Exogenous Activation of the Ethylene Signaling Pathway Enhances the Freezing Tolerance of Young Tea Shoots by Regulating the Plant’s Antioxidant System
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Exogenous Spraying Treatments and Sampling
2.3. Relative Electrolyte Leakage Assay and ACC Measurement
2.4. Measurement of Enzyme Activity
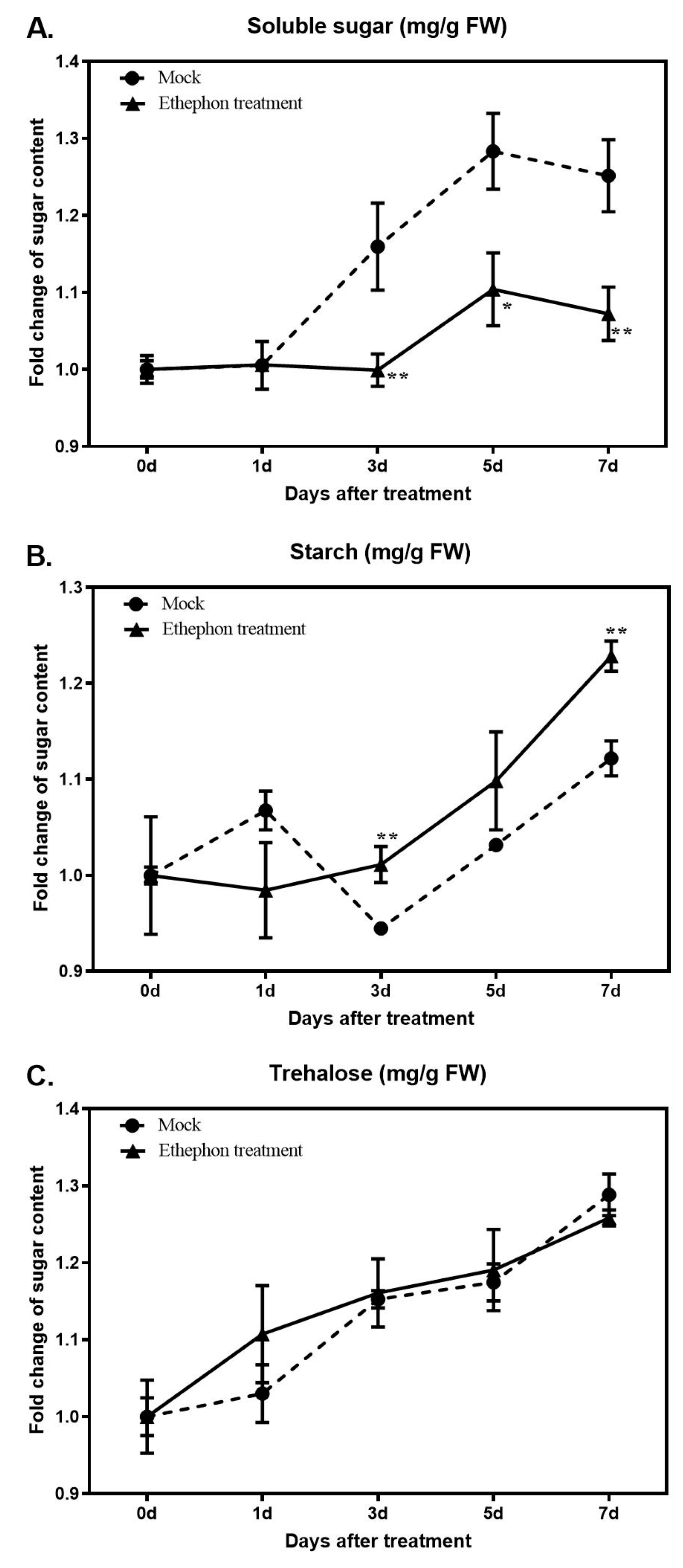
2.5. Measurement of Sugar Contents
2.6. RNA Extraction and Quantitative Real-Time PCR
2.7. Statistical Analysis and Graphing
3. Results
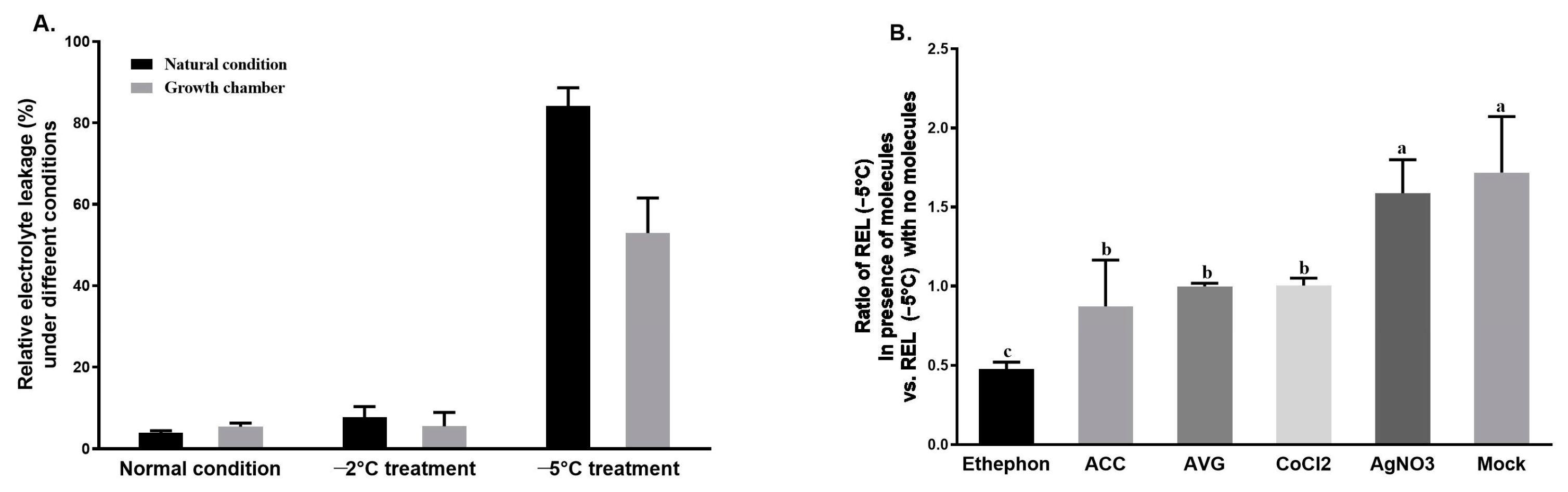
3.1. Determination of REL in Tea Shoots and the Effects of Ethylene Signaling Regulators
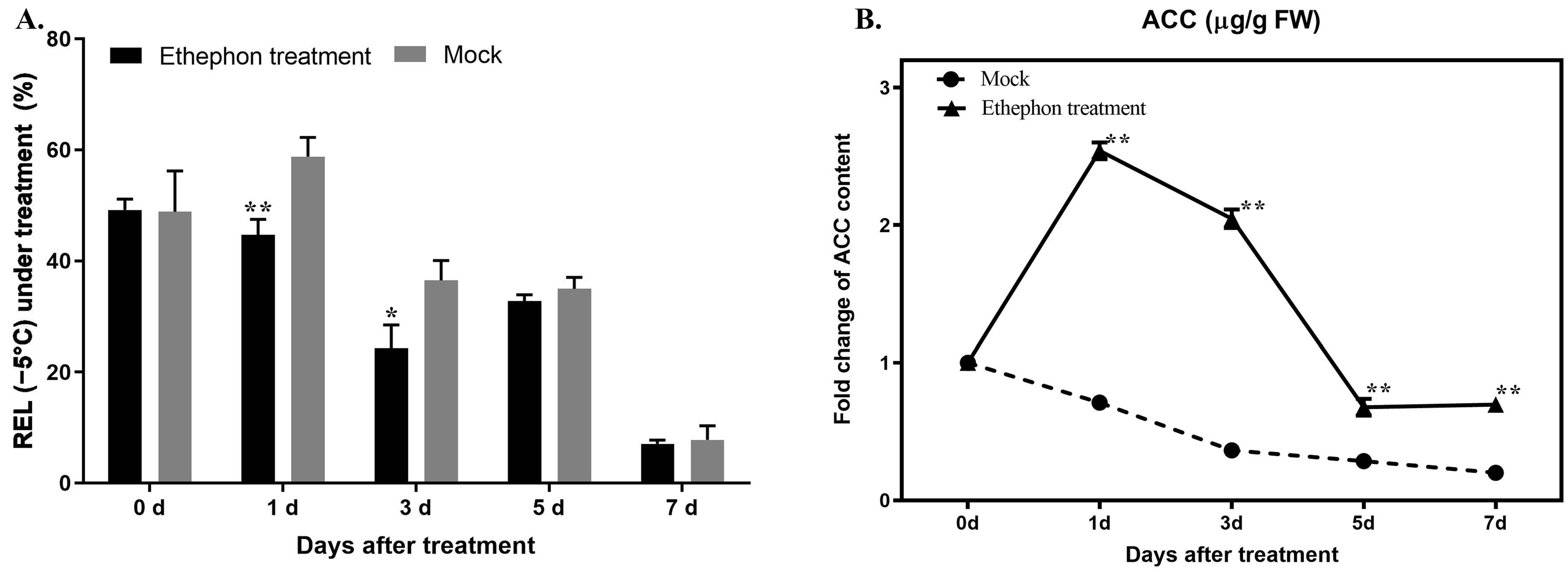
3.2. Time Course of Ethephon Application and the Enhancement of Freezing Resistance
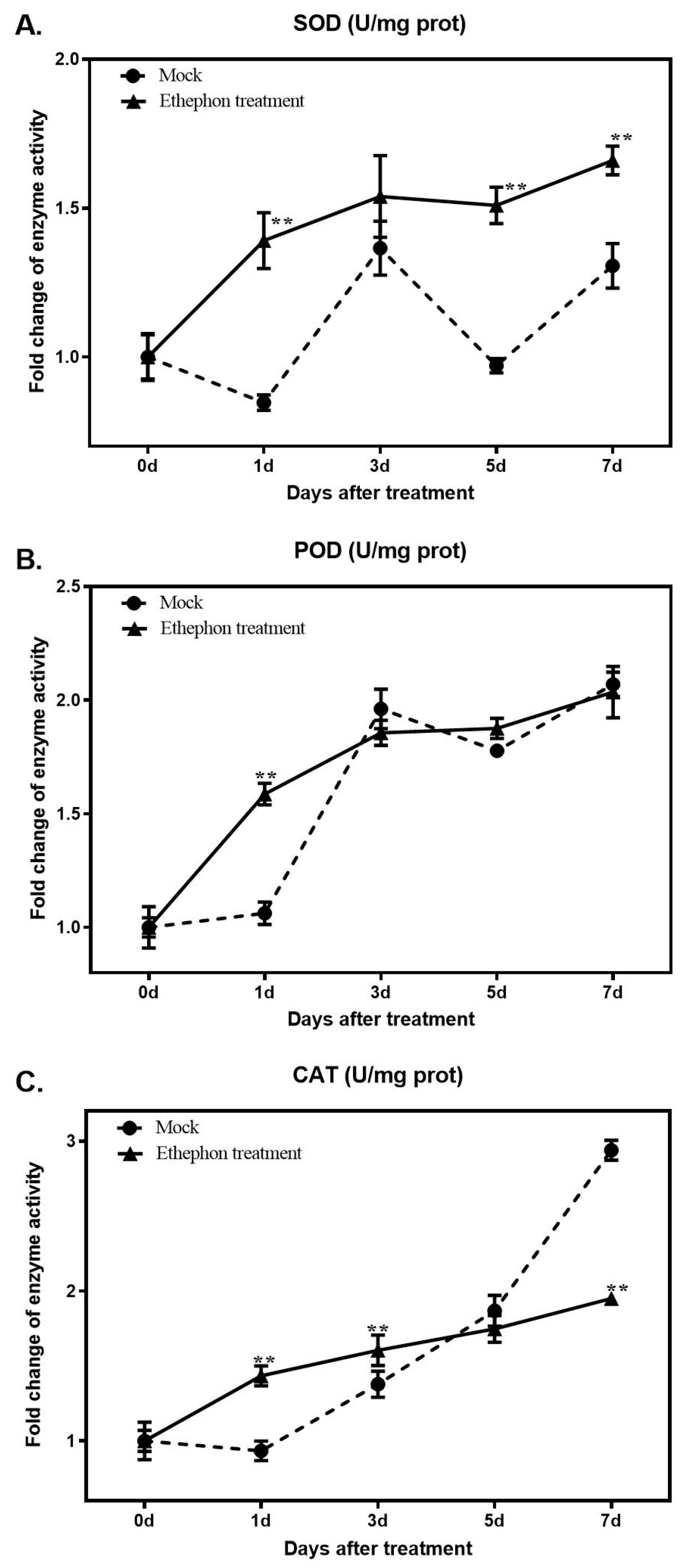
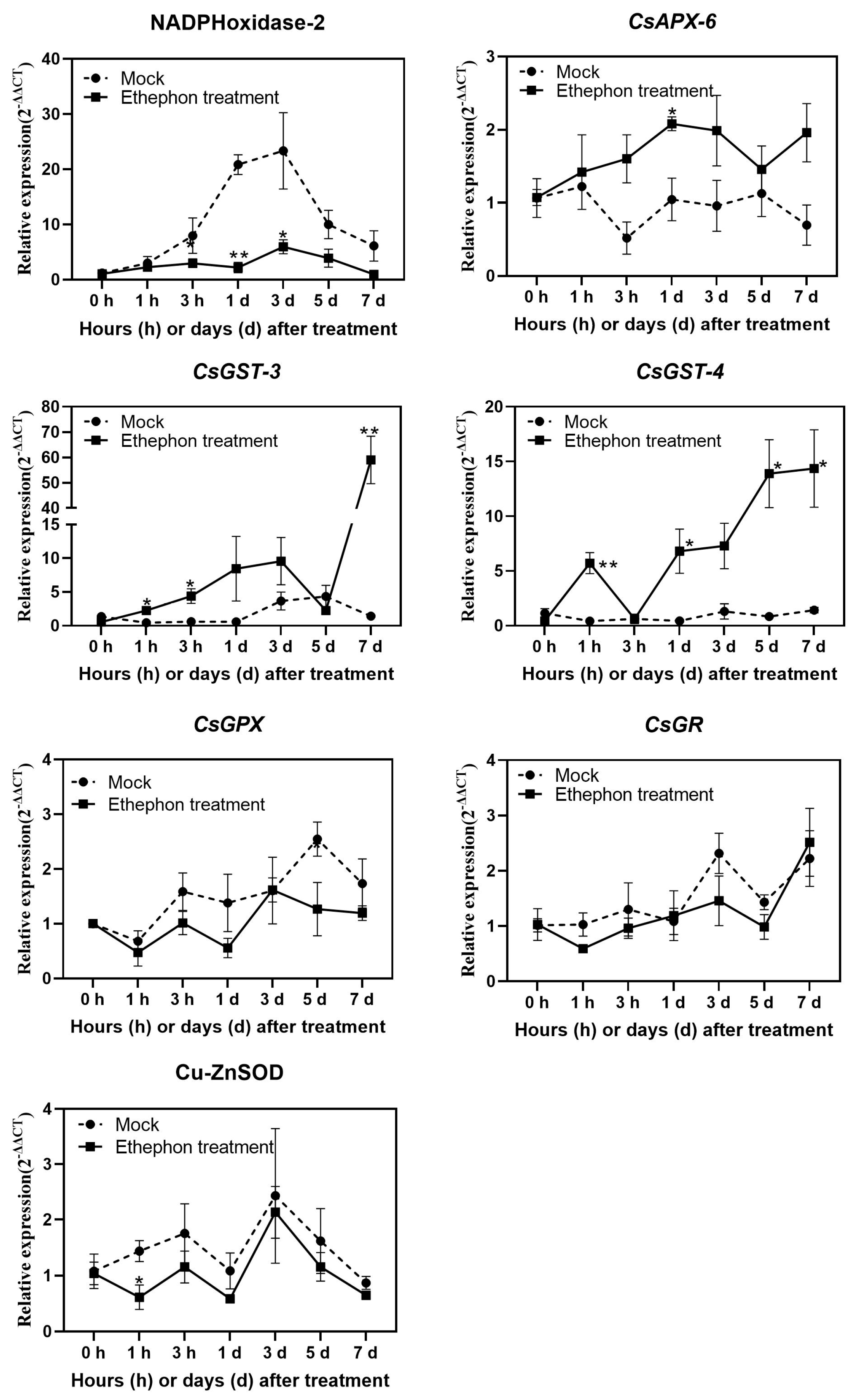
3.3. Changes in Antioxidant Enzyme Activity after Ethephon Application
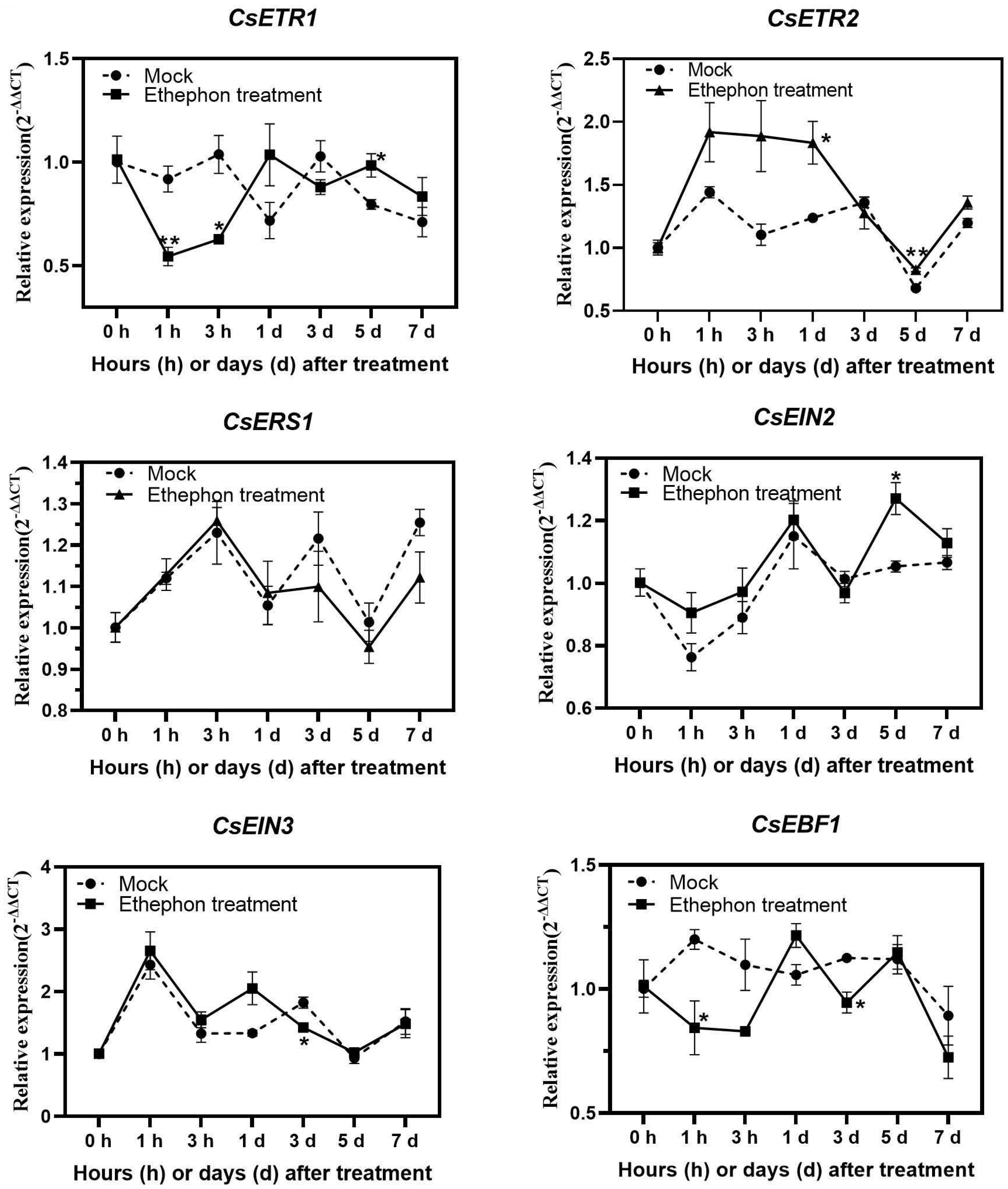
3.4. Analysis of Gene Expression Related to Ethylene Signalling and Antioxidant Activities
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wang, L.; Yao, L.N.; Hao, X.Y.; Li, N.N.; Wang, Y.C.; Ding, C.Q.; Lei, L.; Qian, W.J.; Zeng, J.M.; Yang, Y.J.; et al. Transcriptional and physiological analyses reveal the association of ROS metabolism with cold tolerance in tea plant. Environ. Exp. Bot. 2019, 160, 45–58. [Google Scholar] [CrossRef]
- Wang, X.C.; Zhao, Q.Y.; Ma, C.L.; Zhang, Z.H.; Cao, H.L.; Kong, Y.M.; Yue, C.; Hao, X.Y.; Chen, L.; Ma, J.Q. Global transcriptome profiles of Camellia sinensis during cold acclimation. BMC Genom. 2013, 14, 415. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.K. Abiotic stress signaling and responses in plants. Cell 2016, 167, 313–324. [Google Scholar] [CrossRef] [PubMed]
