Untargeted Metabolomics Analysis of Liquid Endosperm of Cocos nucifera L. at Three Stages of Maturation Evidenced Differences in Metabolic Regulation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Collection and Storage of Samples
2.2. Extraction and Sample Preparation for LC-MS Analyses
2.3. Metabolomic Analysis on UPLC-ESI-MS-QTOF
2.4. Data Analysis
3. Results
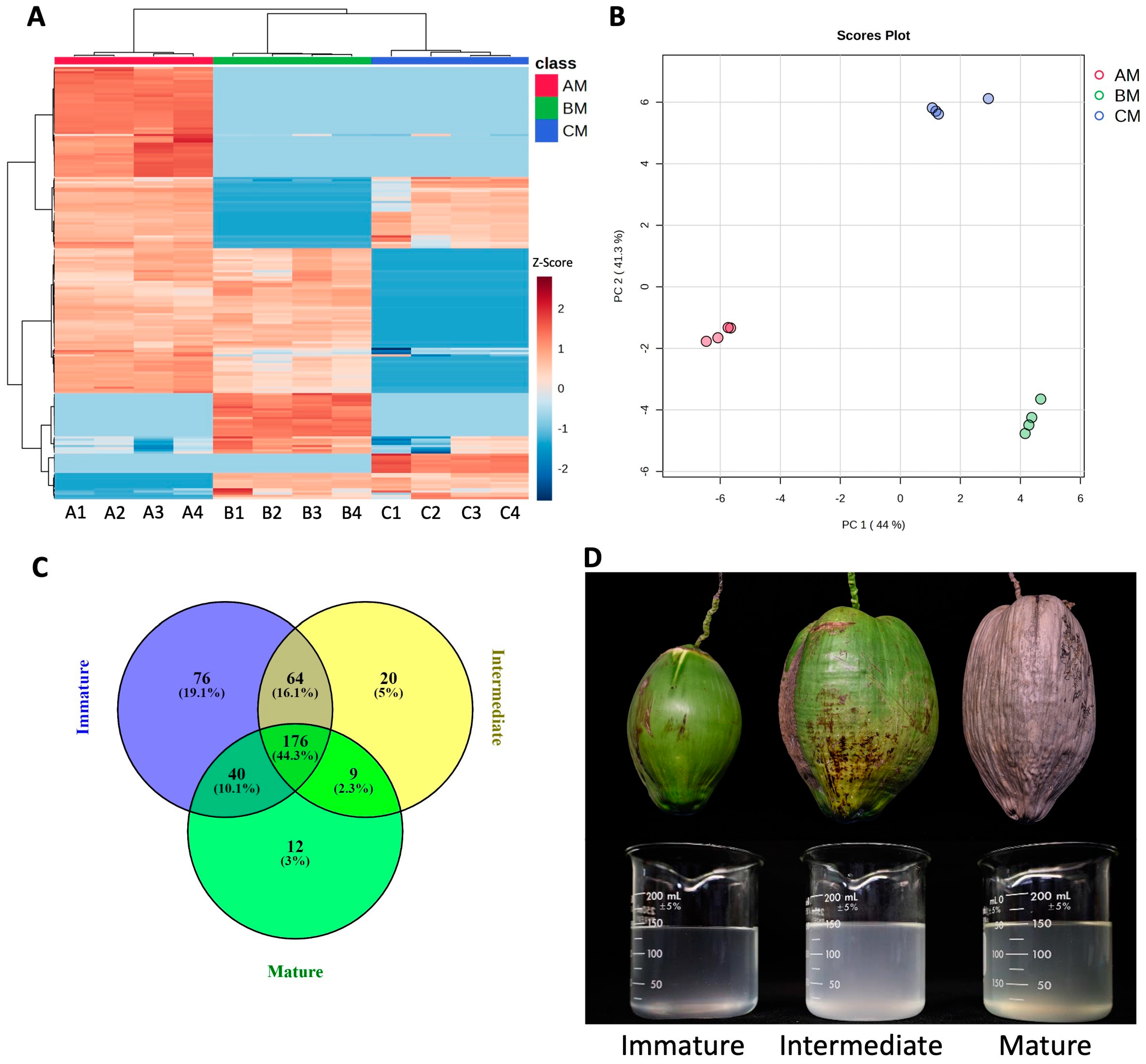
3.1. Cluster Analysis and PCA
3.2. Enrichment of Metabolic Pathways during Ripening
3.3. Annotated Chemical Classes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Beveridge, F.C.; Kalaipandian, S.; Yang, C.; Adkins, S.W. Fruit Biology of Coconut (Cocos nucifera L.). Plants 2022, 11, 3293. [Google Scholar] [CrossRef]
- Sankararaman, S.; Sferra, T.J. Are We Going Nuts on Coconut Oil? Curr. Nutr. Rep. 2018, 7, 107–115. [Google Scholar] [CrossRef]
- Tzec-Simá, M.; Félix, J.W.; Granados-Alegría, M.; Aparicio-Ortiz, M.; Juárez-Monroy, D.; Mayo-Ruiz, D.; Vivas-López, S.; Gómez-Tah, R.; Canto-Canché, B.; Berezovski, M.V.; et al. Potential of Omics to Control Diseases and Pests in the Coconut Tree. Agronomy 2022, 12, 3164. [Google Scholar] [CrossRef]
- Kumar, M.; Saini, S.S.; Agrawal, P.K.; Roy, P.; Sircar, D. Nutritional and Metabolomics Characterization of the Coconut Water at Different Nut Developmental Stages. J. Food Compos. Anal. 2021, 96, 103738. [Google Scholar] [CrossRef]
- Rajesh, M.K.; Sabana, A.A.; Rachana, K.E.; Rahman, S.; Jerard, B.A.; Karun, A. Genetic Relationship and Diversity among Coconut (Cocos nucifera L.) Accessions Revealed through SCoT Analysis. 3 Biotech 2015, 5, 999–1006. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gurr, G.M.; Johnson, A.C.; Ash, G.J.; Wilson, B.A.L.; Ero, M.M.; Pilotti, C.A.; Dewhurst, C.F.; You, M.S. Coconut Lethal Yellowing Diseases: A Phytoplasma Threat to Palms of Global Economic and Social Significance. Front. Plant Sci. 2016, 7, 1521. [Google Scholar] [CrossRef] [Green Version]
- Roongsattham, P.; Morcillo, F.; Fooyontphanich, K.; Jantasuriyarat, C.; Tragoonrung, S.; Amblard, P.; Collin, M.; Mouille, G.; Verdeil, J.-L.; Tranbarger, T.J. Cellular and Pectin Dynamics during Abscission Zone Development and Ripe Fruit Abscission of the Monocot Oil Palm. Front. Plant Sci. 2016, 7, 540. [Google Scholar] [CrossRef] [Green Version]
- Salazar Iribe, A.; Gamboa de Buen, A. Importancia de las pectinas en la dinámica de la pared celular durante el desarrollo vegetal. REB Rev. Educ. Bioquím. 2013, 32, 67–75. [Google Scholar]
- Naresh Kumar, S.; Hebbar, K.; Bai, K.; Rajagopal, V. Physiology and Biochemistry. In The Coconut Palm (Cocos nucifera L.)—Research and Development Perspectives; Springer: Berlin/Heidelberg, Germany, 2018; pp. 443–488. ISBN 9789811327537. [Google Scholar]
