Influence of Different Types of Carbon Sources on Glucosinolate and Phenolic Compounds in Radish Sprouts
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Extraction and Analysis of Desulfo-Glucosinolates
2.3. Extraction and Analysis of Phenolics
2.4. Statistical Analysis
3. Results
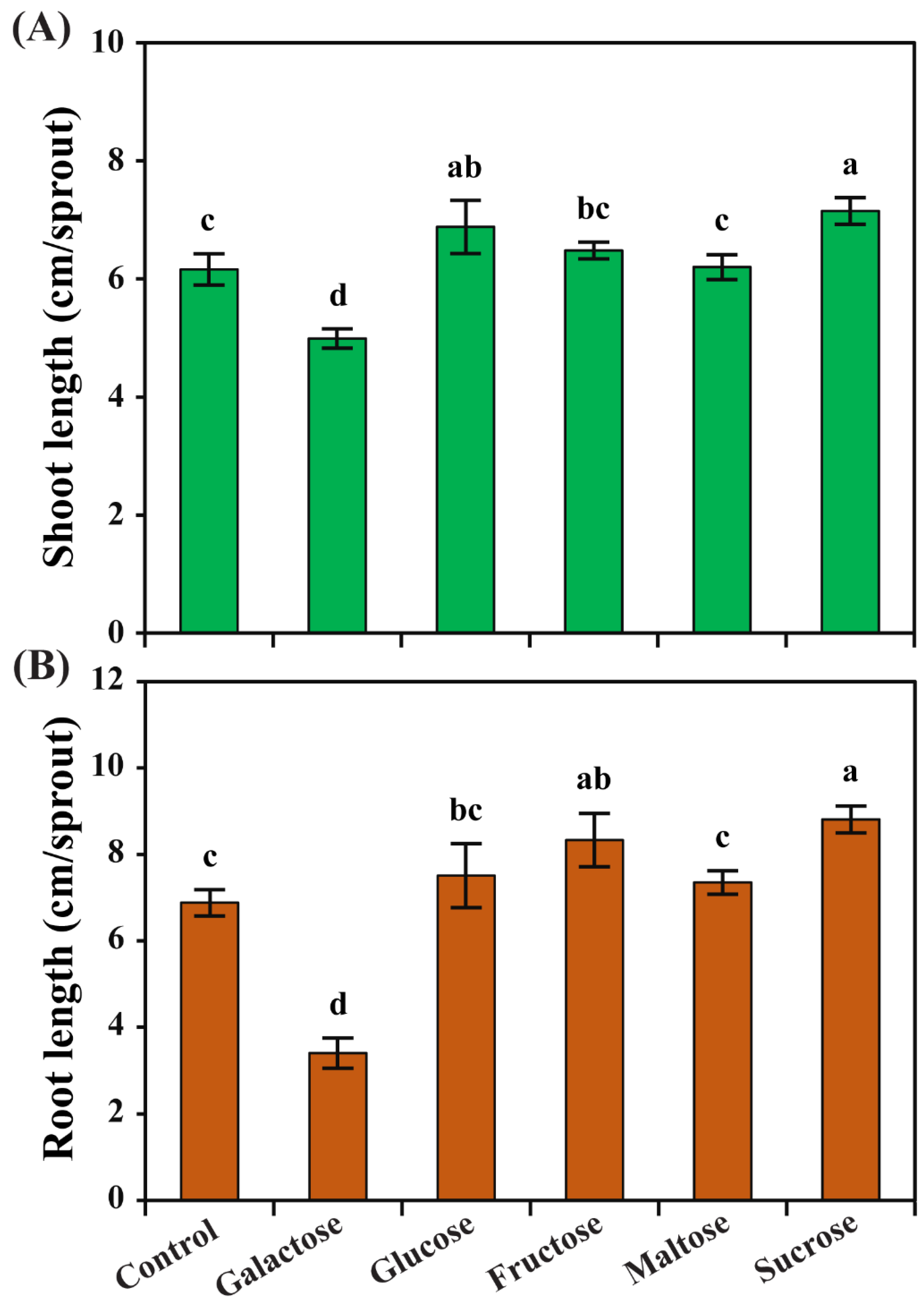
3.1. Effect of Different Carbon Sources on the Growth of Radish Sprouts
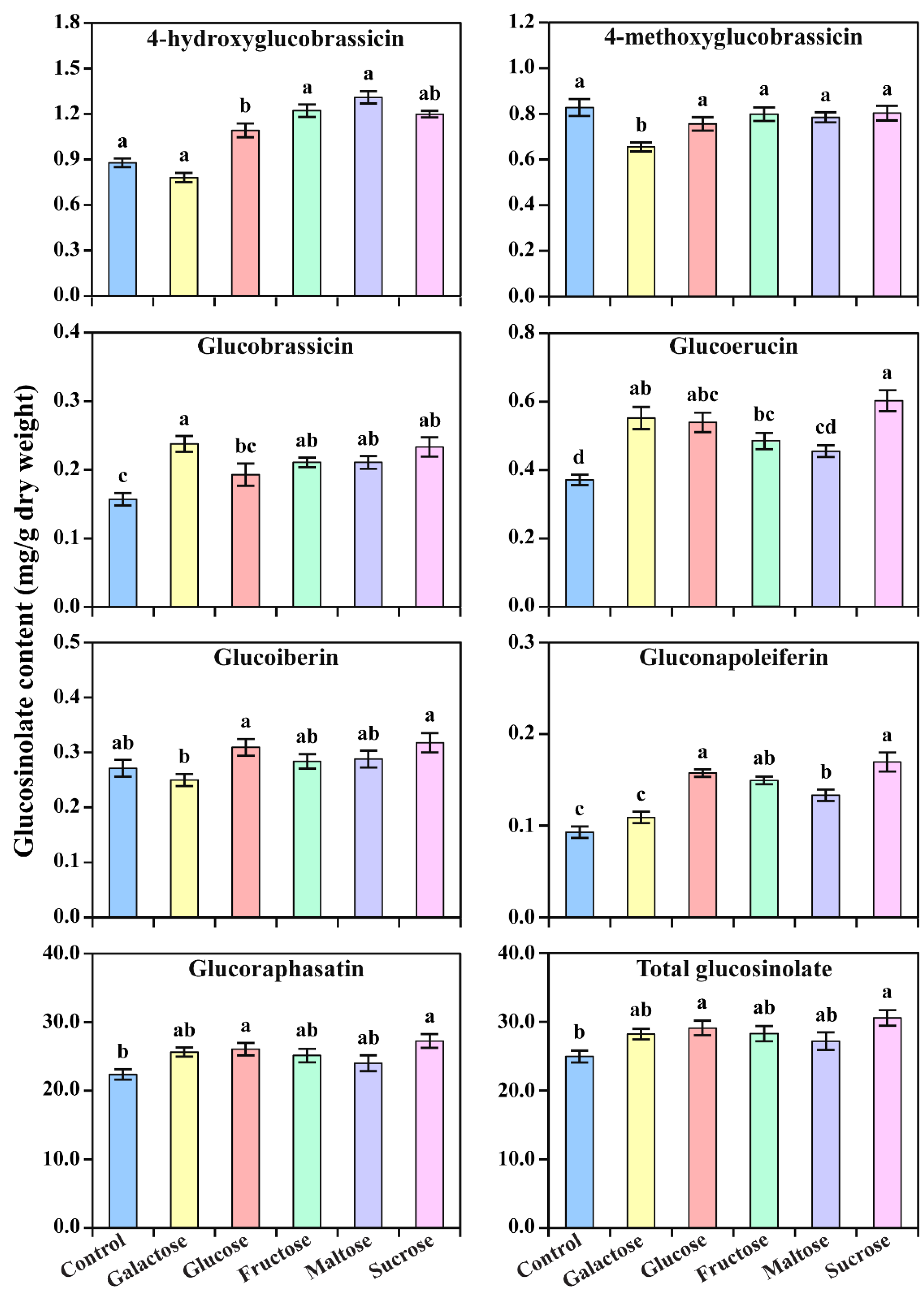
3.2. Accumulation of Glucosinolate in Response to Different Carbon Sources
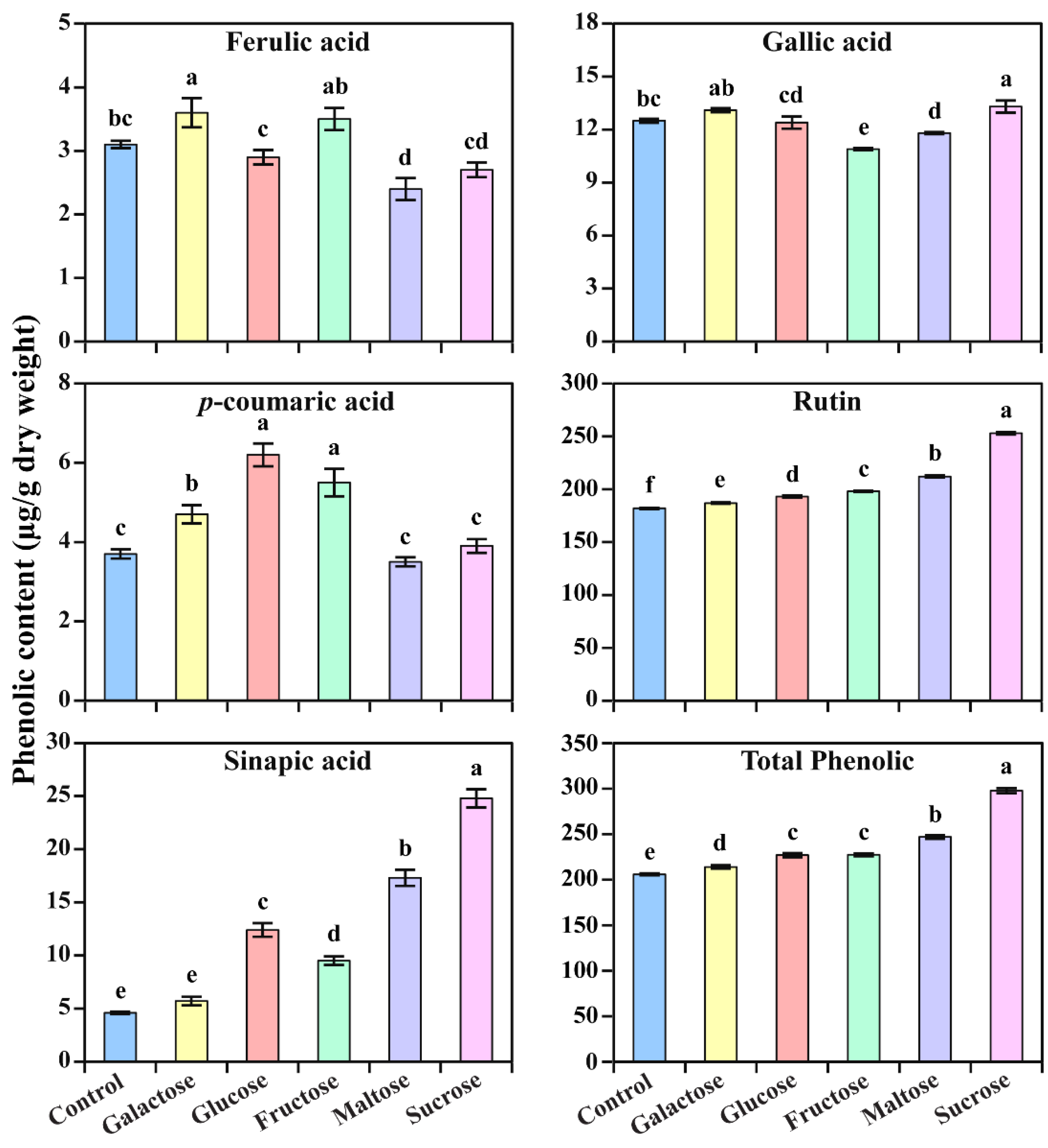
3.3. Accumulation of Phenolics in Response to Different Carbon Sources
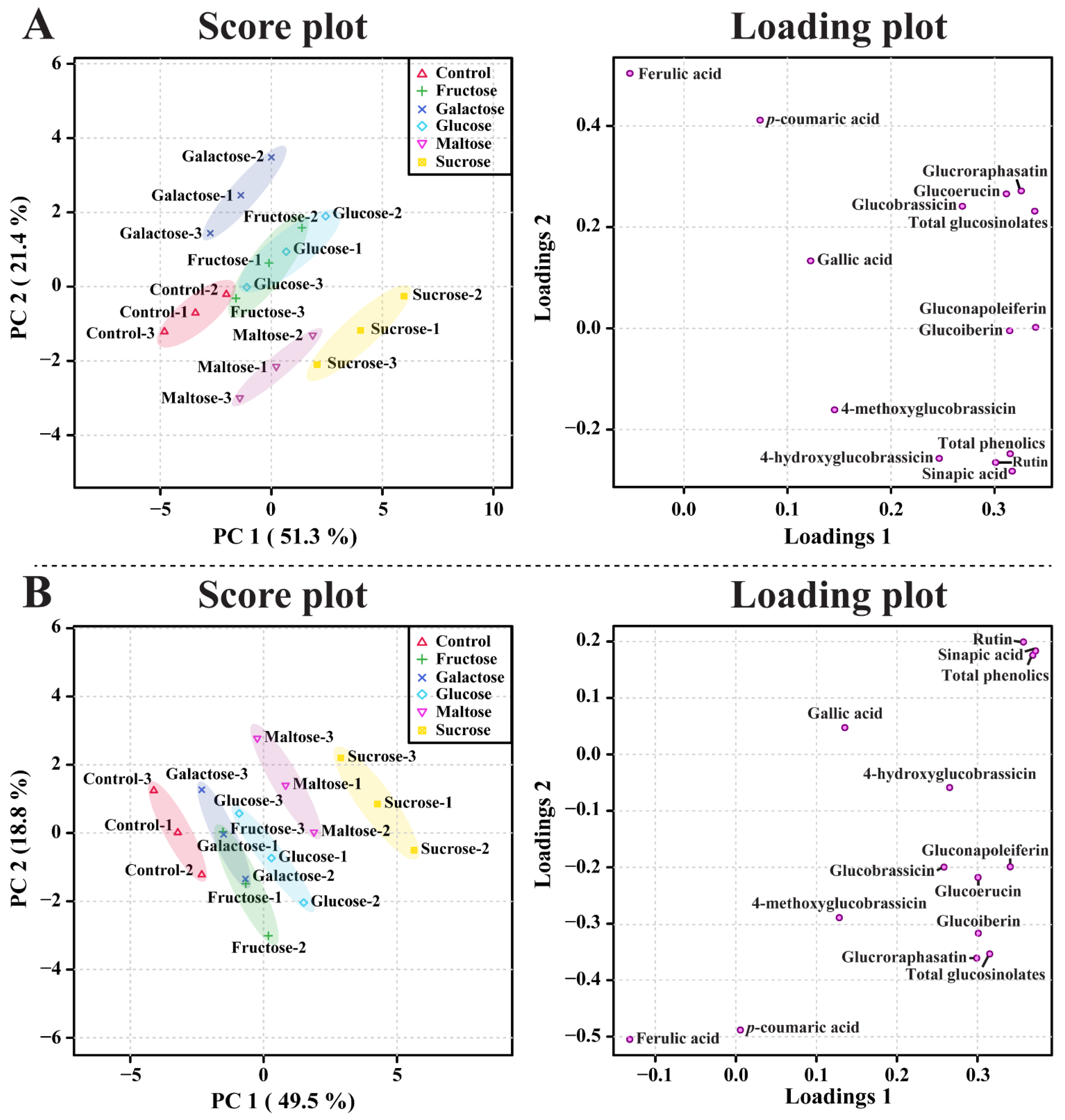
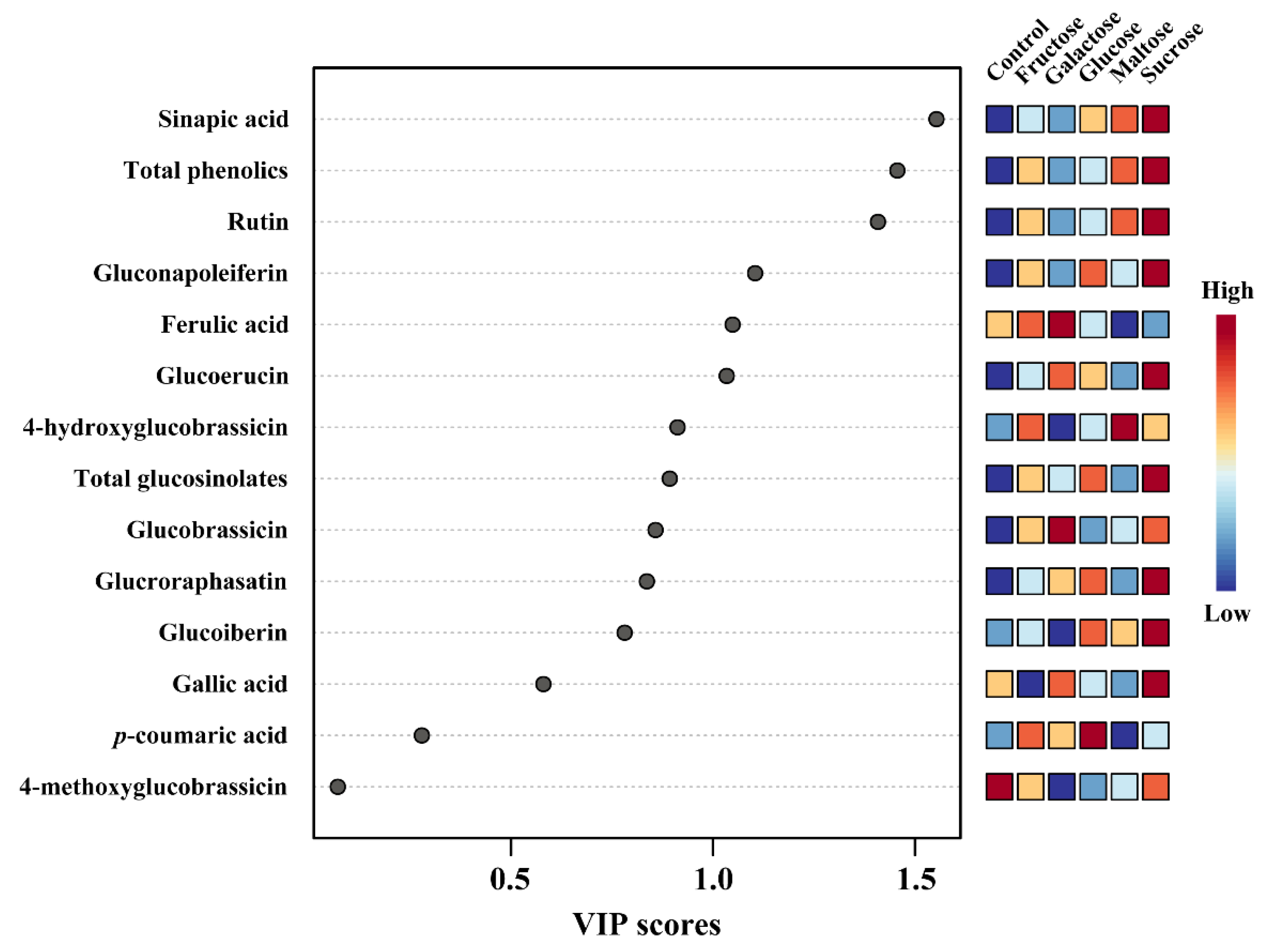
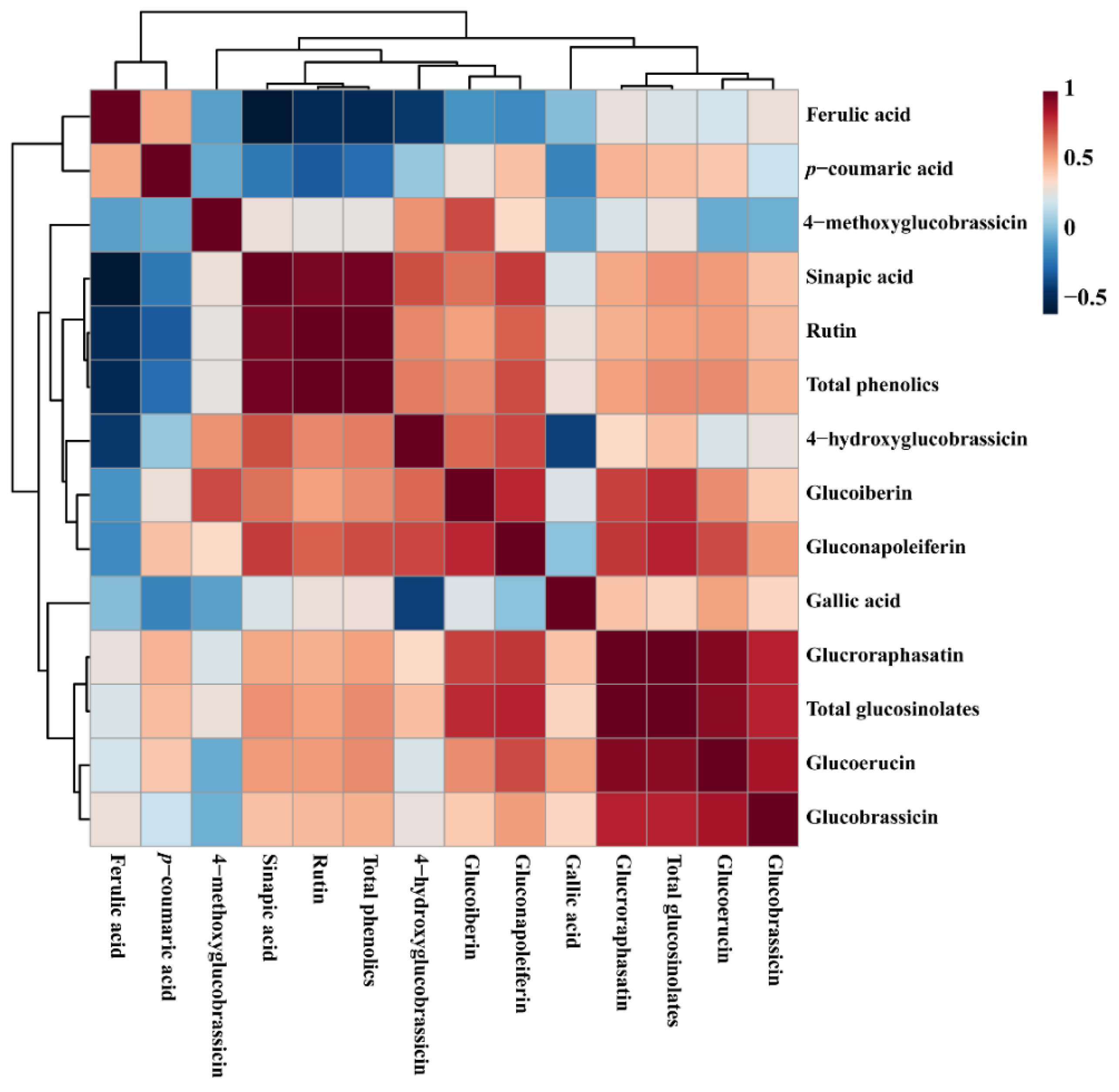
3.4. Metabolic Profiling of Identified Metabolites in Response to Different Carbon Sources
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Carvalho, D.C.d.; Silva, A.L.L.d.; Schuck, M.R.; Purcino, M.; Tanno, G.N.; Biasi, L.A. Fox grape cv. Bordô (Vitis labrusca L.) and grapevine cv. Chardonnay (Vitis vinifera L.) cultivated in vitro under different carbohydrates, amino acids and 6-Benzylaminopurine levels. Braz. Arch. Biol. Technol. 2013, 56, 191–201. [Google Scholar] [CrossRef]
- Park, C.H.; Kim, Y.S.; Li, X.; Kim, H.H.; Arasu, M.V.; Al-Dhabi, N.A.; Lee, S.Y.; Park, S.U. Influence of different carbohydrates on flavonoid accumulation in hairy root cultures of Scutellaria baicalensis. Nat. Prod. Commun. 2016, 11, 799–802. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yaseen, M.; Ahmad, T.; Sablok, G.; Standardi, A.; Hafiz, I.A. Role of carbon sources for in vitro plant growth and development. Mol. Biol. Rep. 2013, 40, 2837–2849. [Google Scholar] [CrossRef] [PubMed]
- Bhagyalakshmi, N.; Thimmaraju, R.; Narayan, M. Various hexoses and di-hexoses differently influence growth, morphology and pigment synthesis in transformed root cultures of red beet (Beta vulgaris). Plant Cell Tissue Organ Cult. 2004, 78, 183–195. [Google Scholar] [CrossRef]
- Rolland, F.; Baena-Gonzalez, E.; Sheen, J. Sugar sensing and signaling in plants: Conserved and novel mechanisms. Annu. Rev. Plant Biol. 2006, 57, 675–709. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El Far, M.M.; Taie, H. Antioxidant activities, total anthocyanins, phenolics and flavonoids contents of some sweetpotato genotypes under stress of different concentrations of sucrose and sorbitol. Aust. J. Basic Appl. Sci. 2009, 3, 3609–3616. [Google Scholar]
