Effects of Moderately-Reduced Water Supply and Picking Time on the Chemical Composition of Pickling Cucumber (Cucumis Sativus L.) in Open Field Cultivation. Agronomy 2020, 10, 1097. [Google Scholar] [CrossRef]
- Thavarajah, D.; Lawrence, T.; Powers, S.; Jones, B.; Johnson, N.; Kay, J.; Bandaranayake, A.; Shipe, E.; Thavarajah, P. Genetic Variation in the Prebiotic Carbohydrate and Mineral Composition of Kale (Brassica Oleracea L. Var. Acephala) Adapted to an Organic Cropping System. J. Food Compos. Anal. 2021, 96, 103718. [Google Scholar] [CrossRef]
- Rossini-Oliva, S.; López-Núñez, R. Potential Toxic Elements Accumulation in Several Food Species Grown in Urban and Rural Gardens Subjected to Different Conditions. Agronomy 2021, 11, 2151. [Google Scholar] [CrossRef]
- Golubkina, N.A.; Seredin, T.M.; Antoshkina, M.S.; Kosheleva, O.V.; Teliban, G.C.; Caruso, G. Yield, Quality, Antioxidants and Elemental Composition of New Leek Cultivars under Organic or Conventional Systems in a Greenhouse. Horticulturae 2018, 4, 39. [Google Scholar] [CrossRef]
- Koca, I.; Tasci, B. Mineral Composition of Leek. In Proceedings of the VII Symposium on Edible Alliaceae, Nigde, Turkey, 21–25 May 2015; pp. 147–152. [Google Scholar]
- Eppendorfer, W.; Eggum, B. Fertilizer Effects on Yield, Mineral and Amino Acid Composition, Dietary Fibre Content and Nutritive Value of Leeks. Plant Foods Hum. Nutr. 1996, 49, 163–174. [Google Scholar] [CrossRef] [PubMed]
- Sarikurkcu, C.; Targan, S.; Ozer, M.S.; Tepe, B. Fatty Acid Composition, Enzyme Inhibitory, and Antioxidant Activities of the Ethanol Extracts of Selected Wild Edible Plants Consumed as Vegetables in the Aegean Region of Turkey. Int. J. Food Prop. 2017, 20, 560–572. [Google Scholar] [CrossRef]

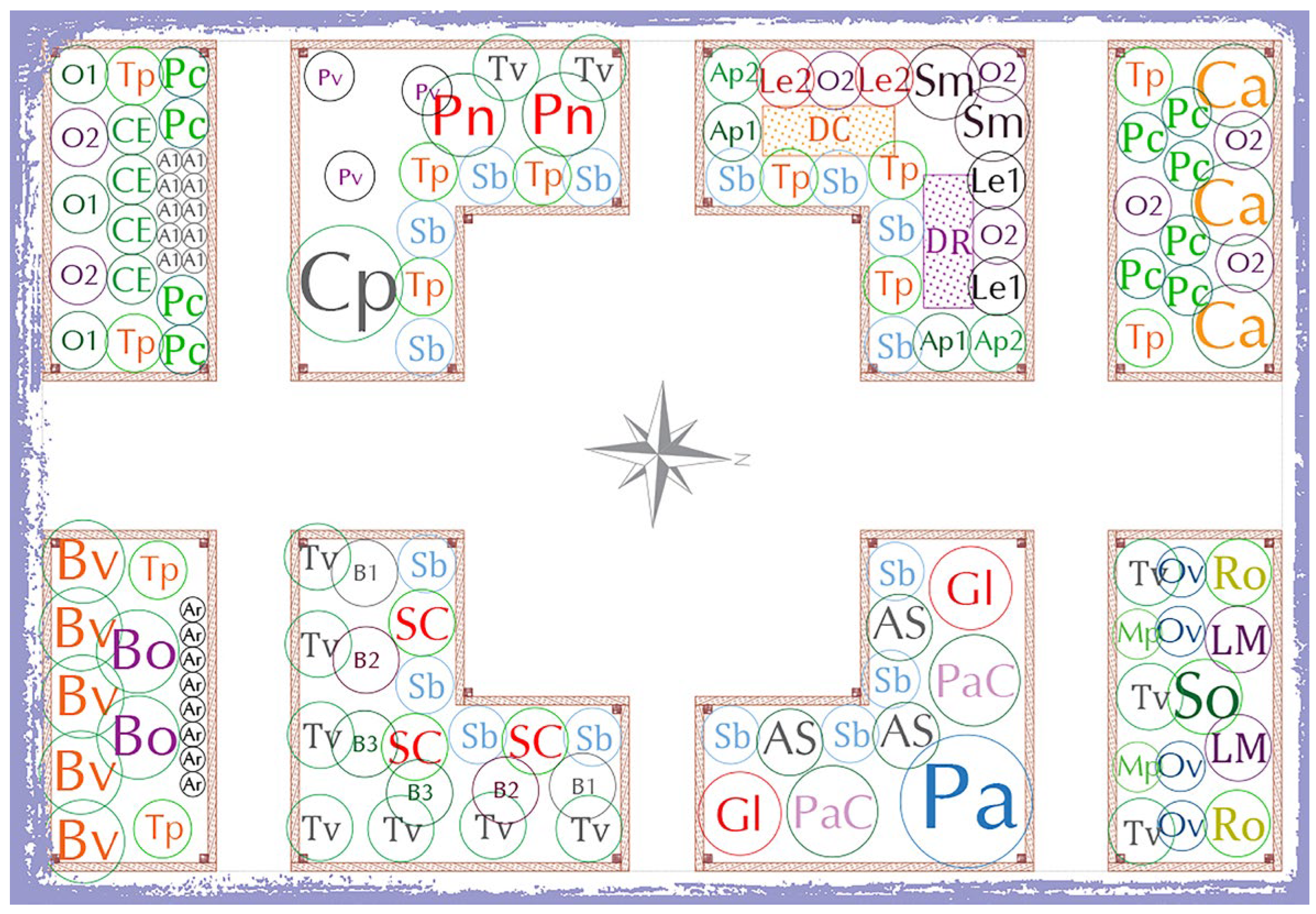
| Abbreviation | Unit | QTY. | Common Name and Cultivar |
|---|---|---|---|
| A1 | pcs. | 10 | white onion ‘Di Parma’ |
| Ap1 | pcs. | 2 | celeriac ‘Giant Prague’ |
| Ap2 | pcs. | 2 | celery ‘Gigante Dorato 2’ |
| Ar | pcs. | 8 | leek ‘Blue de Solaise’ |
| AS | pcs. | 3 | New York aster ‘Starshine’ |
| B1 | pcs. | 2 | kale ‘Nero Di Toscana’ |
| B2 | pcs. | 2 | kale ‘Scarlet’ |
| B3 | pcs. | 2 | kale ‘Kadet’ |
| Bo | pcs. | 2 | cauliflower ‘Clapton F1’ |
| Bv | pcs. | 5 | chard ‘Bright Lights’ |
| Ca | pcs. | 3 | sweet pepper ‘Barbara’ |
| CE | pcs. | 4 | cucumber ‘Ekol’ |
| Cp | pcs. | 1 | patty pan squash ‘Óvári Fehér’ |
| DC | m2 | 0.5 | carrot ‘Cosmic Purple’ |
| DR | m2 | 0.5 | carrot ‘Royal Chantenay’ |
| Gl | pcs. | 2 | butterfly bush ‘Gaudi Red’ |
| Le1 | pcs. | 2 | tomato ‘Black Cherry’ |
| Le2 | pcs. | 2 | tomato ‘Tigerella’ |
| LM | pcs. | 2 | lavender ‘Munstead’ |
| Mp | pcs. | 2 | mint ‘Cinderella’ |
| O1 | pcs. | 3 | basil ‘Italiano Classico Genovese’ |
| O2 | pcs. | 8 | basil ‘Serafim’ |
| Ov | pcs. | 4 | oregano ‘Kreta’ |
| Pa | pcs. | 1 | Russian sage ‘Little Spire’ |
| PaC | pcs. | 2 | fountain grass ‘Cassian’ |
| Pc | pcs. | 10 | parsley ‘Triple Moss Curled’ |
