Abstract
The great economic importance of sugar beet determines the ongoing biotechnological studies conducted worldwide to improve the technology of obtaining doubled haploids (DHs) using the method of unpollinated ovule culture in vitro. To improve the induction of gynogenesis, we tested the effect of thidiazuron (TDZ), temperature bud pretreatment, different concentrations of sucrose, and culturing on liquid or solid medium. Three genotypes were tested in this study. The use of TDZ at a concentration of 0.4 mg/L in solid IMB (induction medium for Beta vulgaris) induction nutrient medium with 3 g/L phytagel, 50 g/L sucrose, 200 mg/L ampicillin and cultivation at 28◦C in the dark produced up to 16.7% induced ovules. The liquid nutrient medium of the same composition induced up to 8% ovules. Increasing TDZ concentration to 0.8 mg/L resulted in reduction or total inhibition of gynogenesis, depending on the genotype. Reducing the sucrose concentration to 20 g/L or increasing it to 80 g/L was not effective. In all three genotypes, the absence of temperature pretreatment of buds (5–6 °C) showed the best results. The plant regeneration with MS nutrient medium of 20 g/L sucrose, 3 g/L phytagel, 1 mg/L 6-benzylaminopurine (BAP) and 0.1 mg/L gibberellic acid (GA3) resulted in up to seven shoots from one induced ovule in the most responsive genotype. We showed by flow cytometry, chromosome counting and chloroplast number assessment that all regenerant plants were haploid (2n = x = 9).
1. Introduction
Sugar beet (Beta vulgaris L. ssp. vulgaris convar. crassa var. altissima) belongs to the Amaranthaceae Juss. family (Chenopodiaceae Vent.) and to the sugar beet group Beta vulgaris L. ssp. vulgaris [1,2]. Nowadays, sugar beet is the most important technical crop grown mainly for sugar production [3] and by-product processing [4], with a possible prospect of use for obtaining alternative fuel: bioethanol and biogas [5,6]. Sugar beet is cultivated from Gibraltar to the Arctic Circle, mainly in warm and temperate climates with little rainfall in the industrialized countries of Western, Central and Eastern Europe, USA, China and Japan. A small proportion of sugar beet is also cultivated in subtropical regions [7]. The sugar beet is thought to be originated from sea beet plants (Beta vulgaris L. ssp. maritima) that are commonly found in Europe along the Mediterranean Sea, Atlantic Ocean, North Sea, and Baltic Sea [8]. The genetic basis for early sugar beet varieties in the mid-18th century is the gene pool of white fodder beets [9,10]. Following the discovery of sugar in fodder beets in 1747 by the German chemist Markgraf, highly productive sugar beet varieties and hybrids were created [11], including the first sugar beet variety Weisse schlesische Rübe [12].
Sugar beet is an allogamous, biennial, and self-incompatible plant [13,14]. During its first year of life, the plant forms a rosette of ovate or heart-shaped dark green and glossy leaves and develops a cone-shaped, white, fleshy root crop 15–35 cm long [15]. Sucrose accumulates in the root crop at a concentration of 15–21% of the beet’s total weight [16]. During the processing of sugar beet, up to 180 kg of sugar is produced from 1 ton of taproots, which are used in the food industry for the production of confectionery, bakery products and canned food [17] By-products of industrial processing of root beet are used for cellulose and pulp, which are useful additives to animal feed and a raw material for the production of pectin, glue and alcohol [7,18]. During the final crystallization stage of the sugar production process, molasses is produced, making up 12.5% of the sugar fraction [19]. More than 25 different food, chemical and perfume industry products are produced from molasses (yeast, alcohols, organic acids) [20]. The chromatographic separation of molasses produces the extraction by-product betaine, which is used in pharmaceuticals and cosmetics [21] and compound feeds for agro-industrial use [22,23]. Sugar beet leaves are a valuable animal feed and are used fresh, siloed and dried. They contain carotene, vitamins, organic and mineral substances. The yield of leaves is 45–55% of the root crop, and in northern areas, the ratio between them reaches 1:1 [20].
According to the latest data of the Food and Agriculture Organization of the United Nations (FAOSTAT 2020), the largest producers of sugar beet in 2020 are European countries, which account for 65% of the total cultivated area and a production volume of 252.9 million tons. The areas with this crop comprise 4.4 million hectares all over the world, while the largest areas of sugar beet are located in Russia—916.6 thousand hectares, which accounts for 20.6% of all sugar beet plantations in Europe with a production volume of 33.9 million tons. The USA, Germany, France and Turkey are also among the leading countries in sugar beet production [24].
