Abstract
Microgreens have a high nutrient density and are beneficial to human health. Even though this class of vegetables have gaining increasing levels of attention in the last year, scientific research on the growth of microgreens in controlled environments under artificial lighting have not been thoroughly characterized. By describing the scientific outputs focused on the impacts of artificial illumination on microgreens, especially from the first two decades of the 21st century, it is therefore possible to detect advancements and research gaps in this research field. This review is divided in two parts: first, a general overview of the scientific production about microgreens; second, a systematic review of scientific studies exploring artificial lighting on the production of microgreens. The overview of scientific production on microgreens and artificial lighting across the Scopus, Web of Science, and Scielo databases, from 2000 to 2021, respectively, indicated three phases, as before 2011 no paper was found: phase 1 (2012 to 2014), six papers; phase 2 (2015 to 2018), fifteen papers; and phase 3 (2019 to 2021), forty-six papers, respectively. Mustard was the most evaluated crop under all production stages. With regard to the second part of this review, studies on artificial lighting with fluorescent lamps (high-pressure sodium light bulbs—HPS), from the supplementation to the replacement of HPS lighting with light emitting diode (LED) lamps, and plant responses with respect to light properties comprise the main works identified. Studies on the distribution of environmental factors under controlled microgreen cultivation present research gaps.
1. Introduction
Microgreens are emerging superfoods with high concentrations of vitamins, minerals, and other bioactive compounds, such as glucosinolates, ascorbic acid, carotenoids, tocopherol, folates, and anthocyanin [1]. They are produced from several commercial crops, such as vegetables, herbs, and grains. Micro vegetables are typically developed between 7 and 21 days after germination, respectively, reaching around 4–8 cm in height, and have different flavors, colors, textures, aromas, and appearances. This kind of food is considered as a new generation of functional foods due to its potential to prevent the occurrence of several diseases, such as cancer, diabetes, anemia, hypertension, inflammatory processes, and other chronic problems.
Microgreens are cultivated under protected or indoor production systems that prioritize sustainability and the optimal utilization of natural resources [2], including energy, water, and fertilizer use efficiency [3]. Indoor production under controlled environments is increasingly being adopted for microgreen cultivation. This production system utilizes LED technology as the primary light source for plant growth [4], offering the flexibility to choose the spectral composition and intensity of light while minimizing radiant heat emissions [5]. Most of the research studies that have been conducted examined the relationship between the intensity of artificial light, particularly in the red and blue ranges [6,7], and the growth of microgreens have shown variability in their responses among different species of micro vegetables [8].
Highlighted as an emerging topic, it is crucial to examine the scientific literature on microgreens, particularly during the first two decades of the 21st century, focusing on the impact of artificial lighting on productivity and quality. Lee et al. [9] and Murphy et al. [10] have emphasized the scarcity of references available on microgreens during the early 2000s. However, the number of research papers on this subject has notably increased since 2010, particularly between the years of 2019 and 2021, respectively. Despite this growth, the number of countries, research groups, and crop varieties studied remains limited [11]. Consequently, microgreens are expected to be a significant area of focus in the upcoming decades, as depicted by Treadwell et al. [11], who referred to them as “A New Specialty Crop”. This paper aims to characterize the progression of scientific research on the use of artificial lighting in microgreen production systems from the years of 2000 to 2021, respectively, highlighting its contributions and advancements, and identifying areas that require further investigation. The review was divided into two parts: first, a general overview of scientific production about microgreens; second, a systematic review of scientific studies exploring artificial lighting on microgreen production. We quantified the overview of scientific production across the Scopus, Web of Science, and Scielo databases from 2000 to 2021, respectively, using the keyword ‘microgreens’, with the last access date on 2 January 2022.
2. Scientific Production on Microgreens from 2000 to 2021: A General Overview
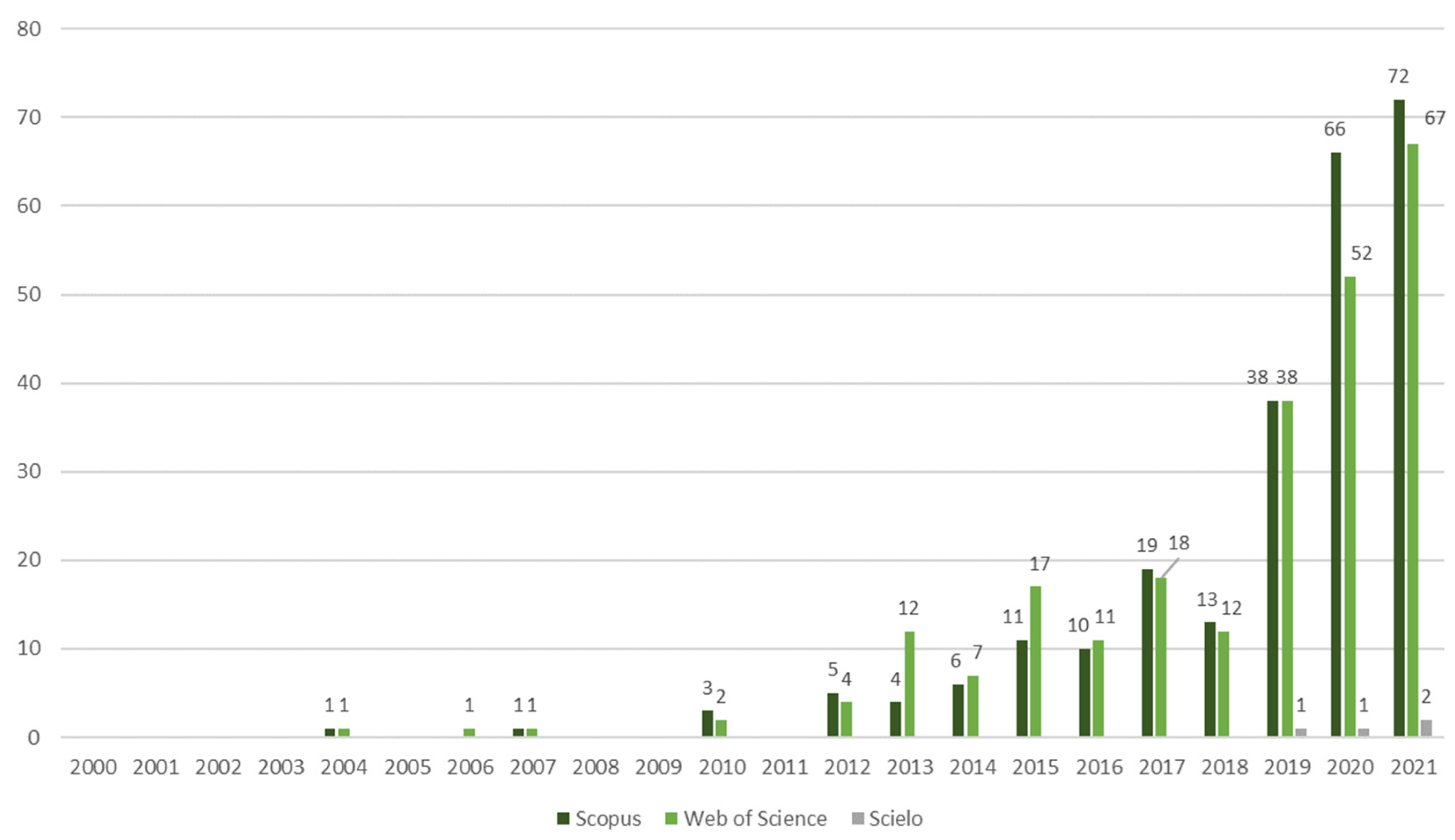
The total number of papers found in the databases based on the keyword ‘microgreens’ were as follows: 249 (Scopus), 243 (Web of Science), and 4 (Scielo), totaling 496 papers, including those repeated across these databases. Resulting in a number of papers below 500 results, there was no need for more keywords or the application of other search filters.
The analysis of the research output in microgreens over the last two decades revealed a notable increase in the number of publications on this subject, which can be categorized into four distinct phases. Phase 1, from 2000 to 2011, respectively, marked the initial stage of microgreens research and returned a very limited number of publications. A total of ten papers were identified, with five found in the Web of Science database and five in Scopus, respectively. Three publications were present in both databases. Phase 2, covering the period from 2012 to 2014, respectively, displayed a diversification of research groups and crops investigated. The subsequent phase, Phase 3 (2015–2018), exhibited an expansion of 192% in the scope of studies that were conducted. Finally, Phase 4, from the years of 2019 to 2021, respectively, exhibited a remarkable annual increase of 204% in the number of publications (as shown in Figure 1).
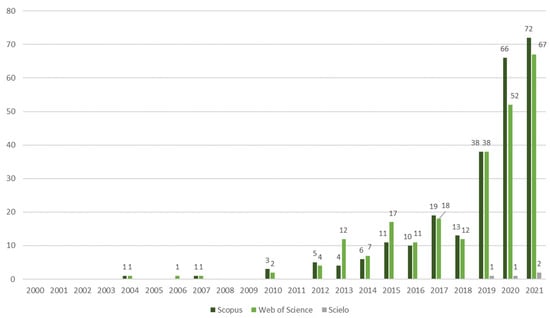
Figure 1.
The number of scientific publications about microgreens based on the Scopus, Web of Science, and Scielo databases from the years of 2000 to 2021, respectively, totaling 496 publications.
The earliest study identified on microgreens, available on both the Scopus and the Web of Science databases, was published in 2004, and was conducted by researchers affiliated with a US institution [9]. This study examined various treatments for beet and chard microgreen seeds. Between the years of 2004 and 2011, respectively, the other studies that were found were predominantly authored by researchers based in the USA [10,12,13], with one exception in 2010 from an institution in the Czech Republic [14]. These studies primarily explored seed treatments, fertilization techniques, and hydroponics, focusing on crops such as beet, buckwheat, rocket, and mustard. During the first decade, it became evident that microgreen research was still relatively limited.