| Pn | nest | 2 | dwarf bean ‘Nano Supernano Giallo’ |
| Pv | nest | 3 | common bean ‘Violeta de Iasi’ |
| Ro | pcs. | 2 | rosemary ‘Green Ginger’ |
| Sb | pcs. | 16 | lamb’s ear ‘Silver Carpet’ |
| SC | pcs. | 3 | woodland sage ‘Caradonna’ |
| Sm | pcs. | 2 | eggplant ‘Black Beauty’ |
| So | pcs. | 1 | common sage ‘Chrestensen’ |
| Tp | pcs. | 12 | French marigold ‘Nana’ |
| Tv | pcs. | 12 | thyme ‘Di Provenza’ |
| Macroelements | V1 and V2 Substrate (d.w.) | V3 Soil (d.w.) |
|---|---|---|
| N | 0.39% | 0.28% |
| P2O5 | 0.24% | 0.32% |
| K2O | 2.05% | 0.21% |
| CaO | 3.12% | 0.41% |
| MgO | 0.60% | 0.27% |
| Microelements | V1 and V2 Substrate (d.w.) | V3 Soil (d.w.) |
|---|---|---|
| MnO | 0.10% | 0.02% |
| Fe2O3 | 0.36% | 0.74% |
| Na2O | 0.58% | 0.39% |
| Cd | 0 ppm | 1.2 ppm |
| Co | 8 ppm | 0 ppm |
| Cr | 80 ppm | 11 ppm |
| Cu | 39 ppm | 43 ppm |
| Mo | 6 ppm | 7 ppm |
| Ni | 35 ppm | 16 ppm |
| Pb | 96 ppm | 74 ppm |
| Zn | 118 ppm | 127 ppm |
| Month and Year | Temperature (°C) | Sunshine Duration (hours) | Rainfall (mm) | Relative Humidity (%) | ||
|---|---|---|---|---|---|---|
| Monthly Average | Monthly Maximum | Monthly Minimum | ||||
| Mar. 2020 | 7.2 | 20.8 | −5.9 | 191.0 | 15.6 | 63.1 |
| Apr. 2020 | 11.1 | 26.9 | −5.9 | 279.8 | 1.6 | 42.0 |
| May. 2020 | 14.4 | 30.1 | 3.5 | 178.2 | 130.5 | 67.0 |
| Jun. 2020 | 21.3 | 33.3 | 6.1 | 235.7 | 99.0 | 71.0 |
| Jul. 2020 | 22.1 | 33.9 | 10.6 | 187.4 | 7.9 | 61.4 |
| Aug. 2020 | 23.6 | 32.3 | 14.9 | 289.6 | 8.8 | 54.1 |
| Sept. 2020 | 19.5 | 31.4 | 8.5 | 264.1 | 24.2 | 59.9 |
| Oct. 2020 | 14.1 | 24.4 | 1.7 | 129.5 | 75.4 | 82.6 |
| Mar. 2021 | 3.5 | 18.1 | −6.4 | 163.4 | 48.4 | 72.9 |
| Apr. 2021 | 8.4 | 24.6 | −3.2 | 183.7 | 40.4 | 68.2 |
| May. 2021 | 15.5 | 27.7 | 3.0 | 212.8 | 62.7 | 69.9 |
| Jun. 2021 | 20.3 | 33.8 | 10.1 | 218.0 | 104.4 | 75.7 |
| Jul. 2021 | 23.3 | 35.9 | 13.4 | 283.7 | 50.3 | 72.8 |
| Aug. 2021 | 21.0 | 34.5 | 11.7 | 265.1 | 132.4 | 73.8 |
| Sept. 2021 | 14.6 | 27.3 | 2.7 | 188.3 | 6.6 | 73.7 |
| Oct. 2021 | 12.3 | 22.6 | −1.4 | 134.5 | 15.8 | 71.2 |
| TOTAL | 15.8 | 28.6 | 4.0 | 3404.8 | 824.0 | 67.5 |
| Cultivar | Dry Weight (%) | Moisture (%) | ||||
|---|---|---|---|---|---|---|
| V1 | V2 | V3 | V1 | V2 | V3 | |
| tomato ‘Tigerella ‘ | 5.99 ± 0.53 ns | 8.75 ± 0.78 ns | 6.34 ± 0.56 ns | 94.01 ± 0.53 ns | 91.25 ± 0.78 ns | 93.66 ± 0.56 ns |
| tomato ‘Black Cherry’ | 8.96 ± 0.80 ns | 9.10 ± 0.81 ns | 9.20 ± 0.82 ns | 91.04 ± 0.80 ns | 90.90 ± 0.81 ns | 90.80 ± 0.82 ns |
| eggplant ‘Black Beauty’ | 7.99 ± 0.71 ns | 7.27 ± 0.65 ns | 7.34 ± 0.66 ns | 92.01 ± 0.71 ns | 92.73 ± 0.65 ns | 92.66 ± 0.66 ns |
| sweet pepper ‘Barbara’ | 8.41 ± 0.75 ab | 6.60 ± 0.59 b | 10.28 ± 0.92 a | 91.59 ± 0.75 ab | 93.40 ± 0.59 a | 89.72 ± 0.92 b |
| cucumber ‘Ekol’ | 4.04 ± 0.36 ns | 3.57 ± 0.32 ns | 4.40 ± 0.39 ns | 95.96 ± 0.36 ns | 96.43 ± 0.32 ns | 95.60 ± 0.39 ns |
| dwarf bean ‘Nano Supernano Gialo’ | 10.08 ± 0.90 ns | 11.3 ± 1.01 ns | 11.80 ± 1.05 ns | 89.92 ± 0.90 ns | 88.7 ± 1.01 ns | 88.2 ± 1.05 ns |