- Sivanandhan, G.; Rajesh, M.; Arun, M.; Jeyaraj, M.; Dev, G.K.; Manickavasagam, M.; Selvaraj, N.; Ganapathi, A. Optimization of carbon source for hairy root growth and withaferin A and withanone production in Withania somnifera. Nat. Prod. Commun. 2012, 7, 1271–1272. [Google Scholar] [CrossRef] [Green Version]
- Guo, R.; Yuan, G.; Wang, Q. Sucrose enhances the accumulation of anthocyanins and glucosinolates in broccoli sprouts. Food Chem. 2011, 129, 1080–1087. [Google Scholar] [CrossRef]
- Guo, R.; Yuan, G.; Wang, Q. Effect of sucrose and mannitol on the accumulation of health-promoting compounds and the activity of metabolic enzymes in broccoli sprouts. Sci. Hortic. 2011, 128, 159–165. [Google Scholar] [CrossRef]
- Baque, M.; Elgirban, A.; Lee, E.-J.; Paek, K.-Y. Sucrose regulated enhanced induction of anthraquinone, phenolics, flavonoids biosynthesis and activities of antioxidant enzymes in adventitious root suspension cultures of Morinda citrifolia (L.). Acta Physiol. Plant. 2012, 34, 405–415. [Google Scholar] [CrossRef]
- Zheng, Y.; Tian, L.; Liu, H.; Pan, Q.; Zhan, J.; Huang, W. Sugars induce anthocyanin accumulation and flavanone 3-hydroxylase expression in grape berries. Plant Growth Regul. 2009, 58, 251–260. [Google Scholar] [CrossRef]
- Wang, Y.; Weathers, P. Sugars proportionately affect artemisinin production. Plant Cell Rep. 2007, 26, 1073–1081. [Google Scholar] [CrossRef] [PubMed]
- Weathers, P.; DeJesus-Gonzalez, L.; Kim, Y.; Souret, F.; Towler, M. Alteration of biomass and artemisinin production in Artemisia annua hairy roots by media sterilization method and sugars. Plant Cell Rep. 2004, 23, 414–418. [Google Scholar] [CrossRef] [PubMed]
- Grant, B.; Beevers, H. Absorption of sugars by plant tissues. Plant Physiol. 1964, 39, 78. [Google Scholar] [CrossRef]
- Abellán, Á.; Domínguez-Perles, R.; Moreno, D.A.; García-Viguera, C. Sorting out the value of cruciferous sprouts as sources of bioactive compounds for nutrition and health. Nutrients 2019, 11, 429. [Google Scholar] [CrossRef] [Green Version]
- Shree, B.; Kumar, S.; Sharma, S.; Katoch, V. Functional significance of underutilized high value cruciferous vegetables-an exotic gleam in the gloomy guise of their functional importance. S. Afr. J. Bot. 2022, 145, 420–437. [Google Scholar] [CrossRef]
- Melim, C.; Lauro, M.R.; Pires, I.M.; Oliveira, P.J.; Cabral, C. The role of glucosinolates from cruciferous vegetables (Brassicaceae) in gastrointestinal cancers: From prevention to therapeutics. Pharmaceutics 2022, 14, 190. [Google Scholar] [CrossRef]
- Avato, P.; Argentieri, M. Brassicaceae: A rich source of health improving phytochemicals. Phytochem. Rev. 2015, 14, 1019–1033. [Google Scholar] [CrossRef]