Sugar is currently produced in 110 countries worldwide, with 80% of its production coming from sugarcane and 20% from sugar beets [25,26]. In 2019, 179.4 million tons of sugar were produced worldwide. The world’s largest sugar producers in 2019 were India—34.3 million tons, Brazil—27.7 million tons, and Thailand—14.8 million tons. Russia ranks sixth on this list with production of 7.3 million tons of sugar [24]. The largest commercial sugar producers in the world are: the Amalgamated Sugar Company LLC (Boise, Idaho, USA), Southern Minnesota Beet Sugar Cooperative (Renville, MN, USA), American Crystal Sugar Company (Moorhead, MN, USA), Michigan Sugar Company (Bay City, MI, USA), the Western Sugar Cooperative (Denver, CO, USA), Tereos (Moussy-Ie-Vieux, France), Nordzucker and Südzucker (Braunschweig, Germany), Prodimex (Krasnogorsk, Russia), Dominant (Moscow, Russia), and Rusagro (Tambov, Russia). In recent years, leading companies have increasingly focused on the development and implementation of innovative solutions, in particular on desugarization—the production of sugar from molasses [27].
Due to the great economic and household importance of sugar beet, breeding institutions in different countries are working hard to create a great variety of forms by creating lines, tetraploid forms (polyploidization) and biotypes with CMS (cytoplasmic male sterility), samples with marker traits. The increase in sugar beet production due to breeding was impressive and occurred at a rapid pace [28]. In the beginning, mass selection was used, followed by schemes based on evaluation of progeny and combinational ability [15]. In modern sugar beet breeding, the leading direction is the creation of highly productive hybrids based on linear source material. The high productivity of new hybrids is based on the effects of heterosis and overdominance in di- and triploid interlinear hybrids. An important step is the creation of homozygous initial lines with high combining ability [29]. Biological features of sugar beet associated with the large genetic diversity of this crop, 2-year cycle of development, inbred depression, polygenic and quantitative inheritance of many important traits formed as a result of allogamy and self- and cross incompatibility make the process of obtaining inbred lines by classical breeding methods labor-intensive [30,31,32]. In traditional sugar beet biennial breeding, inbreeding and selection over at least four to six generations to produce homozygous lines takes 8–12 years [33], and complete homozygosity for allogamous species, such as sugar beet, is not guaranteed [34].
One of the most promising technologies that is intensively developed in many countries around the world and allow significant acceleration in the breeding process is the DH technology [35,36]. The use of in vitro culture methods for obtaining doubled haploids can significantly reduce the sugar beet breeding cycle, in particular the period of obtaining homozygous lines to 1–2 years [37]. Although the most frequently used and successful DH technique is the induction of androgenesis, this approach has not yet been effectively implemented for sugar beet, since only callus or callus and roots have been obtained from microspores [38,39] or anthers [40], respectively. Therefore, gynogenesis is the most commonly used method for obtaining haploids and then doubled haploids of sugar beet [41,42,43,44]. The first successful cultivations of the female gametophyte to produce haploid and doubled haploid sugar beet plants using an in vitro culture of unpollinated ovules was reported by Hosemans and Bossoutrot (1983) [45], D’Halluin and Kelmer (1986) [46] and Van Geyt et al. (1987) [47]. Since then, numerous studies on gynogenesis in sugar beet have been carried out, and they are most often successful.
Studies have already been carried out on the condition, timing, and growth conditions of donor plants [48,49], ovary development stage [42,49,50], genotype [46,47,48,49,51], bud location on the inflorescence [46], type and concentration of growth regulators [51,52], media composition [48,53,54], conditions of ovules cultivation [55], pretreatment of buds with cold [42,49,50], and cultivation of isolated ovules at high temperature [51]. Studies in recent years have already produced doubled sugar beet haploids [46,48,49,55,56,57,58], and protocols for obtaining DH-plants through unpollinated ovule culture in vitro have been published [42,51,59].
Despite numerous studies, the process of obtaining sugar beet haploids by gynogenesis is still associated with some limitations [44]. The current methods provide low efficiency of gynogenesis ranging from 1% to 15%, as well as limited regenerant plant yield (40%) [44,51]. Therefore, protocols for obtaining sugar beet haploids require further development and improvement. To increase the efficiency of sugar beet gynogenesis, the factors stimulating the transition from gametophytic to sporophytic development in unpollinated ovule culture in vitro are currently being investigated. [54,60].
There are a number of factors that can potentially increase gynogenesis efficiency in sugar beet. For instance, a growth regulator, thidiazuron (TDZ), was successfully used in induction and regeneration media to improve gynogenesis in the in vitro culture of carrot and cucumber unpollinated ovules [61,62]. To our knowledge, there are no reports on the use of TDZ as a pretreatment or as a part of the induction nutrient medium for cultivation of sugar beet ovules in vitro. There are only data on somatic embryogenesis of sugar beet in the presence of TDZ [63]. Therefore, we aimed to study the influence of TDZ on gynogenesis induction in combination with bud cold pretreatment, the presence of gelling agent and with different sucrose concentrations. TDZ in combination with other potentially stimulating conditions can improve the technology of sugar beet DH production, which makes this study undoubtedly relevant for this field.
