Asexual Propagation of Greek Salvia officinalis L. Populations Selected for Ornamental Use
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Analysis of Morphological Traits
2.3. Asexual Propagation of Selected S. officinalis Populations
2.3.1. Propagation by Shoot Cuttings
Effect of K-IBA and Season on the Rooting of Cuttings
Effect of Substrate and the Mist or Fog System on Rooting of Cuttings
2.3.2. In Vitro Propagation of S. officinalis
Explant Preparation and Disinfection
Effect of Growth Regulators on the Multiplication of S. officinalis
In Vitro Rooting and Plantlet Acclimatization
2.4. Statistical Analysis
3. Results and Discussion
3.1. Morphological Analysis of Ornamental Traits
3.2. Asexual Propagation of Selected S. officinalis Populations
3.2.1. Propagation by Shoot Cuttings
Effect of K-IBA and Season on Cutting Rootability
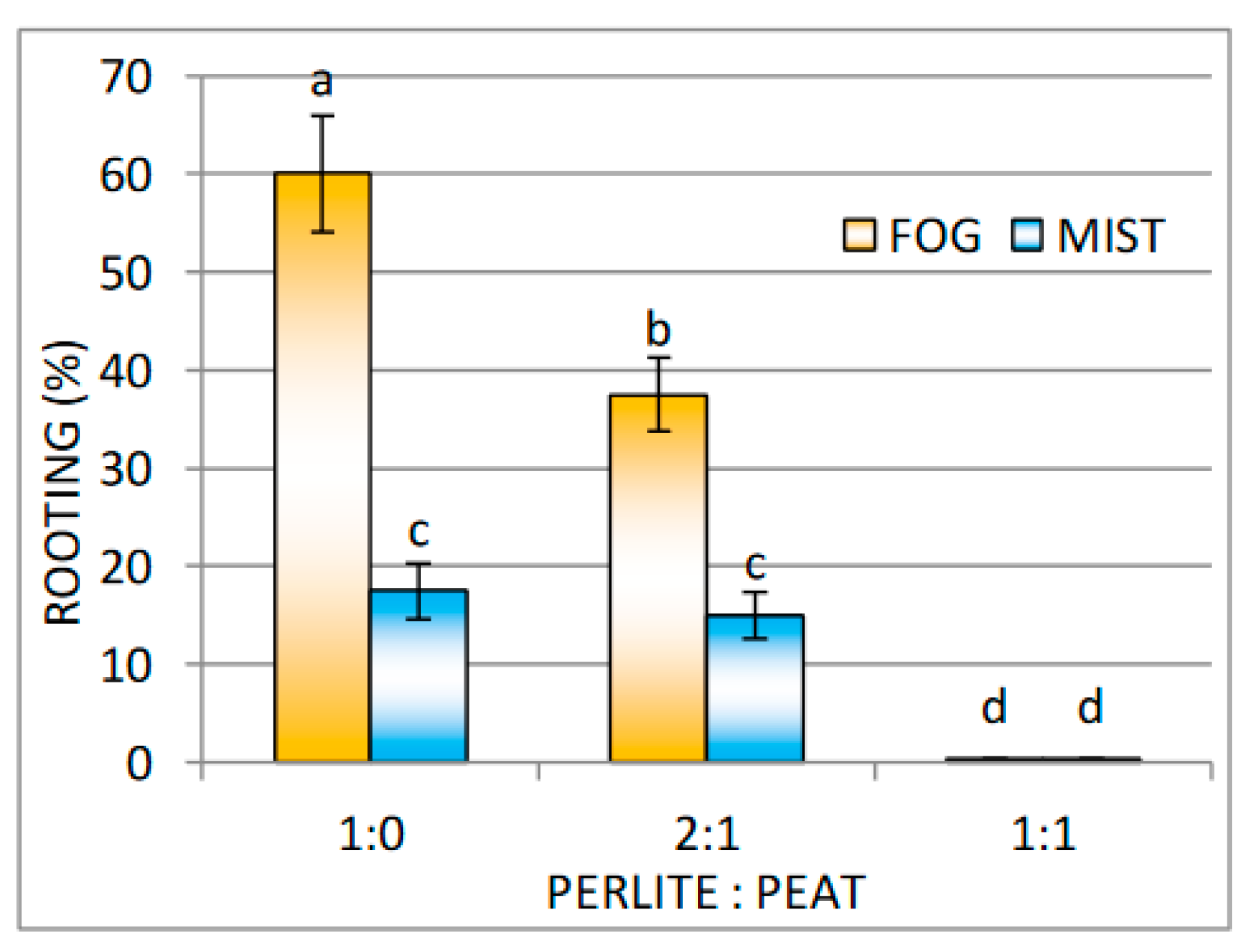
Effect of Substrate and Mist or Fog Systems on the Rooting of Cuttings
3.2.2. In Vitro Propagation of S. officinalis
Effect of Disinfection Treatments on S. officinalis
Effect of Growth Regulators on the Propagation of S. officinalis
Spontaneous Rooting of S. officinalis Explants
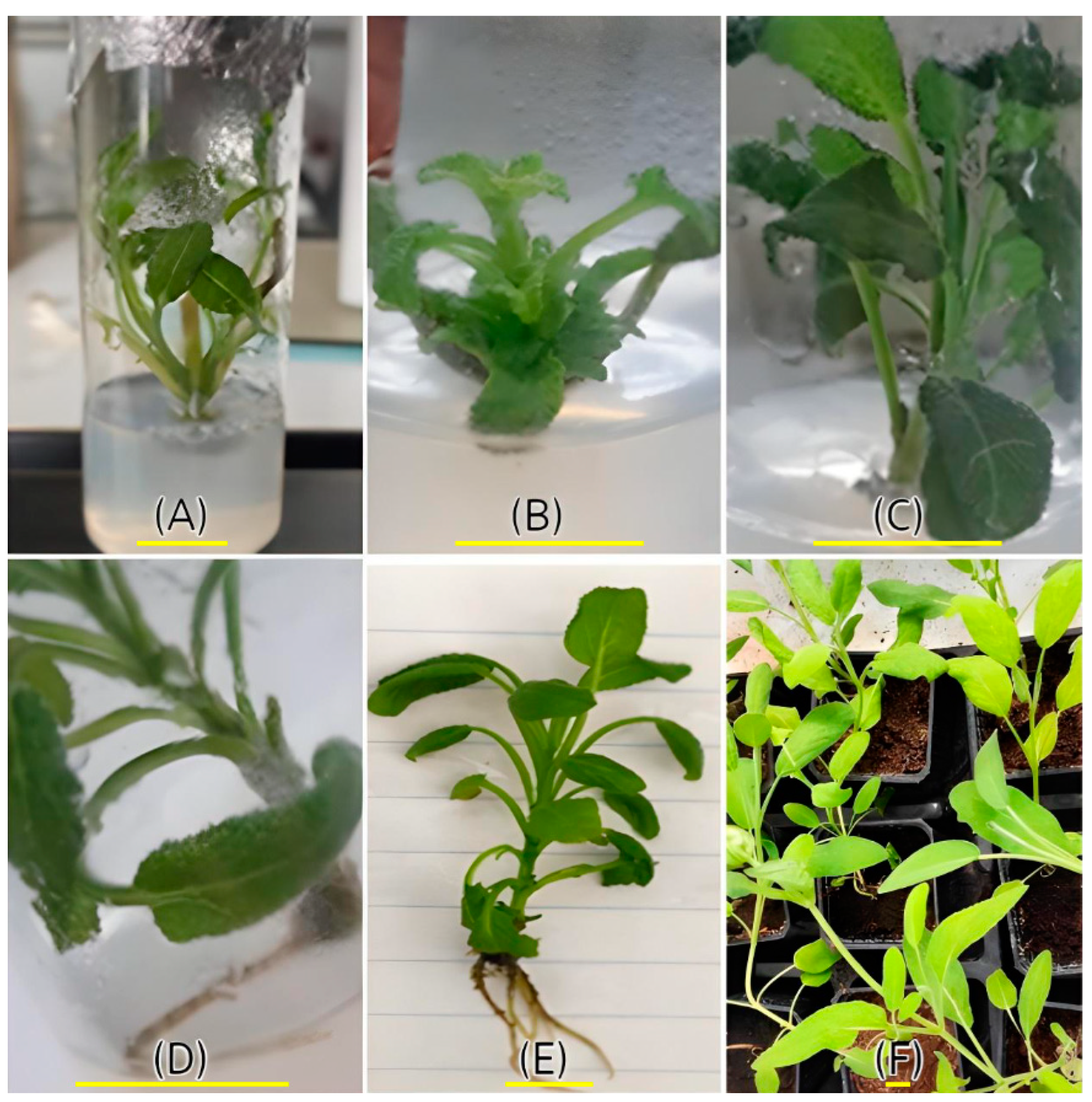
In Vitro Rooting and Plantlet Acclimatization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Barnes, J.; Anderson, L.A.; Phillipson, J.D. Herbal Medicines: A Guide for Healthcare Professionals, 2nd ed.; Pharmaceutical Press: London, UK, 2002; 432p. [Google Scholar]
- Akhondzadeh, S.; Noroozian, M.; Mohammadi, M.; Ohadinia, S.; Jamshidi, A.H.; Khani, M. Salvia officinalis extract in the treatment of patients with mild to moderate Alzheimer’s disease: A double blind, randomized and placebo-controlled trial. J. Clin. Pharm. Ther. 2003, 28, 53–59. [Google Scholar] [CrossRef]
