Silver Nano Chito Oligomer Hybrid Solution for the Treatment of Citrus Greening Disease (CGD) and Biostimulants in Citrus Horticulture
Abstract
1. Introduction
2. Materials and Methods
2.1. Hybrid Solution Preparation
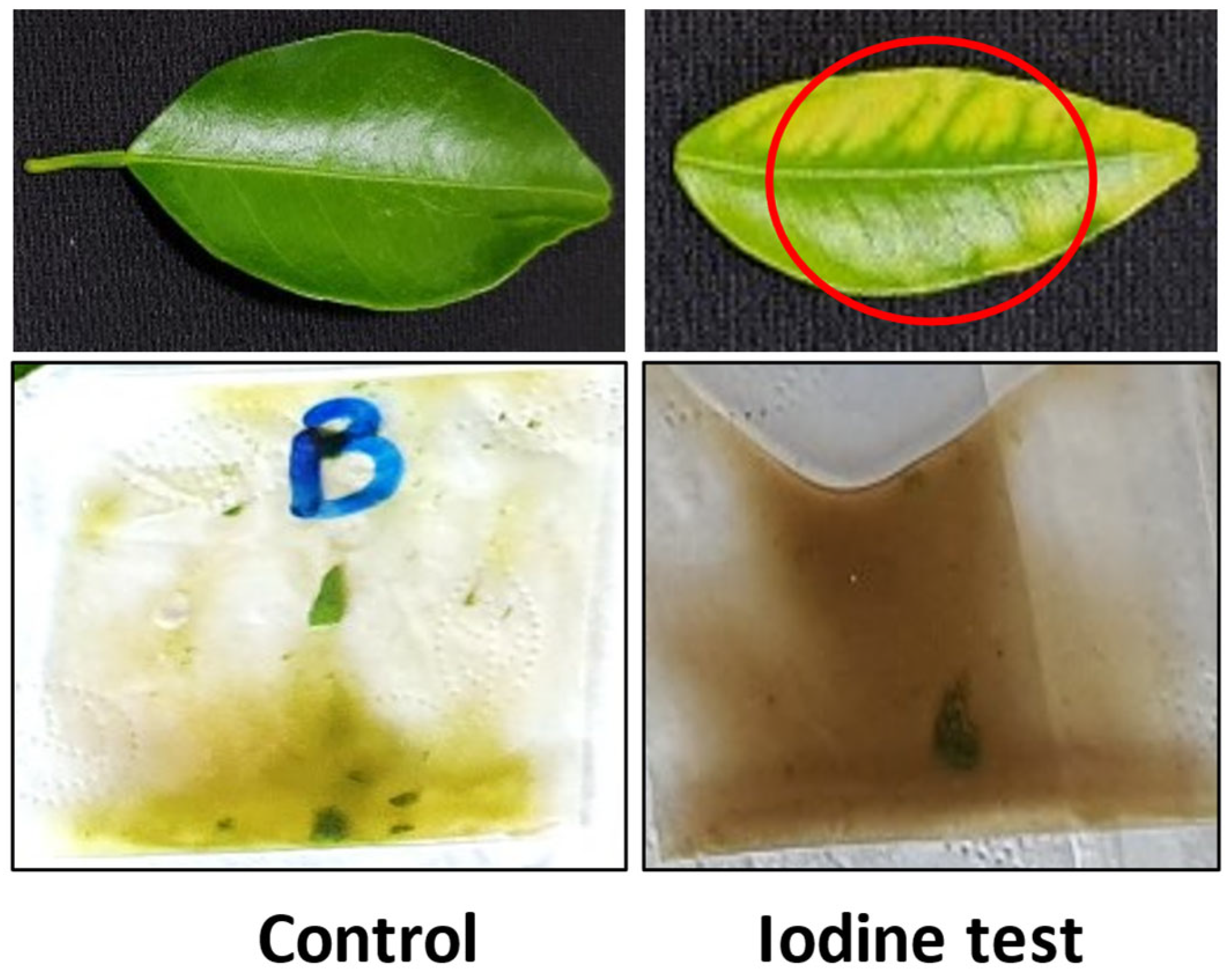
2.2. Citrus Greening Disease Testing (CDG)
2.3. Field Crops Test
2.4. Treatment Determines
2.5. Growth and Yield Analysis
2.6. Statistical Analysis
3. Results
3.1. Physical Properties and Particle Size of Hybrid Solution
3.2. Citrus Greening Disease Assessment
3.3. Field Test Results
3.4. Residual Analysis (LOD)
3.5. Yield of Citrus Fruits
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Office of Agricultural Economics. Available online: https://mis-app.oae.go.th/product (accessed on 11 November 2022).
