Measurement of Dry Matter and Starch in Modern Cassava Genotypes during Long Harvest Cycles
Abstract
1. Introduction
2. Materials and Methods
2.1. Site, Soil, and Climate
2.2. Experimental Design and Treatments
2.3. Soil Tillage, Cassava Planting, and Management
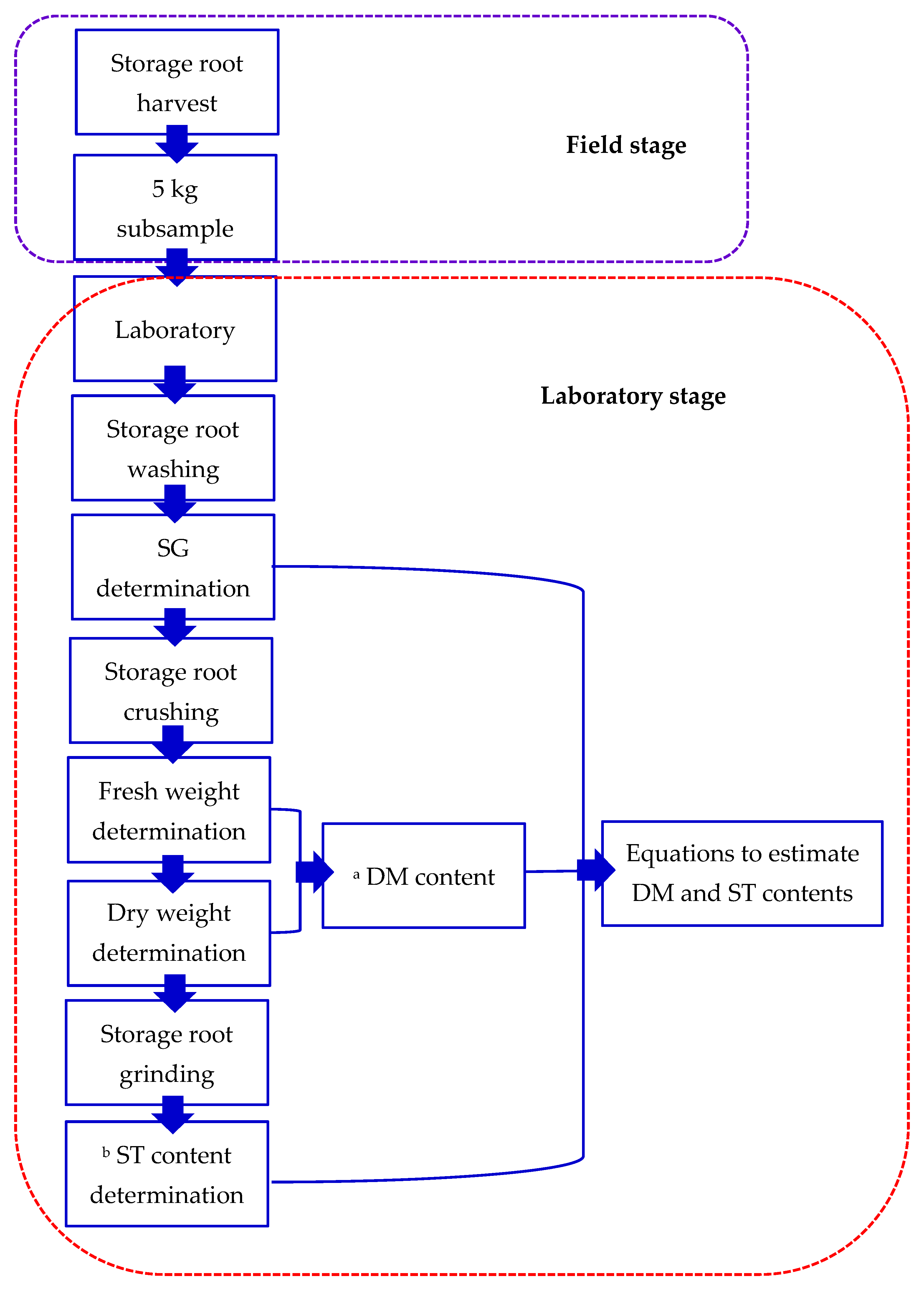
2.4. Storage Root Harvest and Analysis
- (a)
- Empirical equations to obtain DM content
- (b)
- Empirical equations to obtain ST content
2.5. Statistical Analysis
3. Results
3.1. Effect of Genotype on SG and Measured Root DM and ST Content as Related to Plant Age
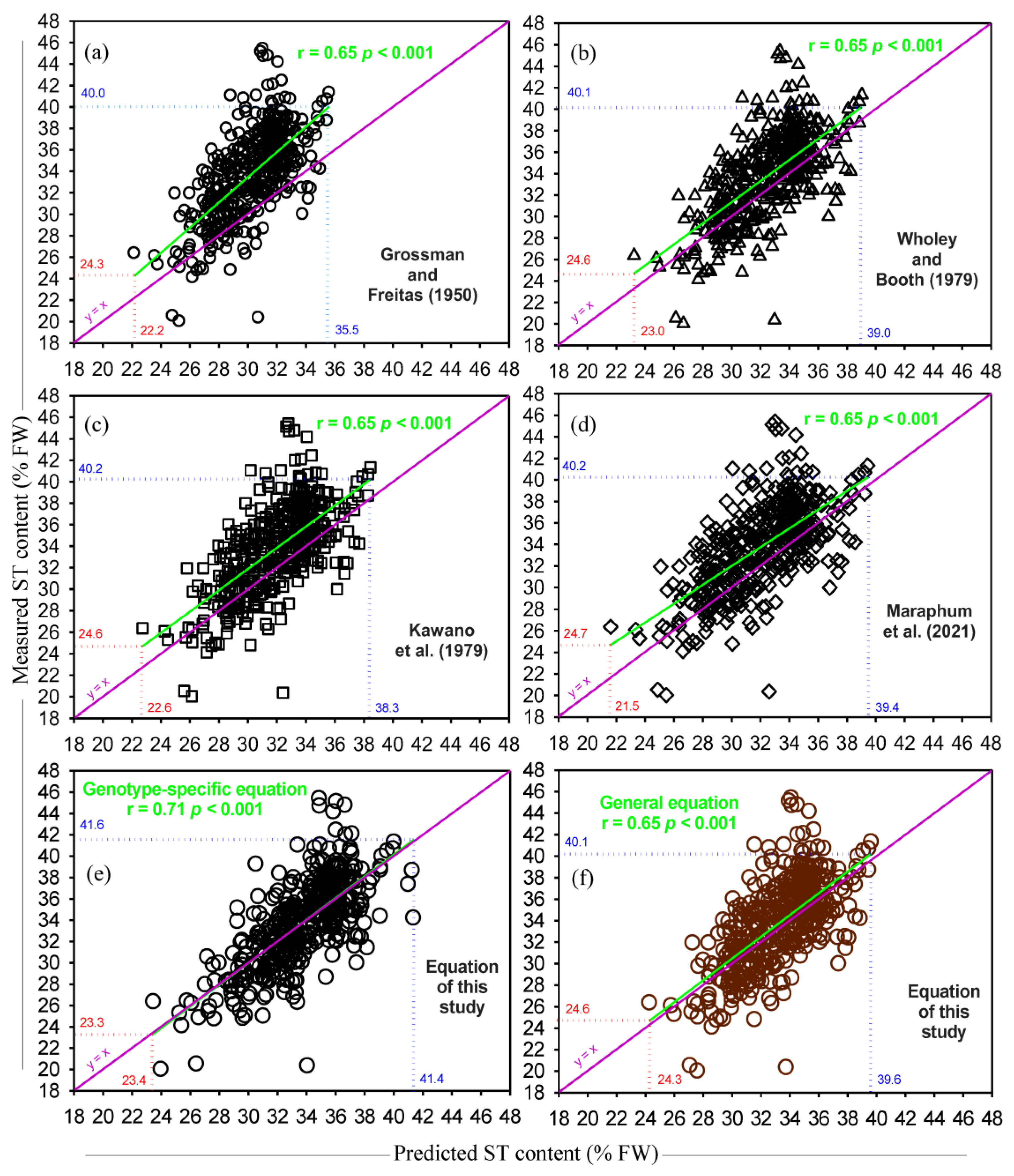
3.2. Estimation of DM and ST Content of Cassava Roots Based on SG
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAOSTAT—Food and Agriculture Organization, Production Cassava All Countries, 1961–2018. 2023. Available online: https://www.fao.org/faostat/en/#data/QV (accessed on 21 April 2023).
- Odedeyi, T.; Rabbi, I.; Poole, C.; Darwazeh, I. Estimation of starch content in cassava based on coefficient of reflection measurement. Front. Food. Sci. Technol. 2022, 2, 878023. [Google Scholar] [CrossRef]
- Teye, E.; Asare, A.P.; Amoah, R.S.; Tetteh, J.P. Determination of the dry matter content of cassava (Manihot Esculenta, Crantz) tubers using specific gravity method. J. Agric. Biol. Sci. 2011, 6, 23–28. [Google Scholar]
- Nassar, N.; Vizzotto, C.S.; Schwartz, C.A.; Pires, O.R. Cassava diversity in Brazil: The case of carotenoid-rich landraces. Genet. Mol. Res. 2007, 6, 116–121. [Google Scholar] [PubMed]
- Bantadjan, Y.; Rittiron, R.; Malithong, K.; Narongwongwattana, S. Rapid Starch Evaluation in Fresh Cassava Root Using a Developed Portable Visible and Near-Infrared Spectrometer. ACS Omega 2020, 5, 11210–11216. [Google Scholar] [CrossRef] [PubMed]
- Moorthy, S.; Padmaja, G. A Rapid Titrimetric Method for the Determination of Starch Content of Cassava Tubers. J. Root Crops 2002, 28, 30–37. [Google Scholar]
- Rangel, M.A.S.; Ringenberg, R.; Santos, W.S.; Vieira, E.A.; Oliveira, M.C.N. Comportamento de Genótipos de Mandioca Quanto às Características Produtivas em Diferentes Épocas de Colheita em Paranavaí, PR; Embrapa Mandioca e Fruticultura: Cruz das Almas, Brasil, 2021; p. 22. [Google Scholar]
- Wholey, D.W.; Booth, R.H. A Comparison of Simple Methods for Estimating Starch Content of Cassava Roots. J. Sci. Food Agric. 1979, 30, 158–164. [Google Scholar] [CrossRef]
- Ikeogu, U.N.; Davrieux, F.; Dufour, D.; Ceballos, H.; Egesi, C.N.; Jannink, J.-L. Rapid Analyses of Dry Matter Content and Carotenoids in Fresh Cassava Roots Using a Portable Visible and near Infrared Spectrometer (Vis/NIRS). PLoS ONE 2017, 12, e0188918. [Google Scholar] [CrossRef] [PubMed]