From 2012, there was a noticeable increase in the number of research papers that were published on microgreens, and a greater diversity of research groups and crops that were investigated. During the period spanning from 2012 to 2021, a total of four papers were identified in Scielo, two hundred and thirty-eight papers in the Web of Science database, and two hundred and forty-four papers in the Scopus database, respectively. The papers retrieved from the Scielo database explored various aspects, including experimentation with different substrates and nutrient solutions for red cabbage [15] and rocket microgreens [16]. These studies were conducted by Brazilian authors affiliated with the Federal University of Rio Grande do Sul (UFRGS). Additionally, Indian researchers contributed to this field by investigating the utilization of microgreens for the production of functional juices [17]. Furthermore, researchers from Turkey focused on evaluating pigments, antioxidants, and bioactive compounds in wheat landraces and cereals [18,19].
3. Scientific Production on Artificial Lighting on Microgreens
3.1. From 2000 to 2021: A General Overview
The second step involved the selection of studies on the effects of artificial lighting on microgreen production, based on information contained in their titles and abstracts. For this, the following criteria were adopted: (i) inclusion of papers published in journals and indexed in databases; (ii) restriction to papers on artificial lighting in microgreens; (iii) exclusion of experiments performed in vitro; and (iv) exclusion of duplicates between the databases, counting only the occurrence in the Scopus database. In the Scielo database, only four papers on microgreens were found, none of which dealt with artificial lighting in microgreens, which is why they were excluded from this review. Ultimately, of the 496 papers counted, 67 remained on artificial lighting in microgreens, found in the Scopus and Web of Science databases, which were considered in this review. For the characterization of the scientific production, the information presented in these included papers was categorized by: the author, the author’s home institution country, the year of publication, and the crops used in the experiments. The organization of this information also made it possible to identify the authors’ research groups and co-authorships in the databases.
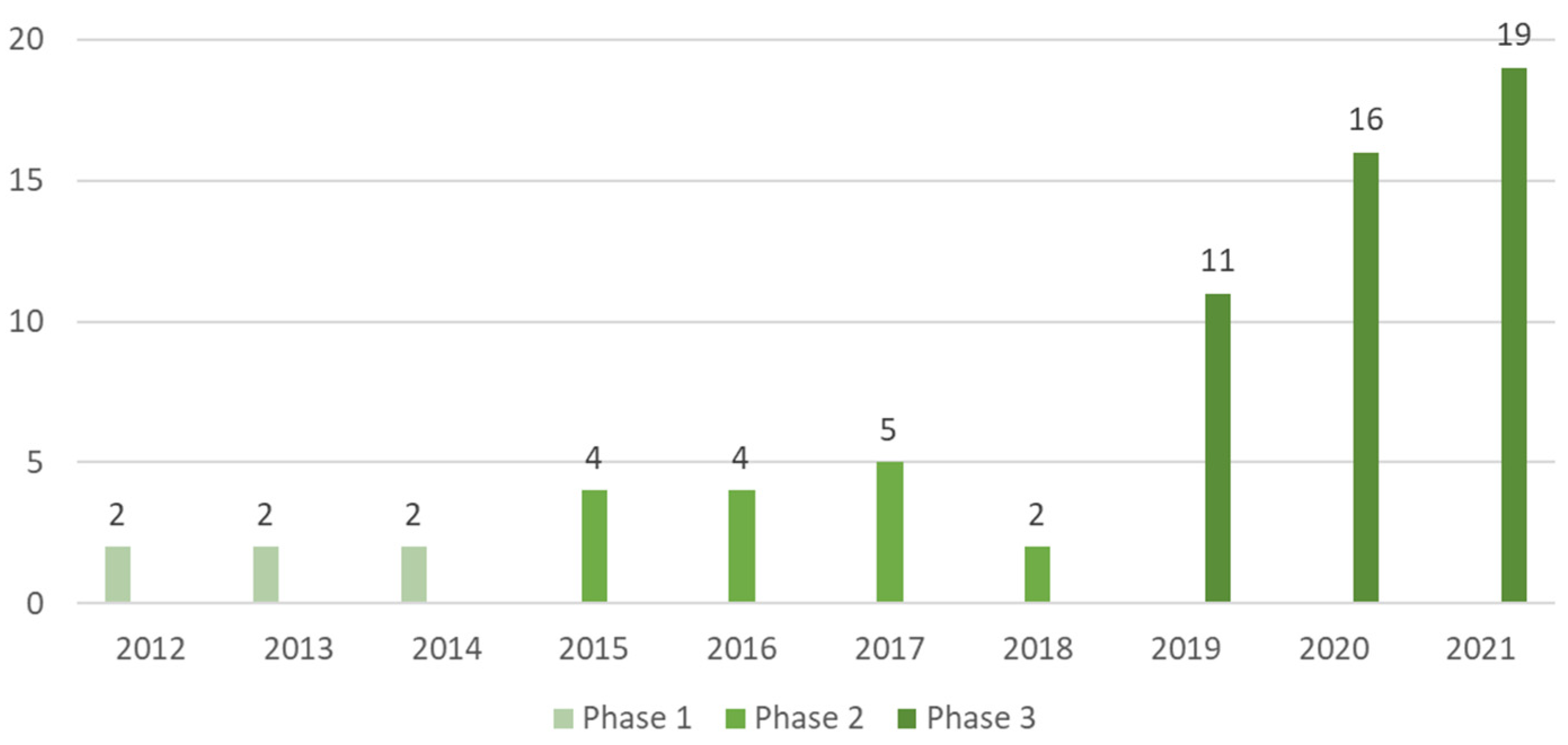
Three phases of scientific production were observed: in phase 1 (2012–2014) six papers were found; in phase 2 (2015–2018), fifteen papers and, in phase 3 (2019–2021), forty-six papers, respectively (as shown in Figure 2). The increase in interest on this subject in the last decade was notorious, going from two papers published in 2012 to nineteen papers in 2021, respectively.
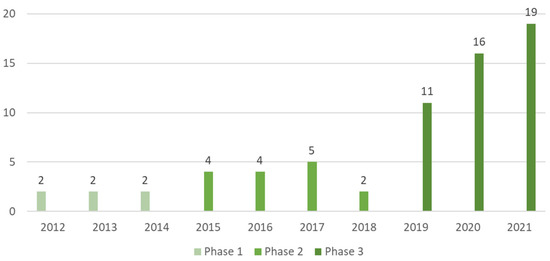
Figure 2.
Number of scientific papers about artificial lighting on microgreens categorized by their year of publication and phase, found on the Scopus and Web of Science databases from the years of 2012 to 2021, respectively totaling 67 publications.
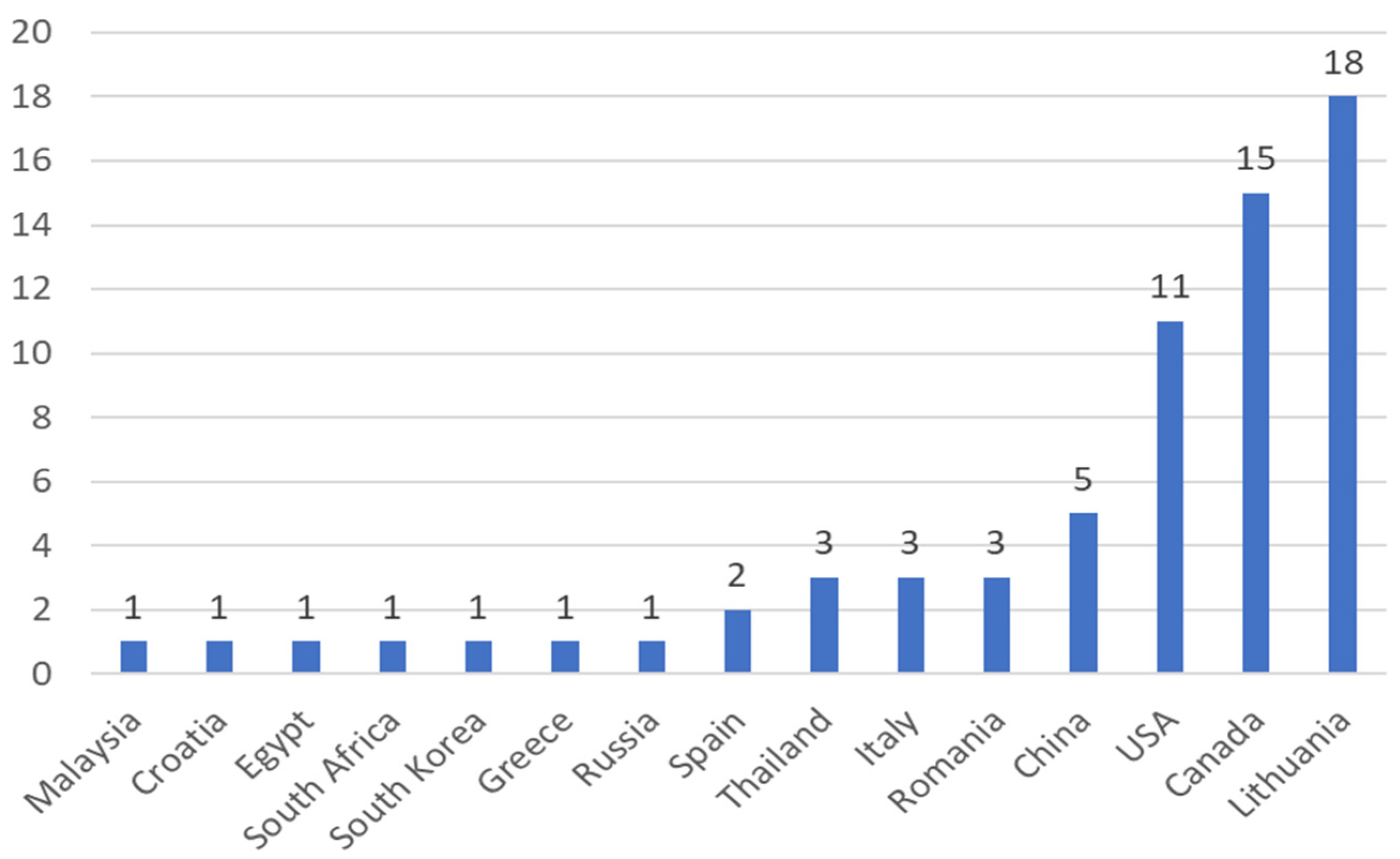
Among the 15 countries that were responsible for these publications on artificial lighting in microgreens in the Scopus and Web of Science databases, Lithuania had the highest number of published papers (Figure 3), responsible for 27% of publications, followed by Canada (22%), and the USA (17%), respectively.