- Poonyapitak, D.; Tantivanich, Y.; Puawongpej, B.; Kositcharoenkul, N. Surveying and Identification Greening Like-Organism in Thailand by Molecular Biology Technic. Plant Protection Research and Development Office. Department of Agriculture. 1858–1862. Available online: file:///C:/Users/User/Desktop/Fulltext%2310_90204.pdf (accessed on 11 November 2022).
- Bendix, C.; Lewis, J.D. The enemy within: Phloem-limited pathogens. Mol. Plant Pathol. 2018, 19, 238–254. [Google Scholar] [CrossRef]
- Food Agriculture Organization of the United Nation (FAO). Managing Huanglongbing/Citrus Greening Disease in the Caribbean. 2013. Available online: https://www.fao.org/3/ax739e/ax739e.pdf (accessed on 26 December 2022).
- Jantasorn, A.; Paradornuwat, A.; Chowpongpang, S.; Chunwongse, J.; Thaveechai, N. Diagnosis of Greening Disease of Citrus spp. in Thailand. In Proceedings of the 45th Kasetsart University Annual Conference, Bangkok, Thailand, 30 January–2 February 2007; pp. 218–225. [Google Scholar]
- Tipu, M.M.H.; Masud, M.; Jahan, R.; Baroi, A.; Hoque, A.K.M.A. Identification of Citrus greening based on visual symptoms. A grower’s diagnostic toolkit. Heliyon 2021, 7, 2–7. [Google Scholar] [CrossRef]
- Whitaker, D.C.; Giurcanu, M.C.; Young, L.J.; Gonzalez, P.; Etxeberria, E.; Roberts, P.; Hendricks, K.; Roman, F. Starch Content of Citrus Leaves. Hortscience 2014, 49, 757–762. [Google Scholar] [CrossRef]
- Stephano-Honedo, J.L.; Torres-Gutie, O.; Toledano-Magan, Y.; Gradilla-Mart, I.; Pestryakov, A.; Sanchez-Gonza, A.; Garc, J.C.; Bogdanchikova, N. ArgovitTM silver nanoparticles to fight Huanglongbing disease in Mexican limes (Citrus aurantifolia Swingle). R. Soc. Chem. 2020, 10, 6146–6155. [Google Scholar]
- Lodhi, G.; Kim, Y.-S.; Hwang, J.-W.; Kim, S.-K.; Jeon, Y.-J.; Je, J.-Y.; Ahn, C.-B.; Moon, S.-H.; Jeon, B.-T.; Park, P.-J. Chitooligosaccharide and its derivatives: Preparation and biological applications. BioMed Res. Int. 2014, 2014, 1–13. [Google Scholar] [CrossRef]
- Liang, S.; Sun, Y.; Dai, X. A review of the preparation, analysis and biological functions of chitooligosaccharide. Int. J. Mol. Sci. 2018, 19, 2197. [Google Scholar] [CrossRef]
- Hadwiger, L.A. Chitosan molecular forms with potential in agriculture and medicine. J. Drug Des. Res. 2017, 4, 1036. [Google Scholar]
- Winker, A.J.; Dominguez-Nuñez, J.A.; Aranaz, I.; Poza-Carrión, C.; Ramonell, K.; Somerville, S.; Berrocal-Lobo, M. Short-Chain Chitin Oligomers: Promoters of Plant Growth. Mar. Drugs 2017, 15, 40. [Google Scholar] [CrossRef] [PubMed]
- Schmitz, C.; Auza, L.G.; Koberidze, D.; Rasche, S.; Fischer, R.; Bortesi, L. Conversion of chitin to defined chitosan oligomers: Current status and future prospects. Mar. Drugs 2019, 17, 452. [Google Scholar] [CrossRef]
- Tariq, M.; Mohammad, K.N.; Ahmed, B.; Siddiqui, M.A.; Lee, J. Biological synthesis of silver nanoparticles and prospects in plant disease management. Molecules 2022, 27, 4754. [Google Scholar] [CrossRef] [PubMed]
- Daniel, S.C.G.K.; Joseph, P.; Sivakumar, M. Biosynthesized silver nanoparticle-based hybrid materials. Nanosci. Nanotechnol. Asia 2017, 7, 1–7. [Google Scholar] [CrossRef]