- Preston, J.C.; Sandve, S.R. Adaptation to seasonality and the winter freeze. Front. Plant Sci. 2013, 4, 167. [Google Scholar] [CrossRef]
- Chinnusamy, V.; Zhu, J.K.; Sunkar, R. Gene regulation During Cold Stress Acclimation in Plants. Methods Mol. Biol. 2010, 639, 39–55. [Google Scholar] [CrossRef]
- Hao, X.Y.; Wang, L.; Zeng, J.M.; Yang, Y.J.; Wang, X.C. Response and adaptation mechanisms of tea plant to low-temperature stress. In Stress Physiology of Tea in the Face of Climate Change; Springer: Singapore, 2018. [Google Scholar] [CrossRef]
- Ruttink, T.; Arend, M.; Morreel, K.; Storme, V.; Rombauts, S.; Fromm, J.; Bhalerao, R.P.; Boerjan, W.; Rohde, A. A molecular timetable for apical bud formation and dormancy induction in poplar. Plant Cell Online 2007, 19, 2370–2390. [Google Scholar] [CrossRef] [PubMed]
- Yu, F. Discussion on the originating place and the originating centre of tea plant. J. Tea Sci. 1986, 6, 1–8. [Google Scholar]
- Luo, T.; Jiang, J.G. Anticancer effects and molecular target of theaflavins from black tea fermentation in vitro and in vivo. J. Agric. Food Chem. 2021, 69, 15052–15065. [Google Scholar] [CrossRef]
- Yi, M.S.; Wu, X.T.; Zhuang, W.; Xia, L.; Chen, Y.; Zhao, R.; Wan, Q.Y.; Du, L.; Zhou, Y. Tea consumption and health outcomes: Umbrella review of meta-analyses of observational studies in humans. Mol. Nutr. Food Res. 2019, 63, e1900389. [Google Scholar] [CrossRef]