- Kaur, K.; Chhikara, N.; Sharma, P.; Garg, M.K.; Panghal, A. Coconut Meal: Nutraceutical Importance and Food Industry Application. Foods Raw Mater. 2019, 7, 419–427. [Google Scholar] [CrossRef]
- Angeles, J.; Lado, J.; Pascual, E.; Cueto, C.; Laurena, A.; Laude, R. Towards the Understanding of Important Coconut Endosperm Phenotypes: Is There an Epigenetic Control? Agronomy 2018, 8, 225. [Google Scholar] [CrossRef] [Green Version]
- Ramanandam, G.; Kumar, K.R.; Padma, E.; Kalpana, M.; Maheswarappa, H.P. Potential Coconut (Cocos nucifera) Hybrids for Yield and Quality for Coastal Region of Andhra Pradesh (India). Indian J. Agric. Sci. 2017, 87, 1073–1076. [Google Scholar] [CrossRef]
- Mardesci, H.; Santosa; Nazir, N.; Hadiguna, R.A. Determination of Value-Added and Contributing Organization in the Development of Coconut Water-Based Agro Industry. IOP Conf. Ser. Earth Environ. Sci. 2021, 709, 012062. [Google Scholar] [CrossRef]
- Arana-Paredes, M.; del Aguila, S.R.; Ruíz, J.R.C. Germinación in vitro de Dianthus caryophyllus en diferentes medios de cultivo. Sagasteguiana 2015, 3, 55–66. [Google Scholar]
- Prando, M.A.S.; Chiavazza, P.; Faggio, A.; Contessa, C. Effect of Coconut Water and Growth Regulator Supplements on in Vitro Propagation of Corylus Avellana L. Sci. Hortic. 2014, 171, 91–94. [Google Scholar] [CrossRef]
- Yong, J.W.H.; Ge, L.; Ng, Y.F.; Tan, S.N. The Chemical Composition and Biological Properties of Coconut (Cocos nucifera L.) Water. Molecules 2009, 14, 5144–5164. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reddy, P.; Kumar, K.; Mohanalakshmi, T.; Kiran, S. Tender Coconut Water Uses, Health Benefits, Good Nutritive Value and Antioxidant Capacity. Indian J. Public Health Res. Dev. 2018, 9, 184. [Google Scholar] [CrossRef]
- Astikasari, N.D. The Effect of Coconut Water Against Decreasing Dehydration Degrees in Children Ages 5-10 Years with Diare. Health Notions 2017, 1, 126–129. [Google Scholar] [CrossRef]
- Valli, S.A.; Gowrie, S.U. Coconut Sprouts as Phytomedicine: An Approach towards the Development of Nutraceuticals. In Phytomedicine; CRC Press: Boca Raton, FL, USA, 2020; ISBN 978-1-00-301489-8. [Google Scholar]
- Patel, R.M.; Jiang, P.; Asplin, J.; Granja, I.; Capretz, T.; Osann, K.; Okhunov, Z.; Landman, J.; Clayman, R.V. Coconut Water: An Unexpected Source of Urinary Citrate. Biomed. Res. Int. 2018, 2018, 3061742. [Google Scholar] [CrossRef] [Green Version]
- Hussein, Z.; Fawole, O.A.; Opara, U.L. Preharvest Factors Influencing Bruise Damage of Fresh Fruits—A Review. Sci. Hortic. 2018, 229, 45–58. [Google Scholar] [CrossRef]
