Zinc Oxide Nanoparticles Enhanced Growth of Tea Trees via Modulating Antioxidant Activity and Secondary Metabolites
Abstract
1. Introduction
2. Materials and Methods
2.1. Overview of the Experimental Site and Plant Materials
2.2. Experiment Design
2.3. Sampling and Analysis
2.4. Determination of Main Biochemical Components
2.5. Determination of Aroma Components in Fresh Tea Leaves Using Headspace Solid-Phase Microextraction Coupled with Gas Chromatography-Mass Spectrometry (HS-SPME/GC-MS)
2.6. Data Collection and Analysis
3. Results
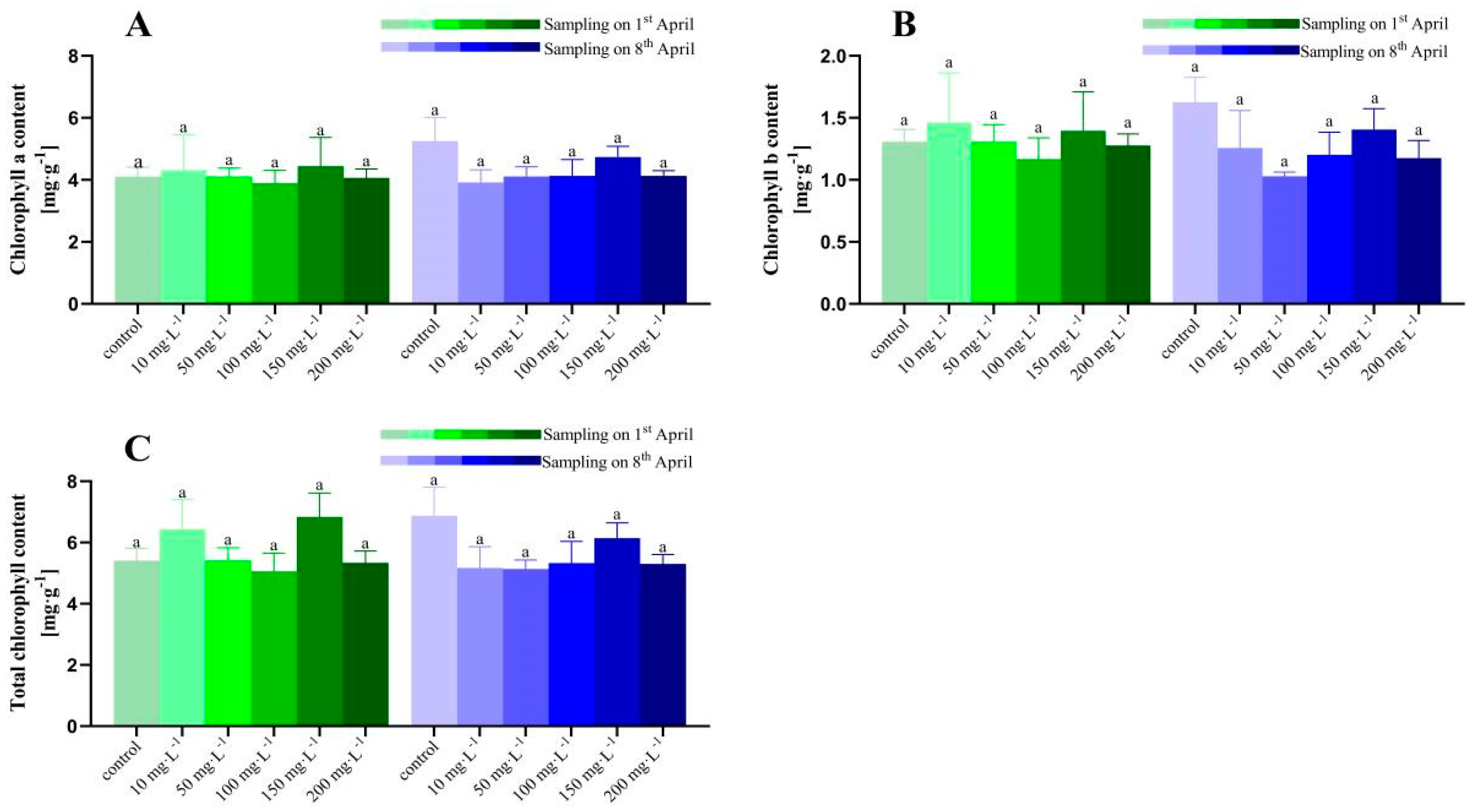
3.1. Chlorophyll Content under Different Concentrations of ZnO-NPs
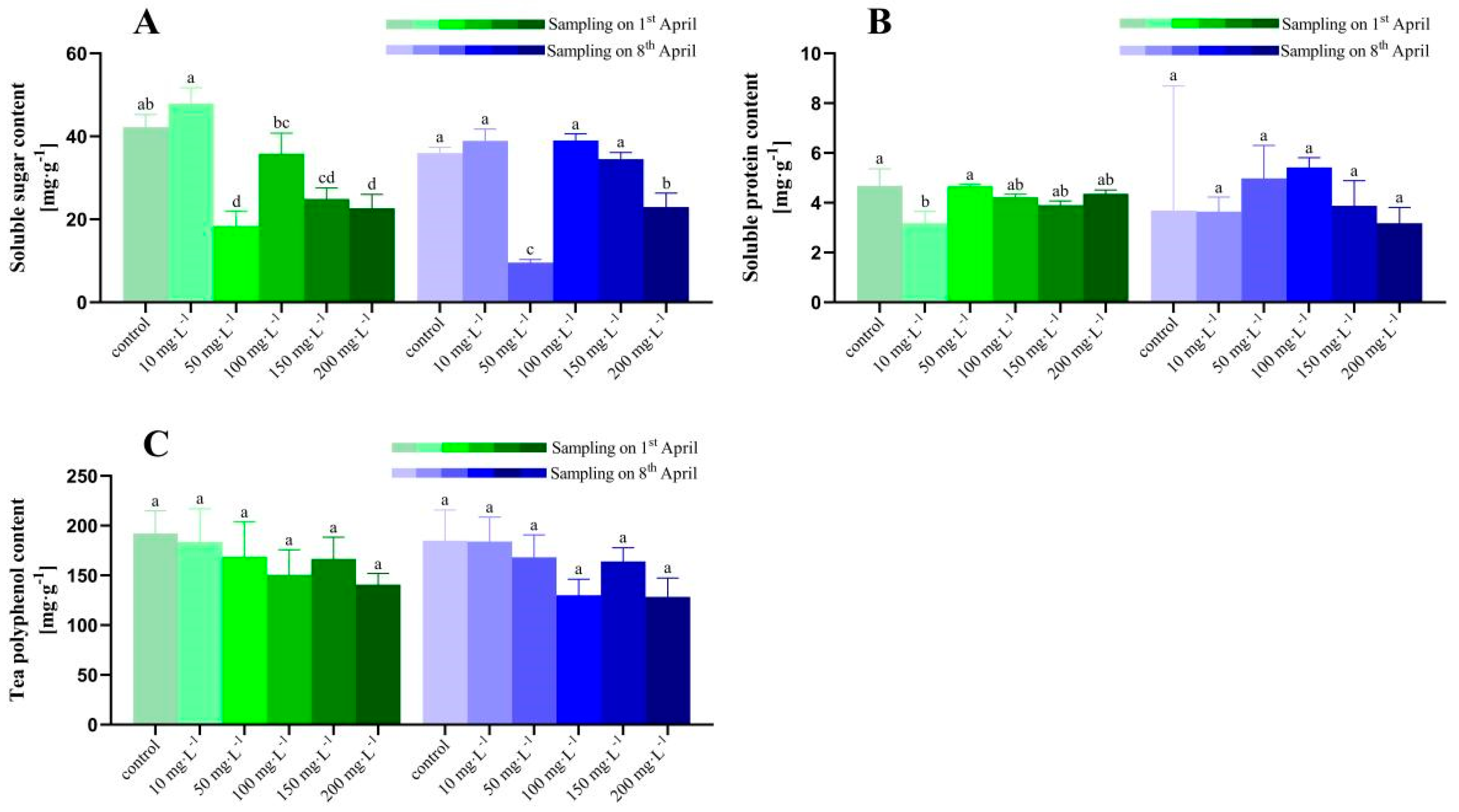
3.2. Tea Nutritional Quality under Different Concentrations of ZnO-NPs
3.3. Biochemical Components of Tea Leaves under Different Concentrations of ZnO-NPs
3.4. Aroma Components of Tea Leaves under Different Concentrations of ZnO-NPs
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Love, J.C.; Estroff, L.A.; Kriebel, J.K.; Nuzzo, R.G.; Whitesides, G.M. Self-assembled monolayers of thiolates on metals as a form of nanotechnology. Chem. Rev. 2005, 105, 1103–1169. [Google Scholar] [CrossRef] [PubMed]
- Stern, S.T.; McNeil, S.E. Nanotechnology safety concerns revisited. Toxic. Sci. 2008, 101, 4–21. [Google Scholar] [CrossRef] [PubMed]
- Corredor, E.; Testillano, P.S.; Coronado, M.J.; Gonzalez-Melendi, P.; Fernandez-Pacheco, R.; Marquina, C.; Ibarra, M.R.; de la Fuente, J.M.; Rubiales, D.; Perez-de-Laque, A.; et al. Nanoparticle penetration and transport in living pumpkin plants: In situ subcellular identification. BMC Plant Biol. 2009, 9, 45. [Google Scholar] [CrossRef] [PubMed]
- Farooqui, A.; Tabassum, H.; Ahmad, A.; Mabood, A.; Ahmad, A.; Ahmad, I.Z. Role of nanoparticles in growth and development of plants: A review. Int. J. Pharma Bio Sci. 2016, 7, 22–37. [Google Scholar] [CrossRef]
- Peng, Y.H.; Tsai, Y.C.; Hsiung, C.E.; Lin, Y.H.; Shih, Y. Influence of water chemistry on the environmental behaviors of commercial ZnO nanoparticles in various water and wastewater samples. J. Hazard. Mater. 2017, 322, 348–356. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, M.; Jana, A.; Sinha, S.; Jothiramajayam, M.; Nag, A.; Chakraborty, A.; Mukherjee, A. Effects of ZnO nanoparticles in plants: Cytotoxicity, genotoxicity, deregulation of antioxidant defenses, and cell-cycle arrest. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2016, 807, 25–32. [Google Scholar] [CrossRef]