- Zhou, J.; Li, R.; Jia, Y.; Wang, Y.; Liu, J.; Panichayupakaranant, P.; Chen, H. Recent progress in matural anticancer agents discovery from tea (Camellia sinensis): A review. Recent Pat. Anti-Cancer Drug Discov. 2022, 17, 343–357. [Google Scholar] [CrossRef]
- Hao, X.Y.; Tang, H.; Wang, B.; Yue, C.; Wang, L.; Zeng, J.M.; Yang, Y.J.; Wang, X.C. Integrative transcriptional and metabolic analyses provide insights into cold spell response mechanisms in young shoots of the tea plant. Tree Physiol. 2018, 38, 1655–1671. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.T.; Wang, B.; Cao, H.L.; Wang, L.; Hao, X.Y.; Wang, X.C.; Yang, Y.J. Effects of exogenous calcium and inhibitors of calcium signaling transduction pathway on cold resistance of tea plant. J. Tea Sci. 2015, 35, 520–526. [Google Scholar] [CrossRef]
- Li, X.; Wei, J.P.; Scott, E.R.; Liu, J.W.; Guo, S.; Li, Y.; Zhang, L.; Han, W.Y. Exogenous melatonin alleviates cold stress by promoting antioxidant defense and redox homeostasis in Camellia sinensis L. Molecules 2018, 23, 165. [Google Scholar] [CrossRef]
- Wang, L.; Di, T.M.; Peng, J.; Li, Y.T.; Li, N.N.; Hao, X.Y.; Ding, C.Q.; Huang, J.Y.; Zeng, J.M.; Yang, Y.J.; et al. Comparative metabolomic analysis reveals the involvement of catechins in adaptation mechanism to cold stress in tea plant (Camellia sinensis var. sinensis). Environ. Exp. Bot. 2022, 201, 104978. [Google Scholar] [CrossRef]
- Eremina, M.; Rozhon, W.; Poppenberger, B. Hormonal control of cold stress responses in plants. Cell. Mol. Life Sci. 2016, 73, 797–810. [Google Scholar] [CrossRef]