- Wu, Q.; Zhao, X.; Chen, C.; Zhang, Z.; Yu, F. Metabolite Profiling and Classification of Developing Styrax Tonkinensis Kernels. Metabolites 2020, 10, 21. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.; Duan, Y.; Hu, Y.; Li, W.; Sun, D.; Hu, H.; Xie, J. Transcriptome Analysis of Atemoya Pericarp Elucidates the Role of Polysaccharide Metabolism in Fruit Ripening and Cracking after Harvest. BMC Plant Biol. 2019, 19, 219. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruan, Y.-L.; Patrick, J.W.; Bouzayen, M.; Osorio, S.; Fernie, A.R. Molecular Regulation of Seed and Fruit Set. Trends Plant Sci. 2012, 17, 656–665. [Google Scholar] [CrossRef] [Green Version]
- Gopala Krishna, A.G.; Gaurav, R.; Bhatnagar, A.; Kumar, P.; Chandrashekar, P. Coconut Oil: Chemistry, Production and Its Applications–A Review. Indian Coconut J. 2010, 53, 15–27. [Google Scholar]
- White, R.A.; Rivas-Ubach, A.; Borkum, M.I.; Köberl, M.; Bilbao, A.; Colby, S.M.; Hoyt, D.W.; Bingol, K.; Kim, Y.-M.; Wendler, J.P.; et al. The State of Rhizospheric Science in the Era of Multi-Omics: A Practical Guide to Omics Technologies. Rhizosphere 2017, 3, 212–221. [Google Scholar] [CrossRef]
- Garcia, C.J.; García-Villalba, R.; Garrido, Y.; Gil, M.I.; Tomás-Barberán, F.A. Untargeted Metabolomics Approach Using UPLC-ESI-QTOF-MS to Explore the Metabolome of Fresh-Cut Iceberg Lettuce. Metabolomics 2016, 12, 138. [Google Scholar] [CrossRef]
- Kumar, S.N. Variability in Coconut (Cocos nucifera L.) Germplasm and Hybrids for Fatty Acid Profile of Oil. J. Agric. Food Chem. 2011, 59, 13050–13058. [Google Scholar] [CrossRef]
- Chen, W.; Zhang, G.; Chen, W.; Zhong, Q.; Chen, H. Metabolomic Profiling of Matured Coconut Water during Post-Harvest Storage Revealed Discrimination and Distinct Changes in Metabolites. RSC Adv. 2018, 8, 31396–31405. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, W.; Chen, H.; Zhong, Q.; Yun, Y.; Chen, W. Metabolomics Analysis of the Deterioration Mechanism and Storage Time Limit of Tender Coconut Water during Storage. Foods 2020, 9, 46. [Google Scholar] [CrossRef] [Green Version]
- Mahayothee, B.; Koomyart, I.; Khuwijitjaru, P.; Siriwongwilaichat, P.; Nagle, M.; Müller, J. Phenolic Compounds, Antioxidant Activity, and Medium Chain Fatty Acids Profiles of Coconut Water and Meat at Different Maturity Stages. Int. J. Food Prop. 2016, 19, 2041–2051. [Google Scholar] [CrossRef] [Green Version]
- Perera, P.I.P.; Hocher, V.; Weerakoon, L.K.; Yakandawala, D.M.D.; Fernando, S.C.; Verdeil, J.-L. Early Inflorescence and Floral Development in Cocos nucifera L. (Arecaceae: Arecoideae). S. Afr. J. Bot. 2010, 76, 482–492. [Google Scholar] [CrossRef] [Green Version]
