Influence of Suboptimal Temperature on Flower Quality and Floral Organ Development in Spray-Type Cut Rose ‘Pink Shine’
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials
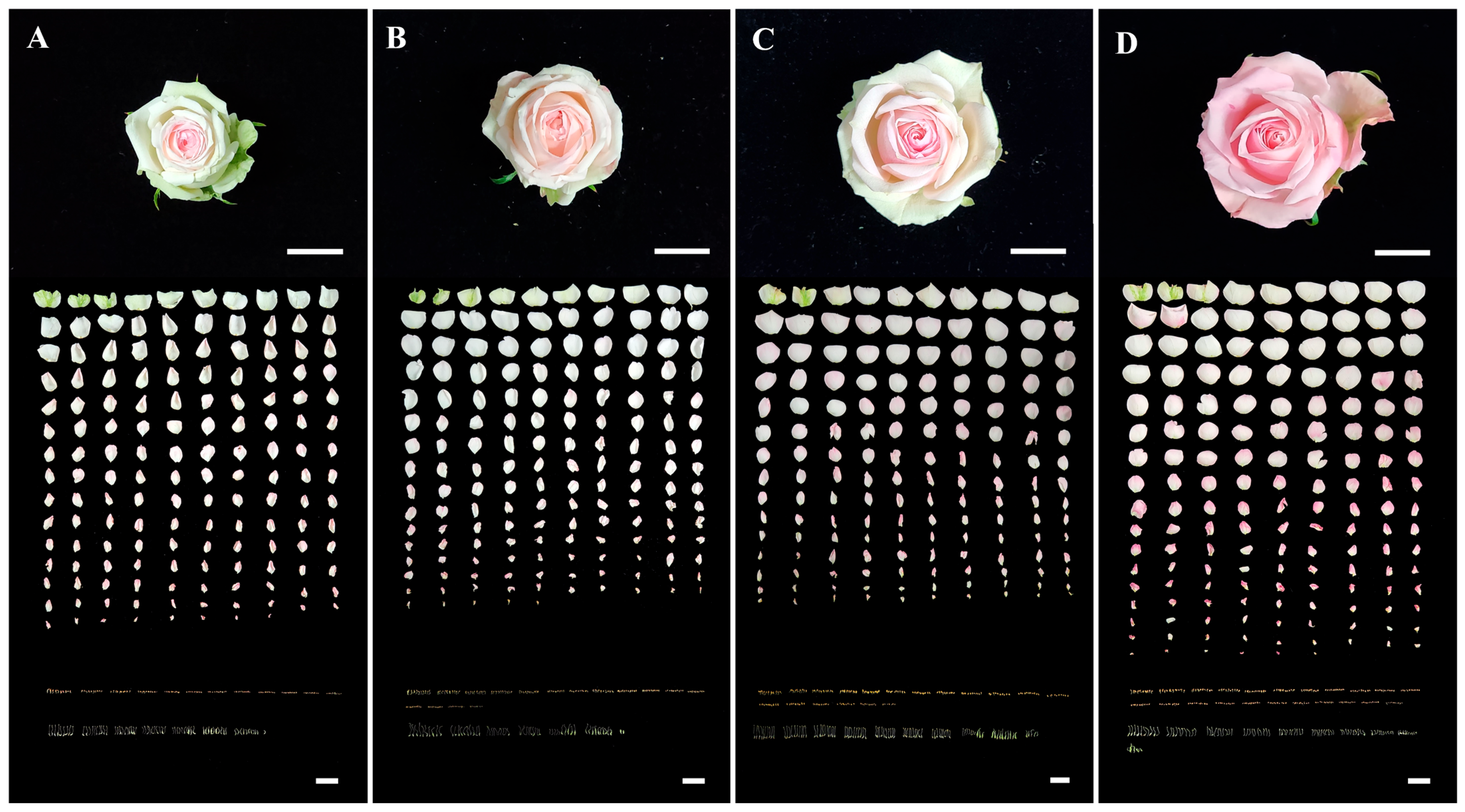
2.2. Temperature Treatment
2.3. Flowering Response and Floral Organ Development
2.4. RNA Extraction and qRT-PCR
2.5. Statistical Analyses
3. Results
3.1. Flowering Response
3.2. Floral Organ Differentiation
3.3. Correlation between Temperature and Flower-Related Traits
3.4. Expression Levels of Flowering-Related Genes
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Shi, L.; He, S.; Wang, Z.; Kim, W.S. Influence of nocturnal supplemental lighting and different irrigation regimes on vase life and vase performance of the hybrid rose ‘Charming Black’. Hortic. Sci. Technol. 2021, 39, 23–36. [Google Scholar] [CrossRef]
- Shin, Y.C.; Hwang, J.Y.; Yeon, J.Y.; Kim, W.S. Changes in floral pigments and scent compounds in garden roses during floral bud development. Flower Res. J. 2022, 30, 26–33. [Google Scholar] [CrossRef]
- Wang, H.; Fan, Y.; Yang, Y.; Zhang, H.; Li, M.; Sun, P.; Zhang, X.; Xue, Z.; Jin, W. Classification of rose petal colors based on optical spectrum and pigment content analyses. Hortic. Environ. Biotechnol. 2022, 64, 153–166. [Google Scholar] [CrossRef]
- Yeon, J.Y.; Kim, M.J.; Shin, Y.C.; Yang, K.R.; Kim, W.S. The efficiency of selecting target flower traits at early seedling stage for new cut rose cultivars. Flower Res. J. 2021, 29, 146–152. [Google Scholar] [CrossRef]
- Yeon, J.Y.; Lee, S.; Lee, K.J.; Kim, W.S. Flowering responses in the cut rose ‘Vital’ to non-optimal temperatures. Hortic. Sci. Technol. 2022, 40, 471–480. [Google Scholar] [CrossRef]
- Chmelnitsky, I.; Azizbekova, N.; Khayat, E.; Zieslin, N. Morphological development of regular and phyllody expressing Rosa hybrida cv. Motrea flowers. Plant Growth Regul. 2002, 37, 215–221. [Google Scholar] [CrossRef]
- Sim, S.; Rowhani, A.; Golino, D. Phyllody in roses. Amer Rose 2004, 39, 32–34. [Google Scholar]
- Dubois, A.; Raymond, O.; Maene, M.; Baudino, S.; Langlade, N.B.; Boltz, V.R.; Vergne, P.; Bendahmane, M. Tinkering with the C-Function: A molecular frame for the selection of double flowers in cultivated roses. PLoS ONE 2010, 5, e9288. [Google Scholar] [CrossRef]