- Lafuent, M.T.; Martı’nez-Téllez, M.A.; Sanchez-Ballesta, M.T.; Dupille, E. Phenylalanine ammonia-lyase as related to ethylene in the development of chilling symptoms during cold storage of citrus fruits. J. Agric. Food Chem. 2001, 49, 6020–6025. [Google Scholar] [CrossRef]
- Lafuente, M.T.; Sala, J.M.; Zacarias, L. Active oxygen detoxifying enzymes and phenylalanine ammonia-lyase in the ethylene-induced chilling tolerance in citrus fruit. J. Agric. Food Chem. 2004, 52, 3606–3611. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, J.Y.; Jiang, Y.M.; Lu, W.J. Cloning and expression analysis of phenylalanine ammonia-lyase in relation to chilling tolerance in harvested banana fruit. Postharvest Biol. Technol. 2007, 44, 34–41. [Google Scholar] [CrossRef]
- Sun, X.; Zhao, T.; Gan, S.; Ren, X.; Fang, L.; Karungo, S.K.; Wang, Y.; Chen, L.; Li, S.; Xin, H. Ethylene positively regulates cold tolerance in grapevine by modulating the expression of ethylene response factor 057. Sci. Rep. 2016, 6, 24066. [Google Scholar] [CrossRef]
- Wang, Y.; Jiang, H.; Mao, Z.; Liu, W.; Jiang, S.; Xu, H.; Su, M.; Zhang, J.; Wang, N.; Zhang, Z.; et al. Ethylene increases the cold tolerance of apple via the MdERF1b-MdCIbHLH1 regulatory module. Plant J. 2021, 106, 379–393. [Google Scholar] [CrossRef]
- Zhao, M.; Liu, W.; Xia, X.; Wang, T.; Zhang, W.H. Cold acclimation induced freezing tolerance of Medicago truncatula seedlings is negatively regulated by ethylene. Physiol. Plant. 2014, 152, 115–129. [Google Scholar] [CrossRef]