- Grossman, J.; Freitas, A.C. Determinação do teor de matéria seca pelo peso específico em mandioca. Rev. Agronômica 1950, 14, 75–80. [Google Scholar]
- Kawano, K.; Fukuda, W.M.G.; Cenpukdee, U. Genetic and Environmental Effects on Dry Matter Content of Cassava Root. Crop Sci. 1987, 27, 69–74. [Google Scholar] [CrossRef]
- Maraphum, K.; Saengprachatanarug, K.; Wongpichet, S.; Phuphuphud, A.; Sirisomboon, P.; Posom, J. Modified Specific Gravity Method for Estimation of Starch Content and Dry Matter in Cassava. Heliyon 2021, 7, e07450. [Google Scholar] [CrossRef] [PubMed]
- Cereda, M.P.; Vilpoux, O.; Takahashi, M. Balança hidrostática como forma de avaliação do teor de massa seca e amido. In Tecnologia, Usos e Potencialidade de Tuberosas Amiláceas Latino-Americanas; Cereda, M.P., Vilpoux, O., Eds.; Fundação Cargil: São Paulo, Brazil, 2003; pp. 30–47. [Google Scholar]
- Conceição, A.J. A Mandioca; Nobel: São Paulo, Brazil, 1981; p. 382. [Google Scholar]
- Fernandes, A.M.; Gazola, B.; da Silva Nunes, J.G.; Garcia, E.L.; Leonel, M. Yield and Nutritional Requirements of Cassava in Response to Potassium Fertilizer in the Second Cycle. J. Plant Nutr. 2017, 40, 2785–2796. [Google Scholar] [CrossRef]
- Mota, L.H.S.O.; Fernandes, A.M.; Assunção, N.S.; Leite, H.M.F. Leaf Area Development and Yield of Cassava in Response to Pruning of Shoots and the Late Supply of Nitrogen and Potassium. Agron. J. 2020, 112, 1406–1422. [Google Scholar] [CrossRef]
- Fernandes, D.S.; Santos, T.P.R.; Fernandes, A.M.; Leonel, M. Harvest time optimization leads to the production of native cassava starches with different properties. Int. J. Biol. Macromol. 2019, 132, 710–721. [Google Scholar] [CrossRef] [PubMed]
- IAC—Instituto Agronômico, Pesquisas da Secretaria de Agricultura de SP Transformaram Produção e Comercialização da Mandioca de Mesa. 2023. Available online: https://www.iac.sp.gov.br/noticiasdetalhes.php?id=1391 (accessed on 5 June 2023).
- Maeda, M.; Dip, T.M. Curvas de porcentagem mássica de água versus peso específico em vegetais in natura–otimização de processos industriais pela seleção via teste da matéria-prima. Food Sci. Technol. 2000, 20, 309–313. [Google Scholar] [CrossRef]
- Carvalho, P.R.N.; Mezette, T.F.; Valle, T.L.; Carvalho, C.R.L.; Feltran, J.C. Avaliação da exatidão, precisão e robustez do método de análise do teor de matéria seca de mandioca (Manihot esculenta, Crantz) por meio da determinação do peso específico (balança hidrostática). Rev. Raízes E Amidos Trop. 2007, 3, 1–4. [Google Scholar]
- Rangel, M.A.S.; Fey, E.; Neubert, E.D.O.; Fidalski, J. Plantio Direto de Mandioca; Embrapa Mandioca e Fruticultura: Cruz das Almas, BA, Brasil, 2018; p. 28. [Google Scholar]