- Ma, N.; Chen, W.; Fan, T.; Tian, Y.; Zhang, S.; Zeng, D.; Li, Y. Low temperature-induced DNA hypermethylation attenuates expression of RhAG, an AGAMOUS homolog, and increases petal number in rose (Rosa hybrida). BMC Plant Biol. 2015, 15, 237. [Google Scholar] [CrossRef]
- Mibus, H.; Heckl, D.; Serek, M. Cloning and characterization of three APETALA1/FRUITFULL-like genes in different flower types of Rosa x hybrida L. J. Plant Growth Regul. 2011, 30, 272–285. [Google Scholar] [CrossRef]
- Zhang, X.; Wu, Q.; Lin, S.; Li, D.; Bao, M.; Fu, X. Identification and characterization of class E genes involved in floral organ development in Dianthus chinensis. Ornam. Plant Res. 2023, 3, 5. [Google Scholar] [CrossRef]
- Litt, A.; Kramer, E.M. The ABC model and the diversification of floral organ identity. Semin. Cell Dev. Biol. 2010, 21, 129–137. [Google Scholar] [CrossRef]
- Breen, K.C.; Tustin, D.S.; Palmer, J.W.; Close, D.C. Method of manipulating floral bud density affects fruit set responses in apple. Sci. Hortic. 2015, 197, 244–253. [Google Scholar] [CrossRef]
- Desta, B.; Tena, N.; Amare, G. Response of rose (Rosa hybrida L.) plant to temperature. Asian J. Plant Soil. Sci. 2022, 7, 93–101. [Google Scholar]
- Hair, C. Roses along the equator: Situating Ecuador and Colombia within the global cut-flower market. South. Q. 2019, 57, 50–67. [Google Scholar]
- Yang, K.R.; Kim, W.H.; Kim, S.J.; Jung, H.H.; Yoo, B.S.; Lee, H.J.; Park, K.Y. Breeding of spray rose cultivar ‘Pink Shine’ with pink color and longer vase life. Flower Res. J. 2020, 28, 210–215. [Google Scholar] [CrossRef]
- Kim, W.S.; Lieth, J.H. Simulation of year-round plant growth and nutrient uptake in Rosa hybrida over flowering cycles. Hortic. Environ. Biotechnol. 2012, 53, 193–203. [Google Scholar] [CrossRef]
- Gapovilla, G.; Schmid, M.; Posé, D. Control of flowering by ambient temperature. J. Exp. Bot. 2015, 66, 59–69. [Google Scholar] [CrossRef]
- Yeon, J.Y.; Kim, W.S. Heat stress to the developing floral buds decreases the synthesis of flowering pigments and scent compounds in the rose petals. Acta Hortic. 2020, 1291, 249–260. [Google Scholar] [CrossRef]