| common bean ‘Violeta de Iasi’ | 10.05 ± 0.89 ns | 10.86 ± 0.97 ns | 11.05 ± 0.98 ns | 89.95 ± 0.89 ns | 89.14 ± 0.97 ns | 88.95 ± 0.98 ns |
| Cultivar | Dry Weight %) | Moisture (%) | ||||
|---|---|---|---|---|---|---|
| V1 | V2 | V3 | V1 | V2 | V3 | |
| kale ‘Nero Di Toscana’ | 13.42 ± 1.20 ns | 13.22 ± 1.18 ns | 12.35 ± 1.10 ns | 86.58 ± 1.20 ns | 86.78 ± 1.18 ns | 87.65 ± 1.10 ns |
| chard ‘Bright Lights’ | 6.53 ± 0.58 ns | 7.21 ± 0.64 ns | 6.96 ± 0.62 ns | 93.47 ± 0.58 ns | 92.79 ± 0.64 ns | 93.04 ± 0.62 ns |
| leek ‘Blue de Solaise’ | 9.97 ± 0.89 ns | 10.87 ± 0.97 ns | 9.26 ± 0.83 ns | 90.03 ± 0.89 ns | 89.13 ± 0.97 ns | 90.74 ± 0.83 ns |
| parsley ‘Triple Moss Curled’ | 15.53 ± 1.39 ns | 17.34 ± 1.55 ns | 20.12 ± 1.8 ns | 84.47 ± 1.39 ns | 82.66 ± 1.55 ns | 79.88 ± 1.80 ns |
| Primary Compounds | T. | Tomato ‘Tigerella’ | Tomato ‘Black Cherry’ | Eggplant ‘Black Beauty’ | Sweet Pepper ‘Barbara’ | Cucumber ‘Ekol’ | Dwarf Bean ‘Nano Supernano Gialo’ | Common Bean ‘Violeta de Iasi’ |
|---|---|---|---|---|---|---|---|---|
| Crude lipid (g/100 g d.w.) | V1 | 11.29 ± 0.98 ns | 11.22 ± 1.00 ns | 11.68 ± 1.23 ns | 11.51 ± 0.91 ns | 9.96 ± 0.95 ns | 12.39 ± 0.99 ns | 12.37 ± 0.67 ns |
| V2 | 11.36 ± 1.02 ns | 11.24 ± 1.00 ns | 11.2 ± 1.00 ns | 11.85 ± 1.06 ns | 10.03 ± 0.99 ns | 12.46 ± 1.03 ns | 13.68 ± 1.22 ns | |
| V3 | 11.85 ± 1.07 ns | 11.36 ± 1.02 ns | 11.24 ± 1.01 ns | 11.2 ± 1.01 ns | 10.52 ± 1.04 ns | 12.95 ± 1.08 ns | 9.99 ± 0.90 ns | |
| Ash (g/100 g d.w.) | V1 | 4.39 ± 0.51 ns | 4.38 ± 0.35 ns | 4.68 ± 0.56 ns | 4.30 ± 0.22 ns | 4.09 ± 0.48 ns | 4.74 ± 0.54 ns | 5.03 ± 0.27 ns |
| V2 | 4.10 ± 0.37 ns | 4.50 ± 0.40 ns | 4.33 ± 0.39 ns | 4.82 ± 0.43 ns | 4.80 ± 0.34 ns | 4.45 ± 0.41 ns | 5.56 ± 0.50 ns | |
| V3 | 4.81 ± 0.43 ns | 4.10 ± 0.37 ns | 4.49 ± 0.40 ns | 4.33 ± 0.39 ns | 4.51 ± 0.40 ns | 5.16 ± 0.46 ns | 4.06 ± 0.36 ns | |
| Crude protein (g/100 g d.w.) | V1 | 28.48 ± 2.42 ns | 28.37 ± 2.57 ns | 29.54 ± 4.29 ns | 35.26 ± 4.77 ns | 27.97 ± 3.32 ns | 32.92 ± 3.37 ns | 32.52 ± 1.77 ns |
| V2 | 28.84 ± 2.58 ns | 28.31 ± 2.53 ns | 25.37 ± 2.27 ns | 31.15 ± 2.79 ns | 26.81 ± 2.79 ns | 31.84 ± 2.85 ns | 35.95 ± 3.22 ns | |
| V3 | 31.14 ± 2,80 ns | 28,83 ± 2,59 ns | 31,3 ± 2,81 ns | 29,36 ± 2,64 ns | 26,16 ± 2,75 ns | 31,14 ± 2,80 ns | 26,25 ± 2,36 ns | |
| Crude fibre (g/100 g d.w.) | V1 | 14.82 ± 1.89 b | 18.66 ± 2.98 ns | 22.14 ± 2.85 ns | 16.09 ± 1.76 ns | 24.82 ± 2.79 ns | 22.80 ± 1.97 b | 24.00 ± 1.31 ns |
| V2 | 13.38 ± 1.02 b | 15.37 ± 1.38 ns | 19.94 ± 1.78 ns | 22.98 ± 2.06 ns | 23.37 ± 2.09 ns | 21.36 ± 1.28 b | 26.52 ± 2.38 ns | |
| V3 | 22.98 ± 2.06 a | 13.38 ± 1.20 ns | 15.37 ± 1.38 ns | 19.93 ± 1.79 ns | 22.98 ± 2.06 ns | 30.97 ± 2.14 a | 19.37 ± 1.74 ns | |
| Dietary fibrfiber 100 g d.w.) | V1 | 8.71 ± 1.58 ns | 8.19 ± 0.36 ab | 8.10 ± 0.89 ns | 7.16 ± 0.32 ns | 6.55 ± 0.53 ns | 9.71 ± 1.59 ns | 8.63 ± 0.47 ns |
| V2 | 6.71 ± 0.60 ns | 9.48 ± 0.85 a | 7.67 ± 0.69 ns | 8.26 ± 0.74 ns | 6.71 ± 0.60 ns | 7.71 ± 0.61 ns | 9.53 ± 0.85 ns | |