- Costa-Pérez, A.; Núñez-Gómez, V.; Baenas, N.; Di Pede, G.; Achour, M.; Manach, C.; Mena, P.; Del Rio, D.; García-Viguera, C.; Moreno, D.A. Systematic review on the metabolic interest of glucosinolates and their bioactive derivatives for human health. Nutrients 2023, 15, 1424. [Google Scholar] [CrossRef]
- Li, Z.; Lee, H.W.; Liang, X.; Liang, D.; Wang, Q.; Huang, D.; Ong, C.N. Profiling of phenolic compounds and antioxidant activity of 12 cruciferous vegetables. Molecules 2018, 23, 1139. [Google Scholar] [CrossRef] [Green Version]
- Wu, J.; Cui, S.; Liu, J.; Tang, X.; Zhao, J.; Zhang, H.; Mao, B.; Chen, W. The recent advances of glucosinolates and their metabolites: Metabolism, physiological functions and potential application strategies. Crit. Rev. Food Sci. Nutr. 2022, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Kamal, R.M.; Abdull Razis, A.F.; Mohd Sukri, N.S.; Perimal, E.K.; Ahmad, H.; Patrick, R.; Djedaini-Pilard, F.; Mazzon, E.; Rigaud, S. Beneficial health effects of glucosinolates-derived isothiocyanates on cardiovascular and neurodegenerative diseases. Molecules 2022, 27, 624. [Google Scholar] [CrossRef] [PubMed]
- Morrison, M.E.; Joseph, J.M.; McCann, S.E.; Tang, L.; Almohanna, H.M.; Moysich, K.B. Cruciferous vegetable consumption and stomach cancer: A case-control study. Nutr. Cancer 2020, 72, 52–61. [Google Scholar] [CrossRef] [PubMed]
- Soundararajan, P.; Kim, J.S. Anti-carcinogenic glucosinolates in cruciferous vegetables and their antagonistic effects on prevention of cancers. Molecules 2018, 23, 2983. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nomura, S.J.; Hwang, Y.-T.; Gomez, S.L.; Fung, T.T.; Yeh, S.-L.; Dash, C.; Allen, L.; Philips, S.; Hilakivi-Clarke, L.; Zheng, Y.-L. Dietary intake of soy and cruciferous vegetables and treatment-related symptoms in Chinese-American and non-Hispanic White breast cancer survivors. Breast Cancer Res. Treat. 2018, 168, 467–479. [Google Scholar] [CrossRef]
- Ağagündüz, D.; Şahin, T.Ö.; Yılmaz, B.; Ekenci, K.D.; Duyar Özer, Ş.; Capasso, R. Cruciferous vegetables and their bioactive metabolites: From prevention to novel therapies of colorectal cancer. Evid. Based Complement. Altern. Med. 2022, 2022, 1534083. [Google Scholar] [CrossRef]
- Connolly, E.L.; Sim, M.; Travica, N.; Marx, W.; Beasy, G.; Lynch, G.S.; Bondonno, C.P.; Lewis, J.R.; Hodgson, J.M.; Blekkenhorst, L.C. Glucosinolates from cruciferous vegetables and their potential role in chronic disease: Investigating the preclinical and clinical evidence. Front. Pharmacol. 2021, 12, 767975. [Google Scholar] [CrossRef]