2. Materials and Methods
2.1. Growing Conditions for Donor Plants
In the present study, sugar beet (Beta vulgaris L. ssp. vulgaris convar. crassa var. altissima) breeding accessions (b.a) 37130 (fertile line—pollinator for Rubin F1), 37131 (fertile line—pollinator for Corvette F1), and 36764 (promising breeding accessions) from the collection of Pervomajskaya breeding and experimental station sugar beets, Gulkevichi, Krasnodar region, Russia was used. Sugar beet taproots of donor plants after five months of vernalization were planted in plastic pots 22 cm in diameter, filled with a mixture of peat and perlite (7:3, v/v). Planting was done on two dates: in February and April. The pots were placed in a growing chamber with an illuminance of 65 μmol m−2 s−1 using Horturion HPS, 600 W 220 V E40 lamps (Osram, Slovenia) for cultivation at a constant temperature of 25 °C and a photoperiod of 16 h/8 h (day/night). Donor plants were watered as needed, adding a liquid commercial fertilizer once a week—Akvarin (Bui, Russia).
2.2. Sterilization of Explants
Inflorescences of sugar beet were collected from donor plants from mid-April to August. Inflorescence fragments of 5–10 cm were kept at 5–6 °C in the dark in a humid chamber for two, four, and eight days; the control inflorescences were not subjected to cold pretreatment. For in vitro culture, the buds were selected from the portion of the spike-like peduncle above the newly opened flower (the inflorescence section, 2 to 5 cm long). Buds with a distance between them less than the size of the buds themselves were not introduced into the culture.
The collected buds were surface-sterilized in 96% ethanol solution for 30 s, then for 15 min in 50% aqueous solution of a commercial bleach preparation containing 10% sodium hypochlorite with the addition of one drop of Tween 20 (Panreac, Barcelona, Spain) per 100 mL solution, then washed three times in sterile distilled water for 10 min. Buds were preserved in sterile 11 cm glass petri dishes with wet filter paper. Isolated ovules were immediately placed in nutrient culture medium.
2.3. In Vitro Culture of Unpollinated Ovules
Ovules from sterilized buds were isolated using dissecting needles in a laminar box under a Stemi 305 stereomicroscope (Carl Zeiss Microscopy GmbH, Jena, Germany) at 10× magnification. For the induction of gynogenesis, nutrient medium IMB (induction medium for Beta vulgaris) on the basis of the mixed composition of the MS and B5 nutrient mediums [64] containing 50 g/L sucrose, 3 g/L phytagel, 200 mg/L ampicillin and 0.4 mg/L TDZ was used. To study the effect of TDZ concentration, nutrient media with 0.8 mg/L TDZ were additionally used. To study the effect of sucrose in concentration, 20 g/L, 50 g/L, and 80 g/L sucrose were used. Isolated ovules were placed in 94×16 mm sterile Petri dishes (Greiner Bio-One GmbH, Frickenhausen, Germany) with 20 mL of solid nutrient medium and in 100 mL culture vessels with 20 mL of liquid nutrient medium. Ovules were cultured in an incubator Binder BF 260 (Binder GmbH, Tuttlingen, Germany) in the dark at 28 °C. Vessels with liquid nutrient medium were grown at 40 rpm on a PSU-10i shaker platform (Biosan, Latvia). Every 3–7 days, the cultivated ovules were observed using a Stemi 508 stereomicroscope with an Axiocam 305 color camera (Carl Zeiss Microscopy GmbH, Jena, Germany).
2.4. Obtaining Regenerant Plants in Unpollinated Ovule Culture
Ovules producing embryoid and callus structures were transferred onto solid MS nutrient medium [65] with 1 mg/L BAP and 0.1 mg/L GA3. Subculturing of embryoid and callus structures was performed for 5–7 weeks until rosettes with leaves were formed. Transplantation of these structures to fresh nutrient media was performed every 3–4 weeks. Microslices with well-developed leaves formed as a result of subculturing were transferred to hormone-free MS medium with 2% sucrose and 3 g/L phytagel to form normally developed rosettes with well-developed 5–7 leaves. To form well-developed roots, microrosettes were transplanted to fresh nutrient media of the same composition every 2–3 weeks.
Cultivation was carried out on the racks with mixed illumination from fluorescent lamps of two types—OSRAM Fluora L36W/77 (predominantly blue and red spectrum) and Philips 36W/54-765 (predominantly white spectrum)—at a total illumination of 24 μmol m−2 s−1, photoperiod 16 h/8 h (day/night) at 25 °C.
2.5. Cultivation of Regenerated Plants
The plants with normally developed leaves and root systems were planted in growing vessels with a mixture of peat and perlite (7:3, v/v) and covered with perforated plastic cups for acclimatization to ex vitro conditions. The regenerated plants were grown in a growing chamber under the same conditions with the same fertilizer application as the donor plants. Well-formed sugar beet roots after three to four months of cultivation were planted in vegetative vessels and placed in a root storage tank for germination under illumination of 16 μmol m −2s−1 using Horturion HPS lamps, 600 W 220 V E40 (Osram, Slovenia) at a constant temperature regime of 4–5 °C and photoperiod 12 h/12 h (day/night).
2.6. Determination of Ploidy Level by Flow Cytometry
A diploid sugar beet donor plant (2n = 2x = 18) was used as a reference standard. For nuclei isolation, about 20 mg of young leaves from a reference standard and regenerant plants were chopped with a razor blade in 500 μL of ice-cold lysis buffer (0.2 M Tris, 4 mM MgCl2, 50 μg/mL RNAase, 0.5% (v/v) TRITON X-100, 0.5% (v/w) polyvinylpyrrolidone K15, and 50 μg/mL propidium iodide, pH 7.5) [66]. Then, plant material was filtered through a 50 μm nylon filter and stained with propidium iodide (PI). PI fluorescence detection and data processing were performed using a CytoFLEX (Beckman Coulter, Inc, 2018, Brea, CA, USA).