- Islas-Flores, I.; Oropeza, C.; Hernández-Sotomayor, S.M.T. Protein Phosphorylation during Coconut Zygotic Embryo Development1. Plant Physiol. 1998, 118, 257–263. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xia, J.; Wishart, D.S. Using MetaboAnalyst 3.0 for Comprehensive Metabolomics Data Analysis. CP Bioinform. 2016, 55, 14.10. 1–14.10. 91. [Google Scholar] [CrossRef] [PubMed]
- Djoumbou Feunang, Y.; Eisner, R.; Knox, C.; Chepelev, L.; Hastings, J.; Owen, G.; Fahy, E.; Steinbeck, C.; Subramanian, S.; Bolton, E.; et al. ClassyFire: Automated Chemical Classification with a Comprehensive, Computable Taxonomy. J. Cheminforma. 2016, 8, 61. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murthy, H.N.; Lee, E.-J.; Paek, K.-Y. Production of Secondary Metabolites from Cell and Organ Cultures: Strategies and Approaches for Biomass Improvement and Metabolite Accumulation. Plant Cell Tiss. Organ Cult. 2014, 118, 1–16. [Google Scholar] [CrossRef]
- Li, X.; Li, C.; Sun, J.; Jackson, A. Dynamic Changes of Enzymes Involved in Sugar and Organic Acid Level Modification during Blueberry Fruit Maturation. Food Chem. 2020, 309, 125617. [Google Scholar] [CrossRef]
- Umer, M.J.; Bin Safdar, L.; Gebremeskel, H.; Zhao, S.; Yuan, P.; Zhu, H.; Kaseb, M.O.; Anees, M.; Lu, X.; He, N.; et al. Identification of Key Gene Networks Controlling Organic Acid and Sugar Metabolism during Watermelon Fruit Development by Integrating Metabolic Phenotypes and Gene Expression Profiles. Hortic. Res. 2020, 7, 193. [Google Scholar] [CrossRef]
- Venskutonis, P.R.; Kraujalis, P. Nutritional Components of Amaranth Seeds and Vegetables: A Review on Composition, Properties, and Uses: Nutritional Components of Amaranth Seeds and Vegetables. Compr. Rev. Food Sci. Food Saf. 2013, 12, 381–412. [Google Scholar] [CrossRef]
- Kumar, M.; Agrawal, P.K.; Roy, P.; Sircar, D. GC-MS-Based Metabolomics Reveals Dynamic Changes in the Nutritionally Important Metabolites in Coconut Meat during Nut Maturation. J. Food Compos. Anal. 2022, 114, 104869. [Google Scholar] [CrossRef]
- Li, J.; Htwe, Y.M.; Wang, Y.; Yang, Y.; Wu, Y.; Li, D.; Kareem, A.; Wang, R. Analysis of Sugars and Fatty Acids during Haustorium Development and Seedling Growth of Coconut. Agron. J. 2019, 111, 2341–2349. [Google Scholar] [CrossRef]
- Pan, Y.-Y.; Chen, Y.-C.; Chang, W.C.-W.; Ma, M.-C.; Liao, P.-C. Visualization of Statistically Processed LC-MS-Based Metabolomics Data for Identifying Significant Features in a Multiple-Group Comparison. Chemom. Intell. Lab. Syst. 2021, 210, 104271. [Google Scholar] [CrossRef]