- Shi, Y.; Tian, S.; Hou, L.; Huang, X.; Zhang, X.; Guo, H.; Yang, S. Ethylene signaling negatively regulates freezing tolerance by repressing expression of CBF and type-a ARR genes in Arabidopsis. Plant Cell. 2012, 24, 2578–2595. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.R.; Zhao, Z.J.; Hao, F.S. NADPH oxidases, essential players of hormone signalings in plant development and response to stresses. Plant Signal. Behav. 2019, 14, 1657343. [Google Scholar] [CrossRef] [PubMed]
- Hao, X.; Horvath, D.P.; Chao, W.S.; Yang, Y.; Wang, X.; Xiao, B. Identification and evaluation of reliable reference genes for quantitative real-time pcr qnalysis in tea plant (Camellia sinensis (L.) O. Kuntze). Int. J. Mol. Sci. 2014, 15, 22155–22172. [Google Scholar] [CrossRef]
- Zolotareva, D.; Zazybin, A.; Belyankova, Y.; Dauletbakov, A.; Tursynbek, S.; Rafikova, K.; Ten, A.; Yu, V.; Bayazit, S.; Basharimova, A.; et al. Increasing sugar content in source for biofuel production using agrochemical and genetic approaches at the stages of bioMass preharvesting and harvesting. Molecules 2022, 27, 5210. [Google Scholar] [CrossRef]
- Yue, C.; Cao, H.L.; Wang, L.; Zhou, Y.H.; Huang, Y.T.; Hao, X.Y.; Wang, Y.C.; Wang, B.; Yang, Y.J.; Wang, X.C. Effects of cold acclimation on sugar metabolism and sugar-related gene expression in tea plant during the winter season. Plant Mol. Biol. 2015, 88, 591–608. [Google Scholar] [CrossRef] [PubMed]
- Toups, H.S.; Cochetel, N.; Gray, D.; Cramer, G.R. VviERF6Ls: An expanded clade in Vitis responds transcriptionally to abiotic and biotic stresses and berry development. BMC Genom. 2020, 21, 472. [Google Scholar] [CrossRef]
- Apelbaum, A.; Burgoon, A.C.; Aanerson, J.D.; Solomos, T.; Lieberman, M. Some characteristics of the system converting 1-aminocyclopropane-1-carboxylic acid to ethylene. Plant Physiol. 1981, 67, 80–84. [Google Scholar] [CrossRef]