- Gostin, I. Effects of different plant hormones on Salvia officinalis cultivated in vitro. Int. J. Botany 2008, 4, 430–436. [Google Scholar] [CrossRef][Green Version]
- Kintzios, S.; Nikolaou, A.; Skoula, M. Somatic embryogenesis and in vitro rosmarinic acid accumulation in Salvia officinalis and S. fruticosa leaf callus cultures. Plant Cell Rep. 1999, 18, 462–466. [Google Scholar] [CrossRef]
- Azimova, S.S.; Glushenkova, A.I. Lipids, Lipophilic Components and Essential Oils from Plant Sources; Springer: London, UK, 2012; 1004p. [Google Scholar] [CrossRef]
- Kontogianni, V.G.; Tomic, G.; Nikolic, I.; Nerantzaki, A.A.; Sayyad, N.; Stosic-Grujicic, S.; Tzakos, A.G. Phytochemical profile of Rosmarinus officinalis and Salvia officinalis extracts and correlation to their antioxidant and anti-proliferative activity. Food Chem. 2013, 136, 120–129. [Google Scholar] [CrossRef]
- Modarres, M.; Esmaeilzadeh Bahabadi, S.; Taghavizadeh Yazdi, M.E. Enhanced production of phenolic acids in cell suspension culture of Salvia leriifolia Benth. using growth regulators and sucrose. Cytotechnology 2018, 70, 741–750. [Google Scholar] [CrossRef] [PubMed]
- Brezoiu, A.M.; Prundeanu, M.; Berger, D.; Deaconu, M.; Matei, C.; Oprea, O.; Vasile, E.; Negreanu-Pîrjol, T.; Muntean, D.; Danciu, C. Properties of Salvia offcinalis L. and Thymus serpyllum L. extracts free and embedded into mesopores of silica and titania Nanomaterials. Nanomaterials 2020, 10, 820. [Google Scholar] [CrossRef]
- Schmiderer, C.; Novak, J. Salvia officinalis L. and Salvia fruticosa Mill.: Dalmatian and three-lobed sage. In Medicinal, Aromatic and Stimulant Plants; Novak, J., Blüthner, W.D., Eds.; Handbook of Plant Breeding; Springer: Cham, Switzerland, 2020; Volume 12, pp. 523–527. [Google Scholar] [CrossRef]
- Laurentin, H. Data analysis for molecular characterization of plant genetic resources. Genet. Resour. Crop Evol. 2009, 56, 277–292. [Google Scholar] [CrossRef]