- Wang, X.; Wu, L.; Qiu, J.; Qian, Y.; Wang, M. Comparative Metabolomic Analysis of the Nutritional Aspects from Ten Cultivars of the Strawberry Fruit. Foods 2023, 12, 1153. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Huang, Y.; Wu, W.; Zhu, C.; Zhang, R.; Chen, J.; Zeng, J. Metabolomics Analysis of the Peels of Different Colored Citrus Fruits (Citrus reticulata Cv. ‘Shatangju’) During the Maturation Period Based on UHPLC-QQQ-MS. Molecules 2020, 25, 396. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, J.; Vidyarthi, S.K.; Bai, W.; Pan, Z. Nutritional Constituents, Health Benefits and Processing of Rosa Roxburghii: A Review. J. Funct. Foods 2019, 60, 103456. [Google Scholar] [CrossRef]
- Yanes, O.; Tautenhahn, R.; Patti, G.J.; Siuzdak, G. Expanding Coverage of the Metabolome for Global Metabolite Profiling. Anal. Chem. 2011, 83, 2152–2161. [Google Scholar] [CrossRef] [Green Version]
- Alseekh, S.; Fernie, A.R. Expanding Our Coverage: Strategies to Detect a Greater Range of Metabolites. Curr. Opin. Plant Biol. 2023, 73, 102335. [Google Scholar] [CrossRef]
- Tang, Y.; Lv, X.; Liu, Y.; Cui, D.; Wu, Y. Metabonomics Study in Mice with Learning and Memory Impairment on the Intervention of Essential Oil Extracted from Cinnamomum Camphora Chvar. Borneol. Front. Pharmacol. 2022, 13, 770411. [Google Scholar] [CrossRef] [PubMed]
- Félix, J.W.; Granados-Alegría, M.I.; Gómez-Tah, R.; Tzec-Simá, M.; Ruíz-May, E.; Canto-Canché, B.; Zamora-Briseño, J.A.; Bojórquez-Velázquez, E.; Oropeza-Salín, C.; Islas-Flores, I. Proteome Landscape during Ripening of Solid Endosperm from Two Different Coconut Cultivars Reveals Contrasting Carbohydrate and Fatty Acid Metabolic Pathway Modulation. Int. J. Mol. Sci. 2023, 24, 10431. [Google Scholar] [CrossRef]
- Henry, R.J.; Furtado, A.; Rangan, P. Pathways of Photosynthesis in Non-Leaf Tissues. Biology 2020, 9, 438. [Google Scholar] [CrossRef]
- Qin, C.; Ahanger, M.A.; Zhou, J.; Ahmed, N.; Wei, C.; Yuan, S.; Ashraf, M.; Zhang, L. Beneficial Role of Acetylcholine in Chlorophyll Metabolism and Photosynthetic Gas Exchange in Nicotiana benthamiana Seedlings under Salinity Stress. Plant Biol. J. 2020, 22, 357–365. [Google Scholar] [CrossRef]
- Toleco, M.; Naake, T.; Zhang, Y.; Heazlewood, J.; Fernie, A.R. Plant Mitochondrial Carriers: Molecular Gatekeepers That Help to Regulate Plant Central Carbon Metabolism. Plants 2020, 9, 117. [Google Scholar] [CrossRef] [Green Version]
- Jackson, J.C.; Gordon, A.; Wizzard, G.; McCook, K.; Rolle, R. Changes in Chemical Composition of Coconut (Cocos nucifera) Water during Maturation of the Fruit. J. Sci. Food Agric. 2004, 84, 1049–1052. [Google Scholar] [CrossRef]