2.7. Determination of Ploidy Level by Chromosome Counting
Chromosome counting was performed on crushed preparations of apical meristems of sugar beet stems stained with the propion–lacmoid method [67]. Plant material was fixed and stained in a standard propion-lacmoid solution for 24 h. The stained tissues were macerated in 40% propionic acid solution by boiling for 1–3 min. Then, the material was placed in a drop of 40% propionic acid on a slide, covered with a coverslip, and the contents of the preparation were crushed. The preparations were viewed using an Axio Imager A2 microscope equipped with an AxioCam MRc5 camera (Carl Zeiss Microscopy GmbH, Jena, Germany) with a 20× and 40× lens. Chromosome counting and imaging were performed using a 100× oil immersion lens. The obtained images were processed and documented using AxioVision software, version 4.8 (Carl Zeiss MicroImaging, Jena, Germany).
2.8. Regenerant Plant Ploidy Determination by Leaf Stomata Guard Cell Chloroplast Counting
The epidermis located on the abaxial side of leaves was removed and washed in distilled water, placed on a slide in a drop of water and covered with a coverslip. The obtained preparations were examined under a fluorescence microscope: Axio Imager A2 with an Axio-Cam MRc5 camera and a set of filters 14: EX BP 510/560, BS FT 580, EM LP 590 (Carl Zeiss Microscopy GmbH, Jena, Germany). Stomata guard cell chloroplasts exhibiting strong red autofluorescence (680 nm) were imaged with 100× oil immersion lens. The number of chloroplasts located in two guard cells was counted in at least 10 stomata for each plant.
2.9. Statistical Analysis
Ovule induction capacity was determined as an average number of induced ovules per 100 cultured ovules. Different durations of cold pretreatment, different concentrations of TDZ, sucrose, and different consistency of nutrient medium were tested. The above conditions were tested in all genotypes in five replicates. In each replication, vessels with 35 ovules were used. The number of regenerated microshoots was determined as the average number of microshoots per induced ovule obtained in five replications for each genotype. Experimental data analysis and statistical evaluation were performed in Microsoft Excel 2016 for Windows 10 and Statistika 7.0.
3. Results
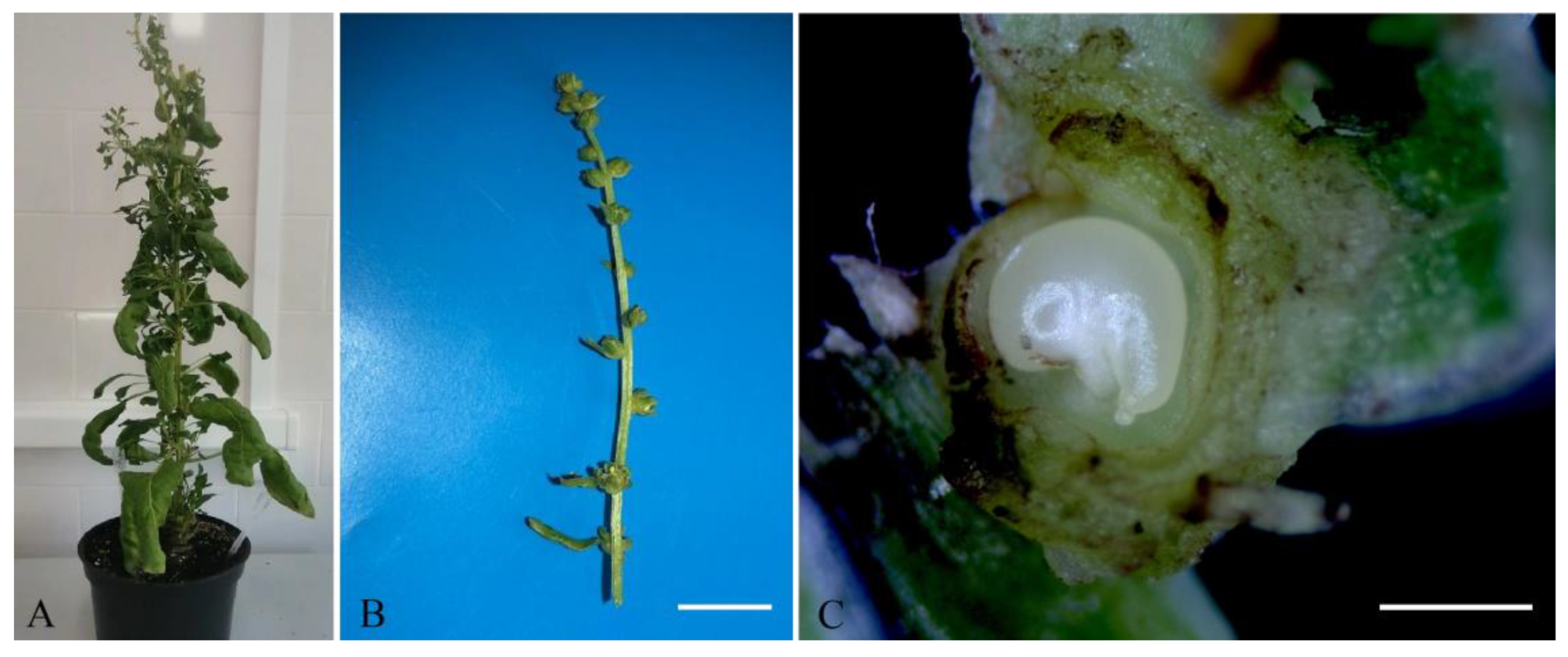
The optimum stage for in vitro unpollinated sugar beet ovule cultivation is when the ovules are comma-shaped and contain an almost or completely mature germinal sac. Such ovules are located in the buds above the opened flower on a 2–5 cm-long spike-like peduncle (Figure 1B,C).