- Lee, S.K.; Kim, W.S. Floral pigmentation and expression of anthocyanin-related genes in bicolored roses ‘Pinky Girl’ as affected by temporal heat stress. Hortic. Sci. Technol. 2015, 33, 923–931. [Google Scholar] [CrossRef][Green Version]
- Kose, C.; Kaya, O. Differential themal analysis reveals the sensitivity of sweet cherry flower organs to low temperatures. Int. J. Biometeorol. 2022, 66, 987–994. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Tang, A.; Wan, H.; Zhang, T.; Cheng, T.; Wang, J.; Yang, W.; Pan, H.; Zhang, Q. An APETALA2 homolog, RcAP2, regulates the number of rose petals derived from stamens and response to temperature fluctuations. Front. Plant Sci. 2018, 9, 481. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Fu, X.; Dong, Y.; Lu, J.; Ren, M.; Zhou, N.; Wang, C. MIKCC-type MADS-box genes in Rosa chinensis: The remarkable expansion of ABCDE model genes and their roles in floral organogenesis. Hortic. Res. 2018, 5, 25. [Google Scholar] [CrossRef] [PubMed]
- Rusanov, K.; Kovacheva, N.; Rusanova, M.; Linde, M.; Debener, T.; Atanassov, I. Genetic control of flower petal number in Rosa x damascena Mill f. trigintipetala. Biotechnol. Biotechnol. Equip. 2019, 33, 597–604. [Google Scholar] [CrossRef]
- Yan, H.; Zhang, H.; Wang, Q.; Jian, H.; Qiu, X.; Baudino, S.; Just, J.; Raymond, O.; Gu, L.; Wang, J.; et al. The Rosa chinensis cv. Viridiflora phyllody phenotype is associated with misexpression of flower organ identity genes. Front. Plant Sci. 2016, 7, 996. [Google Scholar] [CrossRef]
- Kitahara, K.; Hibino, Y.; Adia, R.; Mastumoto, S. Ectopic expression of the rose AGAMOUS-like MADS-box genes ‘MASAKO C1 and D1′ causes similar homeotic transformation of sepal and petal in Arabidopsis and sepal in Torenia. Plant Sci. 2004, 166, 1245–1252. [Google Scholar] [CrossRef]



| Gene | Species | Accession Number | Product Length (bp) | Forward Sequence | Reverse Sequence |
|---|---|---|---|---|---|
| RhAP1 | R. hybrida | FJ970026.27 | 87 | ACAAGATCAACAGGCAGGTC | GAGCATCGCACAAGACAGAG |
| RhAP2 | R. chinensis | MF773425.1 | 103 | CTCCGAAATGGAACCCACAC | GCAGAACTTGACTCCGACC |