| V3 | 8.26 ± 0.74 ns | 6.71 ± 0.60 b | 9.48 ± 0.85 ns | 7.67 ± 0.69 ns | 6.27 ± 0.56 ns | 9.26 ± 0.75 ns | 6.97 ± 0.63 ns | |
| Nitrogen (g/100 g d.w.) | V1 | 37.17 ± 4.16 ns | 43.77 ± 6.21 ns | 48.91 ± 5.50 ns | 39.27 ± 2.16 ns | 32.17 ± 4.11 ns | 42.36 ± 4.24 ns | 52.20 ± 2.84 ns |
| V2 | 35.04 ± 3.14 ns | 37.96 ± 3.40 ns | 46.01 ± 4.12 ns | 50.00 ± 4.47 ns | 30.04 ± 3.09 ns | 40.18 ± 3.19 ns | 57.70 ± 5.17 ns | |
| V3 | 49.98 ± 4.49 ns | 35.03 ± 3.15 ns | 37.95 ± 3.41 ns | 45.99 ± 4.13 ns | 44.98 ± 4.44 ns | 55.12 ± 4.54 ns | 42.13 ± 3.79 ns | |
| Caloric value kJ/g (kcal/100 g d.w.) | V1 | 438.98 ± 41.03 ns | 425.56 ± 36.48 ns | 423.28 ± 38.37 ns | 431.78 ± 42.00 ns | 138.98 ± 14.02 ns | 448.96 ± 42.03 ns | 440.85 ± 24.02 ns |
| V2 | 434.72 ± 38.93 ns | 430.24 ± 38.53 ns | 422.33 ± 37.82 ns | 423.26 ± 37.90 ns | 134.92 ± 12.08 ns | 444.78 ± 39.93 ns | 487.28 ± 43.64 ns | |
| V3 | 432.11 ± 38.83 ns | 434.57 ± 39.06 ns | 430.1 ± 38.65 ns | 432.18 ± 38.84 ns | 122.42 ± 11.00 ns | 422.12 ± 37.94 ns | 355.85 ± 31.98 ns |
| Primary Compounds | T. | Kale ‘Nero Di Toscana’ | Chard ‘Bright Lights’ | Leek ‘Blue de Solaise’ | Parsley ‘Triple Moss Curled’ |
|---|---|---|---|---|---|
| Crude lipid (g/100 g d.w.) | V1 | 13.65 ± 1.25 ns | 13.51 ± 0.93 ns | 9.68 ± 1.21 ns | 9.51 ± 0.89 ns |
| V2 | 13.21 ± 1.02 ns | 13.85 ± 1.08 ns | 9.20 ± 0.98 ns | 9.85 ± 1.04 ns | |
| V3 | 13.23 ± 1.03 ns | 13.20 ± 1.03 ns | 9.24 ± 0.99 ns | 9.20 ± 0.99 ns | |
| Ash (g/100 g d.w.) | V1 | 4.68 ± 0.56 ns | 4.30 ± 0.22 ns | 6.67 ± 0.58 ns | 6.31 ± 0.24 ns |
| V2 | 4.33 ± 0.39 ns | 4.82 ± 0.43 ns | 6.32 ± 0.41 ns | 6.83 ± 0.45 ns | |
| V3 | 4.49 ± 0.40 ns | 4.33 ± 0.39 ns | 6.48 ± 0.42 ns | 6.34 ± 0.41 ns | |
| Crude protein (g/100 g d.w.) | V1 | 32.67 ± 3.27 ns | 32.67 ± 3.02 ns | 30.65 ± 3.25 ns | 31.62 ± 3.04 ns |
| V2 | 31.37 ± 2.65 ns | 29.15 ± 2.77 ns | 29.34 ± 2.63 ns | 31.12 ± 2.79 ns | |
| V3 | 33.30 ± 2.99 ns | 29.36 ± 2.64 ns | 31.30 ± 2.81 ns | 25.36 ± 2.28 ns | |
| Crude fibre (g/100 g d.w.) | V1 | 20.12 ± 2.83 ns | 14.08 ± 1.74 ns | 24.14 ± 2.87 ns | 18.09 ± 1.78 ns |
| V2 | 17.93 ± 1.76 ns | 20.97 ± 2.04 ns | 21.94 ± 1.80 ns | 24.98 ± 2.08 ns | |
| V3 | 13.36 ± 1.36 ns | 16.91 ± 1.77 ns | 17.37 ± 1.40 ns | 21.93 ± 1.81 ns | |
| Dietary fibre (g/100 g d.w.) | V1 | 6.09 ± 0.87 ns | 5.15 ± 0.30 ns | 10.10 ± 0.91 ns | 9.16 ± 0.34 ns |
| V2 | 5.66 ± 0.67 ns | 6.24 ± 0.72 ns | 9.67 ± 0.71 ns | 10.26 ± 0.76 ns | |
| V3 | 7.46 ± 0.83 ns | 5.65 ± 0.67 ns | 11.48 ± 0.87 ns | 9.67 ± 0.71 ns | |
| Nitrogen (g/100 g d.w.) | V1 | 50.93 ± 5.52 ns | 41.29 ± 2.18 ns | 46.91 ± 5.48 ns | 37.27 ± 2.14 ns |
| V2 | 48.03 ± 4.14 ns | 52.02 ± 4.49 ns | 44.01 ± 4.10 ns | 48.00 ± 4.45 ns | |
| V3 | 39.97 ± 3.43 ns | 47.99 ± 4.15 ns | 35.95 ± 3.39 ns | 43.99 ± 4.11 ns | |
| Caloric value kJ/g (kcal/100 g d.w.) | V1 | 420.54 ± 38.02 ns | 429.48 ± 42.24 ns | 424.56 ± 38.06 ns | 433.50 ± 42.28 ns |