- Haris, M.; Hussain, T.; Mohamed, H.I.; Khan, A.; Ansari, M.S.; Tauseef, A.; Khan, A.A.; Akhtar, N. Nanotechnology–A new frontier of nano-farming in agricultural and food production and its development. Sci. Total Environ. 2023, 857, 159639. [Google Scholar] [CrossRef] [PubMed]
- Tibayan, E.B., Jr.; Muflikhun, M.A.; Al Rey, C.V.; Kumar, V.; Santos, G.N.C. Structures and UV resistance of Ag/SnO2 nanocomposite materials synthesized by horizontal vapor phase growth for coating applications. J. Mater. Res. Technol. 2020, 9, 4806–4816. [Google Scholar] [CrossRef]
- Sonseca, A.; Madani, S.; Rodriguez, H.; Hevilla, V.; Echeverria, C.; Fernández-García, M.; Muñoz-Bonilla, A.; Charef, N.; López, D. Multifunctional PLA blends containing chitosan mediated silver nanoparticles: Thermal, mechanical, antibacterial, and degradation properties. Nanomaterials 2020, 10, 22. [Google Scholar] [CrossRef] [PubMed]
- Mei, L.; Zhenlong, X.; Yanmei, S.; Chunlei, L.; Shuyan, J.; Lijun, Z.; Pengxu, L. Multivalent and synergistic chitosan oligosaccharide-Ag nanocomposites for therapy of bacterial infection. Sci. Rep. 2020, 10, 10011. [Google Scholar] [CrossRef]
- Fang, Y.; Hong, C.Q.; Chen, F.R.; Gui, F.Z.; You, Y.X.; Guan, X.; Pan, X.H. Green synthesis of nano silver by tea extract with high antimicrobial activity. Inorg. Chem. Commun. 2021, 132, 108808. [Google Scholar] [CrossRef]
- Muflikhun, M.A.; Santos, G.N.C. A standard method to synthesize Ag, Ag/Ge, Ag/TiO2, SnO2, and Ag/SnO2 nanomaterials using the HVPG technique. MethodsX 2019, 6, 2861–2872. [Google Scholar] [CrossRef]
- Dangtungee, R.; Siengchin, S. Silver Nanopolymer Composites: Production and Efficiency. Mech. Compos. Mater. 2015, 51, 239–244. [Google Scholar] [CrossRef]
- Vatcharakajon, P.; Choengpanya, K.; Susawaengsup, C.; Dangtungee, R. Chitosan oligomer and monomer (COAMs) benefit and its application in innovative organic method for root rot disease treatment in durian crops. Mater. Today Proc. 2023, 77, 1033–1038. [Google Scholar] [CrossRef]
- de Souza Júnior, J.P.; de Mello Prado, R.; Campos CN, S.; Oliveira, D.F.; Cazetta, J.O.; Detoni, J.A. Silicon foliar spraying in the reproductive stage of cotton plays an equivalent role to boron in increasing yield, and combined boron-silicon application, without polymerization, increases fiber quality. Ind. Crops Prod. 2022, 182, 114888. [Google Scholar] [CrossRef]
- Tuoriniemi, J.; Cornelis, G.; Hassellöv, M. Size discrimination and detection capabilities of single-particle ICPMS for environmental analysis of silver nanoparticles. Anal. Chem. 2012, 84, 3965–3972. [Google Scholar] [CrossRef]
- Cheng, X.; Dong, P.; Huang, Z.; Zhang, Y.; Chen, Y.; Nie, X.; Zhang, X. Green synthesis of plasmonic Ag nanoparticles anchored TiO2 nanorod arrays using cold plasma for visible-light-driven photocatalytic reduction of CO2. J. CO2 Util. 2017, 20, 200–207. [Google Scholar] [CrossRef]
- Múgica-Vidal, R.; Sainz-García, E.; Álvarez-Ordóñez, A.; Prieto, M.; González-Raurich, M.; López, M.; López, M.; Rojo-Bezares, B.; Sáenz, Y.; Alba-Elías, F. Production of antibacterial coatings through atmospheric pressure plasma: A promising alternative for combatting biofilms in the food industry. Food Bioprocess Technol. 2019, 12, 1251–1263. [Google Scholar] [CrossRef]