- Durán-Soria, S.; Pott, D.M.; Osorio, S.; Vallarino, J.G. Sugar Signaling During Fruit Ripening. Front. Plant Sci. 2020, 11, 564917. [Google Scholar] [CrossRef] [PubMed]
- Ghaffari, H.; Tadayon, M.R.; Nadeem, M.; Cheema, M.; Razmjoo, J. Proline-Mediated Changes in Antioxidant Enzymatic Activities and the Physiology of Sugar Beet under Drought Stress. Acta Physiol. Plant 2019, 41, 23. [Google Scholar] [CrossRef]
- Zhao, S.; Garcia, D.; Zhao, Y.; Huang, D. Hydro-Electro Hybrid Priming Promotes Carrot (Daucus Carota L.) Seed Germination by Activating Lipid Utilization and Respiratory Metabolism. Int. J. Mol. Sci. 2021, 22, 11090. [Google Scholar] [CrossRef] [PubMed]
- Chew, S.Y.; Chee, W.J.Y.; Than, L.T.L. The Glyoxylate Cycle and Alternative Carbon Metabolism as Metabolic Adaptation Strategies of Candida glabrata: Perspectives from Candida albicans and Saccharomyces cerevisiae. J. Biomed. Sci. 2019, 26, 52. [Google Scholar] [CrossRef] [PubMed]
- Walker, R.P.; Famiani, F. Organic Acids in Fruits: Metabolism, Functions and Contents. In Horticultural Reviews; Warrington, I., Ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2018; pp. 371–430. ISBN 978-1-119-43107-7. [Google Scholar]
- Limbad, M.; Gutierrez-Maddox, N.; Hamid, N.; Kantono, K.; Liu, T.; Young, T. Microbial and Chemical Changes during Fermentation of Coconut Water Kefir Beverage. Appl. Sci. 2023, 13, 7257. [Google Scholar] [CrossRef]
- Hildebrandt, T.M.; Nunes Nesi, A.; Araújo, W.L.; Braun, H.-P. Amino Acid Catabolism in Plants. Mol. Plant 2015, 8, 1563–1579. [Google Scholar] [CrossRef] [Green Version]
- Han, M.; Zhang, C.; Suglo, P.; Sun, S.; Wang, M.; Su, T. L-Aspartate: An Essential Metabolite for Plant Growth and Stress Acclimation. Molecules 2021, 26, 1887. [Google Scholar] [CrossRef]
- Zhang, Z.C.; Yang, T.X.; Wei, Z.M.; Li, Y.; Wei, Y.Q.; Chen, X.M.; Wang, L.Q. Effects of Exogenous Protein-like Precursors on Humification Process during Lignocellulose-like Biomass Composting Amino Acids as the Key Linker to Promote Humification Process. Bioresour. Technol. 2019, 291, 121882. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.-J.; Hou, L.-P.; Bao, J.-J.; Wang, L.-J.; Chen, X.-Y. Sphingolipid Metabolism, Transport, and Functions in Plants: Recent Progress and Future Perspectives. Plant Commun. 2021, 2, 100214. [Google Scholar] [CrossRef]
- Luttgeharm, K.D.; Kimberlin, A.N.; Cahoon, R.E.; Cerny, R.L.; Napier, J.A.; Markham, J.E.; Cahoon, E.B. Sphingolipid Metabolism Is Strikingly Different between Pollen and Leaf in Arabidopsis as Revealed by Compositional and Gene Expression Profiling. Phytochemistry 2015, 115, 121–129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fonseca, A.M.D.; Bizerra, A.M.C.; Souza, J.S.N.D.; Monte, F.J.Q.; Oliveira, M.D.C.F.D.; Mattos, M.C.D.; Cordell, G.A.; Braz-Filho, R.; Lemos, T.L.G. Constituents and Antioxidant Activity of Two Varieties of Coconut Water (Cocos nucifera L.). Rev. Bras. Farm. 2009, 19, 193–198. [Google Scholar] [CrossRef]