- De la Rosa, L.A.; Moreno-Escamilla, J.O.; Rodrigo-García, J.; Alvarez-Parrilla, E. Phenolic compounds. In Postharvest Physiology and Biochemistry of Fruits and Vegetables; Yahia, E., Carrillo-López, A., Eds.; Elsevier Inc.: Amsterdam, The Netherlands, 2018; pp. 253–271. [Google Scholar]
- Choi, M.; Sathasivam, R.; Nguyen, B.V.; Park, N.I.; Woo, S.-H.; Park, S.U. Expression analysis of phenylpropanoid pathway genes and metabolomic analysis of phenylpropanoid compounds in adventitious, hairy, and seedling roots of Tartary buckwheat. Plants 2021, 11, 90. [Google Scholar] [CrossRef]
- Favela-González, K.M.; Hernández-Almanza, A.Y.; De la Fuente-Salcido, N.M. The value of bioactive compounds of cruciferous vegetables (Brassica) as antimicrobials and antioxidants: A review. J. Food Biochem. 2020, 44, e13414. [Google Scholar] [CrossRef]
- Sathasivam, R.; Park, S.U.; Kim, J.K.; Park, Y.J.; Kim, M.C.; Nguyen, B.V.; Lee, S.Y. Metabolic profiling of primary and secondary metabolites in kohlrabi (Brassica oleracea var. gongylodes) sprouts exposed to different light-emitting diodes. Plants 2023, 12, 1296. [Google Scholar] [CrossRef]
- Banihani, S.A. Radish (Raphanus sativus) and diabetes. Nutrients 2017, 9, 1014. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gamba, M.; Asllanaj, E.; Raguindin, P.F.; Glisic, M.; Franco, O.H.; Minder, B.; Bussler, W.; Metzger, B.; Kern, H.; Muka, T. Nutritional and phytochemical characterization of radish (Raphanus sativus): A systematic review. Trends Food Sci. Technol. 2021, 113, 205–218. [Google Scholar] [CrossRef]
- Park, C.H.; Baskar, T.B.; Park, S.-Y.; Kim, S.-J.; Valan Arasu, M.; Al-Dhabi, N.A.; Kim, J.K.; Park, S.U. Metabolic profiling and antioxidant assay of metabolites from three radish cultivars (Raphanus sativus). Molecules 2016, 21, 157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, C.H.; Park, S.-Y.; Park, Y.J.; Kim, J.K.; Park, S.U. Metabolite profiling and comparative analysis of secondary metabolites in Chinese cabbage, radish, and hybrid xBrassicoraphanus. J. Agric. Food Chem. 2020, 68, 13711–13719. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Qiu, X.; Tan, Q.; Xiao, Q.; Mei, S. A comparative metabolomics study of flavonoids in radish with different skin and flesh colors (Raphanus sativus L.). J. Agric. Food Chem. 2020, 68, 14463–14470. [Google Scholar] [CrossRef]
- El-Beltagi, H.S.; Maraei, R.W.; Shalaby, T.A.; Aly, A.A. Metabolites, nutritional quality and antioxidant activity of red radish roots affected by gamma rays. Agronomy 2022, 12, 1916. [Google Scholar] [CrossRef]