| RhFUL | R. hybrida | FJ970028.1 | 130 | ACCAGCCCTACTCTCTTCTC | TGGTGGCATGAGTGTGTTAC |
| RhAP3 | R. rugosa | AB099875 | 107 | CCTCATGGTTTCCTCTTCCG | CCAAAGGTCAATTCCGAGG |
| RhPI | R. rugosa | AB038462 | 139 | TGGAAAGAGGTTATGGGATGC | CAGGTCCACATGGTTCAGAG |
| RhAG | R. hybrida | U43372.1 | 91 | ATCGTCAAGTCACCTTCTGC | ATGAGAGCAACCTCAGCATC |
| RhSHP | R. rugosa | AB025643 | 106 | AATGACAGGGCACAACAGC | CAGGGAGAAAGCTCCTATCG |
| RhSEP | R. rugosa | AB099876.1 | 86 | AGACAAACATGGGAACGTGG | GGCTGGAACATAAGACCCTG |
| RhACT1 (reference) | R. hybrida | KC514918.1 | 116 | GTTCCCAGGAATCGCTGATA | TCCTCCGATCCAAACACTG |
| Treatment | Days to Flowering (Days) | Shoot Length (cm) | Shoot Weight (g FW) | Flower Size z (cm2) | Flower Weight (g FW) | Petal Size (cm2) | Petal Weight (g FW) | Peduncle Length (cm) | No. of Florets y |
|---|---|---|---|---|---|---|---|---|---|
| 25/20 °C | 47.6 c x | 29.7 b | 30.9 b | 13.6 c | 4.2 d | 198.5 c | 3.1 c | 2.6 b | 2.6 b |
| 20/20 °C | 58.8 b | 34.6 a | 65.7 a | 17.4 b | 7.0 c | 309.5 b | 5.4 b | 3.1 a | 3.1 a |
| 20/15 °C | 68.0 a | 30.4 ab | 41.3 b | 18.7 ab | 8.6 b | 335.6 b | 6.4 b | 3.0 ab | 3.0 ab |
| 15/15 °C | 72.6 a | 32.9 ab | 65.9 a | 20.5 a | 10.9 a | 404.6 a | 8.4 a | 3.5 a | 3.5 a |
| Significance | *** | ns | *** | *** | *** | *** | ** | ** | ** |
| Treatment | Sepal | Petal | Petaloid Stamen | Stamen | Carpel | Total |
|---|---|---|---|---|---|---|
| 25/20 °C | 6.1 ± 1.0 z (1.7%) | 95.9 ± 24.1 (27.3%) | 22.8 ± 10.9 (6.4%) | 158.9 ± 20.3 (45.2%) | 68.1 ± 8.9 (19.4%) | 351.8 ± 45.7 (100%) |
| 20/20 °C | 6.4 ± 0.7 (1.5%) | 109.9 ± 20.6 (25.0%) | 30.9 ± 16.3 (7.0%) | 205.8 ± 20.7 (46.7%) | 87.3 ± 18.5 (19.8%) | 440.3 ± 57.2 (100%) |
| 20/15 °C | 6.5 ± 1.1 (1.4%) | 107.0 ± 27.4 (23.0%) | 26.1 ± 12.0 (5.6%) | 212.8 ± 57.3 (45.8%) | 112.5 ± 31.7 (24.2%) | 464.9 ± 106.6 (100%) |
| 15/15 °C | 6.6 ± 1.1 (1.3%) | 108.2 ± 34.2 (22.0%) | 28.7 ± 6.9 (5.8%) | 213.9 ± 35.5 (43.5%) | 134.1 ± 46.2 (27.4%) | 491.5 ± 106.6 (100%) |
| Significance | ns | ns | ns | ** | *** | ** |
| Variable | DAY | SL | SW | FS | PL | Floret | Floral Organ | Sepal | Petal | Petaloid-S | Petal + PS | Stamen | Carpel | DWFO |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TEMPsum | 0.99 *** | 0.26 | 0.45 ** | 0.50 *** | 0.34 * | 0.07 | 0.47 *** | 0.03 | 0.15 | −0.18 | 0.06 | 0.42 ** | 0.66 *** | 0.58 *** |