- Kostas, S.; Hatzilazarou, S.; Pipinis, E.; Vasileiadis, A.; Magklaras, P.; Smyrnioudis, I.; Vasilakis, T.; Chazakis, M.; Anastasiadi, V.; Ziogou, F.-T.; et al. Propagation of Pistacia lentiscus var. Chia genotypes and determination of their ornamental traits combined with a genetic analysis using ISSR markers. Agronomy 2021, 11, 205. [Google Scholar] [CrossRef]
- Kostas, S.; Kaplani, A.; Koulaouzidou, E.; Kotoula, A.A.; Gklavakis, E.; Tsoulpha, P.; Hatzilazarou, S.; Nianiou-Obeidat, I.; Kanellis, A.K.; Economou, A. Sustainable exploitation of greek Rosmarinus officinalis L. populations for ornamental use through propagation by shoot cuttings and in vitro cultures. Sustainability 2022, 14, 4059. [Google Scholar] [CrossRef]
- Nicola, S.; Fontana, E.; Hoeberechts, J.; Saglietti, D. Rooting products and cutting timing on sage (Salvia officinalis L.) propagation. Acta Hortic. 2003, 676, 135–141. [Google Scholar] [CrossRef]
- Kara, N.; Baydar, H.; Erbaş, S. Effects of different cuttings periods and IBA concentrations on rooting ability of some medicinal plant. Derim 2011, 28, 71–81. [Google Scholar]
- Paradikovic, N.; Zelkovic, S.; Tkalec, M.; Vinkovic, T.; Devic, I.; Maric, M. Influence of rooting powder on propagation of sage (Salvia officinalis L.) and rosemary (Rosmarinus officinialis L.) with green cuttings. Poljoprivreda 2013, 19, 10–15. [Google Scholar]
- Loconsole, D.; Cristiano, G.; De Lucia, B. Image analysis of adventitious root quality in wild sage and glossy abelia cuttings after application of different indole-3-butyric acid concentrations. Plants 2022, 11, 290. [Google Scholar] [CrossRef]
- Petrova, M.; Nikolova, M.; Dimitrova, L.; Zayova, E. Micropropagation and evaluation of flavonoid content and antioxidant activity of Salvia officinalis L. Genet. Plant Physiol. 2015, 5, 48–60. Available online: http://www.ifrg-bg.com (accessed on 19 June 2023).