- Park, N.I.; Xu, H.; Li, X.; Jang, I.H.; Park, S.; Ahn, G.H.; Lim, Y.P.; Kim, S.J.; Park, S.U. Anthocyanin accumulation and expression of anthocyanin biosynthetic genes in radish (Raphanus sativus). J. Agric. Food Chem. 2011, 59, 6034–6039. [Google Scholar] [CrossRef]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- International Standards Organisation (ISO). Rapeseed-Determination of Glucosinolates Content—Part 1: Method Using High-Performance Liquid Chromatography; ISO: Geneva, Switzerland, 1992; Volume 9167, pp. 1–9. [Google Scholar]
- Lee, S.Y.; Kwon, H.; Kim, J.K.; Park, C.H.; Sathasivam, R.; Park, S.U. Comparative analysis of glucosinolate and phenolic compounds in green and red kimchi cabbage (Brassica rapa L. ssp. pekinensis) hairy roots after exposure to light and dark conditions. Horticulturae 2023, 9, 466. [Google Scholar] [CrossRef]
- Do, T.M.H.; Choi, M.; Kim, J.K.; Kim, Y.J.; Park, C.; Park, C.H.; Park, N.I.; Kim, C.; Sathasivam, R.; Park, S.U. Impact of light and dark treatment on phenylpropanoid pathway genes, primary and secondary metabolites in Agastache rugosa transgenic hairy root cultures by overexpressing Arabidopsis transcription factor AtMYB12. Life 2023, 13, 1042. [Google Scholar] [CrossRef]
- Chae, S.C. Influence of carbon sources on shoot organogenesis in Echinacea angustifolia DC. Life Sci. 2013, 10, 1300–1303. [Google Scholar]
- Kim, J.K.; Baskar, T.B.; Park, S.U. Effect of carbon sources and sucrose concentrations on shoot organogenesis of Aloe saponaria. Biosci. Biotechnol. Res. Asia 2016, 13, 925–930. [Google Scholar] [CrossRef] [Green Version]
- Alina, T.; Magdalena, J.; Andrzej, T. The effect of carbon source on callus induction and regeneration ability in Pharbitis nil. Acta Physiol. Plant. 2006, 28, 619–626. [Google Scholar] [CrossRef]
- Cuenca, B.; Vieitez, A. Influence of carbon source on shoot multiplication and adventitious bud regeneration in in vitro beech cultures. Plant Growth Regul. 2000, 32, 1–12. [Google Scholar] [CrossRef]
- Tang, D.; Ishii, K.; Ohba, K. In vitro regeneration of Alnus cremastogyne Burk from epicotyl explants. Plant Cell Rep. 1996, 15, 658–661. [Google Scholar] [CrossRef]
- Welander, M.; Welander, N.; Brackman, A.-S. Regulation of in vitro shoot multiplication in Syringa, Alnus and Malus by different carbon sources. J. Hortic. Sci. 1989, 64, 361–366. [Google Scholar] [CrossRef]