- AOAC. Official Methods of Analysis of AOAC International, 17th ed.; AOAC International: Rockville, MD, USA, 2000. [Google Scholar]
- Ferreira, D.F. Sisvar: A Computer Statistical Analysis System. Ciênc. Agrotec. 2011, 35, 1039–1042. [Google Scholar] [CrossRef]
- Weiß, C.H. StatSoft, Inc., Tulsa, OK.: STATISTICA, Version 8. AStA Adv. Stat. Anal. 2007, 91, 339–341. [Google Scholar] [CrossRef]
- Sriroth, K.; Santisopasri, V.; Petchalanuwat, C.; Kurotjanawong, K.; Piyachomkwan, K.; Oates, C.G. Cassava Starch Granule Structure–Function Properties: Influence of Time and Conditions at Harvest on Four Cultivars of Cassava Starch. Carbohydr. Polym. 1999, 38, 161–170. [Google Scholar] [CrossRef]
- Buddhakulsomsiri, J.; Parthanadee, P.; Praneetpholkrang, P. Determining Appropriate Production and Inbound Logistics Practices for a Cassava Supply Chain in Thailand. Agric. Nat. Resour. 2015, 49, 937–950. [Google Scholar]
- Buddhakulsomsiri, J.; Parthanadee, P.; Pannakkong, W. Prediction Models of Starch Content in Fresh Cassava Roots for a Tapioca Starch Manufacturer in Thailand. Comput. Electron. Agric. 2018, 154, 296–303. [Google Scholar] [CrossRef]

| Properties | SY1 (São Pedro do Turvo) | SY2 (Paraguaçú Paulista) |
|---|---|---|
| pH (CaCl2) | 3.8 | 4.1 |
| Organic Matter (g dm−3) | 10.0 | 10.0 |
| Presin (mg dm−3) | 5.0 | 1.0 |
| K (mmolc dm−3) | 0.82 | 0.91 |
| Ca (mmolc dm−3) | 2.0 | 3.0 |
| Mg (mmolc dm−3) | 1.0 | 1.0 |
| Al (mmolc dm−3) | 11.0 | 4.0 |
| H+Al (mmolc dm−3) | 39.0 | 32.0 |
| Cation exchange capacity (mmolc dm−3) | 43.0 | 36.0 |
| Base saturation (%) | 10 | 12 |
| S (mg dm−3) | 11.0 | 4.0 |
| B (mg dm−3) | 0.14 | 0.35 |
| Cu (mg dm−3) | 0.30 | 0.20 |
| Fe (mg dm−3) | 103.0 | 19.0 |
| Mn (mg dm−3) | 3.5 | 1.4 |
| Zn (mg dm−3) | 0.1 | 0.1 |
| MAP (Month) | IAC 14 | IAC 90 | BRS CS01 | BRS 419 | BRS 420 | BRS Ocauçú | BRS Boitatá | 1097/13 | 2011 02-43 | Mean |
|---|---|---|---|---|---|---|---|---|---|---|
| 5 (March) | 1.115 abcd | 1.120 ab | 1.119 ab | 1.109 cde | 1.124 a | 1.104 e | 1.112 bcde | 1.115 abc | 1.106 de | 1.114 |
| 7 (May) | 1.141 abc | 1.138 a | 1.146 a | 1.130 c | 1.137 abc | 1.146 ab | 1.137 bc | 1.144 a | 1.131 abc | 1.139 |
| 9 (July) | 1.139 ab | 1.135 ab | 1.146 ab | 1.133 b | 1.135 ab | 1.147 ab | 1.143 ab | 1.146 a | 1.126 ab | 1.139 |
| 10 (August) | 1.142 ab | 1.135 bc | 1.143 ab | 1.126 d | 1.136 ab | 1.138 ab | 1.127 cd | 1.144 a | 1.119 d | 1.134 |
| 11 (September) | 1.119 ef | 1.115 f | 1.136 bc | 1.130 cd | 1.126 de | 1.146 a | 1.139 ab | 1.133 bcd | 1.119 ef | 1.129 |
| 12 (October) | 1.120 ab | 1.110 abcd | 1.111 d | 1.102 cd | 1.111 abcd | 1.118 bcd | 1.117 abc | 1.121 a | 1.102 cd | 1.112 |