- Fiorati, A.; Bellingeri, A.; Punta, C.; Corsi, I.; Venditti, I. Silver nanoparticles for water pollution monitoring and treatments: Ecosafety challenge and cellulose-based hybrids solution. Polymers 2020, 12, 1635. [Google Scholar] [CrossRef] [PubMed]
- Haroon, M.; Zaidi, A.; Ahmed, B.; Rizvi, A.; Khan, M.S.; Musarrat, J. Effective inhibition of phytopathogenic microbes by eco-friendly leaf extract mediated silver nanoparticles (AgNPs). Indian J. Microbiol. 2019, 59, 273–287. [Google Scholar] [CrossRef]
- Akpinar, I.; Unal, M.; Sar, T. Potential antifungal effects of silver nanoparticles (AgNPs) of different sizes against phytopathogenic Fusarium oxysporum f. sp. radicis-lycopersici (FORL) strains. SN Appl. Sci. 2021, 3, 506. [Google Scholar] [CrossRef]
- Hashmi, S.S.; Abbasi, B.H.; Rahman, L.; Zaka, M.; Zahir, A. Phytosynthesis of organo-metallic silver nanoparticles and their anti-phytopathogenic potency against soil borne Fusarium spp. Mater. Res. Express 2019, 6, 1150a9. [Google Scholar] [CrossRef]
- Matthews, S.; Ali, A.; Siddiqui, Y.; Supramaniam, C.V. Plant bio-stimulant: Prospective, safe and natural resources. J. Soil Sci. Plant Nutr. 2022, 22, 2570–2586. [Google Scholar] [CrossRef]
- Salachna, P.; Byczyńska, A.; Zawadzińska, A.; Piechocki, R.; Mizielińska, M. Stimulatory effect of silver nanoparticles on the growth and flowering of potted oriental lilies. Agronomy 2019, 9, 610. [Google Scholar] [CrossRef]
- Saha, N.; Dutta Gupta, S. Promotion of shoot regeneration of Swertia chirata by biosynthesized silver nanoparticles and their involvement in ethylene interceptions and activation of antioxidant activity. Plant Cell Tissue Organ Cult. (PCTOC) 2018, 134, 289–300. [Google Scholar] [CrossRef]
- Guzmán-Báez, G.A.; Trejo-Téllez, L.I.; Ramírez-Olvera, S.M.; Salinas-Ruíz, J.; Bello-Bello, J.J.; Alcántar-González, G.; Hidalgo-Contreras, J.V.; Gómez-Merino, F.C. Silver nanoparticles increase nitrogen, phosphorus, and potassium concentrations in leaves and stimulate root length and number of roots in tomato seedlings in a hormetic manner. Dose-Response 2021, 19, 15593258211044576. [Google Scholar] [CrossRef] [PubMed]





| Group | Treatment |
|---|---|
| 1 | Ampicillin |
| 2 | AgNPs |
| 3 | AgNPs + Gibberellic acid |
| 4 | The hybrid solution |
| 5 | The hybrid solution + Gibberellic acid |
| Physical Leaves Criteria | Determination |
|---|---|
| Shoot length | Centimeter (cm) |
| Number of leaves | Number of leaves |
| Leaf area | Centimeter square (cm2) |
| Greenness of leaves | Greenness SPAD index |
| Measurement | Z Average (d.nm) | PdI (Mn) |
|---|---|---|
| 1 | 278.8 | 0.314 |
| 2 | 284.3 | 0.203 |
| 3 | 284.8 | 0.186 |
| 4 | 283.6 | 0.234 |
| 5 | 277.3 | 0.339 |
| 6 | 286.1 | 0.200 |
| Treatment | January–March 2022 | March–May 2022 | ||
|---|---|---|---|---|
| Shoot Length (cm) | The Number of Leaves (no.) | Shoot Length (cm) | The Number of Leaves (no.) | |
| 1. Ampicillin | 9.62 ± 0.13 | 7.09 ± 0.15 | 9.78 ± 0.35 | 42.7 ± 0.14 |
| 2. AgNPs | 10.20 ± 0.21 | 7.23 ± 0.22 | 10.22 ± 0.28 | 7.57 ± 0.13 |