- Zhang, Z.; Huang, R. Enhanced tolerance to freezing in tobacco and tomato overexpressing transcription factor TEERF2/LeERF2 is modulated by ethylene biosynthesis. Plant Mol. Biol. 2010, 73, 241–249. [Google Scholar] [CrossRef]
- Mou, W.; Kao, Y.T.; Michard, E.; Simon, A.A.; Li, D.; Wudick, M.M.; Lizzio, M.A.; Feijó, J.A.; Chang, C. Ethylene-independent signaling by the ethylene precursor ACC in Arabidopsis ovular pollen tube attraction. Nat. Commun. 2020, 11, 4082. [Google Scholar] [CrossRef]
- Yu, W.; Sheng, J.; Zhao, R.; Wang, Q.; Ma, P.; Shen, L. Ethylene biosynthesis is involved in regulating chilling tolerance and SlCBF1 gene expression in tomato fruit. Postharvest Biol. Technol. 2019, 149, 139–147. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, H.; Quan, R.; Wang, X.C.; Huang, R. Transcriptional regulation of the ethylene response factor LeERF2 in the expression of ethylene biosynthesis genes controls ethylene production in tomato and tobacco. Plant Physiol. 2009, 150, 365–377. [Google Scholar] [CrossRef]
- Khan, M.; Hu, J.; Dahro, B.; Ming, R.; Zhang, Y.; Wang, Y.; Alhag, A.; Li, C.; Liu, J.H. ERF108 from poncirus trifoliata (L.) Raf. functions in cold tolerance by modulating raffinose synthesis through transcriptional regulation of Ptrrafs. Plant J. 2021, 108, 705–724. [Google Scholar] [CrossRef]
- Hu, Z.; Huang, X.; Amombo, E.; Liu, J.; Fan, A.; Bi, A.; Ji, K.; Xin, H.; Chen, L.; Fu, J. The ethylene responsive factor CdERFerf1 from bermudagrass (Cynodon dactylon) positively regulates cold tolerance. Plant Sci. 2020, 294, 110432. [Google Scholar] [CrossRef] [PubMed]