| DAY | 0.25 | 0.45 ** | 0.51 *** | 0.35 * | 0.06 | 0.47 *** | 0.03 | 0.16 | −0.17 | 0.07 | 0.43 ** | 0.67 *** | 0.59 *** | |
| SL | 0.66 *** | 0.12 | 0.32 * | 0.61 *** | 0.29 * | −0.08 | 0.25 | 0.01 | 0.21 | 0.34 * | 0.19 | 0.24 | ||
| SW | 0.32 | 0.55 *** | 0.76 *** | 0.47 *** | 0.01 | 0.34 * | 0.20 | 0.37 * | 0.39 ** | 0.46 ** | 0.43 ** | |||
| FS | 0.34 | 0.05 | 0.08 | 0.20 | −0.14 | 0.11 | −0.08 | 0.07 | 0.18 | 0.57 *** | ||||
| PL | 0.40 ** | 0.36 * | −0.13 | 0.23 | 0.28 | 0.30 * | 0.29 * | 0.33 * | 0.28 | |||||
| Floret | 0.25 | −0.13 | 0.28 | 0.20 | 0.31 * | 0.25 | 0.10 | 0.15 | ||||||
| Floral organ | −0.05 | 0.80 *** | 0.29 * | 0.79 *** | 0.89 *** | 0.90 *** | 0.61 *** | |||||||
| Sepal | −0.26 | 0.15 | −0.16 | 0.01 | −0.03 | 0.19 | ||||||||
| Petal | 0.23 | 0.93 *** | 0.56 *** | 0.65 *** | 0.31 * | |||||||||
| Petaloid-S | 0.57 *** | 0.15 | 0.08 | 0.33 * | ||||||||||
| Petal + PS | 0.53 *** | 0.58 *** | 0.38 ** | |||||||||||
| Stamen | 0.71 *** | 0.51 *** | ||||||||||||
| Carpel | 0.67 *** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shin, Y.C.; Yeon, J.Y.; Kim, W.S. Influence of Suboptimal Temperature on Flower Quality and Floral Organ Development in Spray-Type Cut Rose ‘Pink Shine’. Horticulturae 2023, 9, 861. https://doi.org/10.3390/horticulturae9080861
Shin YC, Yeon JY, Kim WS. Influence of Suboptimal Temperature on Flower Quality and Floral Organ Development in Spray-Type Cut Rose ‘Pink Shine’. Horticulturae. 2023; 9(8):861. https://doi.org/10.3390/horticulturae9080861
Chicago/Turabian StyleShin, Yeong Chan, Je Yeon Yeon, and Wan Soon Kim. 2023. "Influence of Suboptimal Temperature on Flower Quality and Floral Organ Development in Spray-Type Cut Rose ‘Pink Shine’" Horticulturae 9, no. 8: 861. https://doi.org/10.3390/horticulturae9080861
APA StyleShin, Y. C., Yeon, J. Y., & Kim, W. S. (2023). Influence of Suboptimal Temperature on Flower Quality and Floral Organ Development in Spray-Type Cut Rose ‘Pink Shine’. Horticulturae, 9(8), 861. https://doi.org/10.3390/horticulturae9080861