- Kriebel, R.; Drew, B.; González-Gallegos, J.; Celep, F.; Heeg, L.; Malik, M.; Sytsma, K. Pollinator shifts, contingent evolution, and evolutionary constraint drive floral disparity in Salvia (Lamiaceae): Evidence from morphometrics and phylogenetic comparative methods. Evolution 2020, 74, 1335–1355. [Google Scholar] [CrossRef] [PubMed]
- Gokdogan, E.Y.; Burun, B. The studies on seed germination and in vitro cultures of Salvia L. species from turkish Flora. Nat. Prod. Biotechnol. 2022, 2, 60–73. [Google Scholar]
- Grzegorczyk, I.; Bilichowski, I.; Mikiciuk-Olasik, E.; Wysokińska, H. In vitro cultures of Salvia officinalis L. as a source of antioxidant compounds. Acta Soc. Bot. Pol. Pol. Tow. Bot. 2005, 74, 17–21. [Google Scholar] [CrossRef]
- Grzegorczyk-Karolak, I.; Matkowski, A.; Wysokińska, H. Antioxidant activity of extracts from in vitro cultures of Salvia officinalis L. Food Chem. 2007, 104, 536–541. [Google Scholar] [CrossRef]
- Santos-Gomes, P.C.; Seabra, R.M.; Andrade, P.B.; Fernandes-Ferreira, M. Phenolic antioxidant compounds produced by in vitro shoots of sage (Salvia officinalis L.). Plant Sci. 2002, 162, 981–987. [Google Scholar] [CrossRef]
- Santos-Gomes, P.C.; Fernandes-Ferreira, M. Essential oils produced by in vitro shoots of sage (Salvia officinalis L.). J. Agric. Food Chem. 2003, 51, 2260–2266. [Google Scholar] [CrossRef]
- Wielgus, K.; Luwanska, A.; Szalata, M.; Mielcarek, S.; Gryszczynska, A.; Lipinski, D.; Slomski, R. Phytochemical estimation of Sage (Salvia officinalis L.) cultivated in vitro-flavonoids and phenolic acids. Acta Fytotech. Zootech 2011, 14, 8–11. [Google Scholar]
- Bolta, I.; Evi, D.B.; Bohanec, B.; Andren, S. A preliminary investigation of ursolic acid in cell suspension culture of Salvia officinalis. Plant Cell Tissue Organ Cult. 2000, 62, 57–63. [Google Scholar] [CrossRef]
- Mehalaine, S.; Chenchouni, H. New insights for the production of medicinal plant materials: Ex vitro and in vitro propagation of valuable Lamiaceae species from northern Africa. Curr. Plant Biol. 2021, 27, 100216. [Google Scholar] [CrossRef]
- Zewdinesh, D.Z.; Beemnet, M.K. Effect of cutting size and position on propagation ability of Sage (Salvia officinalis L.). Int. J. Adv. Biol. Biomed. Res. 2016, 4, 68–76. [Google Scholar]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Esmaeili, G.; Fatemi, H.; Baghani avval, M.; Azizi, M.; Arouiee, H.; Vaezi, J.; Fujii, Y. Diversity of chemical composition and morphological traits of eight iranian wild Salvia species during the first step of domestication. Agronomy 2022, 12, 2455. [Google Scholar] [CrossRef]
- Herraiz-Peñalver, D.; Asensio, S.; Manzanera, M.C.; Herrero, B.; Martin, H.; Santiago, Y.; Zalacaín, A.; Berruga, M.I.; Sánchez-Vioque, R. Evaluation of the morphological variability in Iberian Salvia lavandulifolia Vahl accessions. Genet. Resour. Crop Evol. 2017, 64, 1797–1806. [Google Scholar] [CrossRef]
- Mossi, A.J.; Cansian, R.L.; Paroul, N.; Toniazzo, G.; Oliveira, J.V.; Pierozan, M.K.; Pauletti, G.; Rota, L.; Santos, A.C.A.; Serafini, L.A. Morphological characterisation and agronomical parameters of different species of Salvia sp. (Lamiaceae). Braz. J. Biol. 2011, 71, 121–129. [Google Scholar] [CrossRef]
- Leontaritou, P.; Lamari, F.N.; Papasotiropoulos, V.; Iatrou, G. Exploration of genetic, morphological and essential oil variation reveals tools for the authentication and breeding of Salvia pomifera subsp. calycina (Sm.) Hayek. Phytochemistry 2021, 191, 112900. [Google Scholar] [CrossRef]