- Yu, X.; Reed, B.M. Improved shoot multiplication of mature hazelnut (Corylus avellana L.) in vitro using glucose as a carbon source. Plant Cell Rep. 1993, 12, 256–259. [Google Scholar] [CrossRef]
- Harada, H.; Murai, Y. Micropropagation of Prunus mume. Plant Cell Tissue Organ Cult. 1996, 46, 265–267. [Google Scholar] [CrossRef]
- Hsia, C.-N.; Korban, S.S. Factors affecting in vitro establishment and shoot proliferation of Rosa hybrida L. and Rosa chinensis minima. Vitr. Cell. Dev. Biol.-Plant 1996, 32, 217–222. [Google Scholar] [CrossRef]
- Romano, A.; Noronha, C.; Martins-Loucao, M. Role of carbohydrates in micropropagation of cork oak. Plant Cell Tissue Organ Cult. 1995, 40, 159–167. [Google Scholar] [CrossRef]
- Fotopoulos, S.; Sotiropoulos, T. In vitro propagation of the peach rootstock: The effect of different carbon sources and types of sealing material on rooting. Biol. Plant. 2004, 48, 629–631. [Google Scholar] [CrossRef]
- Bogunia, H.; Przywara, L. Rola cukrowców w roslinnych kulturach in vitro. (Sugars in plant tissue culture.). Wiad. Bot. 1999, 43, 25–36. [Google Scholar]
- Kim, S.-J.; Uddin, M.R.; Park, S.U. Glucosinolate accumulation in three important radish (‘Raphanus sativus’) cultivars. Aust. J. Crop Sci. 2013, 7, 1843–1847. [Google Scholar]
- Hanlon, P.R.; Barnes, D.M. Phytochemical composition and biological activity of 8 varieties of radish (Raphanus sativus L.) sprouts and mature taproots. J. Food Sci. 2011, 76, C185–C192. [Google Scholar] [CrossRef] [PubMed]
- Yuan, G.; Wang, X.; Guo, R.; Wang, Q. Effect of salt stress on phenolic compounds, glucosinolates, myrosinase and antioxidant activity in radish sprouts. Food Chem. 2010, 121, 1014–1019. [Google Scholar] [CrossRef]
- Ahn, M.; Moon, J.; Park, C.; Bang, H.; Kim, G.O.; Kim, S.-J.; Kim, K.-h.; Shin, T. Chungpihongsim radish (Raphanus sativus L. cv. Chungpihongsim) ameliorates ethanol-induced gastric injury in rats. Orient. Pharm. Exp. Med. 2016, 16, 37–43. [Google Scholar] [CrossRef]
- Al-Dhabi, N.A.; Arasu, M.V.; Kim, S.J.; RomijUddin, M.; Park, W.T.; Lee, S.Y.; Park, S.U. Methyl jasmonate-and light-induced glucosinolate and anthocyanin biosynthesis in radish seedlings. Nat. Prod. Commun. 2015, 10, 1211–1214. [Google Scholar] [CrossRef] [Green Version]
- Pająk, P.; Socha, R.; Gałkowska, D.; Rożnowski, J.; Fortuna, T. Phenolic profile and antioxidant activity in selected seeds and sprouts. Food Chem. 2014, 143, 300–306. [Google Scholar] [CrossRef]
- Park, H.; Shin, Y.; Kim, Y.-J. Antioxidant contents and activities of twelve varieties of vegetable sprouts. Korean J. Food Sci. Technol. 2019, 51, 207–213. [Google Scholar]