- Sirelkhatim, A.; Shahrom, M.; Azman, S.; Noor, H.M.K.; Chuo, A.L.; Siti, K.M.B.; Habsah, H.; Dasmawati, M. Review on zinc oxide nanoparticles: Antibacterial activity and toxicity mechanism. Micro. Nano. Lett. 2015, 7, 219–242. [Google Scholar] [CrossRef]
- Zhao, L.J.; Peralta-Videa, J.R.; Rico, C.M.; Hernandez-Viezcas, J.A.; Sun, Y.P.; Niu, G.H.; Servin, A.; Nunez, J.E.; Duarte-Gardea, M.; Gardea-Torresdey, J.L. CeO2 and ZnO nanoparticles change the nutritional qualities of cucumber (Cucumis sativus). J. Agricul. Food Chem. 2014, 62, 2752–2759. [Google Scholar] [CrossRef]
- Singh, N.B.; Amist, N.; Yadav, K.; Singh, D.; Pandey, J.K.; Singh, S.C. Zinc oxide nanoparticles as fertilizer for the germination, growth and metabolism of vegetable crops. J. Nanoeng. 2013, 3, 1–12. [Google Scholar] [CrossRef]
- Rajiv, P.; Vanathi, P. Effect of parthenium based vermicompost and zinc oxide nanoparticles on growth and yield of Arachis hypogaea L. in zinc deficient soil. Biocat. Agri. Biotech. 2018, 13, 251–257. [Google Scholar] [CrossRef]
- Prasad, T.N.V.K.V.; Sudhakar, P.; Sreenivasulu, Y.; Latha, P.; Munaswamy, V.; Raja Reddy, K.; Sreeprasad, T.S.; Sajanlal, P.R.; Pradeep, T. Effect of nanoscale zinc oxide particles on the germination, growth and yield of peanut. J. Plant Nutri. 2012, 35, 905–927. [Google Scholar] [CrossRef]
- Khodakovskaya, M.; Dervishi, E.; Mahmood, M.; Xu, Y.; Li, Z.R.; Watanabe, F.; Biris, A.S. Carbon nanotubes are able to penetrate plant seed coat and dramatically affect seed germination and plant growth. ACS Nano. 2009, 3, 3221–3227. [Google Scholar] [CrossRef] [PubMed]
- Torabian, S.; Zahedi, M.; Khoshgoftarmanesh, A. Effect of foliar spray of zinc oxide on some antioxidant enzymes activity of sunflower under salt stress. J. Agri. Sci. Tech. 2016, 18, 1013–1025. [Google Scholar]
- Pokharel, S.S.; Shen, F.; Parajulee, M.N.; Wang, Y.H.; Chen, F.J. Effects of elevated atmospheric CO2 concentration on tea quality and insect pests’ occurrences: A review. Glob. Ecol. Conserv. 2021, 27, e01553. [Google Scholar] [CrossRef]