| 13 (November) | 1.104 ab | 1.101 ab | 1.096 bc | 1.084 c | 1.099 b | 1.100 ab | 1.101 ab | 1.112 a | 1.097 bc | 1.099 |
| 14 (December) | 1.120 ab | 1.113 ab | 1.112 bc | 1.106 bc | 1.117 ab | 1.120 c | 1.123 ab | 1.116 a | 1.114 ab | 1.116 |
| 15 (January) | 1.130 ab | 1.132 ab | 1.124 b | 1.121 b | 1.128 b | 1.136 ab | 1.137 b | 1.133 a | 1.121 b | 1.129 |
| 17 (March) | 1.143 a | 1.139 ab | 1.136 b | 1.128 ab | 1.136 b | 1.146 ab | 1.143 b | 1.145 a | 1.128 ab | 1.138 |
| 19 (May) | 1.145 ab | 1.143 bc | 1.140 bc | 1.140 bc | 1.138 bc | 1.144 c | 1.148 c | 1.158 a | 1.129 bc | 1.143 |
| 21 (July) | 1.159 a | 1.166 a | 1.156 ab | 1.141 c | 1.148 bc | 1.159 a | 1.164 a | 1.165 a | 1.138 c | 1.155 |
| Mean | 1.132 | 1.128 | 1.133 | 1.123 | 1.128 | 1.135 | 1.134 | 1.138 | 1.120 |
| MAP (Month) | IAC 14 | IAC 90 | BRS CS01 | BRS 419 | BRS 420 | BRS Ocauçú | BRS Boitatá | 1097 -13 | 2011 02-43 | Mean |
|---|---|---|---|---|---|---|---|---|---|---|
| DM content (% FW) | ||||||||||
| 5 (March) | 30.9 bc | 32.8 b | 32.1 b | 30.3 bc | 36.8 a | 32.5 b | 30.7 bc | 33.3 b | 28.3 c | 32.0 |
| 7 (May) | 38.7 a | 37.5 a | 40.5 a | 38.4 a | 38.4 a | 40.1 a | 40.5 a | 38.8 a | 36.0 a | 38.8 |
| 9 (July) | 39.5 ab | 39.1 b | 41.2 ab | 38.7 b | 39.2 b | 41.4 a | 41.1 ab | 39.5 ab | 39.7 ab | 39.5 |
| 10 (August) | 40.6 ab | 38.0 cd | 38.4 bcd | 37.5 cd | 38.7 bc | 39.5 bc | 37.6 cd | 42.6 a | 36.2 d | 38.8 |
| 11 (September) | 34.0 c | 34.6 c | 39.8 ab | 37.1 abc | 36.0 abc | 40.4 a | 40.2 a | 35.1 bc | 36.3 abc | 37.1 |
| 12 (October) | 35.9 ab | 35.8 ab | 36.6 ab | 34.9 b | 34.1 b | 37.3 ab | 36.2 ab | 36.3 a | 33.5 b | 35.6 |
| 13 (November) | 33.8 a | 32.4 ab | 30.7 abc | 29.0 bc | 28.4 c | 33.2 a | 31.7 abc | 33.9 a | 29.1 bc | 31.4 |
| 14 (December) | 33.1 ab | 34.0 a | 33.8 ab | 34.2 a | 33.6 ab | 32.8 ab | 30.3 b | 34.0 a | 31.7 ab | 33.7 |
| 15 (January) | 37.1 a | 37.5 a | 37.4 a | 38.4 a | 38.0 a | 37.3 a | 39.3 a | 38.2 a | 34.0 a | 37.5 |
| 17 (March) | 38.9 a | 39.1 a | 40.2 a | 39.6 a | 39.1 a | 40.3 a | 40.7 a | 41.1 a | 36.1 a | 39.5 |
| 19 (May) | 40.1 a | 40.3 a | 41.5 a | 40.0 a | 39.9 a | 41.4 a | 41.4 a | 43.2 a | 37.7 a | 40.6 |
| 21 (July) | 43.1 a | 43.5 a | 44.3 a | 38.8 a | 41.8 a | 44.5 a | 44.1 a | 45.5 a | 40.1 a | 42.9 |
| Mean | 37.4 | 36.8 | 38.3 | 36.6 | 37.0 | 38.7 | 38.5 | 38.6 | 34.8 | 37.4 |
| ST content (% FW) | ||||||||||
| 5 (March) | 31.3 ab | 32.5 ab | 32.3 ab | 29.9 bc | 34.9 a | 32.5 ab | 30.1 bc | 33.0 ab | 27.3 c | 31.5 |
| 7 (May) | 34.9 a | 34.5 a | 36.9 a | 34.4 a | 34.7 a | 35.1 a | 36.4 a | 34.6 a | 33.2 a | 35.0 |
| 9 (July) | 36.0 ab | 33.4 b | 37.3 ab | 34.1 b | 34.4 b | 34.5 ab | 37.0 a | 33.7 b | 32.8 b | 34.8 |