- Cunha, A.G.; Alves Filho, E.G.; Silva, L.M.A.; Ribeiro, P.R.V.; Rodrigues, T.H.S.; Brito, E.S.D.; Miranda, M.R.A.D. Chemical Composition of Thermally Processed Coconut Water Evaluated by GC–MS, UPLC-HRMS, and NMR. Food Chem. 2020, 324, 126874. [Google Scholar] [CrossRef] [PubMed]
- Serra, J.L.; Rodrigues, A.M.D.C.; De Freitas, R.A.; Meirelles, A.J.D.A.; Darnet, S.H.; Silva, L.H.M.D. Alternative Sources of Oils and Fats from Amazonian Plants: Fatty Acids, Methyl Tocols, Total Carotenoids and Chemical Composition. Food Res. Int. 2019, 116, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Rogowska, A.; Stpiczyńska, M.; Pączkowski, C.; Szakiel, A. The Influence of Exogenous Jasmonic Acid on the Biosynthesis of Steroids and Triterpenoids in Calendula officinalis Plants and Hairy Root Culture. Int. J. Mol. Sci. 2022, 23, 12173. [Google Scholar] [CrossRef]
- Alatawi, K.A.; Alshubaily, F.A. Coconut Products Alleviate Hyperglycaemic, Hyperlipidimic and Nephropathy Indices in Streptozotocin-Induced Diabetic Wistar Rats. Saudi J. Biol. Sci. 2021, 28, 4224–4231. [Google Scholar] [CrossRef]
- Zhang, Y.; Butelli, E.; Martin, C. Engineering Anthocyanin Biosynthesis in Plants. Curr. Opin. Plant Biol. 2014, 19, 81–90. [Google Scholar] [CrossRef]
- Karouw, S.; Mawardi, S.; Miftahorrachman; Pandin, D.S.; Kumaunang, J.; Matana, Y.; Santosa, B.; Manaroinsong, E.; Tenda, E.T. Physicochemical and Phytochemical Characteristics of Exotic Cungap Red Coconut. IOP Conf. Ser. Earth Environ. Sci. 2020, 418, 012036. [Google Scholar] [CrossRef]
- Wei, J.; Zhao, M.; Meng, K.; Xia, G.; Pan, Y.; Li, C.; Zhang, W. The Diuretic Effects of Coconut Water by Suppressing Aquaporin and Renin–Angiotensin–Aldosterone System in Saline-Loaded Rats. Front. Nutr. 2022, 9, 930506. [Google Scholar] [CrossRef]
- Shen, N.; Wang, T.; Gan, Q.; Liu, S.; Wang, L.; Jin, B. Plant Flavonoids: Classification, Distribution, Biosynthesis, and Antioxidant Activity. Food Chem. 2022, 383, 132531. [Google Scholar] [CrossRef]
- Tian, Q.; Wang, G.; Ma, X.; Shen, Q.; Ding, M.; Yang, X.; Luo, X.; Li, R.; Wang, Z.; Wang, X.; et al. Riboflavin Integrates Cellular Energetics and Cell Cycle to Regulate Maize Seed Development. Plant Biotechnol. J. 2022, 20, 1487–1501. [Google Scholar] [CrossRef]