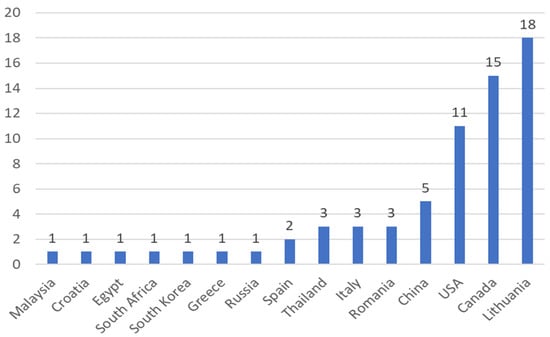
Figure 3.
The number of scientific papers about artificial lighting on microgreens categorized by their year of publication and phase, found on the Scopus and Web of Science databases from the years of 2012 to 2021, respectively, totaling 67 publications.
Despite the variety of countries researching the topic of artificial lighting in microgreens, a concentration of production by certain research groups in these countries was observed (Table 1). The authors Brazaitytė, Vaštakaitė, Samuolienė, and Viršile (and co-authors) led research in Lithuania on artificial lighting in microgreens, all being members of the Lithuanian Research Center for Agriculture and Forestry. For Canada, the authors from the University of Guelph group; and, in the case of the United States, the following institutions were listed: the University of Tennessee, Purdue University—Indiana, and the University of Florida.

Table 1.
Related publications on artificial lighting in microgreens, categorized by author, year of publication, and country, according to the Scopus and Web of Science for the period 2011–2021.
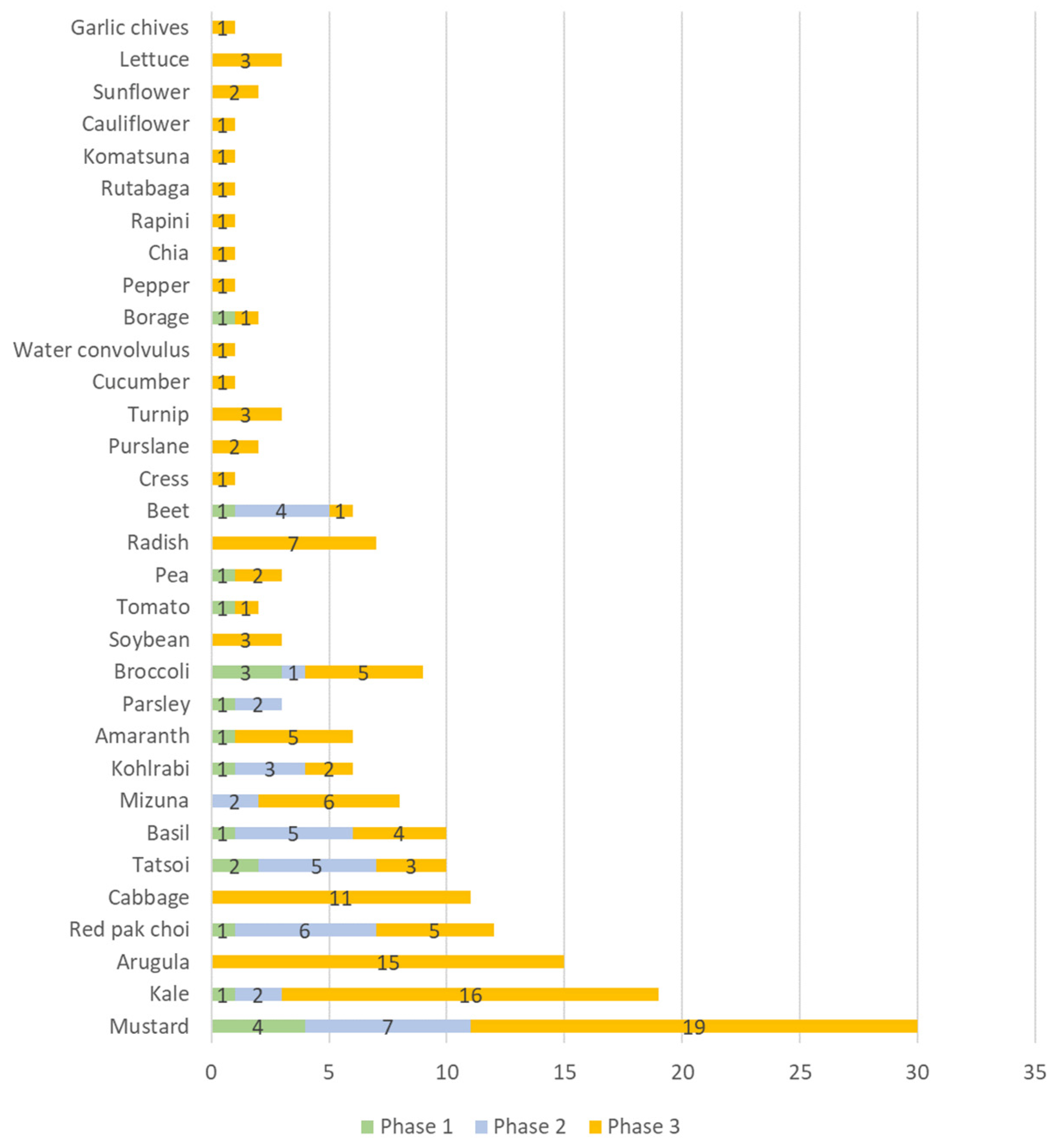
The categorization of the results of the scientific production by crops allowed the identification of 36 species of microgreens studied. With several papers having used more than one crop in their experiments, this totaled 187 observations (as displayed in Figure 4).
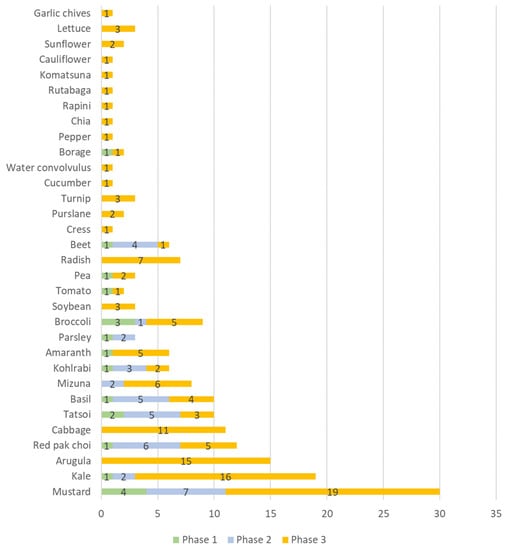
Figure 4.
Number of scientific publications categorized by species on the use of artificial lighting in microgreens found in the Scopus and Web of Science databases for the period 2012–2021.
The microgreen species that were most studied in terms of artificial lighting in each phase of scientific production were as follows, in order of greatest occurrence: in phase 1, mustard, broccoli, and tatsoi; in phase 2, mustard, red pak choi, tatsoi, basil, beetroot, kohlrabi kale, parsley, and kale; and in phase 3, mustard, kale, rocket, cabbage, radish, mizuna, red pack choi, amaranth, broccoli, basil, kohlorabi, tomato, and pea.
The most recent publications on artificial lighting on microgreens have pointed towards the diversification of plants in their experiments. Kamal [27] included thirteen crops in his study; meanwhile, Graham [69] and Bantis [82] worked with six microgreen crops in their trials. Between 2011–2018, 42% of the included studies used three crops in their experiments; and between 2019–2021, 31% of the total number of publications displayed the results of their experiments with four crops, respectively. On the other hand, more specific studies with a single crop totaled 35% of publications in phase 3 (17 papers), while in phases 1 and 2 they corresponded to 19% of publications on this subject, respectively. This trend seems to indicate the deepening of analyzes by research groups that have studied the subject for a longer period of time, as was the case in Samuolienė et al. [83] and Brazaityte et al. [77].
The bibliographic review on artificial lighting in microgreens from 2000 to 2021 allowed for the establishment of the following categories for an in-depth discussion of scientific production: lighting with cold, white, and incandescent fluorescent lamps (high-pressure sodium light bulbs—HPS); from complementing to replacing HPS lighting with LED lamps; studies on light intensity (photon flux density); light quality (wavelengths); combination of light intensity and quality; duration of light (photoperiod); and frequency (pulsed light).
3.2. Lighting with Cold, White, and Incandescent Fluorescent Lamps
The first studies on artificial lighting in microgreens were performed using cold, white, and incandescent fluorescent lamps [20]. Kopsell et al. [20] noted that a change in light management can be an effective way for growers to increase the levels of antioxidants and carotenoids in mustard microgreens. Plants were cultivated in a controlled environment, with cold white (160 W) and incandescent (60 W) fluorescent lamps under 275 µmol m−2 s−1, with a photoperiod of 14 h. After the emergence of the first true leaf, 463 μmol m−2 s−1 of light intensity was applied for a duration of 36 h. The authors observed a decrease in the concentrations of chlorophyll a and b, indicating high light stress levels, as well as a decline in the levels of β-carotene and neoxanthin. However, the concentrations of lutein remained unchanged, while increases in the zeaxanthin and antheraxanthin levels were observed.
3.3. From Complementing to Replacing HPS Lighting for LED Lamps
The spectral composition analysis of HPS lamps revealed a concentration of light emission in the yellow and orange spectrum range, with additional peaks detected in the blue, green, and red regions [84]. Thus, from understanding that this type of lighting may not fully satisfy the plant’s requirements, a research line was started to explore the supplementation of HPS lighting with LED lamps [22,58]. Samuolienė et al. [22] highlighted that red light seemed to be more effective in terms of anthocyanin production [85], increasing phenol concentration [86], and positively influenced the antioxidant activity [87]. Samuolienė et al. [22] and Brazaitytė et al. [58] assessed the short-term application (3 days in the pre-harvest stage) of light with red LEDs (638 nm), and observed that this effect was dependent on the species and the natural amount of antioxidant compounds in each crop.