- Li, Z.X.; Yang, W.J.; Ahammed, G.J.; Shen, C.; Yan, P.; Li, X.; Han, W.Y. Developmental changes in carbon and nitrogen metabolism affect tea quality in different leaf position. Plant Physiol. Biochem. 2016, 106, 327–335. [Google Scholar] [CrossRef]
- Li, X.; Ahammed, G.J.; Li, Z.; Zhang, L.; Wei, J.; Yan, P.; Zhang, L.; Han, W. Freezing stress deteriorates tea quality of new flush by inducing photosynthetic inhibition and oxidative stress in mature leaves. Sci. Hortic. 2018, 230, 155–160. [Google Scholar] [CrossRef]
- Mao, A.J.; Su, H.; Fang, S.M.; Chen, X.; Ning, J.M.; Ho, C.T.; Wan, X.C. Effects of roasting treatment on non-volatile compounds and taste of green tea. Int. J. Food Sci. Technol. 2018, 53, 2586–2594. [Google Scholar] [CrossRef]
- Li, X.; Li, M.H.; Deng, W.W.; Ahammed, G.J.; Wei, J.P.; Yan, P.; Zhang, L.P.; Fu, J.Y.; Han, W.Y. Exogenous melatonin improves tea quality under moderate high temperatures by increasing epigallocatechin-3-gallate and theanine biosynthesis in Camellia sinensis L. J. Plant Physiol. 2020, 253, 153273. [Google Scholar] [CrossRef]
- Cheng, Y.C.; Cheng, Y.X.; Zheng, H.; Song, Y.X.; Li, R.Z.; Wan, F.T.; Li, J.L. Evaluation and comparison of the toxic effects of MgO NPs, ZnO NPs, α-Fe2O3 NPs, γ-Fe2O3 NPs, and Fe3O4 NPs on the remediation for cadmium-related effects in wheat seedlings. Water Air Soil Poll. 2020, 231, 471. [Google Scholar] [CrossRef]
- Concepción, G.; Sandra, G.; Francisca, A.F.; Demetrio, G.; Mar, B.; Dolores, M. Effects of aged ZnO NPs and soil type on Zn availability, accumulation and toxicity to pea and beet in a greenhouse experiment. Ecotox. Environ. Safe. 2018, 160, 222–230. [Google Scholar]
- Li, H.S. The Principles and Technologies for Plant Physiology and Biochemistry Experiments; High Education Press: Beijing, China, 2006. (In Chinese) [Google Scholar]
- Wu, Q.K.; Cao, Y.Y.; Zhao, X.; Zhang, Z.H.; Yu, F.Y.; Guy, R.D. A comparative study of seed reserve accumulation in five Styrax species with potential for biofuel production. Trees 2020, 34, 891–902. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Ranieri, A.; Castagna, A.; Baldan, B.; Soldatini, G.F. Iron deficiency differently affects peroxidase isoforms in sunflower. J. Exp. Bot. 2001, 52, 25–35. [Google Scholar] [CrossRef] [PubMed]