- Leontaritou, P.; Lamari, F.N.; Papasotiropoulos, V.; Iatrou, G. Morphological, genetic and essential oil variation of greek sage (Salvia fruticosa Mill.) populations from Greece. Ind. Crops Prod. 2020, 150, 112346. [Google Scholar] [CrossRef]
- Zigene, Z.D.; Asfaw, B.T.; Bitima, T.D. Phenotypic diversity of rosemary (Salvia rosmarinus Schleid.) accessions for qualitative characters. Heliyon 2022, 8, e11895. [Google Scholar] [CrossRef] [PubMed]
- Araghi, A.M.; Nemati, H.; Azizi, M.; Moshtaghi, N.; Shoor, M.; Hadian, J. Assessment of phytochemical and agro-morphological variability among different wild accessions of Mentha longifolia L. cultivated in field condition. Ind. Crops Prod. 2019, 140, 111698. [Google Scholar] [CrossRef]
- Devi, A.; Iqbal, T.; Wani, I.A.; Sharma, G.; Verma, S.; Noureldeen, A.; Darwish, H. Assessment of variability among morphological and molecular characters in wild populations of mint [Mentha longifolia (L.) L.] germplasm. Saudi J. Biol. Sci. 2022, 29, 3528–3538. [Google Scholar] [CrossRef]
- Ayanoglu, F.; Özkan, C. Change in tissue mineral elemental concentration during root initiation and development of Salvia officinalis L. cuttings and effects. Turk. J. Agric. For. 2000, 24, 677–682. [Google Scholar]
- Davies, F.; Geneve, R.; Wilson, S.; Hartmann, H.; Kester, D. Hartmann and Kester’s Plant Propagation: Principles and Practices, 9th ed.; Pearson Prentice Hall: Upper Saddle River, NJ, USA, 2017; 915p. [Google Scholar]
- Kacar, O.; Azkan, N.; Çöplü, N. Effects of different rooting media and indole butyric acid on rooting of stem cuttings in sage (Salvia officinalis L. and Salvia triloba L.). J. Food Agric. Environ. 2009, 7, 349–352. [Google Scholar]
- Martini, A.N.; Bertsouklis, K.F.; Vlachou, G.; Dariotis, E.; Papafotiou, M. Comparative evaluation of rooting cuttings of five Mediterranean sage species (Salvia sp.) native to Greece. Acta Hortic. 2020, 1298, 587–592. [Google Scholar] [CrossRef]
- Vârban, D.I.; Vârban, R.; Duda, M.M.; Muntean, S.; Moldovan, C. The influence of substrates on rooting of Salvia officinalis L. cuttings. Hop Med. Plants 2014, 24, 52–56. [Google Scholar]
- Lemraski, M.G.; Eftekhari, M.; Faraji, M.; Zarrini, S.S. Study of callus induction in common sage (Salvia officinalis L.). Int. J. Agric. Crop Sci. (IJACS) 2014, 7, 386–389. [Google Scholar]
- Ioja-Boldura, O.M.; Radu, F.; Popescu, S.; Borozan, A. Regeneration, micropropagation, callus cultures and somatic embryogenesis of common sage (Salvia officinalis L.). Bull. Univ. Agric. Sci. Vet. Med. Cluj-Napoca. Hortic. 2010, 67, 308–313. [Google Scholar]
- Villegas-Sánchez, E.; Macías-Alonso, M.; Osegueda-Robles, S.; Herrera-Isidrón, L.; Nuñez-Palenius, H.; González-Marrero, J. In vitro culture of Rosmarinus officinalis L. in a temporary immersion system: Influence of two phytohormones on plant growth and carnosol production. Pharmaceuticals 2021, 14, 747. [Google Scholar] [CrossRef]
- Tsoulpha, P.; Alexandri, S.; Tsaktsira, M. Critical factors affecting an efficient micropropagation protocol for Pyrus spinosa Forskk. J. Appl. Hortic. 2018, 20, 190–195. [Google Scholar] [CrossRef]
- Tawfik, A.A.; Mohamed, M.F. Regeneration of salvia (Salvia officinalis L.) via induction of meristematic callus. In Vitro Cell. Dev. Biol. Plant 2007, 43, 21–27. [Google Scholar] [CrossRef]