| V2 | 420.31 ± 37.80 ns | 420.24 ± 37.79 ns | 424.33 ± 37.84 ns | 425.26 ± 37.83 ns | |
| V3 | 438.07 ± 39.53 ns | 420.17 ± 37.92 ns | 442.09 ± 39.57 ns | 425.19 ± 37.96 ns |
| Carotenoids | T. | Tomato ‘Tigerella’ | Tomato ‘Black Cherry’ | Eggplant ‘Black Beauty’ | Sweet Pepper ‘Barbara’ |
|---|---|---|---|---|---|
| Lycopene (µg/mg d.w.) | V1 | 8.62 ± 0.16 b | 8.77 ± 0.08 a | 2.46 ± 0.09 ns | 3.78 ± 0.11 c |
| V2 | 9.54 ± 0.06 a | 5.60 ± 0.14 b | 2.46 ± 0.12 ns | 4.83 ± 0.05 a | |
| V3 | 9.74 ± 0.09 a | 8.93 ± 0.01 a | 2.55 ± 0.14 ns | 4.56 ± 0.01 b | |
| ß-carotene (µg/mg d.w.) | V1 | 7.57 ± 0.06 ns | 7.55 ± 0.09 ns | 2.32 ± 0.04 b | 6.51 ± 0.00 b |
| V2 | 7.51 ± 0.03 ns | 7.52 ± 0.03 ns | 2.54 ± 0.04 a | 6.59 ± 0.02 a | |
| V3 | 7.61 ± 0.02 ns | 7.46 ± 0.06 ns | 2.57 ± 0.05 a | 6.64 ± 0.03 a |
| Macroelements and Microelements | T. | Tomato ‘Tigerella’ | Tomato ‘Black Cherry’ | Eggplant ‘Black Beauty’ | Sweet Pepper ‘Barbara’ | Cucumber ‘Ekol’ | Dwarf Bean ‘Nano Supernano Gialo’ | Common Bean ‘Violeta de Iasi’ |
|---|---|---|---|---|---|---|---|---|
| Fe (ppm) | V1 | 1.81 ± 0.23 ns | 1.65 ± 0.09 ns | 1.39 ± 0.08 ns | 1.17 ± 0.07 ns | 0.99 ± 0.05 ns | 3.00 ± 0.16 ns | 2.53 ± 0.14 ns |
| V2 | 1.62 ± 0.14 ns | 1.82 ± 0.16 ns | 1.54 ± 0.14 ns | 1.30 ± 0.12 ns | 1.09 ± 0.10 ns | 3.31 ± 0.29 ns | 2.79 ± 0.25 ns | |
| V3 | 1.58 ± 0.14 ns | 1.33 ± 0.12 ns | 1.12 ± 0.10 ns | 0.95 ± 0.08 ns | 0.80 ± 0.07 ns | 2.42 ± 0.22 ns | 2.04 ± 0.18 ns | |
| Ca (ppm) | V1 | 62.68 ± 4.67 ns | 72.67 ± 3.96 ns | 61.26 ± 3.34 ns | 51.64 ± 2.81 ab | 43.53 ± 2.37 ab | 73.01 ± 3.98 ab | 61.55 ± 3.35 a |
| V2 | 65.31 ± 5.85 ns | 80.32 ± 7.19 ns | 67.71 ± 6.06 ns | 57.08 ± 5.11 a | 48.12 ± 4.31 a | 80.70 ± 7.23 a | 68.03 ± 6.09 a | |
| V3 | 69.58 ± 6.26 ns | 58.66 ± 5.27 ns | 49.45 ± 4.44 ns | 41.68 ± 3.75 b | 35.14 ± 3.16 b | 58.93 ± 5.30 b | 49.68 ± 4.47 b | |
| Mg (ppm) | V1 | 28.12 ± 4.41 ns | 22.28 ± 1.21 ns | 18.79 ± 1.02 ns | 15.84 ± 0.86 ns | 13.35 ± 0.73 ns | 26.78 ± 1.46 ns | 22.58 ± 1.23 ns |
| V2 | 23.35 ± 2.09 ns | 24.63 ± 2.21 ns | 20.77 ± 1.86 ns | 17.50 ± 1.57 ns | 14.76 ± 1.32 ns | 29.60 ± 2.65 ns | 24.96 ± 2.23 ns | |
| V3 | 21.34 ± 1.92 ns | 17.99 ± 1.62 ns | 15.17 ± 1.36 ns | 12.79 ± 1.15 ns | 10.78 ± 0.97 ns | 21.62 ± 1.94 ns | 18.22 ± 1.64 ns | |
| Zn (ppm) | V1 | 0.52 ± 0.12 ns | 0.55 ± 0.03 ns | 0.47 ± 0.03 ns | 0.40 ± 0.02 ns | 0.33 ± 0.02 ns | 0.59 ± 0.03 ns | 0.49 ± 0.03 ns |
| V2 | 0.33 ± 0.03 ns | 0.61 ± 0.05 ns | 0.52 ± 0.04 ns | 0.43 ± 0.04 ns | 0.37 ± 0.03 ns | 0.65 ± 0.06 ns | 0.54 ± 0.05 ns | |
| V3 | 0.53 ± 0.05 ns | 0.45 ± 0.04 ns | 0.38 ± 0.03 ns | 0.32 ± 0.03 ns | 0.27 ± 0.02 ns | 0.47 ± 0.04 ns | 0.40 ± 0.03 ns | |
| K (ppm) | V1 | 1.88 ± 0.23 ns | 1.21 ± 0.07 ns | 1.02 ± 0.06 ns | 0.86 ± 0.05 ns | 0.72 ± 0.04 ns | 1.60 ± 0.09 ns | 1.35 ± 0.07 ns |
| V2 | 1.72 ± 0.15 ns | 1.34 ± 0.12 ns | 1.13 ± 0.10 ns | 0.95 ± 0.09 ns | 0.80 ± 0.07 ns | 1.77 ± 0.16 ns | 1.49 ± 0.13 ns | |
| V3 | 1.16 ± 0.10 ns | 0.98 ± 0.09 ns | 0.82 ± 0.08 ns | 0.69 ± 0.06 ns | 0.59 ± 0.05 ns | 1.29 ± 0.12 ns | 1.09 ± 0.10 ns | |