The investigation into the replacement of HPS lamps with LED lighting began when it was observed that HPS lamps generated a higher energy input through thermal radiation and increased temperatures on the lamp surface [28,36,54]. Consequently, maintaining a safe distance between the lamps and the plants became necessary to avoid leaf burn [5].
In their studies, Kopsell et al. [28] and Kopsell et al. [54] compared incandescent fluorescent light with LEDs and made several observations. In the case of broccoli microgreens (21 days), the application of fluorescent incandescent light resulted in a lower fresh mass (FM) and lower concentrations of most metabolites in the plant tissue [28]. However, for Chinese cabbage (30 days) subjected to the same treatment, significantly higher levels of fresh and dry mass were obtained [54]. These differences were attributed to the effects of the narrowband LED light on more mature plants that have established hormone synthesis pathways [54].
Vaštakaitė and Viršilė [36] investigated whether different types of lamps (HPS at 150 μmol m−2 s−1 as the control; LED at 150 μmol m−2 s−1; and LED at 250 μmol m−2 s−1, respectively) alter the nutritional quality of microgreens of the Brassicaceae family. The light spectra of these lamps consisted of eight violet (420–430 nm), sixteen blue (460–470 nm), eight orange (610–615 nm), three red (620–630 nm), fifty-six red (660–670 nm), and eight white LEDs (with blue, 400–500 nm, green, 500–600 nm, and red, 600–700 nm, respectively). The physiological response to artificial light varied among the Brassicaceae species. Under 150 and 250 LED light, shorter, and with smaller hypocotyls microgreens, and higher contents of total anthocyanin and mineral elements (Ca, K, Mg, Na, P, Fe, and Zn) were observed. Moreover, plants under the 250 LED light treatment displayed higher contents of phytochemicals (including ascorbic acid, total phenols, and flavonols), DPPH (2, 2- diphenyl-1-picrylhydrazyl), and ABTS (3-ethylbenzothiazoline-6-sulphonic acid) radical scavenging activity that showed oxidative stress.
The introduction of LEDs revolutionized the potential applications for indoor plant growth [52]. Their key advantages can be summarized as follows: (i) emission of light within a narrow range of the spectrum; (ii) high energy efficiency compared to traditional lamps, resulting in reduced energy consumption and costs; (iii) operate at low voltages; (iv) capability to regulate the photosynthetic photon flux density (PPFD), enabling fine-tuning of the light intensity for optimal plant growth; and (v) low emission of heat during their operation [5].
3.4. Effects of Light Intensity and Quality on Microgreen Production
Research began to seek evidence on the effect of light properties on microgreen species. Studies were found on intensity (PPFD), quality (wavelengths), frequency (pulsed and continuous light), and duration (photoperiod), both individually and in combination.
The PPFD has been widely studied for the assessment of the growth, nutritional quality, and antioxidant properties of microgreens [8,26,36]. While low light (less than 110 μmol m−2 s−1) drives lower photosynthetic rates [88] and nitrate accumulation [89], high levels (greater than 545 μmol m−2 s−1) can create mild photostress and activate photoprotective mechanisms that influence the production of primary and secondary metabolites in plants, such as antioxidants, important for human nutrition [26].
Studies have shown that insufficient levels of PPFD (110 μmol m−2 s−1) suppressed normal growth, leading to elongation of the hypocotyl, reduction in the dry mass and leaf area, as well as a decreased nutritional value in the microgreens from the Brassicaceae family [26]. Additionally, elevated levels of nitrate were observed. In contrast, the treatment with a PPFD range of 330–440 μmol m−2 s−1 resulted in an increased leaf surface area, reduced nitrate content, and higher levels of total anthocyanins. Interestingly, exposing the microgreens to high light levels of light (545 μmol m−2 s−1) did not exert significant positive effects on most of the investigated parameters. However, mild oxidative photostress occurred, leading to pigment degradation.
Jones-Baumgardt et al. [70] assessed the effects of the PPFD (between 100 and 600 μmol·m−2 s−1) of LEDs, with 15% blue light and 85% red light, respectively, on the phytochemical profiles, growth, and morphological attributes of microgreens from kale, cabbage, rocket, and mustard. For all species, there was an increase in the concentration of ascorbate (total and reduced) and total anthocyanin proportional to the increase in the PPFD. Total phenolic concentrations were increased in all species at high PPFDs, while chlorophyll concentrations decreased in the rocket, cabbage, and mustard microgreens. Xonti et al. [41] conducted a study where they observed stunted growth in the cabbage and radish microgreens when exposed to a light intensity treatment of 270 μmol m−2 s−1. However, this light intensity led to an increase in the levels of starch and antioxidants present in the plants. Additionally, when the higher intensity treatment (270 μmol m−2 s−1) was applied to kale, it resulted in higher levels of ascorbic acid content.
Vetchinnikov et al. [6] also assessed the effects of light intensity (50, 100, and 200 μmol m−2 s−1) with blue and red LED lamps (450 nm and 660 nm) on the radish, cabbage, and basil microgreens. They observed that intensities greater than 100 µmol m−2 s−1 decreased plant growth and led to a higher energy consumption, with 100 μmol m−2 s−1 being found as the optimal intensity for basil, and 50 µmol m−2 s−1 for cabbage and radish, respectively.
Gao et al. [49] investigated the effects of red, blue, and green LED lights in a 1:1:1 ratio on broccoli microgreens, while varying the lighting intensities at 30, 50, 70, and 90 μmol m−2 s−1, respectively. The researchers observed that the plants grown under the intensity of 50 μmol m−2 s−1 exhibited a higher fresh mass, dry mass, and moisture content. However, they also found that their phytochemical aspects were at their lowest under this intensity. Conversely, the intensity of 70 μmol m−2 s−1 resulted in the production of plants containing the highest phytochemical contents.
Regarding the light quality, within the visible spectrum, blue light (400–500 nm) and red light (600–700 nm) are absorbed by chlorophyll and carotenoids for photosynthesis. Red light influences various growth and development processes, albeit in a way that depends on the light intensity and/or photoperiod. These include germination, inhibition of hypocotyl elongation and petiole growth, cotyledon opening and expansion, and biomass accumulation in many plant species [90]. Similarly, blue light plays an important role in hypocotyl growth and cotyledon expansion, and also acts on other phototrophic responses, such as leaf expansion and positioning, and stomatal opening and conductance, as well as on the proportion of leaf mass and chlorophyll per leaf area [70,90,91]. Graham et al. [69] also supported previous findings in that responses to light are often species specific.
Several studies have investigated the impact of combining blue and red LED lights on the concentration of pigments in the shoot tissue of microgreens. Unlike photosynthesis, which is primarily influenced by light intensity, the photoresponse is predominantly wavelength dependent [24,28,42,54]. Both red and blue light play crucial roles in optimizing photosynthesis. The action spectrum of the biosynthetic wavelengths that are essential for the production of plant pigments differs between chlorophylls, which absorb strongly in the red (663 and 642 nm) and blue (430 and 453 nm) regions of the spectrum, and the carotenoids, lutein, and b-carotene, which absorb in the blue region (448 and 454 nm). Previous studies have shown that the concentration of phenols can be improved by supplementation with red light and the carotenoids, while ascorbic acid content can be increased by supplementation with blue light, respectively. Far-red LED supplementation promotes carotenoid suppression [42]. Samuolienė et al. showed that intrinsic (such as genotype) or extrinsic (such as environmental conditions) factors can drastically influence the quantity or quality of phytochemicals [42]. However, Ying et al. [72] concluded that the impact of varying the proportions of these light qualities on the composition of various phytochemicals in the microgreens grown indoors is still unclear. The authors showed that 20% blue light provided by LED arrays is ideal for achieving optimal levels of reduced and total ascorbate in all microgreen species that they studied (arugula—Eruca sativa L., ‘Red Russian’ kale—Brassica napus L. subsp. napus var. pabularia (DC.) Alef., and ‘Mizuna’ mustard—Brassica juncea L.), except red cabbage (Brassica oleracea L. var. capitata f. rubra). They also showed that 30% blue light promotes the highest accumulation of total anthocyanin in microgreens of the Brassicaceae family grown indoors, except for mustard.
The light spectral quality also affect other parameters, such as the growth, yield, and nutritional quality of plants [7,30,40]. The blue light stimulates the photomorphogenesis and mechanisms of the stomata opening/closing regulation, as well as biomass accumulation [88]. Kong et al. [75] showed that monochromatic blue light stimulated plant elongation for several species. Ying et al. [30] also observed that blue light promoted hypocotyl elongation and increased the yield and cotyledon area of mustard. The combination of red and far-red spectra along with the particular spectra of blue light can benefit normal plant growth [52].
Wollaeger and Runkle [30] found that plants grown under 25% or more blue light had a lower height than those grown under red light. These, under red light, obtained a greater leaf area and fresh mass compared to those cultivated under 25% or more under blue light. Brazaitytė et al. [40] observed that red light at 638 and 665 nm caused hypocotyl elongation, increased the fresh mass, and decreased the leaf area; although, there was variation between the species observed. Cultivation at 638 nm, compared to 665 nm, promoted better nutritional quality and displayed a greater accumulation of antioxidants, especially of ascorbic acid and β-carotene. Lobiuc et al. [50] found that lighting with blue LEDs induces a greater plant growth and higher concentrations of compounds, such as chlorophyll and anthocyanins. Kyriacou et al. [64] concluded that plant growth parameters, including lutein and beta carotene, are favored by the combination of blue and red LEDs. Monochromatic lights, on the other hand, resulted in the accumulation of some minerals and reduced of the concentration of others.
Kyriacou et al. [64], however, explained that the relationship between plants and their response to light is species dependent, including aspects such as mineral component content. This finding aligns with the observations made by Brazaityté et al. [7] regarding the effects of the different proportions of blue and red light. These studies indicate that the responses of the growth parameters, such as the leaf area, fresh and dry mass, and optical reflectance properties, like chlorophyll, flavonoids, anthocyanins, and carotenoids, vary among the different plant species.