- Ahmad, T.; Abbasi, N.A.; Hafiz, I.A.; Ali, A. Comparison of sucrose and sorbitol as main carbon energy sources in microprogation of peach rootstock GF-677. Pak. J. Bot. 2007, 39, 1269. [Google Scholar]
- Fuentes, S.R.; Calheiros, M.B.; Manetti-Filho, J.; Vieira, L.G. The effects of silver nitrate and different carbohydrate sources on somatic embryogenesis in Coffea canephora. Plant Cell Tissue Organ Cult. 2000, 60, 5–13. [Google Scholar] [CrossRef]
- Thompson, M.R.; Thorpe, T.A. Metabolic and non-metabolic roles of carbohydrates. In Cell and Tissue Culture in Forestry; Bong, J.M., Durzan, D.J., Eds.; Martinus Nijhoff Publisher: Dardrecht, The Netherlands, 1987; pp. 89–112. [Google Scholar]
- Amiri, S.; Kazemitabar, S. Enhancement of callus induction and regeneration efficiency from embryo cultures of Datura stramonium by adjusting carbon sources and concentrations. Afr. J. Biotechnol. 2011, 10, 10101–10107. [Google Scholar]
- Jheng, F.-Y.; Do, Y.-Y.; Liauh, Y.-W.; Chung, J.-P.; Huang, P.-L. Enhancement of growth and regeneration efficiency from embryogenic callus cultures of Oncidium ‘Gower Ramsey’ by adjusting carbohydrate sources. Plant Sci. 2006, 170, 1133–1140. [Google Scholar] [CrossRef]
- Swedlund, B.; Locy, R.D. Sorbitol as the primary carbon source for the growth of embryogenic callus of maize. Plant Physiol. 1993, 103, 1339–1346. [Google Scholar] [CrossRef] [Green Version]
- Thwe, A.A.; Chae, S.C.; Chung, S.-O.; Park, S.U. Enhancement of the in vitro root regeneration efficiency of Rehmannia glutinosa Libosch. stem explants by different carbon sources. Life Sci. 2013, 10, 579–582. [Google Scholar]
- Luo, J.; He, G.-Y. Optimization of elicitors and precursors for paclitaxel production in cell suspension culture of Taxus chinensis in the presence of nutrient feeding. Process Biochem. 2004, 39, 1073–1079. [Google Scholar] [CrossRef]
- Sivanandhan, G.; Arun, M.; Mayavan, S.; Rajesh, M.; Mariashibu, T.; Manickavasagam, M.; Selvaraj, N.; Ganapathi, A. Chitosan enhances withanolides production in adventitious root cultures of Withania somnifera (L.) Dunal. Ind. Crops Prod. 2012, 37, 124–129. [Google Scholar] [CrossRef]
- Jung, K.; Kwak, S.S.; Kim, S.W.; Lee, H.; Choi, C.; Liu, J.R. Improvement of the catharanthine productivity in hairy root cultures ofCatharanthus roseus by using monosaccharides as a carbon source. Biotechnol. Lett. 1992, 14, 695–700. [Google Scholar] [CrossRef]
- Masoumian, M.; Arbakariya, A.; Syahida, A.; Maziah, M. Flavonoids production in Hydrocotyle bonariensis callus tissues. J. Med. Plants Res. 2011, 5, 1564–1574. [Google Scholar]