- Ngomuo, M.; Mneney, E.; Ndakidemi, P.A. The in vitro propagation techniques for producing banana using shoot tip cultures. Am. J. Plant Sci. 2014, 5, 1614–1622. [Google Scholar] [CrossRef]
- Rout, G.R.; Samantaray, S.; Das, P. In vitro manipulation and propagation of medicinal plants. Biotechnol. Adv. 2000, 18, 91–120. [Google Scholar] [CrossRef]
- Mohamed, M.A.H.; Aly, M.K.; Ahmed, E.T.; Abd El-latif, S.A.H. Effect of plant growth regulators on organogenesis of Salvia officinalis L. plants. Minia J. Agric. Res. Dev. 2019, 3, 401–414. [Google Scholar]
- Cuenca, S.; Amo-Marco, J.B. In vitro propagation of two spanish endemic species of salvia through bud proliferation. In Vitro Cell. Dev. Biol. Plant 2000, 36, 225–229. [Google Scholar] [CrossRef]
- Arikat, N.A.; Jawad, F.M.; Karam, N.S.; Shibli, R.A. Micropropagation and accumulation of essential oils in wild sage (Salvia fruticosa Mill.). Sci. Hortic. 2004, 100, 193–202. [Google Scholar] [CrossRef]
- Mascarello, C.; Mantovani, E.; Ruffoni, B. In vitro culture of several ornamental and medicinal Salvia species. Acta Hortic. 2006, 723, 375–380. [Google Scholar] [CrossRef]
- van Staden, A.B.; Lall, N. Medicinal plants as alternative treatments for progressive macular hypomelanosis. In Medicinal Plants for Holistic Health and Well-Being, 1st ed.; Lall, N., Ed.; Elsevier Academic Press: London, UK, 2018; pp. 145–182. [Google Scholar]
- Zuzarte, Μ.R.; Dinis, A.M.; Cavaleiro, C.; Salgueiro, L.R.; Canhoto, J.M. Trichomes, essential oils and in vitro propagation of Lavandula pedunculata (Lamiaceae). Ind. Crops Prod. 2010, 32, 580–587. [Google Scholar] [CrossRef]
- Ghanbar, T.; Hosseini, B.; Jabbarzadeh, Z.; Farokhzad, A.; Sharafi, A. High-frequency in vitro direct shoots regeneration from axillary nodal and shoot tip explants of clary sage (Salvia sclarea L.). Bulg. J. Agric. Sci. 2016, 22, 73–78. [Google Scholar]
- Jafari, S.; Daneshvar, M.H.; Salmi, M.S.; Abdi, L.J. Indirect organogenesis and plant regeneration in common sage (Salvia officinalis L.): An important medicinal plant of Iran. Mod Appl. Sci. 2017, 11, 22–29. [Google Scholar] [CrossRef][Green Version]



| Population | Latitude (North) | Longitude (East) | Number of Plants per Population | |
|---|---|---|---|---|
| 1 | ARISTI | 39.933689 | 20.679384 | 21 |
| 2 | ARNISSA | 40.798127 | 21.828355 | 9 |
| 3 | ELAFOTOPOS | 39.901731 | 20.692177 | 26 |
| 4 | IGOUMENITSA | 39.485731 | 20.264105 | 10 |
| 5 | KEFALOVRYSO | 40.003702 | 20.558476 | 11 |
| 6 | KALPAKI | 39.902897 | 20.641573 | 18 |
| 7 | KALYBIA | 39.902781 | 20.641872 | 15 |
| 8 | KATO PEDINA | 39.877418 | 20.670230 | 31 |
| 9 | KERKYRA | 39.770129 | 19.697890 | 10 |
| 10 | MAYROBOUNI | 39.954294 | 20.619267 | 18 |
| 11 | MESOBOUNI | 39.942593 | 20.646485 | 26 |
| 12 | MIKROBALTOS | 40.078276 | 21.872652 | 22 |
| Morphological Traits | Description |
|---|---|
| 1. Leaf number | Number of leaves per branch, 20 terminal branches (15 cm from the shoot tip) per population |
| 2. Leaf length | In cm, measured from the base to the tip of adult /mature leaf, 50 leaves per population |
| 3. Leaf width | In cm, measured at the widest part of adult leaf/mature, 50 leaves per population |
| 4. Inflorescence length | In cm, measured from the base to the tip of inflorescence, 20 inflorescences per population |
| 5. Node number per inflorescence | Number of nodes per inflorescence, 20 inflorescences per population |
| 6. Flower number | Number of flowers per inflorescence, 20 inflorescences per population |
| 7. Branch number | Number of terminal branches per plant, was measured in all plants |