The influence of light quality on growth parameters, particularly hypocotyl elongation, has been studied in the literature [7,19,25,29,31,43,45,73]. These studies explored the potential to manipulate plant growth to either promote elongation for improved harvest or inhibit it to create more compact plants. Kong et al. [19] explained that the regulation of elongation growth involves at least two photoreceptor systems: phytochromes and cryptochromes. Phytochromes comprise red and far-red light receptors (activated by red light and deactivated by far-red light, respectively), and cryptochromes are one of the blue light receptors.
Ying et al. [31] found that fresh and dry yields were not affected by the percentage of blue light for the species tested, except for cabbage, and that responses to the percentage of blue light varied with the plant characteristics and microgreen species. Accordingly, Ying et al. [43] showed that in general, blue light promoted microgreen elongation without compromising the yield and the quality. Kong and Zheng [45] concluded that blue light compared to red can promote elongation as a response to avoid shading. However, Brazaityté et al. [7] observed that increasing the percentage of blue light during plant growth was associated with the reduced elongation of the mustard (Brassica juncea) and kale (Brassica napus) microgreens.
Other studies evaluated the influence of light quality, with emphasis placed on the levels of vitamins, minerals, and/or nutrient absorption in microgreens [7,27,51,53,55,61,68,82]. Brazaityté et al. [7] emphasized that red light has no effect on the mineral nutrient concentration in microgreens. However, they observed that the blue and red light spectra, when combined, have been more effective for the concentration of mineral elements, although the mechanisms that regulate their absorption need to be better clarified.
Kamal et al. [27] pointed to the combination of 20% blue: 10% green: 70% red as the one that presented the best results for the growth of microgreens; while treatment with 80% blue: 20% red are favorable for increasing the vitamin and mineral concentrations. Brazaityté et al. [7] observed that increasing the percentage of blue light during plant growth showed an increase in the accumulation of most nutrients. Toscano et al. [51] showed that blue lighting caused differences between microgreens with regard to the accumulation of phytochemicals, and that the sugar content varied due to the species and not the treatments themselves. Ferrón-Carrillo et al. [61] also emphasized that, among other findings, some responses were species dependent.
Contributions of green wave bands have been less studied in the context of plant development [44,46,56]. Carvalho and Folta [44] assessed whether green wave bands (525 nm), in combination with blue (470 nm) or far-red (730 nm) wavelengths, can modulate the accumulation of anthocyanins in the microgreens. They showed that this effect is dependent on the PPFD and varies depending on the genotype. This evidence corroborates with the findings made by Gerovac et al. [46] and Craver et al. [56], who observed that regardless of the light quality, and under increasing light intensities, the total carotenoid concentrations were lower for the mizuna and mustard microgreens, while anthocyanin concentrations were higher for kohlrabi.
Pioneeringly, the amber LED was evaluated in the biosynthesis of carotenoids, being put forward as a potential new approach for the production of antioxidant-rich microgreens [63]. Alrifai et al. [63] assessed whether combined red, blue, and amber LED light ratios affect carotenoid accumulation in Brassicaceae microgreens. Total and individual carotenoid concentrations (of β-carotene, lutein, α-carotene, neoxanthin, and violaxanthin) increased by 20–44% and 10–55%, respectively, under amber-blue light dependent on the amount of red in most microgreens.
In general, the literature indicates that the absence of ultraviolet radiation (UV-A) negatively affects the nutritional quality of plants, mainly by decreasing the content of phenolic compounds [92,93,94]. However, the use of these wavelengths under controlled environments is still in its early stages [93,94], and research on microgreens has since been conducted to investigate their effects [37,59,62,67,71,77].
Brazaityte et al. [77] observed that, in general, UV-A LED parameters can improve the nutritional quality of mustard microgreens without causing adverse effects on plant growth. However, the impact of UV-A radiation on the investigated substances is species dependent: it negatively affected the accumulation of mineral elements in all plants, except for mizuna; decreased nitrate assimilation in kohlrabi, mizuna, red pak choi, but increased in the broccoli and tatsoi microgreens [32]. In contrast, Gao et al. [67] applied selenium and UV-A radiation treatments to broccoli microgreens, and found that all treatments showed positive results for their evaluated criteria. For the case of treatment with UV-A radiation alone, higher levels of chlorophylls, soluble proteins, and total phenolic compounds were observed. For the combination of both treatments, it was possible to observe the highest levels of anthocyanins, glucoraphanin, total aliphatic glucosinolates, and total glucosinolates. Zhang et al. [71] showed that blue and UV-A lights increased the concentration of total phenolic compounds, flavonoids, and antioxidants when compared to the results obtained with white light.
Lu et al. [59] worked with UV-B lighting, and together with the application of calcium chloride spray, found that treatment with UV-B radiation provided an increase in glucosinolates, along with maintaining the quality of the postharvest-stored plant.
3.5. Effects of the Photoperiod on Microgreen Production
The effect of the photoperiod has also been studied in the context of microgreen cultivation [33], including with different wavelengths, such as the spectrum of UV-A radiation [77], or light blue [79].
Brazaityte et al. [77] investigated whether photoperiods of 10 h or 16 h of light, associated with the light supplementation with UV-A LEDs (366 nm, 390 nm, and 402 nm), affect the growth and phytochemical concentrations of the mustard microgreens. They observed that lutein/zeaxanthin and β-carotene concentrations increased under 366 nm at 10 h, and under 390 nm at 16 h, respectively. The highest accumulation of mineral elements, except for iron, was observed under 402 nm at 16 h. Mlinaric et al. [23] evaluated whether 24 h or 48 h photoperiods affect the levels of antioxidants in chia microgreens, and concluded that lighting for 48 h increased the concentration of bioactive compounds and antioxidants in plants.
Kong et al. [79] investigated whether 16 h of light, compared to a continuous photoperiod (24 h), can alter the effects of blue light on microgreen elongation. The authors showed that stretching was greater under 24 h in many cases, with the exception of mustard, which showed the lowest sensitivity in response to stretching, regardless of the photoperiod. In order to promote the hypocotyl elongation of the rocket and sunflower microgreens, Kong and Zheng [80] applied a period of 5 days without light after germination, followed by 7 and 2 days, respectively, of exposure to a combination of light (85% red: 15% blue and 100 μmol m−2 s−1) with a photoperiod of 24 h. The treatment with the dark period promoted hypocotyl growth for both plants, increased sunflower productivity, and decreased the productivity and cotyledon size for the rocket microgreen.
3.6. Effects of Light Frequency on Microgreen Production
Considered another crucial parameter, the frequency (pulsed light) can be varied in an attempt to reduce energy costs, or the heat generated in lighting, as well as to maintain temperature management [35,48,60,65].
Vaštakaite et al. [48,60] and Vaštakaitė -Kairienė et al. [35] agreed that the effects of pulsed light depend on the frequencies, wavelengths, and microgreen species. Song et al. [65] showed that with shorter pulses (1 h on/1 h off), photomorphogenic development was impaired. However, within seconds (1 s on/1 s off), seedlings developed as if they had received the full 12 h treatments. The dark period, when extended to at least 10 s, did not affect seedling development, whereas the extension to 20 s exhibited only small effects on the characteristics of the seedling. Pulsed light with prolonged dark periods can decrease the energy input from at least 30% to 50%, respectively, without affecting the visible characteristics of the seedlings.
These studies have shown that the implementation of pulsed light strategies has great potential in producing microgreens under artificial lighting. More studies are still needed to investigate the benefits of this technique beyond reducing the energy costs.
4. Conclusions
The analysis of research on the effects of artificial lighting on microgreen growth from the period 2000 to 2021 reveals three distinct phases. Phase 0 (2000–2011) represented the period for which no publications were found, indicating a lack of focus on this topic at that time. Phase 1 (2012–2014) marked the beginning of research on this topic, with six papers. Phase 2 (2015–2018) showed consolidation, with 15 papers and expanding knowledge. Phase 3 (2019–2021) experienced significant growth, with 46 papers, reflecting an increased scientific interest. Lithuania, Canada, and the USA were the leading countries in research output, and mustard was the most studied crop.
The research in the field of artificial lighting for microgreens has covered various aspects, including the use of cold, white, and incandescent fluorescent lamps (HPS), supplementation and replacement of HPS lighting with LED lamps, and the effects of different light properties, such as the quantity (PPFD), quality (wavelengths), frequency (pulsed and continuous light), and photoperiod on the plant responses. Even though these studies have contributed to further understanding the relationship between light and microgreen growth, there are still research gaps present, particularly in the distribution of the environmental factors under the controlled cultivation of these microgreens.
It is clear that microgreens and the application of artificial lighting are research topics on the rise. In particular, in the period from 2019 to 2021, respectively, an optimistic scenario can be seen for the advancement of scientific knowledge on the cultivation of microgreens.
Author Contributions
Conceptualization, H.B.P. and T.Q.Z.C.; methodology, H.B.P. and T.Q.Z.C.; validation, all authors; formal analysis, H.B.P. and T.Q.Z.C.; investigation, H.B.P. and T.Q.Z.C.; resources, H.B.P. and T.Q.Z.C.; data curation, H.B.P. and T.Q.Z.C.; writing—original draft preparation, H.B.P. and T.Q.Z.C.; writing—review and editing, all authors; visualization, all authors; supervision, T.Q.Z.C.; project administration, T.Q.Z.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior—Brasil (CAPES)—Finance Code 001.