| Na (ppm) | V1 | 1.33 ± 0.11 ns | 1.41 ± 0.08 ns | 1.19 ± 0.07 ns | 1.00 ± 0.06 ns | 0.84 ± 0.04 ns | 1.43 ± 0.08 ns | 1.20 ± 0.07 ns |
| V2 | 1.35 ± 0.12 ns | 1.56 ± 0.14 ns | 1.31 ± 0.12 ns | 1.11 ± 0.10 ns | 0.93 ± 0.08 ns | 1.58 ± 0.14 ns | 1.33 ± 0.12 ns | |
| V3 | 1.35 ± 0.12 ns | 1.14 ± 0.10 ns | 0.96 ± 0.09 ns | 0.81 ± 0.07 ns | 0.68 ± 0.06 ns | 1.15 ± 0.10 ns | 0.97 ± 0.09 ns |
| Macroelements and Microelements | T. | Kale ‘Nero Di Toscana’ | Chard ‘Bright Lights’ | Leek ‘Blue de Solaise’ | Parsley ‘Triple Moss Curled’ |
|---|---|---|---|---|---|
| Fe (ppm) | V1 | 0.70 ± 0.04 ns | 1.61 ± 0.16 b | 2.13 ± 0.12 ns | 1.79 ± 0.10 ns |
| V2 | 0.78 ± 0.07 ns | 1.58 ± 0.14 b | 2.35 ± 0.21 ns | 1.98 ± 0.18 ns | |
| V3 | 0.57 ± 0.05 ns | 2.87 ± 0.26 a | 1.72 ± 0.15 ns | 1.45 ± 0.13 ns | |
| Ca (ppm) | V1 | 30.94 ± 1.69 ns | 66.56 ± 4.88 ns | 51.89 ± 2.83 ns | 43.74 ± 2.38 a |
| V2 | 34.20 ± 3.06 ns | 69.60 ± 6.24 ns | 57.35 ± 5.14 ns | 48.35 ± 4.33 a | |
| V3 | 24.97 ± 2.25 ns | 69.91 ± 6.28 ns | 41.88 ± 3.76 ns | 35.30 ± 3.17 b | |
| Mg (ppm) | V1 | 9.49 ± 0.52 ns | 22.80 ± 2.60 ns | 19.03 ± 1.04 ab | 16.05 ± 0.87 ns |
| V2 | 10.49 ± 0.94 ns | 21.35 ± 1.91 ns | 21.04 ± 1.88 a | 17.74 ± 1.59 ns | |
| V3 | 7.66 ± 0.69 ns | 25.64 ± 2.30 ns | 15.36 ± 1.38 b | 12.95 ± 1.16 ns | |
| Zn (ppm) | V1 | 0.24 ± 0.01 ns | 0.37 ± 0.04 ns | 0.42 ± 0.02 ns | 0.35 ± 0.02 ns |
| V2 | 0.26 ± 0.02 ns | 0.53 ± 0.05 ns | 0.46 ± 0.04 ns | 0.39 ± 0.03 ns | |
| V3 | 0.19 ± 0.02 ns | 0.56 ± 0.05 ns | 0.34 ± 0.03 ns | 0.28 ± 0.03 ns | |
| K (ppm) | V1 | 0.51 ± 0.03 ns | 1.56 ± 0.30 ns | 1.14 ± 0.06 ns | 0.96 ± 0.05 ns |
| V2 | 0.57 ± 0.05 ns | 1.16 ± 0.10 ns | 1.25 ± 0.11 ns | 1.06 ± 0.10 ns | |
| V3 | 0.42 ± 0.04 ns | 1.53 ± 0.14 ns | 0.92 ± 0.08 ns | 0.77 ± 0.07 ns | |
| Na (ppm) | V1 | 0.60 ± 0.03 ns | 1.35 ± 0.12 ns | 1.02 ± 0.06 ns | 0.86 ± 0.05 ns |
| V2 | 0.66 ± 0.06 ns | 1.35 ± 0.12 ns | 1.12 ± 0.10 ns | 0.95 ± 0.08 ns | |
| V3 | 0.48 ± 0.04 ns | 1.37 ± 0.12 ns | 0.82 ± 0.08 ns | 0.69 ± 0.06 ns |
| Anti-Nutritive Compounds | T. | Tomato ‘Tigerella’ | Tomato ‘Black Cherry’ | Eggplant ‘Black Beauty’ | Sweet Pepper ‘Barbara’ | Cucumber ‘Ekol’ | Dwarf Bean ‘Nano Supernano Gialo’ | Common Bean ‘Violeta de Iasi’ |
|---|---|---|---|---|---|---|---|---|
| Phytate (g/100 g d.w.) | V1 | 3.69 ± 0.38 ns | 3.40 ± 0.19 ns | 3.15 ± 0.17 ns | 4.70 ± 0.47 ns | 4.61 ± 0.25 ab | 3.39 ± 0.19 ns | 3.27 ± 0.29 ns |
| V2 | 3.58 ± 0.32 ns | 3.74 ± 0.33 ns | 3.48 ± 0.31 ns | 4.58 ± 0.41 ns | 5.10 ± 0.46 a | 3.72 ± 0.33 ns | 3.75 ± 0.33 ns | |
| V3 | 3.42 ± 0.31 ns | 3.58 ± 0.32 ns | 2.54 ± 0.23 ns | 4.42 ± 0.4 ns | 3.72 ± 0.33 b | 3.54 ± 0.32 ns | 3.59 ± 0.31 ns | |
| Tannin (g/100 g d.w.) | V1 | 3.53 ± 0.75 a | 1.96 ± 0.69 b | 2.16 ± 0.12 ns | 0.81 ± 0.22 ns | 0.50 ± 0.03 ns | 1.76 ± 0.67 b | 1.18 ± 0.10 c |
| V2 | 2.45 ± 0.22 ab | 3.95 ± 0.36 a | 2.38 ± 0.21 ns | 0.45 ± 0.04 ns | 0.55 ± 0.05 ns | 3.94 ± 0.36 a | 3.96 ± 0.34 a | |