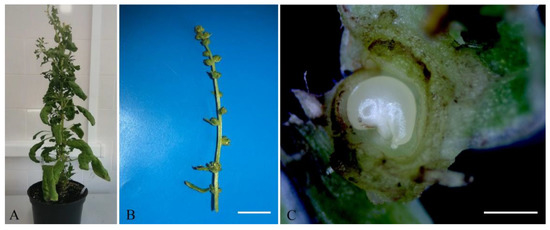
Figure 1.
The appearance of sugar beet plants b.a. 37130: (A) a donor sugar beet plant grown in an environmental chamber; (B) a spike-like inflorescence of sugar beet with buds containing ovules for induction of gynogenesis in vitro, scale bar = 1 cm; (C) a comma-shaped sugar beet ovule in a bud suitable for induction of gynogenesis, scale bar = 1 mm.
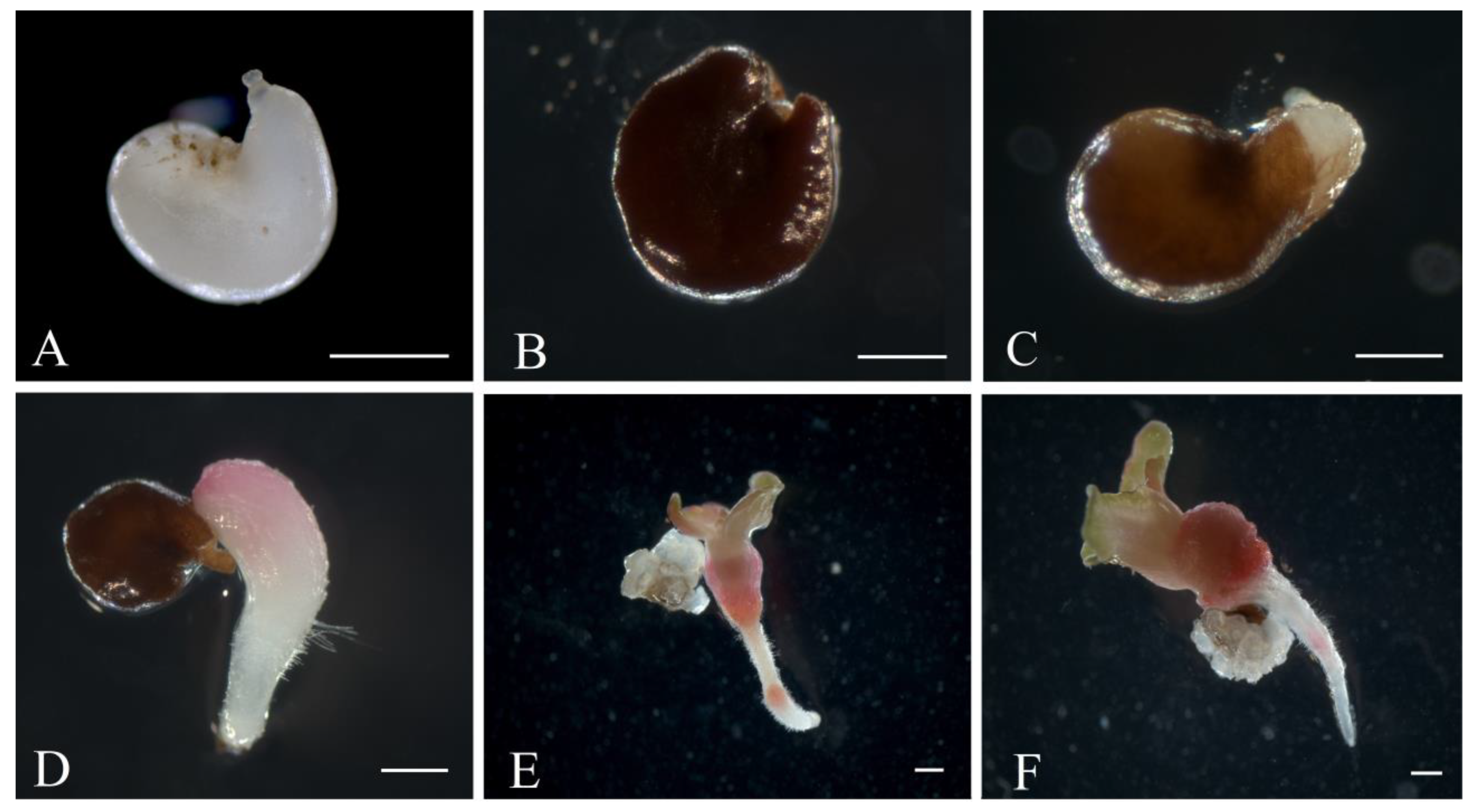
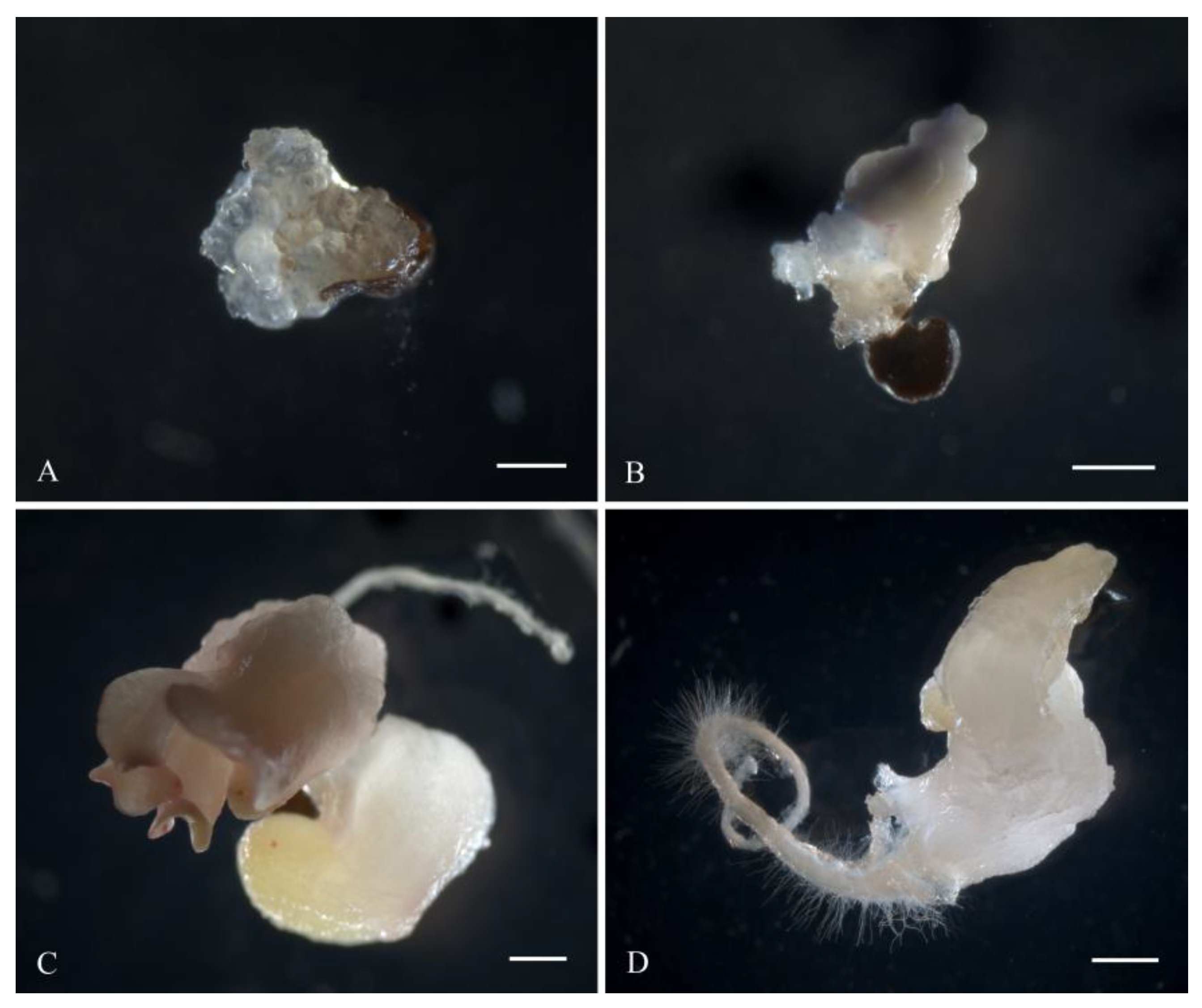
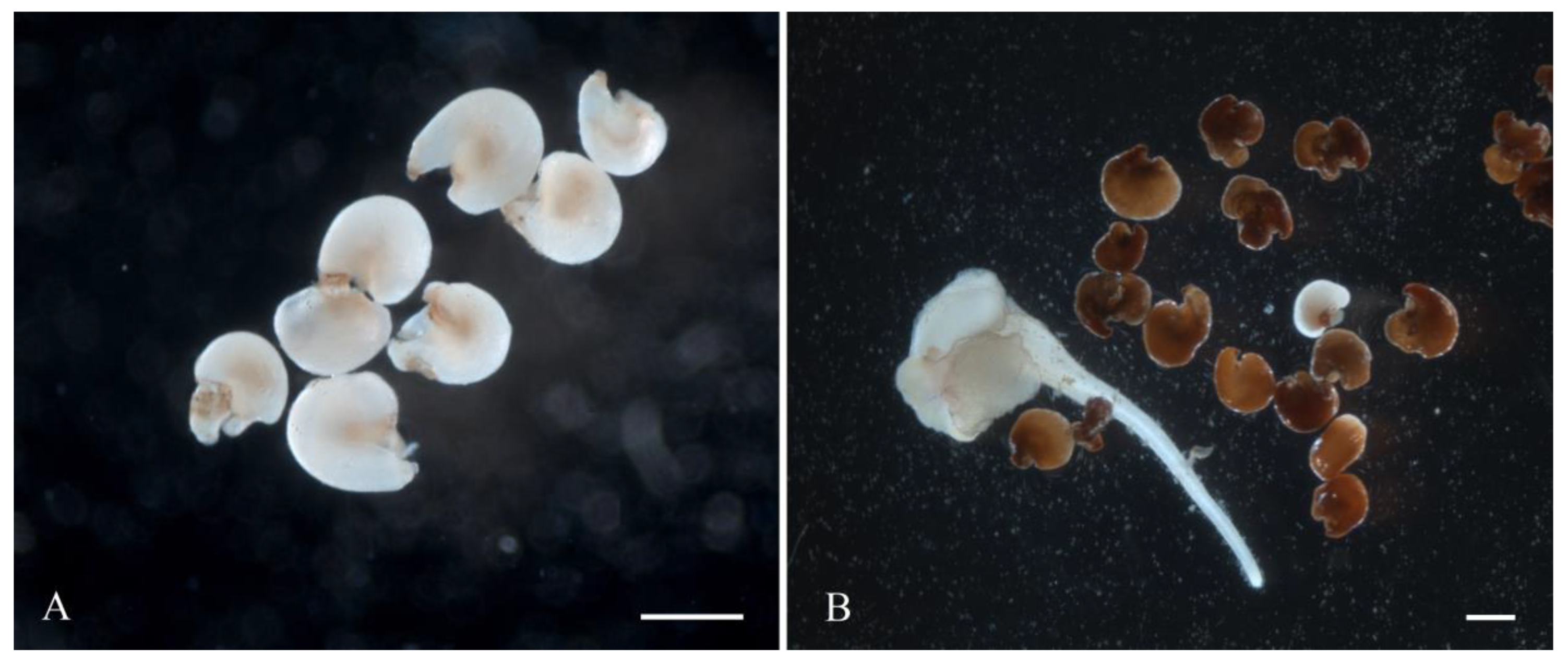
White sugar beet ovules (Figure 2A) changed their color to light brown in three weeks of cultivation. After four weeks, the ovules further darkened to brown (Figure 2B). At the fifth week of cultivation, a rupture of the induced ovule occurred in the micropylar end, and the protrusion of white dense