| 10 (August) | 38.1 a | 36.6 ab | 38.0 a | 31.3 c | 37.5 a | 37.2 a | 36.5 ab | 39.1 a | 34.1 bc | 36.5 |
| 11 (September) | 31.3 ab | 29.4 ab | 31.7 ab | 29.6 ab | 30.5 ab | 31.6 ab | 34.0 a | 28.4 b | 30.0 ab | 30.7 |
| 12 (October) | 30.8 ab | 31.2 ab | 31.9 ab | 29.3 bc | 28.0 c | 32.1 ab | 30.2 ab | 32.5 a | 29.5 abc | 30.3 |
| 13 (November) | 31.0 ab | 33.2 a | 29.9 abc | 24.7 d | 24.2 d | 30.1 abc | 28.9 bc | 30.3 ab | 26.4 cd | 28.7 |
| 14 (December) | 32.8 ab | 31.1 ab | 32.3 ab | 30.4 ab | 28.3 b | 31.0 ab | 33.1 a | 31.9 ab | 27.7 b | 30.9 |
| 15 (January) | 35.0 a | 36.6 a | 35.5 a | 33.4 ab | 30.5 b | 34.8 a | 35.4 a | 35.3 a | 30.8 ab | 34.2 |
| 17 (March) | 37.3 ab | 38.2 ab | 37.6 ab | 36.3 abc | 32.3 c | 38.6 ab | 39.5 a | 38.5 ab | 35.1 bc | 36.5 |
| 19 (May) | 38.1 ab | 38.8 ab | 37.9 ab | 36.7 bc | 33.3 c | 38.1 ab | 40.7 a | 37.2 abc | 32.7 c | 36.9 |
| 21 (July) | 33.4 c | 38.7 ab | 34.8 abc | 34.8 abc | 34.1 bc | 36.1 abc | 36.8 abc | 39.9 a | 32.6 c | 35.7 |
| Mean | 34.5 | 34.8 | 35.1 | 32.4 | 31.7 | 34.2 | 35.1 | 34.8 | 30.8 | |
| Genotype | SG × DM Content | SG × ST Content | ||
|---|---|---|---|---|
| Regression Equation | R2 | Regression Equation | R2 | |
| IAC 14 | y = −192.0112 + 202.7012 *** x | 0.69 | y = −109.5272 + 127.0557 ** x | 0.37 |
| IAC 90 | y = −153.1717 + 168.5676 *** x | 0.72 | y = −117.4801 + 134.6916 *** x | 0.48 |
| BRS CS01 | y = −176.9792 + 190.0264 ** x | 0.75 | y = −97.3650 + 117.0525 * x | 0.42 |
| BRS 419 | y = −194.5820 + 205.7919 *** x | 0.62 | y = −151.2487 + 163.2803 *** x | 0.64 |
| BRS 420 | y = −228.2490 + 235.0584 *** x | 0.71 | y = −216.7411 + 220.5523 *** x | 0.56 |
| BRS Ocauçú | y = −179.0768 + 192.3948 *** x | 0.82 | y = −102.9561 + 121.5908 *** x | 0.35 |
| BRS Boitatá | y = −237.0694 + 243.2902 *** x | 0.70 | y = −152.0970 + 166.1325 *** x | 0.36 |
| 1097/13 | y = −208.8248 + 217.7174 *** x | 0.82 | y = −146.2954 + 159.4273 ** x | 0.58 |
| 2011 02-43 | y = −238.1149 + 244.0085 *** x | 0.77 | y = −177.9014 + 186.8521 *** x | 0.60 |
| All genotypes | y = −191.2806 + 202.4617 *** x | 0.92 | y = −140.9525 + 154.4288 *** x | 0.77 |
| Genotype | (DM %FW) a | MAP | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 5 | 7 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 17 | 19 | 21 | |||
| IAC 14 | Measured | 30.9 b | 38.2 a | 39.2 a | 40.6 a | 34.0 a | 35.9 a | 33.8 a | 33.2 b | 37.4 a | 39.0 b | 40.3 a | 43.1 a | |
| Predicted | IN | 34.1 a | 38.8 a | 38.5 a | 39.6 a | 34.0 a | 34.8 a | 31.9 b | 34.7 a | 37.2 a | 39.8 ab | 40.1 a | 42.3 c | |
| CM | 34.7 a | 39.2 a | 38.9 a | 39.8 a | 36.3 a | 35.3 a | 32.6 b | 35.1 a | 37.7 a | 40.2 a | 40.6 a | 42.7 b | ||