- Jiang, M.Y.; Zhang, J.H. Water stress -induced abscisic acid accumulation triggers the increased generation of reactive oxygen species and up-regulated the activities of antioxidant enzymes in the maize leaves. J. Exp. Bot. 2002, 53, 2401–2410. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Chen, H.; Ni, M.; Yu, F.Y. A study on petal morphological and physiological characteristics of Styrax japonicus during the flowering period. Agronomy 2021, 11, 1498. [Google Scholar] [CrossRef]
- Ji, X.Y. Comparative analysis of volatile organic compounds and bioactive compounds in typical coniferous and broad-leaved tree species. J. Essent. Oil Bear. 2020, 23, 1105–1117. [Google Scholar] [CrossRef]
- Faizan, M.; Hayat, S. Effect of foliar spray of ZnO-NPs on the physiological parameters and antioxidant systems of Lycopersicon esculentum. Pol. J. Nat. Sci. 2019, 34, 87–105. [Google Scholar]
- Ahmed, R.; Uddin, M.K.; Quddus, M.A.; Samad, M.Y.A.; Hossain, M.M.; Haque, A.N.A. Impact of foliar application of zinc and zinc oxide nanoparticles on growth, yield, nutrient uptake and quality of tomato. Horticulturae 2023, 9, 162. [Google Scholar] [CrossRef]
- Faizan, M.; Faraz, A.; Yusuf, M.; Khan, S.T.; Hayat, S. Zinc oxide nanoparticle-mediated changes in photosynthetic efficiency and antioxidant system of tomato plants. Photosynthetica 2018, 56, 678–686. [Google Scholar] [CrossRef]
- Faizan, M.; Faraz, A.; Mir, A.R.; Hayat, S. Role of zinc oxide nanoparticles in countering negative effects generated by cadmium in Lycopersicon esculentum. J. Plant Growth Regul. 2020, 40, 101–115. [Google Scholar] [CrossRef]
- Faizan, M.; Bhat, J.A.; Chen, C.; Alyemeni, M.N.; Wijaya, L.; Ahmad, P.; Yu, F.Y. Zinc oxide nanoparticles (ZnO-NPs) induce salt tolerance by improving the antioxidant system and photosynthetic machinery in tomato. Plant Physiol. Biochem. 2021, 161, 122–130. [Google Scholar] [CrossRef]
- Wang, X.P.; Yang, X.Y.; Chen, S.Y.; Li, Q.Q.; Wang, W.; Hou, C.J.; Gao, X.; Wang, L.; Wang, S.C. Zinc oxide nanoparticles affect biomass accumulation and photosynthesis in Arabidopsis. Front. Plant Sci. 2016, 6, 1243. [Google Scholar] [CrossRef] [PubMed]
- Guzel, S.; Terzi, R. Exogenous hydrogen peroxide increases dry matter production, mineral content and level of osmotic solutes in young maize leaves and alleviates deleterious effects of copper stress. Bot. Stud. 2013, 54, 1182. [Google Scholar] [CrossRef] [PubMed]