- Guo, X.; Luo, T.; Han, D.; Zhu, D.; Li, Z.; Wu, Z.; Wu, Z. Multi-Omics Analysis Revealed Room Temperature Storage Affected the Quality of Litchi by Altering Carbohydrate Metabolism. Sci. Hortic. 2022, 293, 110663. [Google Scholar] [CrossRef]
- Zhao, L.; Zhang, B.; Huang, H.; Huang, W.; Zhang, Z.; Wang, Q.; Luo, H.; An, B. Metabolomic and Transcriptomic Analyses Provide Insights into Metabolic Networks during Cashew Fruit Development and Ripening. Food Chem. 2023, 404, 134765. [Google Scholar] [CrossRef] [PubMed]
- Dossou, S.S.K.; Xu, F.; Cui, X.; Sheng, C.; Zhou, R.; You, J.; Tozo, K.; Wang, L. Comparative Metabolomics Analysis of Different Sesame (Sesamum Indicum L.) Tissues Reveals a Tissue-Specific Accumulation of Metabolites. BMC Plant Biol. 2021, 21, 352. [Google Scholar] [CrossRef] [PubMed]
- Arrutia, F.; Binner, E.; Williams, P.; Waldron, K.W. Oilseeds beyond Oil: Press Cakes and Meals Supplying Global Protein Requirements. Trends Food Sci. Technol. 2020, 100, 88–102. [Google Scholar] [CrossRef]
- Zhou, L.; Lu, L.; Chen, C.; Zhou, T.; Wu, Q.; Wen, F.; Chen, J.; Pritchard, H.W.; Peng, C.; Pei, J.; et al. Comparative Changes in Sugars and Lipids Show Evidence of a Critical Node for Regeneration in Safflower Seeds during Aging. Front. Plant Sci. 2022, 13, 1020478. [Google Scholar] [CrossRef]
- Ribbenstedt, A.; Ziarrusta, H.; Benskin, J.P. Development, Characterization and Comparisons of Targeted and Non-Targeted Metabolomics Methods. PLoS ONE 2018, 13, e0207082. [Google Scholar] [CrossRef] [Green Version]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gómez-Tah, R.; Islas-Flores, I.; Félix, J.W.; Granados-Alegría, M.I.; Tzec-Simá, M.; Guerrero-Analco, J.A.; Monribot-Villanueva, J.L.; Canto-Canché, B. Untargeted Metabolomics Analysis of Liquid Endosperm of Cocos nucifera L. at Three Stages of Maturation Evidenced Differences in Metabolic Regulation. Horticulturae 2023, 9, 866. https://doi.org/10.3390/horticulturae9080866
Gómez-Tah R, Islas-Flores I, Félix JW, Granados-Alegría MI, Tzec-Simá M, Guerrero-Analco JA, Monribot-Villanueva JL, Canto-Canché B. Untargeted Metabolomics Analysis of Liquid Endosperm of Cocos nucifera L. at Three Stages of Maturation Evidenced Differences in Metabolic Regulation. Horticulturae. 2023; 9(8):866. https://doi.org/10.3390/horticulturae9080866
Chicago/Turabian StyleGómez-Tah, Rufino, Ignacio Islas-Flores, Jean Wildort Félix, María Inés Granados-Alegría, Miguel Tzec-Simá, José Antonio Guerrero-Analco, Juan Luis Monribot-Villanueva, and Blondy Canto-Canché. 2023. "Untargeted Metabolomics Analysis of Liquid Endosperm of Cocos nucifera L. at Three Stages of Maturation Evidenced Differences in Metabolic Regulation" Horticulturae 9, no. 8: 866. https://doi.org/10.3390/horticulturae9080866