| IAC 90 | Measured | 32.8 b | 39.1 a | 39.1 b | 38.0 a | 34.6 a | 36.4 a | 32.4 b | 34.0 a | 37.8 a | 39.3 a | 40.8 a | 43.5 a | |
| Predicted | IN | 35.6 a | 39.4 a | 39.2 b | 37.6 a | 34.9 a | 34.0 b | 33.2 a | 34.7 a | 37.5 a | 39.1 a | 39.4 a | 43.0 a | |
| CM | 35.6 a | 40.0 a | 39.8 a | 38.6 a | 35.7 a | 33.6 b | 32.0 b | 34.4 a | 37.8 a | 39.6 a | 40.0 a | 43.8 a | ||
| BRS CS01 | Measured | 32.1 b | 39.4 a | 40.1 a | 38.4 a | 39.8 a | 35.7 a | 30.7 a | 32.9 a | 36.5 a | 39.2 a | 39.8 a | 44.3 a | |
| Predicted | IN | 35.1 a | 40.0 a | 40.1 a | 39.7 a | 40.3 a | 33.3 b | 30.8 a | 33.6 a | 35.7 a | 37.5 a | 39.6 a | 43.4 b | |
| CM | 35.4 a | 39.9 a | 40.0 a | 40.0 a | 39.0 a | 32.8 b | 31.1 a | 33.0 a | 35.3 a | 37.2 a | 39.5 a | 42.2 c | ||
| BRS 419 | Measured | 30.3 c | 37.9 a | 38.5 a | 37.5 ab | 37.1 a | 34.6 a | 29.0 a | 34.2 a | 38.2 a | 40.4 a | 41.0 a | 38.8 a | |
| Predicted | IN | 34.9 a | 38.3 a | 38.4 a | 38.2 a | 37.9 a | 33.1 b | 30.2 a | 34.1 a | 36.7 a | 39.3 a | 40.7 a | 39.8 a | |
| CM | 33.4 b | 37.8 a | 37.9 a | 36.8 b | 38.1 a | 32.7 b | 28.7 a | 33.7 a | 36.3 a | 38.8 a | 40.2 a | 39.9 a | ||
| BRS 420 | Measured | 36.8 a | 38.2 a | 38.8 a | 38.7 a | 36.0 b | 34.4 a | 28.4 b | 31.9 b | 37.6 a | 38.3 a | 40.1 a | 41.8 a | |
| Predicted | IN | 36.5 a | 39.3 a | 38.9 a | 39.4 a | 36.8 ab | 32.9 c | 30.5 ab | 33.5 a | 36.3 a | 37.7 a | 39.2 a | 40.4 a | |
| CM | 36.4 a | 39.2 a | 38.9 a | 38.7 a | 37.5 a | 33.7 b | 31.7 a | 34.2 a | 36.6 a | 37.8 a | 39.1 a | 40.9 a | ||
| BRS Ocauçú | Measured | 32.5 a | 40.6 a | 41.5 a | 39.5 a | 40.4 a | 35.9 a | 33.2 a | 32.8 a | 35.5 b | 39.3 b | 39.6 a | 44.5 a | |
| Predicted | IN | 33.3 a | 40.3 a | 40.6 ab | 39.8 a | 40.7 a | 34.4 b | 32.6 a | 33.3 a | 38.0 a | 40.4 a | 39.0 a | 42.9 a | |
| CM | 32.6 a | 39.6 a | 39.9 b | 39.1 a | 40.6 a | 33.4 c | 31.8 a | 32.2 a | 37.1 a | 39.7 ab | 38.2 a | 42.8 a | ||
| BRS Boitatá | Measured | 30.7 b | 39.3 a | 39.3 a | 37.6 a | 40.2 a | 34.7 a | 31.7 a | 30.3 b | 37.9 a | 39.4 a | 39.9 a | 44.1 a | |
| Predicted | IN | 33.3 a | 38.4 b | 40.0 a | 37.6 a | 40.4 a | 34.3 a | 30.2 a | 34.2 a | 36.7 a | 38.3 a | 38.9 a | 43.8 a | |
| CM | 34.1 a | 38.0 b | 39.3 a | 37.0 a | 39.4 a | 34.5 a | 32.0 a | 34.4 a | 36.6 a | 37.9 a | 38.4 a | 43.4 a | ||
| 1097/13 | Measured | 33.3 b | 38.8 a | 40.5 a | 42.6 a | 35.1 a | 37.3 a | 33.9 a | 34.0 b | 37.7 b | 40.9 a | 42.7 a | 45.5 a | |
| Predicted | IN | 33.6 ab | 40.6 a | 40.7 a | 40.4 b | 37.9 a | 35.3 b | 32.8 a | 35.6 a | 39.1 a | 40.7 a | 42.6 a | 44.1 a | |
| CM | 34.7 a | 40.6 a | 40.8 a | 40.3 b | 38.5 a | 35.7 b | 34.1 a | 36.0 a | 39.3 a | 40.7 a | 42.5 a | 43.6 a | ||