tissue was observed (Figure 2C). After five to six weeks of cultivation, embryoids were formed from the induced ovules in all genotypes as a result of germ sac cell proliferation and dedifferentiation. Embryoid formation could be observed and identified under a stereomicroscope by light pink (Figure 2D) or white structures (Figure 3D), which subsequently increased in size to form a primary root and cotyledon leaves (Figure 2E). After transferring the embryoid structures for cultivation at a photoperiod of 16/8 h (day/night), greening of the cotyledon and hypocotyl sections of the embryoids and further growth and increase in size were observed (Figure 2F). In rare cases, callus structures developed independently from a single ovule (Figure 3A), and the origin of the callus cells could not be determined. The formation of embryoid structures was often accompanied by the simultaneous development of callus sections from a single ovule (Figure 3B). In some cases, the callus structures stopped growing and underwent developmental depression followed by cell death. Occasionally, polyembryony was observed in a single ovule, resulting in the formation of fused embryoids that subsequently failed to develop and died off (Figure 3C).
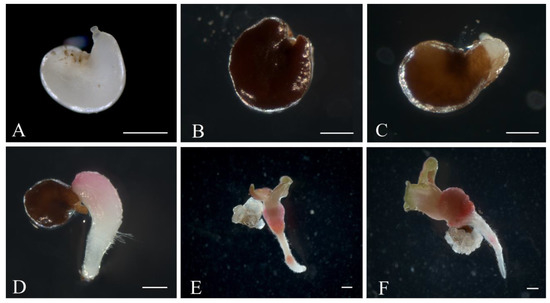
Figure 2.
Embryoid induction in an unpollinated sugar beet ovule culture: (A) sugar beet ovules immediately after isolation in growth medium; (B,C) ovules after 4–5 weeks of cultivation; (D) embryoid formation at the micropylar end of the ovule; (E,F) embryoid structure developing from the ovules after six weeks of cultivation. Scale bars = 1000 µm.