- Raliya, R.; Tarafdar, J.C. ZnO nanoparticle biosynthesis and its effect on phosphorous-mobilizing enzyme secretion and gum contents in cluster bean (Cyamopsis tetragonoloba L.). Agr. Res. 2013, 2, 48–57. [Google Scholar] [CrossRef]
- Mukherjee, A.; Sun, Y.; Morelius, E.; Tamez, C.; Bandyopadhyay, S.; Niu, G.; White, J.C.; Peralta-Videa, J.R.; Gardea-Torresdey, J.L. Differential toxicity of bare and hybrid ZnO nanoparticles in Green Pea (Pisum sativum L.): A life cycle study. Front. Plant Sci. 2016, 12, 1242. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.M.; Du, X.; Nie, C.N.; Zhang, X.; Tan, X.Q.; Li, Q. Evaluation of sensory and safety quality characteristics of “high mountain tea”. Food Sci. Nutr. 2022, 10, 3338–3354. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.S.; Lin, X.M.; Qiao, R.Y.; Zheng, X.Q.; Lu, J.L.; Ye, J.H.; Liang, Y.R. Effect of fluoride treatment on gene expression in tea plant (Camellia sinensis). Sci. Rep. 2017, 7, 9847. [Google Scholar] [CrossRef]
- Tripathy, B.C.; Oelmuller, R. Reactive oxygen species generation and signaling in plants. Plant Signal. Behav. 2014, 7, 1621–1633. [Google Scholar] [CrossRef]
- Gill, S.S.; Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef]
- Ali, B.; Hasan, S.A.; Hayat, S.; Hayat, Q.; Yadav, S.; Fariduddin, Q.; Ahmad, A. A role for brassinosteroids in the amelioration of aluminium stress through antioxidant system in mung bean (Vigna radiata L. Wilczek). Environ. Exp. Bot. 2008, 62, 153–159. [Google Scholar] [CrossRef]
- Khan, J.; Brennan, D.M.; Bradley, N.; Gao, B.; Bruckdorfer, R.; Jacobs, M. 3 Nitrotyrosine in the proteins of human plasma determined by an ELISA method. Biochem. J. 1998, 330, 795–801. [Google Scholar] [CrossRef]
- Zeng, L.T.; Watababe, N.; Yang, Z.Y. Understanding the biosyntheses and stress response mechanisms of aroma compounds in tea (Camellia sinensis) to safely and effectively improve tea aroma. Crit. Rev. Food Sci. Nutr. 2019, 59, 2321–2334. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.C.; Zhu, Y.; Yan, H.; Chen, M.; Xie, D.C.; Wang, M.Q.; Ni, D.J.; Lin, Z. Identification of aroma composition and key odorants contributing to aroma characteristics of white teas. Molecules 2020, 25, 6050. [Google Scholar] [CrossRef]
- Li, F.X.; Yao, X.Z.; Lu, L.T.; Jiao, Y.J. Preparation of Zn-gly and Se-gly and their effects on the nutritional quality of tea (Camellia sinensis). Plants 2023, 12, 1049. [Google Scholar] [CrossRef] [PubMed]