| 2011 02-43 | Measured | 28.3 c | 39.2 a | 39.8 a | 36.2 a | 36.3 a | 34.1 a | 29.1 b | 33.4 a | 37.0 a | 38.4 a | 38.8 a | 40.1 a | |
| Predicted | IN | 32.0 b | 39.3 a | 39.1 a | 34.7 a | 34.9 a | 31.7 c | 29.9 ab | 34.2 a | 36.9 a | 39.1 a | 39.2 a | 37.9 a | |
| CM | 33.0 a | 38.9 a | 38.7 a | 35.4 a | 36.3 a | 32.6 b | 31.1 a | 34.7 a | 36.9 a | 38.7 a | 38.8 a | 39.4 a | ||
| Genotype | (ST %FW) a | MAP | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 5 | 7 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 17 | 19 | 21 | |||
| IAC 14 | Measured | 31.3 a | 34.8 a | 35.6 a | 38.1 a | 31.3 a | 30.8 a | 31.0 b | 32.8 a | 35.2 a | 37.3 a | 38.0 a | 33.4 b | |
| Predicted | IN | 34.1 a | 35.1 a | 34.9 a | 38.7 a | 31.3 a | 32.6 a | 32.3 a | 32.6 a | 34.2 a | 35.8 b | 36.0 b | 33.3 b | |
| CM | 33.0 a | 34.9 a | 34.6 a | 37.5 a | 29.5 a | 31.8 a | 31.1 b | 31.8 a | 33.7 a | 35.6 b | 35.9 b | 36.4 a | ||
| IAC 90 | Measured | 32.5 b | 35.2 b | 34.5 a | 36.6 a | 29.4 a | 31.2 a | 33.2 b | 31.5 a | 36.5 a | 38.2 a | 38.7 a | 38.7 a | |
| Predicted | IN | 36.3 a | 36.4 a | 36.2 a | 37.0 a | 28.9 a | 32.1 a | 35.3 a | 32.6 a | 34.9 ab | 36.1 ab | 36.4 b | 39.0 a | |
| CM | 33.8 b | 35.5 ab | 35.3 a | 36.5 a | 28.8 a | 30.6 a | 30.6 c | 31.2 a | 33.8 b | 35.1 b | 35.4 c | 37.6 a | ||
| BRS CS01 | Measured | 32.3 c | 36.5 a | 36.8 a | 38.0 ab | 31.7 a | 31.9 ab | 29.9 b | 31.6 ab | 35.4 a | 37.6 a | 37.9 a | 34.8 | |
| Predicted | IN | 36.1 a | 36.3 a | 36.4 a | 39.8 a | 32.2 a | 32.2 a | 32.6 a | 32.3 a | 33.6 b | 34.8 a | 36.1 a | 34.1 | |
| CM | 33.6 b | 35.4 a | 35.5 a | 37.7 b | 32.4 a | 29.9 b | 29.8 b | 30.1 b | 31.9 c | 33.4 a | 35.1 a | 35.9 | ||
| BRS 419 | Measured | 29.9 b | 34.0 a | 33.6 a | 31.3 b | 29.6 a | 29.1 a | 24.7 a | 30.6 a | 32.8 a | 36.2 a | 36.7 a | 34.8 a | |
| Predicted | IN | 32.0 a | 33.5 a | 33.6 a | 34.9 a | 31.9 a | 29.4 a | 27.9 a | 30.2 a | 32.3 a | 34.3 a | 35.4 a | 33.5 a | |
| CM | 31.9 a | 33.8 a | 33.9 a | 34.9 a | 31.4 a | 29.9 a | 27.7 a | 30.6 a | 32.6 a | 34.5 a | 35.6 a | 33.4 a | ||
| BRS 420 | Measured | 34.9 a | 34.2 a | 34.0 a | 37.5 a | 30.5 a | 27.4 b | 24.2 b | 28.4 a | 30.1 b | 32.3 a | 33.8 a | 34.1 a | |
| Predicted | IN | 32.3 a | 34.3 a | 34.0 a | 35.3 a | 29.7 a | 28.3 b | 26.2 b | 28.9 a | 31.5 ab | 32.8 a | 34.2 a | 34.1 a | |
| CM | 34.6 a | 34.8 a | 34.6 a | 36.5 a | 30.7 a | 30.6 a | 30.3 a | 31.0 a | 32.9 a | 33.8 a | 34.7 a | 34.5 a | ||
| BRS Ocauçú | Measured | 32.5 a | 35.0 a | 36.5 a | 37.2 a | 31.6 a | 32.1 a | 30.1 a | 30.1 a | 36.2 a | 38.6 a | 38.1 a | 36.1 a | |
| Predicted | IN | 30.7 a | 35.7 a | 35.9 a | 37.1 a | 33.7 a | 32.0 a | 29.9 a | 31.2 a | 34.2 a | 35.8 a | 34.9 a | 35.3 a | |