| V3 | 1.48 ± 0.13 b | 2.45 ± 0.22 ab | 1.74 ± 0.16 ns | 0.48 ± 0.04 ns | 0.40 ± 0.04 ns | 2.43 ± 0.22 ab | 2.46 ± 0.21 b | |
| Oxalate (g/100 g d.w.) | V1 | 1.91 ± 0.39 b | 3.47 ± 0.85 a | 1.20 ± 0.07 ns | 3.2 ± 0.23 ns | 4.00 ± 0.22 ns | 3.57 ± 0.86 a | 4.00 ± 0.35 a |
| V2 | 1.36 ± 0.12 b | 2.13 ± 0.19 ab | 1.32 ± 0.12 ns | 3.36 ± 0.30 ns | 4.42 ± 0.40 ns | 2.11 ± 0.19 ab | 2.14 ± 0.18 b | |
| V3 | 3.83 ± 0.34 a | 1.36 ± 0.12 b | 0.97 ± 0.09 ns | 3.83 ± 0.34 ns | 3.23 ± 0.29 ns | 1.34 ± 0.12 b | 1.36 ± 0.12 b | |
| Saponin (g/100 g d.w.) | V1 | 2.35 ± 0.49 b | 3.06 ± 0.45 a | 1.45 ± 0.08 ns | 0.63 ± 0.05 b | 0.97 ± 0.05 ns | 3.04 ± 0.45 a | 3.24 ± 0.28 a |
| V2 | 1.65 ± 0.15 b | 2.62 ± 0.23 ab | 1.6 ± 0.14 ns | 0.65 ± 0.06 ab | 1.07 ± 0.10 ns | 2.61 ± 0.23 ab | 2.63 ± 0.23 ab | |
| V3 | 3.93 ± 0.36 a | 1.65 ± 0.15 b | 1.17 ± 0.10 ns | 0.93 ± 0.08 a | 0.78 ± 0.07 ns | 1.64 ± 0.15 b | 1.66 ± 0.14 b | |
| Trypsin Inhibitors (TUI/mg d.w.) | V1 | 9.42 ± 0.67 ns | 9.34 ± 0.88 ns | 8.72 ± 0.48 ns | 6.42 ± 0.40 ns | 7.01 ± 0.38 a | 9.32 ± 0.88 ns | 9.42 ± 0.82 ns |
| V2 | 9.91 ± 0.89 ns | 9.22 ± 0.83 ns | 9.64 ± 0.87 ns | 6.92 ± 0.62 ns | 7.75 ± 0.69 a | 9.25 ± 0.83 ns | 9.25 ± 0.80 ns | |
| V3 | 9.71 ± 0.87 ns | 9.91 ± 0.89 ns | 7.04 ± 0.64 ns | 6.71 ± 0.6 ns | 5.66 ± 0.51 b | 9.94 ± 0.89 ns | 9.95 ± 0.87 ns | |
| α-Amylase Inhibitors IC50 (mg/mL d.w.) | V1 | 0.67 ± 0.16 ns | 0.60 ± 0.03 ab | 0.38 ± 0.02 ns | 0.67 ± 0.16 a | 0.36 ± 0.02 ns | 0.62 ± 0.03 ab | 0.54 ± 0.05 b |
| V2 | 0.43 ± 0.04 ns | 0.77 ± 0.07 a | 0.42 ± 0.04 ns | 0.43 ± 0.04 ab | 0.40 ± 0.04 ns | 0.75 ± 0.07 a | 0.77 ± 0.07 a | |
| V3 | 0.35 ± 0.03 ns | 0.43 ± 0.04 b | 0.31 ± 0.03 ns | 0.35 ± 0.03 b | 0.30 ± 0.03 ns | 0.44 ± 0.04 b | 0.43 ± 0.04 b |
| Anti-Nutritive Compounds | T. | Kale ‘Nero Di Toscana’ | Chard ‘Bright Lights’ | Leek ‘Blue de Solaise’ | Parsley ‘Triple Moss Curled’ |
|---|---|---|---|---|---|
| Phytate (g/100 g d.w.) | V1 | 3.90 ± 0.21 ns | 3.29 ± 0.18 ns | 3.74 ± 0.20 ns | 3.54 ± 0.36 ns |
| V2 | 4.31 ± 0.39 ns | 3.64 ± 0.33 ns | 4.13 ± 0.37 ns | 3.42 ± 0.31 ns | |
| V3 | 3.15 ± 0.28 ns | 2.66 ± 0.24 ns | 3.01 ± 0.27 ns | 3.26 ± 0.29 ns | |
| Tannin (g/100 g d.w.) | V1 | 4.12 ± 0.23 ns | 3.47 ± 0.19 ns | 2.56 ± 0.14 ns | 2.18 ± 0.48 ns |
| V2 | 4.56 ± 0.41 ns | 3.84 ± 0.34 ns | 2.83 ± 0.25 ns | 1.48 ± 0.13 ns | |
| V3 | 3.33 ± 0.30 ns | 2.81 ± 0.25 ns | 2.06 ± 0.18 ns | 1.18 ± 0.11 ns | |
| Oxalate (g/100 g d.w.) | V1 | 2.22 ± 0.12 ns | 1.88 ± 0.10 ns | 1.42 ± 0.08 ns | 2.05 ± 0.59 b |
| V2 | 2.46 ± 0.22 ns | 2.07 ± 0.18 ns | 1.57 ± 0.14 ns | 3.83 ± 0.34 a | |
| V3 | 1.79 ± 0.16 ns | 1.51 ± 0.14 ns | 1.15 ± 0.10 ns | 3.98 ± 0.36 a | |
| Saponin (g/100 g d.w.) | V1 | 2.73 ± 0.15 ns | 2.30 ± 0.12 ns | 1.72 ± 0.09 ns | 2.29 ± 0.52 b |
| V2 | 3.02 ± 0.27 ns | 2.55 ± 0.23 ns | 1.90 ± 0.17 ns | 3.93 ± 0.35 a | |
| V3 | 2.21 ± 0.20 ns | 1.86 ± 0.17 ns | 1.39 ± 0.13 ns | 3.23 ± 0.29 ab | |
| Trypsin Inhibitors (TUI/mg d.w.) | V1 | 9.63 ± 0.52 ns | 8.12 ± 0.44 a | 10.35 ± 0.57 ab | 9.86 ± 0.94 ns |