- Bolouri-Moghaddam, M.R.; Le Roy, K.; Xiang, L.; Rolland, F.; Van den Ende, W. Sugar signalling and antioxidant network connections in plant cells. FEBS J. 2010, 277, 2022–2037. [Google Scholar] [CrossRef]
- Mittler, R. ROS are good. Trends Plant Sci. 2017, 22, 11–19. [Google Scholar] [CrossRef] [PubMed]
| Gene | Primer | Primer Sequence (5′-3′) | Product Size |
|---|---|---|---|
| (bp) | |||
| CsETR1 | qRT-CsETR1-F | CACGTACTGGGCTAGAGCTTCAACTTTC | 192 |
| qRT-CsETR1-R | CAACCACCTCTCCGGGCATGTATTTTC | ||
| CsETR2 | qRT-CsETR2-F | TCTCTTGGCTCGGCTGTCTCTTCGTT | 154 |
| qRT-CsETR2-R | CATCTTCATCGCCACTTGCTGTCA | ||
| CsERS1 | qRT-CsERS1-F | CCGAAATAATGGTGGTGCTGGTCTGG | 166 |
| qRT-CsERS1-R | CTGCATTGTTGGTTCATTTGGGCTATTG | ||
| CsEIN2 | qRT-CsEIN2-F | GGAGAGGGCTGTGTTTGGAGAGTGGA | 199 |
| qRT-CsEIN2-R | GCAGGCAGAAGCAGGGGGTCATT | ||
| CsEIN3 | qRT-CsEIN3-F | ATGGTGGCCTCAATTGGGTCTTCC | 198 |
| qRT-CsEIN3-R | GTGGCACTCTCCTTCGCTGTCATCTTAT | ||
| CsEBF1 | qRT-CsEBF1-F | GACCTCTGCCAGTGTCCTTC | 191 |
| qRT-CsEBF1-R | CCCTGATCCCCAACATGAGG | ||
| CsNADPHoxidase-2 | qRT-CsNADPHoxidase-2-F | TGGGAAAGCAAGTGAGTGACAATAGC | 169 |
| qRT-CsNADPHoxidase-2-R | TAAGCAGAGAAAGACCAAACCAAGAGTG | ||
| CsAPX-6 | qRT-CsAPX-6-F | TTGTTGCATTATCCGGGGCT | 151 |
| qRT-CsAPX-6-R | GCACGATCTGAAGGAAGACCA | ||
| CsGST-3 | qRT-CsGST-3-F | GAAGTTGTTGGGACATTGGGC | 186 |
| qRT-CsGST-3-R | GACTCGCAAATGGGCTTTCC | ||
| CsGST-4 | qRT-CsGST-4-F | AGCTTCTAGGTGCATCGCC | 165 |
| qRT-CsGST-4-R | CACTATGGATGAGAACCGGCA | ||
| CsGPX | qRT-CsGPX-F | GCTCCCTTTTGCAGTGGTTT | 161 |
| qRT-CsGPX-R | ATTGTGCATGCGGTTTCCTG | ||
| CsGR | qRT-CsGR-F | ATGGTGAAGAAATAAGGGCTGATGC | 152 |