| 3. AgNPs + Gibberellic acid | 16.85 ± 0.51 | 8.65 ± 0.21 | 17.37 ± 0.17 | 8.99 ± 0.11 |
| 4. The hybrid solution | 11.58 ± 0.43 | 7.53 ± 0.26 | 12.43 ± 0.11 | 8.29 ± 0.17 |
| 5. The hybrid solution + Gibberellic acid | 17.10 ± 0.25 | 8.45 ± 0.14 | 17.10 ± 0.23 | 8.78 ± 0.21 |
| F-test | ** | ns | ** | ns |
| c.v. % | 5.19 ± 0.01 | 1.11 ± 0.02 | 17.56 ± 0.04 | 21.82 ± 0.03 |
| Treatment | January–March 2022 | March–May 2022 | ||||
|---|---|---|---|---|---|---|
| Leaf Area (cm2) | Greenness Index (SPAD) | Iodine Test Kit (−/+) | Leaf Area (cm2) | Greenness Index (SPAD) | Iodine Test Kit (−/+) | |
| 1. Ampicillin | 10.30 ± 0.34 | 59.92 ± 0.32 | negative | 10.42 ± 0.11 | 60.42 ± 0.13 | negative |
| 2. AgNPs | 9.87 ± 0.21 | 54.59 ± 0.14 | negative | 9.94 ± 0.18 | 54.99 ± 0.11 | negative |
| 3. AgNPs + Gibberellic acid | 8.87 ± 0.12 | 54.97 ± 0.16 | negative | 9.27 ± 0.24 | 55.54 ± 0.28 | negative |
| 4. The hybrid solution | 10.51 ± 0.36 | 54.26 ± 0.27 | negative | 10.75 ± 0.19 | 54.86 ± 0.21 | negative |
| 5. The hybrid solution + Gibberellic acid | 6.87 ± 0.87 | 59.28 ± 0.17 | negative | 6.93 ± 0.28 | 59.91 ± 0.11 | negative |
| F-test | ns | ns | - | ns | ns | - |
| c.v. % | 6.41 ± 0.12 | 20.55 ± 0.21 | - | 25.07 ± 0.13 | 8.06 ± 0.11 | - |
| Test Item | Results | Unit | Method |
|---|---|---|---|
| Silver | Not found 1 | mg/kg | AOAC (2019), 999.10 detected by ICP-OES |
| Criteria of Comparison | Ampicillin | AgNPs-COAMs | AgNPs-COAMs |
|---|---|---|---|
| Application method | Trunk injection | Trunk injection | Foliar application |
| Yield weight per Citrus tree (kg) | 95 | 110 | 115 |
| % Of incomplete fruit | 25% | 5% | 5% |
| Net of Yield (kg) | 71.25 | 104.50 | 109.25 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vatcharakajon, P.; Sornsaket, A.; Choengpanya, K.; Susawaengsup, C.; Sornsakdanuphap, J.; Boonplod, N.; Bhuyar, P.; Dangtungee, R. Silver Nano Chito Oligomer Hybrid Solution for the Treatment of Citrus Greening Disease (CGD) and Biostimulants in Citrus Horticulture. Horticulturae 2023, 9, 725. https://doi.org/10.3390/horticulturae9060725
Vatcharakajon P, Sornsaket A, Choengpanya K, Susawaengsup C, Sornsakdanuphap J, Boonplod N, Bhuyar P, Dangtungee R. Silver Nano Chito Oligomer Hybrid Solution for the Treatment of Citrus Greening Disease (CGD) and Biostimulants in Citrus Horticulture. Horticulturae. 2023; 9(6):725. https://doi.org/10.3390/horticulturae9060725
Chicago/Turabian StyleVatcharakajon, Pakpoom, Ampol Sornsaket, Khuanjarat Choengpanya, Chanthana Susawaengsup, Jirapong Sornsakdanuphap, Nopporn Boonplod, Prakash Bhuyar, and Rapeephun Dangtungee. 2023. "Silver Nano Chito Oligomer Hybrid Solution for the Treatment of Citrus Greening Disease (CGD) and Biostimulants in Citrus Horticulture" Horticulturae 9, no. 6: 725. https://doi.org/10.3390/horticulturae9060725
APA StyleVatcharakajon, P., Sornsaket, A., Choengpanya, K., Susawaengsup, C., Sornsakdanuphap, J., Boonplod, N., Bhuyar, P., & Dangtungee, R. (2023). Silver Nano Chito Oligomer Hybrid Solution for the Treatment of Citrus Greening Disease (CGD) and Biostimulants in Citrus Horticulture. Horticulturae, 9(6), 725. https://doi.org/10.3390/horticulturae9060725