Data Availability Statement
All data are available in scientific databases.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Bhaswant, M.; Shanmugam, D.K.; Miyazawa, T.; Abe, C.; Miyazawa, T. Microgreens—A Comprehensive Review of Bioactive Molecules and Health Benefits. Molecules 2023, 28, 867. [Google Scholar] [CrossRef] [PubMed]
- Purquerio, L.F.V.; Calori, A.H.; Moraes, L.A.S.d.; Factor, T.L.; Tivelli, S.W. Produção de baby leaf em bandejas utilizadas para produção de mudas e em hidroponia NFT. In Produção de Mudas de Hortaliças; Nascimento, W.M., Pereira, R.B., Eds.; Embrapa: Brasília, Brazil, 2016; pp. 221–253. ISBN 9788570355799. [Google Scholar]
- Loconsole, D.; Cocetta, G.; Santoro, P.; Ferrante, A. Optimization of LED Lighting and Quality Evaluation of Romaine Lettuce Grown in an Innovative Indoor Cultivation System. Sustainability 2019, 11, 841. [Google Scholar] [CrossRef]
- Silva, L.M.; Cruz, L.P.; Pacheco, V.S.; Machado, E.C.; Purquerio, L.F.V.; Ribeiro, R.V. Energetic Efficiency of Biomass Production Is Affected by Photoperiod in Indoor Lettuce Cultivation. Theor. Exp. Plant Physiol. 2022, 34, 265–276. [Google Scholar] [CrossRef]
- Morrow, R.C. LED Lighting in Horticulture. HortScience 2008, 43, 1947–1950. [Google Scholar] [CrossRef]
- Vetchinnikov, A.A.; Filatov, D.A.; Olonina, S.I.; Kazakov, A.V.; Olonin, I.Y. Influence of the Radiation Intensity of LED Light Sources of the Red-Blue Spectrum on the Yield and Energy Consumption of Microgreens. IOP Conf. Ser. Earth Environ. Sci. 2021, 723, 032046. [Google Scholar] [CrossRef]
- Brazaitytė, A.; Miliauskienė, J.; Vaštakaitė-Kairienė, V.; Sutulienė, R.; Laužikė, K.; Duchovskis, P.; Małek, S. Effect of Different Ratios of Blue and Red Led Light on Brassicaceae Microgreens under a Controlled Environment. Plants 2021, 10, 801. [Google Scholar] [CrossRef] [PubMed]
- Jones-Baumgardt, C.; Llewellyn, D.; Ying, Q.; Zheng, Y. Intensity of Sole-Source Light-Emitting Diodes Affects Growth, Yield, and Quality of Brassicaceae Microgreens. HortScience 2019, 54, 1168–1174. [Google Scholar] [CrossRef]
- Lee, J.S.; Pill, W.G.; Cobb, B.B.; Olszewski, M. Seed Treatments to Advance Greenhouse Establishment of Beet and Chard Microgreens. J. Hortic. Sci. Biotechnol. 2004, 79, 565–570. [Google Scholar] [CrossRef]
- Murphy, C.J.; Llort, K.F.; Pill, W.G. Factors Affecting the Growth of Microgreen Table Beet. Int. J. Veg. Sci. 2010, 16, 253–266. [Google Scholar] [CrossRef]
- Treadwell, D.; Hochmuth, R.; Landrum, L.; Laughlin, W. Microgreens: A New Specialty Crop. Available online: https://edis.ifas.ufl.edu/publication/HS1164 (accessed on 27 February 2022).
- Brentlinger, D.J. New Trends in Hydroponic Crop Production in the U.S. Acta Hortic. 2007, 742, 31–34. [Google Scholar] [CrossRef]
- Roe, N.E. Growing Microgreens: Maybe the Ultimate Specialty Crop! Proc. Fla. State Hortic. Soc. 2006, 119, 289–290. [Google Scholar]
- Janovská, D.; Štočková, L.; Stehno, Z. Evaluation of Buckwheat Sprouts as Microgreens. Acta Agric. Slov. 2010, 95, 157–162. [Google Scholar] [CrossRef]
- Wieth, A.R.; Pinheiro, W.D.; Duarte, T.D.S. Purple Cabbage Microgreens Grown in Different Substrates and Nutritive Solution Concentrations. Rev. Caatinga 2019, 32, 976–985. [Google Scholar] [CrossRef]
- Wieth, A.R.; Pinheiro, W.D.; Da Silva Duarte, T. Commercial Substrates and Nutrient Concentrations in the Production of Arugula Microgreens. Agron. Colomb. 2021, 39, 82–88. [Google Scholar] [CrossRef]
- Sharma, P.; Sharma, A.; Rasane, P.; Dey, A.; Choudhury, A.; Singh, J.; Kaur, S.; Dhawan, K.; Kaur, D. Optimization of a Process for Microgreen and Fruit-Based Functional Beverage. An. Acad. Bras. Cienc. 2020, 92, e20190596. [Google Scholar] [CrossRef] [PubMed]
- Altuner, F.; Tuncturk, R.; Oral, E.; Tuncturk, M. Evaluation of Pigment, Antioxidant Capacity and Bioactive Compounds in Microgreens of Wheat Landraces and Cereals. Chil. J. Agric. Res. 2021, 81, 643–654. [Google Scholar] [CrossRef]
- Kong, Y.; Schiestel, K.; Zheng, Y. Maximum Elongation Growth Promoted as a Shade-Avoidance Response by Blue Light Is Related to Deactivated Phytochrome: A Comparison with Red Light in Four Microgreen Species. Can. J. Plant Sci. 2020, 100, 314–326. [Google Scholar] [CrossRef]
- Kopsell, D.A.; Pantanizopoulos, N.I.; Sams, C.E.; Kopsell, D.E. Shoot Tissue Pigment Levels Increase in “Florida Broadleaf” Mustard (Brassica juncea L.) Microgreens Following High Light Treatment. Sci. Hortic. 2012, 140, 96–99. [Google Scholar] [CrossRef]
- Brazaityte, A.; Vaštakaite, V.; Jankauskiene, J.; Viršile, A.; Samuolienė, G.; Sakalauskiene, S.; Novičkovas, A.; Miliauskiene, J.; Duchovskis, P. Comparison of LED and HPS Illumination Effects on Cultivation of Red Pak Choi Microgreens under Indoor and Greenhouse Conditions. Acta Hortic. 2020, 1287, 395–402. [Google Scholar] [CrossRef]
- Samuolienė, G.; Brazaityte, A.; Sirtautas, R.; Sakalauskiene, S.; Jankauskiene, J.; Duchovskis, P.; Novičkovas, A. The Impact of Supplementary Short-Term Red LED Lighting on the Antioxidant Properties of Microgreens. Acta Hortic. 2012, 956, 649–655. [Google Scholar] [CrossRef]
- Mlinarić, S.; Gvozdić, V.; Vuković, A.; Varga, M.; Vlašiček, I.; Cesar, V.; Begović, L. The Effect of Light on Antioxidant Properties and Metabolic Profile of Chia Microgreens. Appl. Sci. 2020, 10, 5731. [Google Scholar] [CrossRef]
- Kopsell, D.A.; Sams, C.E. Increases in Shoot Tissue Pigments, Glucosinolates, and Mineral Elements in Sprouting Broccoli after Exposure to Short-Duration Blue Light from Light Emitting Diodes. J. Am. Soc. Hortic. Sci. 2013, 138, 31–37. [Google Scholar] [CrossRef]
- Ying, Q.; Kong, Y.; Zheng, Y. Growth and Appearance Quality of Four Microgreen Species under Light- emitting Diode Lights with Different Spectral Combinations. HortScience 2020, 55, 1406–1410. [Google Scholar] [CrossRef]
- Samuolienė, G.; Brazaityte, A.; Jankauskiene, J.; Viršile, A.; Sirtautas, R.; Novičkovas, A.; Sakalauskiene, S.; Sakalauskaite, J.; Duchovskis, P. LED Irradiance Level Affects Growth and Nutritional Quality of Brassica Microgreens. Cent. Eur. J. Biol. 2013, 8, 1241–1249. [Google Scholar] [CrossRef]
- Kamal, K.Y.; Khodaeiaminjan, M.; El-Tantawy, A.A.; Moneim, D.A.; Salam, A.A.; Ash-shormillesy, S.M.A.I.; Attia, A.; Ali, M.A.S.; Herranz, R.; El-Esawi, M.A.; et al. Evaluation of Growth and Nutritional Value of Brassica Microgreens Grown under Red, Blue and Green LEDs Combinations. Physiol. Plant. 2020, 169, 625–638. [Google Scholar] [CrossRef] [PubMed]
- Kopsell, D.A.; Sams, C.E.; Barickman, T.C.; Morrow, R.C. Sprouting Broccoli Accumulate Higher Concentrations of Nutritionally Important Metabolites under Narrow-Band Light-Emitting Diode Lighting. J. Am. Soc. Hortic. Sci. 2014, 139, 469–477. [Google Scholar] [CrossRef]
- Ying, Q.; Kong, Y.; Zheng, Y. Overnight Supplemental Blue, Rather than Far-Red, Light Improves Microgreen Yield and Appearance Quality without Compromising Nutritional Quality during Winter Greenhouse Production. HortScience 2020, 55, 1468–1474. [Google Scholar] [CrossRef]
- Wollaeger, H.M.; Runkle, E.S. Growth of Impatiens, Petunia, Salvia, and Tomato Seedlings under Blue, Green, and Red Light-Emitting Diodes. HortScience 2014, 49, 734–740. [Google Scholar] [CrossRef]
- Ying, Q.; Kong, Y.; Jones-Baumgardt, C.; Zheng, Y. Responses of Yield and Appearance Quality of Four Brassicaceae Microgreens to Varied Blue Light Proportion in Red and Blue Light-Emitting Diodes Lighting. Sci. Hortic. 2020, 259, 108857. [Google Scholar] [CrossRef]
- Brazaityte, A.; Viršile, A.; Jankauskiene, J.; Sakalauskiene, S.; Samuolienė, G.; Sirtautas, R.; Novičkovas, A.; Dabašinskas, L.; Miliauskiene, J.; Vaštakaite, V.; et al. Effect of Supplemental UV-A Irradiation in Solid-State Lighting on the Growth and Phytochemical Content of Microgreens. Int. Agrophys. 2015, 29, 13–22. [Google Scholar] [CrossRef]