| 8. Branch length | Length of branches per plant, in cm, 20 terminal branches per population |
| Treatment No. | (%) EtOH v/v * | Ascorbic and Citric Acid ** | NaOCl (%) | Time Duration (min) |
|---|---|---|---|---|
| D1 | 70 | 0.06 | 10 | |
| D2 | 70 | + | 0.06 | 11 |
| D3 | 70 | 0.06 | 12 | |
| D4 | 60 | + | 0.06 | 11 |
| D5 | 50 | + | 0.06 | 12 |
| D6 | 70 | 0.08 | 7 | |
| D7 | 70 | 0.04 | 17 |
| Medium | IAA (mg·L−1) | BAP (mg·L−1) | TDZ (mg·L−1) |
|---|---|---|---|
| MS 1 | 0 | 0 | 0 |
| MS 2 | 0.1 | - | - |
| MS 3 | 0.1 | 0.2 | - |
| MS 4 | 0.1 | 0.5 | - |
| MS 5 | 0.1 | 0.8 | - |
| MS 6 | 0.1 | 1.1 | - |
| MS 7 | 0.1 | - | 0.2 |
| MS 8 | 0.1 | - | 0.5 |
| MS 9 | 0.1 | - | 0.8 |
| MS 10 | 0.1 | - | 1.1 |
| K-IBA g·L−1 | Number of Roots | Length of Roots (cm) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Winter | Spring | Summer | Autumn | Winter | Spring | Summer | Autumn | ||
| Kefalovryso | 0 | - | 2.5 ± 0.3 d,* | - | - | - | 5.8 ± 0.5 a | - | - |
| 0.5 | - | 3.8 ± 0.1 b,c,** | - | 3.4 ± 0.3 c | - | 5.1 ± 0.2 b | - | 4.8 ± 0.2 b,c | |
| 1.0 | 4.2 ± 0.2 a,b | 4.4 ± 0.3 a | - | 4.2 ± 0.3 a,b | 4.2 ± 0.4 d | 4.4 ± 0.4 c,d | - | 4.6 ± 0.3 b,c | |
| Igoumenitsa | 0 | - | - | - | - | - | - | - | - |
| 0.5 | 3.8 ± 0.3 b | 3.9 ± 0.3 b | - | 4.3 ± 0.2 a,b | 4.6 ± 0.2 b,c | 5.3 ± 0.2 a | - | 4.7 ± 0.4 a,b | |
| 1.0 | 4.4 ± 0.1 a | 4.7 ± 0.2 a | - | 4.8 ± 0.4 a | 4.1 ± 0.4 c | 4.8 ± 0.3 a,b | - | 4.8 ± 0.3 a,b | |
| Aristi | 0 | - | 3.1 ± 0.1 c | - | 2.9 ± 0.1 c | - | 5.9 ± 0.4 a | - | 5.5 ± 0.4 a,b |
| 0.5 | 4.7 ± 0.3 b | 4.7 ± 0.2 b | - | 4.6 ± 0.4 b | 4.7 ± 0.3 b,c | 5.0 ± 0.2 b | - | 4.7 ± 0.5 b,c | |
| 1.0 | - | 5.3 ± 0.2 a | 2.2 ± 0.1 d | 5.4 ± 0.2 a | - | 4.9 ± 0.3 b | 3.4 ± 0.2 d | 4.2 ± 0.3 c | |
| Fog | Mist | |||
|---|---|---|---|---|
| Perlite:Peat | Number of Roots | Length of Roots (cm) | Number of Roots | Length of Roots (cm) |
| 1:0 | 4.2 ± 0.3 a,*,** | 3.9 ± 0.4 a | 3.7 ± 0.7 a | 4.0 ± 0.5 a |
| 2:1 | 4.8 ± 0.6 a | 4.7 ± 0.5 a | 4.3 ± 0.5 a | 3.8 ± 0.4 a |
| 1:1 | - | - | - | - |
| Treatment | Percentage of Survival | ||
|---|---|---|---|
| Aristi | Kefalovryso | Igoumenitsa | |
| D1 | 80.0 ± 9 a,b,*,** | 75.0 ± 9 a | 65.0 ± 10 a |
| D2 | 95.0 ± 5 a | 85.0 ± 8 a | 75.0 ± 9 a |
| D3 | 80.0 ± 8 a,b | 65.0 ± 10 a,b | 60.0 ± 11 a |
| D4 | 65.0 ± 10 b | 60.0 ± 11 b | 65.0 ± 4 a |
| D5 | 45.0 ± 11 c | 30.0 ± 10 c | 35.0 ± 10 b |
| D6 | 30.0 ± 10 c | 20.0 ± 11 c | 25.0 ± 9 b |
| D7 | 40.0 ± 11 c | 40.0 ± 11 c | 35.0 ± 10 b |
| Nutrient Medium | Number of Shoots | Length of Shoots (cm) | ||||
|---|---|---|---|---|---|---|
| Aristi | Kefalovryso | Igoumenitsa | Aristi | Kefalovryso | Igoumenitsa | |
| MS1 | 1.28 ± 0.43 b,*,** | 0.57 ± 0.25 c | 0.20 ± 0.16 b | 0.28 ± 0.09 c | 0.27 ± 0.15 d | 0.42 ± 0.22 b |
| MS2 | 1.21 ± 0.36 b | 0.78 ± 0.31 c | 0.50 ± 0.25 a,b | 0.38 ± 0.21 c | 0.72 ± 0.33 c,d | 0.44 ± 0.22 b |
| MS3 | 1.42 ± 0.44 b | 1.07 ± 0.32 b,c | 0.42 ± 0.25 a,b | 0.53 ± 0.21 c | 0.88 ± 0.35 c,d | 0.90 ± 0.47 a,b |
| MS4 | 1.64 ± 0.42 b | 1.14 ± 0.37 b,c | 0.57 ± 0.30 a,b | 1.25 ± 0.38 b,c | 1.17 ± 0.46 b,c | 1.10 ± 0.59 a,b |
| MS5 | 3.21 ± 0.40 a | 2.30 ± 0.43 a | 1.00 ± 0.37 a | 3.20 ± 0.50 a | 2.77 ± 0.54 a | 2.17 ± 0.80 a |
| MS6 | 1.21 ± 0.48 b | 2.00 ± 0.49 a,b | 0.57 ± 0.27 a,b | 0.95 ± 0.39 b,c | 0.87 ± 0.34 c,d | 1.17 ± 0.52 a,b |
| MS7 | 0.78 ± 0.28 b | 0.70 ± 0.30 c | 0.28 ± 0.19 b | 1.50 ± 0.46 b,c | 0.97 ± 0.38 c,d | 0.42 ± 0.28 b |