- Cai, B.B. Effects of Zinc on Aroma Metabolism of Tea Tree (Camellia sinensis). Master’s Thesis, Anhui Agricultural University, Hefei, China, 2020. (In Chinese). [Google Scholar]
- Yang, Z.Y.; Baldermann, S.; Watanabe, N. Recent studies of the volatile compounds in tea. Food Res. Int. 2013, 2, 585–599. [Google Scholar] [CrossRef]
- Fu, X.M.; Chen, Y.Y.; Mei, X.; Katsuno, T.; Kobayashi, E.; Dong, F.; Watanabe, N.; Yang, Z.Y. Regulation of formation of volatile compounds of tea (Camellia sinensis) leaves by single light wavelength. Sci. Rep. 2015, 5, 16858. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.P.; Yu, F.Y. Corolla structure and fragrance components in Styrax tonkinensis. Trees 2015, 29, 1127–1134. [Google Scholar] [CrossRef]
- Chen, C.; Cao, Y.Y.; Chen, H.; Ni, M.; Yu, F.Y. Floral scent compounds and emission patterns of three Styrax species. Dendrobiology 2021, 85, 30–38. [Google Scholar] [CrossRef]

| Relative Content (%) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Treatments | ||||||||
| No. | Compounds | RI | CK | 10 mg·L−1 | 50 mg·L−1 | 100 mg·L−1 | 150 mg·L−1 | 200 mg·L−1 |
| 1 | Ethanolamine | 698.92 | 6.53 ± 0.36 a | 4.47 ± 0.50 a | 3.68 ± 1.28 b | - | 5.65 ± 0.26 a | - |
| 2 | H-Ala-Beta-Ala-Oh | 805.50 | - | 4.81 ± 0.66 a | 1.96 ± 0.48 b | - | - | - |
| 3 | Sarcosine | 839.45 | - | - | 1.02 ± 0.65 | - | - | - |
| 4 | Benzene | 953.66 | 54.78 ± 13.23 b | 49.93 ± 8.45 c | 49.4 ± 6.59 c | 30.84 ± 4.98 d | 62.14 ± 14.21 a | 28.72 ± 5.32 d |
| 5 | Bestatin | 1030.41 | - | 17.78 ± 3.45 a | 5.98 ± 2.37 b | - | 3.28 ± 1.07 c | - |
| 6 | Ketorolac | 1031.09 | 11.83 ± 0.69 | - | - | 11.8 ± 1.02 | - | - |
| 7 | γ-Terpinene | 1058.32 | - | - | 0.52 ± 0.18 | - | - | - |
| 8 | Ocimene | 1259.86 | - | - | - | 8.92 ± 4.12 | - | - |
| 9 | Ocimene mixture of isomers | 1276.41 | - | - | 0.51 ± 0.26 | - | 0.6 ± 0.23 | - |
| 10 | Esculetin | 1319.01 | 1.04 ± 0.57 a | 1.8 ± 1.23 a | 0.63 ± 0.21 b | - | - | - |
| 11 | Anisole | 1325.15 | - | - | - | 2.66 ± 1.21 | 2.09 ± 1.02 | - |
| 12 | trans-3-hexen-1-ol | 1331.08 | - | 3.64 ± 2.26 a | 1.57 ± 0.75 b | 1.3 ± 0.52 b | - | - |
| 13 | cis-3-hexenyl acetate | 1336.21 | 8.98 ± 3.12 a | 3.78 ± 1.21 c | - | 4.95 ± 2.21 b | 6.07 ± 2.25 b | - |