- Chattopadhyay, S.; Srivastava, A.K.; Bhojwani, S.S.; Bisaria, V.S. Production of podophyllotoxin by plant cell cultures of Podophyllum hexandrum in bioreactor. J. Biosci. Bioeng. 2002, 93, 215–220. [Google Scholar] [CrossRef]
- Gibson, S.I. Plant sugar-response pathways. Part of a complex regulatory web. Plant Physiol. 2000, 124, 1532–1539. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jang, J.-C.; Sheen, J. Sugar sensing in higher plants. Plant Cell 1994, 6, 1665–1679. [Google Scholar] [PubMed] [Green Version]
- Vitrac, X.; Larronde, F.; Krisa, S.; Decendit, A.; Deffieux, G.; Mérillon, J.-M. Sugar sensing and Ca2+–calmodulin requirement in Vitis vinifera cells producing anthocyanins. Phytochemistry 2000, 53, 659–665. [Google Scholar] [CrossRef] [PubMed]
- Hara, M.; Oki, K.; Hoshino, K.; Kuboi, T. Enhancement of anthocyanin biosynthesis by sugar in radish (Raphanus sativus) hypocotyl. Plant Sci. 2003, 164, 259–265. [Google Scholar] [CrossRef]
- Blanc, G.; Lardet, L.; Martin, A.; Jacob, J.-L.; Carron, M.-P. Differential carbohydrate metabolism conducts morphogenesis in embryogenic callus of Hevea brasiliensis (Mull. Arg.). J. Exp. Bot. 2002, 53, 1453–1462. [Google Scholar] [CrossRef] [Green Version]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, C.H.; Choi, M.; Park, Y.E.; Yeo, H.J.; Kim, J.K.; Kim, Y.B.; Sankaranarayanan, S.; Sathasivam, R.; Park, S.U. Influence of Different Types of Carbon Sources on Glucosinolate and Phenolic Compounds in Radish Sprouts. Horticulturae 2023, 9, 679. https://doi.org/10.3390/horticulturae9060679
Park CH, Choi M, Park YE, Yeo HJ, Kim JK, Kim YB, Sankaranarayanan S, Sathasivam R, Park SU. Influence of Different Types of Carbon Sources on Glucosinolate and Phenolic Compounds in Radish Sprouts. Horticulturae. 2023; 9(6):679. https://doi.org/10.3390/horticulturae9060679
Chicago/Turabian StylePark, Chang Ha, Minsol Choi, Ye Eun Park, Hyeon Ji Yeo, Jae Kwang Kim, Yeon Bok Kim, Subramanian Sankaranarayanan, Ramaraj Sathasivam, and Sang Un Park. 2023. "Influence of Different Types of Carbon Sources on Glucosinolate and Phenolic Compounds in Radish Sprouts" Horticulturae 9, no. 6: 679. https://doi.org/10.3390/horticulturae9060679
APA StylePark, C. H., Choi, M., Park, Y. E., Yeo, H. J., Kim, J. K., Kim, Y. B., Sankaranarayanan, S., Sathasivam, R., & Park, S. U. (2023). Influence of Different Types of Carbon Sources on Glucosinolate and Phenolic Compounds in Radish Sprouts. Horticulturae, 9(6), 679. https://doi.org/10.3390/horticulturae9060679