| MS8 | 1.78 ± 0.49 b | 1.07 ± 0.33 b,c | 0.57 ± 0.30 a,b | 1.39 ± 0.49 b,c | 1.25 ± 0.46 b,c | 0.77 ± 0.41 b |
| MS9 | 3.35 ± 0.55 a | 2.40 ± 0.40 a | 0.92 ± 0.40 a | 2.23 ± 0.50 a,b | 1.94 ± 0.47 a | 1.50 ± 0.65 a,b |
| MS10 | 1.28 ± 0.39 b | 1.07 ± 0.33 b,c | 0.50 ± 0.27 a,b | 1.49 ± 0.50 b,c | 1.60 ± 0.44 a,b | 1.07 ± 0.56 a,b |
| Average | 1.71 ± 0.87 | 1.31 ± 0.67 | 0.55 ± 0.25 | 1.32 ± 0.89 | 1.24 ± 0.71 | 0.99 ± 0.55 |
| Nutrient Medium | Rooting Formation (%) | Length of Roots (cm) | ||||
|---|---|---|---|---|---|---|
| Aristi | Kefalovryso | Igoumenitsa | Aristi | Kefalovryso | Igoumenitsa | |
| MS1 | 0 b | 0 b | 0 b | - | - | - |
| MS2 | 0 b | 0 b | 0 b | - | - | - |
| MS3 | 0 b | 0 b | 0 b | - | - | - |
| MS4 | 7 ± 7 b,*,** | 7 ± 7 b | 0 b | 0.94 ± 0.21 c | 0.91 ± 0.19 c | - |
| MS5 | 64 ± 13 a | 57 ± 13 a | 28 ± 12 a | 4.01 ± 0.51 a | 3.03 ± 0.40 a | 1.00 ± 0.18 b |
| MS6 | 7 ± 7 b | 7 ± 7 b | 7 ± 7 b | 1.21 ± 0.19 c | 1.00 ± 0.17 c | 0.57 ± 0.13 c |
| MS7 | 0 b | 0 b | 0 b | - | - | - |
| MS8 | 0 b | 0 b | 0 b | - | - | - |
| MS9 | 21 ± 11 b | 14 ± 9 b | 14 ± 9 a,b | 3.05 ± 0.41 b | 2.02 ± 0.35 b | 1.92 ± 0.30 a |
| MS10 | 7 ± 7 b | 7 ± 7 b | 7 ± 7 b | 1.01 ± 0.23 c | 0.90 ± 0.20 c | 0.85 ± 0.18 b,c |
| Average | 10.6 ± 19.8 | 9.2 ± 17.4 | 5.6 ± 9.2 | 2.32 ± 1.45 | 1.73 ± 0.99 | 1.08 ± 0.58 |
| Population | Propagation Medium | Rooting Medium | Rooting (%) | Acclimatization (%) |
|---|---|---|---|---|
| Aristi | MS5 | R1 | 16.6 ± 11 d,*,** | 100 a |
| MS5 | R2 | 33.3 ± 14 b,c,d | 100 a | |
| MS5 | R3 | 100 a | 92.0 ± 5 a | |
| MS5 | - *** | 64.0 ± 13 b | 88.8 ± 7 a | |
| MS9 | R1 | 41.6 ± 14 b,c | 100 a | |
| MS9 | R2 | 41.6 ± 14 b,c | 100 a | |
| MS9 | R3 | 66.7 ± 14 b | 100 a | |
| MS9 | - | 21.0 ± 11 c,d | 100 a | |
| Kefalovryso | MS5 | R1 | 33.3 ± 14 b,c,d | 100 a |
| MS5 | R2 | 33.3 ± 14 b,c,d | 100 a | |
| MS5 | R3 | 66.7 ± 14 b | 87.5 ± 6 a | |
| MS5 | - | 57.0 ± 13 b | 100 a | |
| MS9 | R1 | 25 ± 13 c,d | 100 a | |
| MS9 | R2 | 41.6 ± 14 b,c | 100 a | |
| MS9 | R3 | 41.6 ± 14 b,c | 100 a | |
| MS9 | - | 14.0 ± 9 d | 100 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nanos, C.; Tsoulpha, P.; Kostas, S.; Hatzilazarou, S.; Michail, I.; Anastasiadi, V.; Pipinis, E.; Gklavakis, E.; Kanellis, A.K.; Nianiou-Obeidat, I. Asexual Propagation of Greek Salvia officinalis L. Populations Selected for Ornamental Use. Horticulturae 2023, 9, 847. https://doi.org/10.3390/horticulturae9070847
Nanos C, Tsoulpha P, Kostas S, Hatzilazarou S, Michail I, Anastasiadi V, Pipinis E, Gklavakis E, Kanellis AK, Nianiou-Obeidat I. Asexual Propagation of Greek Salvia officinalis L. Populations Selected for Ornamental Use. Horticulturae. 2023; 9(7):847. https://doi.org/10.3390/horticulturae9070847
Chicago/Turabian StyleNanos, Christos, Parthena Tsoulpha, Stefanos Kostas, Stefanos Hatzilazarou, Ioanna Michail, Vasiliki Anastasiadi, Elias Pipinis, Evangelos Gklavakis, Angelos K. Kanellis, and Irini Nianiou-Obeidat. 2023. "Asexual Propagation of Greek Salvia officinalis L. Populations Selected for Ornamental Use" Horticulturae 9, no. 7: 847. https://doi.org/10.3390/horticulturae9070847
APA StyleNanos, C., Tsoulpha, P., Kostas, S., Hatzilazarou, S., Michail, I., Anastasiadi, V., Pipinis, E., Gklavakis, E., Kanellis, A. K., & Nianiou-Obeidat, I. (2023). Asexual Propagation of Greek Salvia officinalis L. Populations Selected for Ornamental Use. Horticulturae, 9(7), 847. https://doi.org/10.3390/horticulturae9070847