| 14 | trans-3-hexenyl acetate | 1351.08 | - | - | 12.33 ± 6.24 a | 4.78 ± 0.85 b | - | - |
| 15 | Leaf alcohol | 1351.25 | 1.8 ± 1.25 b | - | 1.52 ± 0.64 b | - | 6.53 ± 2.25 a | - |
| 16 | trans-2-hexenyl acetate | 1354.77 | - | - | 0.42 ± 0.14 | - | 0.69 ± 0.29 | - |
| 17 | 3-(Chloromethyl)heptane | 1476.84 | - | 0.88 ± 0.24 b | - | 1.27 ± 1.07 a | - | - |
| 18 | Fema 3498 | 1494.38 | 0.93 ± 0.35 b | - | 1.03 ± 0.45 b | 3.26 ± 1.22 a | - | - |
| 19 | cis-alpha,alpha,5-trimethyl-5-vinyltetrahydrofuran-2-methanol | 1524.89 | 1.51 ± 0.96 b | 3.69 ± 1.82 a | - | - | 0.78 ± 0.26 c | - |
| 20 | Linalool | 1545.78 | 5.97 ± 1.24 b | 4.09 ± 1.08 c | - | 5.77 ± 1.35 b | 6.75 ± 2.21 a | - |
| 21 | cis-3-Hexenyl 2-methylbutanoate | 1657.69 | - | - | 1.08 ± 0.37 b | 3.45 ± 1.12 a | 0.56 ± 0.17 c | - |
| 22 | methyl (Z)-3,7-dimethylocta-2,6-dienoate | 1657.75 | 0.77 ± 0.24 b | - | - | 1.02 ± 0.65 a | - | - |
| 23 | Methyl salicylate | 1662.75 | 0.97 ± 0.47 | - | - | - | - | - |
| 24 | cis-3-Hexenyl hexanoate | 1665.66 | 1.54 ± 1.21 a | 0.94 ± 0.27 b | 1.7 ± 0.28 a | - | - | - |
| 25 | cis-3-Hexenyl butyrate | 1665.69 | 1.11 ± 0.56 b | - | 1.37 ± 0.49 b | 2.92 ± 0.98 a | - | - |
| 26 | Farnesene | 1768.47 | - | - | - | 3.94 ± 0.74 | - | - |
| 27 | 1-Hexadecanol | 1839.39 | 0.89 ± 0.64 b | 1.52 ± 0.89 a | - | - | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, C.; Lai, J.; Chen, H.; Yu, F. Zinc Oxide Nanoparticles Enhanced Growth of Tea Trees via Modulating Antioxidant Activity and Secondary Metabolites. Horticulturae 2023, 9, 631. https://doi.org/10.3390/horticulturae9060631
Chen C, Lai J, Chen H, Yu F. Zinc Oxide Nanoparticles Enhanced Growth of Tea Trees via Modulating Antioxidant Activity and Secondary Metabolites. Horticulturae. 2023; 9(6):631. https://doi.org/10.3390/horticulturae9060631
Chicago/Turabian StyleChen, Chen, Jiaying Lai, Hong Chen, and Fangyuan Yu. 2023. "Zinc Oxide Nanoparticles Enhanced Growth of Tea Trees via Modulating Antioxidant Activity and Secondary Metabolites" Horticulturae 9, no. 6: 631. https://doi.org/10.3390/horticulturae9060631
APA StyleChen, C., Lai, J., Chen, H., & Yu, F. (2023). Zinc Oxide Nanoparticles Enhanced Growth of Tea Trees via Modulating Antioxidant Activity and Secondary Metabolites. Horticulturae, 9(6), 631. https://doi.org/10.3390/horticulturae9060631