- Meas, S.; Luengwilai, K.; Thongket, T. Enhancing Growth and Phytochemicals of Two Amaranth Microgreens by LEDs Light Irradiation. Sci. Hortic. 2020, 265, 109204. [Google Scholar] [CrossRef]
- Brazaityte, A.; Sakalauskiene, S.; Samuolienė, G.; Jankauskiene, J.; Viršile, A.; Novičkovas, A.; Sirtautas, R.; Miliauskiene, J.; Vaštakaite, V.; Dabašinskas, L.; et al. The Effects of LED Illumination Spectra and Intensity on Carotenoid Content in Brassicaceae Microgreens. Food Chem. 2015, 173, 600–606. [Google Scholar] [CrossRef] [PubMed]
- Vaštakaitė-Kairienė, V.; Brazaitytė, A.; Viršilė, A.; Samuolienė, G.; Miliauskienė, J.; Jankauskienė, J.; Duchovskis, P. Pulsed Light-Emitting Diodes for Higher Contents of Mineral Elements in Mustard Microgreens. Acta Hortic. 2020, 1271, 149–154. [Google Scholar] [CrossRef]
- Vaštakaite, V.; Viršile, A. Light—Emitting Diodes (LEDs) for Higher Nutritional Quality of Brassicaceae Microgreens. Res. Rural Dev. 2015, 1, 111–117. [Google Scholar]
- Brazaityte, A.; Viršile, A.; Samuolienė, G.; Vaštakaite-Kairiene, V.; Miliauskiene, J.; Jankauskiene, J.; Duchovskis, P. Effect of Supplemental UV-A Radiation on Mineral Elements Content and Nitrate Variation in Brassica Microgreens. Acta Hortic. 2020, 1296, 675–681. [Google Scholar] [CrossRef]
- Vastakaite, V.; Viršile, A.; Brazaityte, A.; Samuolienė, G.; Jankauskiene, J.; Sirtautas, R.; Novickovas, A.; Dabasinskas, L.; Sakalauskiene, S.; Miliauskiene, J.; et al. The Effect of Blue Light Dosage on Growth and Antioxidant Properties of Brassicaceae Microgreens. In Nordic View to Sustainable Rural Development; Nordic Association of Agricultural Science: Riga, Latvia, 2015; p. 104. [Google Scholar]
- Jones-Baumgardt, C.; Llewellyn, D.; Zheng, Y. Different Microgreen Genotypes Have Unique Growth and Yield Responses to Intensity of Supplemental PAR from Light-Emitting Diodes during Winter Greenhouse Production in Southern Ontario, Canada. HortScience 2020, 55, 156–163. [Google Scholar] [CrossRef]
- Brazaityte, A.; Sakalauskiene, S.; Viršile, A.; Jankauskiene, J.; Samuolienė, G.; Sirtautas, R.; Vastakaite, V.; Miliauskiene, J.; Duchovskis, P.; Noviekovas, A.; et al. The Effect of Short-Term Red Lighting on Brassicaceae Microgreens Grown Indoors. Acta Hortic. 2016, 1123, 177–183. [Google Scholar] [CrossRef]
- Xonti, A.; Hunter, E.; Kulu, N.; Maboeei, P.; Stander, M.; Kossmann, J.; Peters, S.; Loedolff, B. Diversification of Health-Promoting Phytochemicals in Radish (Raphanus raphanistrum) and Kale (Brassica oleracea) Micro-Greens Using High Light Bio-Fortification. Funct. Foods Health Dis. 2020, 10, 65–81. [Google Scholar] [CrossRef]
- Samuolienė, G.; Brazaityte, A.; Viršile, A.; Jankauskiene, J.; Sakalauskiene, S.; Duchovskis, P. Red Light-Dose or Wavelength-Dependent Photoresponse of Antioxidants in Herb Microgreens. PLoS ONE 2016, 11, e0163405. [Google Scholar] [CrossRef]
- Ying, Q.; Kong, Y.; Zheng, Y. Applying Blue Light Alone, or in Combination with Far-Red Light, during Nighttime Increases Elongation without Compromising Yield and Quality of Indoor-Grown Microgreens. HortScience 2020, 55, 876–881. [Google Scholar] [CrossRef]
- Carvalho, S.D.; Folta, K.M. Green Light Control of Anthocyanin Production in Microgreens. Acta Hortic. 2016, 1134, 13–18. [Google Scholar] [CrossRef]
- Kong, Y.; Zheng, Y. Growth and Morphology Responses to Narrow-Band Blue Light and Its Co-Action with Low-Level UVB or Green Light: A Comparison with Red Light in Four Microgreen Species. Environ. Exp. Bot. 2020, 178, 104189. [Google Scholar] [CrossRef]
- Gerovac, J.R.; Craver, J.K.; Boldt, J.K.; Lopez, R.G. Light Intensity and Quality from Sole-Source Light-Emitting Diodes Impact Growth, Morphology, and Nutrient Content of Brassica Microgreens. HortScience 2016, 51, 497–503. [Google Scholar] [CrossRef]
- Johnson, R.E.; Kong, Y.; Zheng, Y. Elongation Growth Mediated by Blue Light Varies with Light Intensities and Plant Species: A Comparison with Red Light in Arugula and Mustard Seedlings. Environ. Exp. Bot. 2020, 169, 103898. [Google Scholar] [CrossRef]
- Vaštakaite, V.; Viršile, A.; Brazaityte, A.; Samuolienė, G.; Jankauskiene, J.; Novičkovas, A.; Duchovskis, P. Pulsed Light-Emitting Diodes for a Higher Phytochemical Level in Microgreens. J. Agric. Food Chem. 2017, 65, 6529–6534. [Google Scholar] [CrossRef]
- Gao, M.; He, R.; Shi, R.; Zhang, Y.; Song, S.; Su, W.; Liu, H. Differential Effects of Low Light Intensity on Broccoli Microgreens Growth and Phytochemicals. Agronomy 2021, 11, 537. [Google Scholar] [CrossRef]
- Lobiuc, A.; Vasilache, V.; Pintilie, O.; Stoleru, T.; Burducea, M.; Oroian, M.; Zamfirache, M.M. Blue and Red LED Illumination Improves Growth and Bioactive Compounds Contents in Acyanic and Cyanic Ocimum basilicum L. Microgreens. Molecules 2017, 22, 2111. [Google Scholar] [CrossRef]
- Toscano, S.; Cavallaro, V.; Ferrante, A.; Romano, D.; Patané, C. Effects of Different Light Spectra on Final Biomass Production and Nutritional Quality of Two Microgreens. Plants 2021, 10, 1584. [Google Scholar] [CrossRef]
- Samuolienė, G.; Viršilė, A.; Brazaitytė, A.; Jankauskienė, J.; Sakalauskienė, S.; Vaštakaitė, V.; Novičkovas, A.; Viškelienė, A.; Sasnauskas, A.; Duchovskis, P. Blue Light Dosage Affects Carotenoids and Tocopherols in Microgreens. Food Chem. 2017, 228, 50–56. [Google Scholar] [CrossRef]
- Maina, S.; Ryu, D.H.; Cho, J.Y.; Jung, D.S.; Park, J.E.; Nho, C.W.; Bakari, G.; Misinzo, G.; Jung, J.H.; Yang, S.H.; et al. Exposure to Salinity and Light Spectra Regulates Glucosinolates, Phenolics, and Antioxidant Capacity of Brassica Carinata l. Microgreens. Antioxidants 2021, 10, 1183. [Google Scholar] [CrossRef]
- Kopsell, D.A.; Sams, C.E.; Morrow, R.C. Interaction of Light Quality and Fertility on Biomass, Shoot Pigmentation and Xanthophyll Cycle Flux in Chinese Kale. J. Sci. Food Agric. 2017, 97, 911–917. [Google Scholar] [CrossRef]
- Giménez, A.; Martínez-Ballesta, M.D.C.; Egea-Gilabert, C.; Gómez, P.A.; Artés-Hernández, F.; Pennisi, G.; Orsini, F.; Crepaldi, A.; Fernández, J.A. Combined Effect of Salinity and Led Lights on the Yield and Quality of Purslane (Portulaca oleracea L.) Microgreens. Horticulturae 2021, 7, 180. [Google Scholar] [CrossRef]
- Craver, J.K.; Gerovac, J.R.; Lopez, R.G.; Kopsell, D.A. Light Intensity and Light Quality from Sole-Source Light-Emitting Diodes Impact Phytochemical Concentrations within Brassica Microgreens. J. Am. Soc. Hortic. Sci. 2017, 142, 3–12. [Google Scholar] [CrossRef]
- Byrdwell, W.C.; Kubzdela, N.; Goldschmidt, R. Changes in Compositions of Galactolipids, Triacylglycerols, and Tocopherols of Lettuce Varieties (Lactuca sativa L.) with Type, Age, and Light Source. J. Food Compos. Anal. 2021, 100, 103631. [Google Scholar] [CrossRef]
- Brazaitytė, A.; Vaštakaitė, V.; Viršilė, A.; Jankauskienė, J.; Samuolienė, G.; Sakalauskienė, S.; Novičkovas, A.; Miliauskienė, J.; Duchovskis, P. Changes in Mineral Element Content of Microgreens Cultivated under Different Lighting Conditions in a Greenhouse. Acta Hortic. 2018, 1227, 507–515. [Google Scholar] [CrossRef]
- Lu, Y.; Dong, W.; Yang, T.; Luo, Y.; Chen, P. Preharvest UVB Application Increases Glucosinolate Contents and Enhances Postharvest Quality of Broccoli Microgreens. Molecules 2021, 26, 3247. [Google Scholar] [CrossRef] [PubMed]
- Vaštakaitė, V.; Viršilė, A.; Brazaitytė, A.; Samuolienė, G.; Miliauskienė, J.; Jankauskienė, J.; Duchovskis, P. Pulsed LED Light Increases the Phytochemical Level of Basil Microgreens. Acta Hortic. 2018, 1227, 579–584. [Google Scholar] [CrossRef]
- Ferrón-Carrillo, F.; Guil-Guerrero, J.L.; González-Fernández, M.J.; Lyashenko, S.; Battafarano, F.; da Cunha-Chiamolera, T.P.L.; Urrestarazu, M. LED Enhances Plant Performance and Both Carotenoids and Nitrates Profiles in Lettuce. Plant Foods Hum. Nutr. 2021, 76, 210–218. [Google Scholar] [CrossRef]