| qRT-CsGR-R | TATGCTTGGTATGTTTGTGCGAGAGT | ||
| CsCu-ZnSOD | qRT-CsCu-ZnSOD-F | TGGGATTGTTGGTTTCTCGGT | 214 |
| qRT-CsCu-ZnSOD-R | GGCAAAGCTGATGCTCAACC | ||
| CsPTB1 | qRT-CsPTB1-F | TGACCAAGCACACTCCACACTATCG | 107 |
| qRT-CsPTB1-R | TGCCCCCTTATCATCATCCACAA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.; Tang, J.; Ren, H.; Li, Y.; Li, C.; Wang, H.; Wang, L.; Yang, Y.; Wang, X.; Hao, X. Exogenous Activation of the Ethylene Signaling Pathway Enhances the Freezing Tolerance of Young Tea Shoots by Regulating the Plant’s Antioxidant System. Horticulturae 2023, 9, 875. https://doi.org/10.3390/horticulturae9080875
Chen Y, Tang J, Ren H, Li Y, Li C, Wang H, Wang L, Yang Y, Wang X, Hao X. Exogenous Activation of the Ethylene Signaling Pathway Enhances the Freezing Tolerance of Young Tea Shoots by Regulating the Plant’s Antioxidant System. Horticulturae. 2023; 9(8):875. https://doi.org/10.3390/horticulturae9080875
Chicago/Turabian StyleChen, Yao, Junwei Tang, Hengze Ren, Yuteng Li, Congcong Li, Haoqian Wang, Lu Wang, Yajun Yang, Xinchao Wang, and Xinyuan Hao. 2023. "Exogenous Activation of the Ethylene Signaling Pathway Enhances the Freezing Tolerance of Young Tea Shoots by Regulating the Plant’s Antioxidant System" Horticulturae 9, no. 8: 875. https://doi.org/10.3390/horticulturae9080875
APA StyleChen, Y., Tang, J., Ren, H., Li, Y., Li, C., Wang, H., Wang, L., Yang, Y., Wang, X., & Hao, X. (2023). Exogenous Activation of the Ethylene Signaling Pathway Enhances the Freezing Tolerance of Young Tea Shoots by Regulating the Plant’s Antioxidant System. Horticulturae, 9(8), 875. https://doi.org/10.3390/horticulturae9080875