| CM | 31.1 a | 35.1 a | 35.3 a | 36.9 a | 34.2 a | 30.4 b | 30.4 a | 29.5 a | 33.3 a | 35.2 a | 34.1 a | 36.5 a | ||
| BRS Boitatá | Measured | 30.1 b | 36.4 a | 38.6 a | 36.5 a | 34.0 a | 31.0 a | 28.9 b | 34.0 a | 36.8 a | 39.5 a | 40.7 a | 36.8 a | |
| Predicted | IN | 32.1 a | 36.0 a | 37.1 a | 36.6 a | 33.6 a | 33.2 a | 29.0 ab | 33.1 a | 34.9 a | 35.9 ab | 36.4 b | 37.7 a | |
| CM | 32.4 a | 33.9 b | 34.9 a | 35.1 b | 32.9 a | 31.3 a | 30.6 a | 31.2 a | 32.8 a | 33.8 b | 34.2 b | 37.2 a | ||
| 1097/13 | Measured | 33.0 a | 35.3 a | 34.2 b | 39.1 a | 28.4 a | 32.5 a | 30.3 b | 32.0 a | 35.9 a | 38.4 a | 37.2 a | 39.9 a | |
| Predicted | IN | 33.0 a | 36.3 a | 36.5 a | 38.4 a | 32.0 a | 32.4 a | 32.4 a | 32.7 a | 35.3 a | 36.4 b | 37.8 a | 38.8 b | |
| CM | 33.0 a | 35.9 a | 36.1 a | 38.0 a | 31.8 a | 32.2 a | 32.5 a | 32.4 a | 34.9 a | 36.0 b | 37.4 a | 37.5 c | ||
| 2011 02-43 | Measured | 27.3 c | 36.6 a | 34.3 a | 34.1 a | 30.0 a | 29.5 a | 26.4 c | 29.1 b | 33.1 a | 35.0 a | 33.9 a | 32.6 a | |
| Predicted | IN | 29.7 b | 34.6 b | 34.3 a | 32.2 a | 28.8 a | 28.7 a | 27.7 b | 30.6 ab | 32.7 a | 34.4 a | 34.4 a | 32.6 a | |
| CM | 31.5 a | 34.6 b | 34.5 a | 33.6 a | 29.4 a | 29.8 a | 29.8 a | 31.4 a | 33.1 a | 34.5 a | 34.5 a | 32.8 a | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva, R.M.d.; Fernandes, A.M.; Leonel, M.; Pelvine, R.A.; Figueiredo, R.T.d.; Rangel, M.A.S.; Ringenberg, R.; Oliveira, L.A.d.; Santos, V.d.S.; Vieira, E.A. Measurement of Dry Matter and Starch in Modern Cassava Genotypes during Long Harvest Cycles. Horticulturae 2023, 9, 733. https://doi.org/10.3390/horticulturae9070733
Silva RMd, Fernandes AM, Leonel M, Pelvine RA, Figueiredo RTd, Rangel MAS, Ringenberg R, Oliveira LAd, Santos VdS, Vieira EA. Measurement of Dry Matter and Starch in Modern Cassava Genotypes during Long Harvest Cycles. Horticulturae. 2023; 9(7):733. https://doi.org/10.3390/horticulturae9070733
Chicago/Turabian StyleSilva, Rudieli Machado da, Adalton Mazetti Fernandes, Magali Leonel, Raíra Andrade Pelvine, Ricardo Tajra de Figueiredo, Marco Antonio Sedrez Rangel, Rudiney Ringenberg, Luciana Alves de Oliveira, Vanderlei da Silva Santos, and Eduardo Alano Vieira. 2023. "Measurement of Dry Matter and Starch in Modern Cassava Genotypes during Long Harvest Cycles" Horticulturae 9, no. 7: 733. https://doi.org/10.3390/horticulturae9070733
APA StyleSilva, R. M. d., Fernandes, A. M., Leonel, M., Pelvine, R. A., Figueiredo, R. T. d., Rangel, M. A. S., Ringenberg, R., Oliveira, L. A. d., Santos, V. d. S., & Vieira, E. A. (2023). Measurement of Dry Matter and Starch in Modern Cassava Genotypes during Long Harvest Cycles. Horticulturae, 9(7), 733. https://doi.org/10.3390/horticulturae9070733