- Zhang, X.; Wei, J.; Tian, J.; Li, N.; Jia, L.; Shen, W.; Cui, J. Enhanced Anthocyanin Accumulation of Immature Radish Microgreens by Hydrogen-Rich Water under Short Wavelength Light. Sci. Hortic. 2019, 247, 75–85. [Google Scholar] [CrossRef]
- Alrifai, O.; Hao, X.; Liu, R.; Lu, Z.; Marcone, M.F.; Tsao, R. LED-Induced Carotenoid Synthesis and Related Gene Expression in Brassica Microgreens. J. Agric. Food Chem. 2021, 69, 4674–4685. [Google Scholar] [CrossRef]
- Kyriacou, M.C.; El-Nakhel, C.; Pannico, A.; Graziani, G.; Soteriou, G.A.; Giordano, M.; Zarrelli, A.; Ritieni, A.; De Pascale, S.; Rouphael, Y. Genotype-Specific Modulatory Effects of Select Spectral Bandwidths on the Nutritive and Phytochemical Composition of Microgreens. Front. Plant Sci. 2019, 10, 1501. [Google Scholar] [CrossRef] [PubMed]
- Song, S.; Kusuma, P.; Carvalho, S.D.; Li, Y.; Folta, K.M. Manipulation of Seedling Traits with Pulsed Light in Closed Controlled Environments. Environ. Exp. Bot. 2019, 166, 103803. [Google Scholar] [CrossRef]
- Harakotr, B.; Srijunteuk, S.; Rithichai, P.; Tabunhan, S. Effects of Light-Emitting Diode Light Irradiance Levels on Yield, Antioxidants and Antioxidant Capacities of Indigenous Vegetable Microgreens. Sci. Technol. Asia 2019, 24, 59–66. [Google Scholar] [CrossRef]
- Gao, M.; He, R.; Shi, R.; Li, Y.; Song, S.; Zhang, Y.; Su, W.; Liu, H. Combination of Selenium and Uva Radiation Affects Growth and Phytochemicals of Broccoli Microgreens. Molecules 2021, 26, 4646. [Google Scholar] [CrossRef]
- Tantharapornrerk, N.; Vichitsoonthonkul, T.; Techavuthiporn, C.; Photchanachai, S. Growth and Antioxidant System of Chinese Kale Microgreens in Response to Different Illumination of Light Sources. N. Z. J. Crop Hortic. Sci. 2023, 51, 108–122. [Google Scholar] [CrossRef]
- Graham, T.; Yorio, N.; Zhang, P.; Massa, G.; Wheeler, R. Early Seedling Response of Six Candidate Crop Species to Increasing Levels of Blue Light. Life Sci. Sp. Res. 2019, 21, 40–48. [Google Scholar] [CrossRef]
- Jones-Baumgardt, C.; Ying, Q.; Zheng, Y.; Bozzo, G.G. The Growth and Morphology of Microgreens Is Associated with Modified Ascorbate and Anthocyanin Profiles in Response to the Intensity of Sole-Source Light-Emitting Diodes. Can. J. Plant Sci. 2021, 101, 212–228. [Google Scholar] [CrossRef]
- Zhang, X.; Bian, Z.; Li, S.; Chen, X.; Lu, C. Comparative Analysis of Phenolic Compound Profiles, Antioxidant Capacities, and Expressions of Phenolic Biosynthesis-Related Genes in Soybean Microgreens Grown under Different Light Spectra. J. Agric. Food Chem. 2019, 67, 13577–13588. [Google Scholar] [CrossRef]
- Ying, Q.; Jones-Baumgardt, C.; Zheng, Y.; Bozzo, G. The Proportion of Blue Light from Light-Emitting Diodes Alters Microgreen Phytochemical Profiles in a Species-Specific Manner. HortScience 2021, 56, 13–20. [Google Scholar] [CrossRef]
- Kong, Y.; Schiestel, K.; Zheng, Y. Pure Blue Light Effects on Growth and Morphology Are Slightly Changed by Adding Low-Level UVA or Far-Red Light: A Comparison with Red Light in Four Microgreen Species. Environ. Exp. Bot. 2019, 157, 58–68. [Google Scholar] [CrossRef]
- Truzzi, F.; Whittaker, A.; Roncuzzi, C.; Saltari, A.; Levesque, M.P.; Dinelli, G. Microgreens: Functional Food with Antiproliferative Cancer Properties Influenced by Light. Foods 2021, 10, 1690. [Google Scholar] [CrossRef]
- Lau, T.Q.; Tang, V.T.H.; Kansedo, J. Influence of Soil and Light Condition on the Growth and Antioxidants Content of Amaranthus Cruentus (Red amaranth) Microgreen. IOP Conf. Ser. Mater. Sci. Eng. 2019, 495, 012051. [Google Scholar] [CrossRef]
- Rusu, T.; Moraru, P.I.; Mintas, O.S. Influence of Environmental and Nutritional Factors on the Development of Lettuce (Lactuca sativa L.) Microgreens Grown in a Hydroponic System: A Review. Not. Bot. Horti Agrobot. Cluj Napoca 2021, 49, 12427. [Google Scholar] [CrossRef]
- Brazaitytė, A.; Viršilė, A.; Samuolienė, G.; Vaštakaitė-Kairienė, V.; Jankauskienė, J.; Miliauskienė, J.; Novičkovas, A.; Duchovskis, P. Response of Mustard Microgreens to Different Wavelengths and Durations of UV-A LEDs. Front. Plant Sci. 2019, 10, 1153. [Google Scholar] [CrossRef] [PubMed]
- Rusu, T.; Cowden, R.J.; Moraru, P.I.; Maxim, M.A.; Ghaley, B.B. Overview of Multiple Applications of Basil Species and Cultivars and the Effects of Production Environmental Parameters on Yields and Secondary Metabolites in Hydroponic Systems. Sustainability 2021, 13, 11332. [Google Scholar] [CrossRef]
- Kong, Y.; Kamath, D.; Zheng, Y. Blue versus Red Light Can Promote Elongation Growth Independent of Photoperiod: A Study in Four Brassica Microgreens Species. HortScience 2019, 54, 1955–1961. [Google Scholar] [CrossRef]
- Kong, Y.; Zheng, Y. Early-Stage Dark Treatment Promotes Hypocotyl Elongation Associated with Varying Effects on Yield and Quality in Sunflower and Arugula Microgreens. Can. J. Plant Sci. 2021, 101, 954–961. [Google Scholar] [CrossRef]
- Brazaitytė, A.; Vaštakaitė-Kairienė, V.; Jankauskienė, J.; Viršilė, A.; Samuolienė, G.; Sakalauskienė, S.; Novičkovas, A.; Miliauskienė, J.; Duchovskis, P. Effect of Blue Light Percentage on Mineral Elements Content in Brassica Microgreens. Acta Hortic. 2020, 1271, 119–125. [Google Scholar] [CrossRef]
- Bantis, F. Light Spectrum Differentially Affects the Yield and Phytochemical Content of Microgreen Vegetables in a Plant Factory. Plants 2021, 10, 2182. [Google Scholar] [CrossRef]
- Samuolienė, G.; Miliauskienė, J.; Kazlauskas, A.; Viršilė, A. Growth Stage Specific Lighting Spectra Affect Photosynthetic Performance, Growth and Mineral Element Contents in Tomato. Agronomy 2021, 11, 901. [Google Scholar] [CrossRef]
- Cathey, H.M.; Campbell, L.E. Security Lighting and Its Impact on the Landscape. Arboric. Urban For. 1975, 1, 181–187. [Google Scholar] [CrossRef]
- Zhou, Y.; Singh, B.R. Red Light Stimulates Flowering and Anthocyanin Biosynthesis in American Cranberry. Plant Growth Regul. 2002, 38, 165–171. [Google Scholar] [CrossRef]
- Li, Q.; Kubota, C. Effects of Supplemental Light Quality on Growth and Phytochemicals of Baby Leaf Lettuce. Environ. Exp. Bot. 2009, 67, 59–64. [Google Scholar] [CrossRef]
- Wu, M.C.; Hou, C.Y.; Jiang, C.M.; Wang, Y.T.; Wang, C.Y.; Chen, H.H.; Chang, H.M. A Novel Approach of LED Light Radiation Improves the Antioxidant Activity of Pea Seedlings. Food Chem. 2007, 101, 1753–1758. [Google Scholar] [CrossRef]
- Ilieva, I.; Ivanova, T.; Naydenov, Y.; Dandolov, I.; Stefanov, D. Plant Experiments with Light-Emitting Diode Module in Svet Space Greenhouse. Adv. Sp. Res. 2010, 46, 840–845. [Google Scholar] [CrossRef]
- Anjana, S.U.; Iqbal, M. Factors responsible for nitrate accumulation: A review. In Sustainable Agriculture; Lichtfouse, E., Navarrete, M., Debaeke, P., Véronique, S., Alberola, C., Eds.; Springer: Cham, Switzerland, 2009; pp. 533–554. ISBN 9789048126668. [Google Scholar]
- Kami, C.; Lorrain, S.; Hornitschek, P.; Fankhauser, C. Light-Regulated Plant Growth and Development. Curr. Top. Dev. Biol. 2010, 91, 29–66. [Google Scholar] [CrossRef]
- Hogewoning, S.W.; Trouwborst, G.; Maljaars, H.; Poorter, H.; van Ieperen, W.; Harbinson, J. Blue Light Dose-Responses of Leaf Photosynthesis, Morphology, and Chemical Composition of Cucumis Sativus Grown under Different Combinations of Red and Blue Light. J. Exp. Bot. 2010, 61, 3107–3117. [Google Scholar] [CrossRef]
- Iwai, M.; Ohta, M.; Tsuchiya, H.; Suzuki, T. Enhanced Accumulation of Caffeic Acid, Rosmarinic Acid and Luteolin-Glucoside in Red Perilla Cultivated under Red Diode Laser and Blue LED Illumination Followed by UV-A Irradiation. J. Funct. Foods 2010, 2, 66–70. [Google Scholar] [CrossRef]
- Wargent, J.J.; Jordan, B.R. From Ozone Depletion to Agriculture: Understanding the Role of UV Radiation in Sustainable Crop Production. New Phytol. 2013, 197, 1058–1076. [Google Scholar] [CrossRef]
- Neugart, S.; Schreiner, M. UVB and UVA as Eustressors in Horticultural and Agricultural Crops. Sci. Hortic. 2018, 234, 370–381. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).