| V2 | 10.64 ± 0.95 ns | 8.97 ± 0.80 a | 11.44 ± 1.02 a | 9.71 ± 0.87 ns | |
| V3 | 7.77 ± 0.70 ns | 6.55 ± 0.59 b | 8.35 ± 0.75 b | 9.38 ± 0.84 ns | |
| α-Amylase Inhibitors IC50 (mg/mL d.w.) | V1 | 0.80 ± 0.04 ns | 0.68 ± 0.04 ns | 0.45 ± 0.03 ns | 0.41 ± 0.06 ns |
| V2 | 0.89 ± 0.08 ns | 0.75 ± 0.07 ns | 0.50 ± 0.04 ns | 0.35 ± 0.03 ns | |
| V3 | 0.65 ± 0.06 ns | 0.55 ± 0.05 ns | 0.36 ± 0.03 ns | 0.54 ± 0.05 ns |
| Cultivar | Average Yield (g) | ||
|---|---|---|---|
| V1 | V2 | V3 | |
| tomato ‘Tigerella’ | 1313 ± 118 ns | 1389 ± 462 ns | 1229 ± 422 ns |
| tomato ’Black Cherry’ | 1068 ± 96 a | 923 ± 83 ab | 685 ± 62 b |
| sweet pepper ‘Barbara’ | 2116 ± 191 a | 1329 ± 120 b | 692 ± 108 c |
| cucumber ’Ekol’ | 1928 ± 174 a | 1166 ± 105 b | 1003 ± 90 b |
| common bean ’Violeta de Iasi’ | 4000 ± 374 a | 3673 ± 316 b | 3202 ± 286 c |
| dwarf bean ’Nano Supernano Giallo’ | 1097 ± 116 ns | 1015 ± 98 ns | 866 ± 74 ns |
| Cultivar | Average Yield (g) | ||
|---|---|---|---|
| V1 | V2 | V3 | |
| white onion ‘Di Parma’ | 1208 ± 109 ns | 939 ± 85 ns | 852 ± 77 ns |
| leek ‘Blue de Solaise’ | 4562 ± 413 a | 3792 ± 343 ab | 2813 ± 254 b |
| celery ‘Gigante Dorato 2’ | 1551 ± 140 ns | 1559 ± 141 ns | 1194 ± 108 ns |
| celeriac ‘Giant Prague’ | 1782 ± 161 b | 1589 ± 143 b | 4065 ± 368 a |
| chard ‘Bright Lights’ | 13026 ± 1179 a | 9261 ± 838 ab | 6630 ± 600 b |
| carrot ‘Cosmic Purple’ | 440 ± 39 ns | 365 ± 33 ns | 491 ± 77 ns |
| carrot ‘Royal Chantenay’ | 2183 ± 197 ns | 1748 ± 274 ns | 1896 ± 297 ns |
| kale ‘Kadet’ | 3356 ± 304 a | 1145 ± 103 b | 1210 ± 109 b |
| kale ‘Nero Di Toscana’ | 2295 ± 207 a | 1745 ± 158 ab | 1458 ± 132 b |
| kale ‘Scarlet’ | 2671 ± 241 a | 1338 ± 121 b | 1410 ± 127 b |
| mint ‘Cinderella’ | 1491 ± 135 ab | 955 ± 86 b | 1963 ± 177 a |
| oregano ‘Kreta’ | 1023 ± 92 ns | 955 ± 86 ns | 915 ± 82 ns |
| parsley ‘Triple Moss Curled’ | 2454 ± 222 ns | 2299 ± 208 ns | 2063 ± 186 ns |
| thyme ‘Di Provenza’ | 1130 ± 102 ns | 934 ± 84 ns | 813 ± 73 ns |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Istrate, A.-M.-R.; Cojocariu, M.; Teliban, G.-C.; Cojocaru, A.; Stoleru, V. Quality and Yield of Edible Vegetables from Landscape Design. Horticulturae 2023, 9, 615. https://doi.org/10.3390/horticulturae9060615
Istrate A-M-R, Cojocariu M, Teliban G-C, Cojocaru A, Stoleru V. Quality and Yield of Edible Vegetables from Landscape Design. Horticulturae. 2023; 9(6):615. https://doi.org/10.3390/horticulturae9060615
Chicago/Turabian StyleIstrate, Ana-Maria-Roxana, Mirela Cojocariu, Gabriel-Ciprian Teliban, Alexandru Cojocaru, and Vasile Stoleru. 2023. "Quality and Yield of Edible Vegetables from Landscape Design" Horticulturae 9, no. 6: 615. https://doi.org/10.3390/horticulturae9060615
APA StyleIstrate, A.-M.-R., Cojocariu, M., Teliban, G.-C., Cojocaru, A., & Stoleru, V. (2023). Quality and Yield of Edible Vegetables from Landscape Design. Horticulturae, 9(6), 615. https://doi.org/10.3390/horticulturae9060615