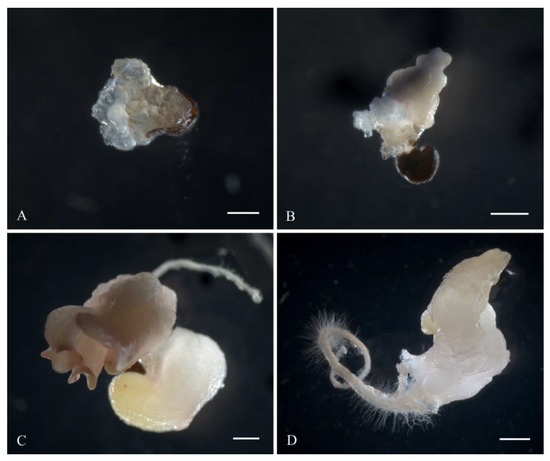
Figure 3.
Variants of gynogenic development from induced beet ovules in vitro: (A) formation of callus structures; (B) simultaneous development of callus and embryo-like structures from one induced ovule; (C) polyembryony; (D) formation of white embryoids. Scale bars = 1000 µm.
3.1. Testing Effects of Different Medium Component Concentration and Temperature Stress Pretreatment on Gynogenesis in Different Genotypes
We tested TDZ effects on gynogenic activity of sugar beet ovules. We added TDZ to solid induction medium IMB at the concentrations of 0.4 mg/L or 0.8 mg/L. The use of TDZ at a concentration of 0.4 mg/L in solid IMB medium resulted in the highest number of induced ovules in all genotypes, with the b.a. 37130 genotype having the highest induction rate of 16.7% (Table 1).

Table 1.
The effect of TDZ on the induction capacity of sugar beet ovules.
When TDZ was used at a higher concentration of 0.8 mg/L, it caused a statistically significant decrease in gynogenesis induction in two sugar beet genotypes: b.a. 37130 and b.a. 37131. The induction capacity decreased by 1.9 in b.a. 37130 and was completely absent in b.a. 37131. The results also showed a trend for a lower gynogenesis induction in b.a. 36764 (1.5-fold decrease). Two-factor ANOVA confirmed a significant effect of TDZ concentration and genotype on induction in sugar beet unpollinated ovules in vitro. The proportion of the TDZ concentration effect was 31.5%.
Next, we studied the impact of different sucrose concentrations on gynogenesis. The induction efficiency of all tested genotypes depended on the concentration of sucrose in the IMB nutrient medium. We tested 2%, 5%, and 8% sucrose concentrations and demonstrated that the highest ovule induction was achieved when 5% sucrose concentration was used. The results were consistent for all tested genotypes. When 5% sucrose was used, induction efficiencies ranged from 4.7% in b.a. 37131 and 6% in b.a. 36764 to the highest induction level of 12.7% in b.a. 37130 (Table 2).

Table 2.
Effect of sucrose concentration on the induction capacity of sugar beet ovules on IMB medium with 0.4 mg/L TDZ in an in vitro culture of unpollinated ovules.
In all genotypes, 5% sucrose concentration had a positive statistically significant effect on the number of induced ovules. Two-factor ANOVA confirmed a significant effect of sucrose concentration and genotype on induction in sugar beet unpollinated ovules in vitro. The effects from sucrose concentration and genotype factors were 63.1% and 10.8%, respectively. Genotypic specificity has a significant effect when sucrose is used at concentrations of 2% and 5%, which was confirmed statistically, and no significant differences between genotypes were found at sucrose concentration of 8%.
Reducing sucrose concentration to 2% or increasing it to 8% had negative effects on gynogenesis. The reduction in sucrose concentration to 2% resulted in either a 6.3-fold decrease in the number of responsive ovules in b.a. 37130 or a complete suppression of induction in b.a. 37131 and b.a. 36764. Increasing the concentration to 8% resulted in a 4.6-fold decrease in the number of responsive ovules in b.a. 36764 or a complete suppression of induction in b.a. 37130 and b.a. 37130.
We tested the efficiency of the bud pretreatment with cold temperature and found that the highest gynogenesis induction in all genotypes was achieved in the control without pretreatment. The best result of induction (16.0%) was observed for genotype b.a. 37130 (Table 3). The lack of stimulating effect of temperature pretreatment of buds on ovule induction activity was statistically significant in all genotypes. Genotypic specificity was observed in experiments without temperature pretreatment of buds and in cases of cold pretreatment of buds for four days. Bud pretreatment at low positive temperatures (5–6 °C) for four days can be used to induce gynogenesis, but in this case a decrease in the proportion of induced ovules was observed in genotype b.a. 37130 by a factor of 2.4, for genotype b.a. 36764 by 4, and in genotype b.a. 37131 complete absence of induction of gynogenesis. Application of bud pretreatment with cold (5–6 °C) for two and six days had no stimulating effect on the ovule induction capacity of any genotypes. Two-factor ANOVA confirmed a significant effect of the bud pretreatment with cold temperature and genotype on induction in sugar beet unpollinated ovules in vitro. The effects from the cold pretreatment and genotype factors were 47.2% and 18.6%, respectively.

Table 3.
Effect of bud pretreatment with cold (5–6 °C) on induction capacity of sugar beet ovules on IMB medium with 0.4 mg/L TDZ in unpollinated ovule culture in vitro.
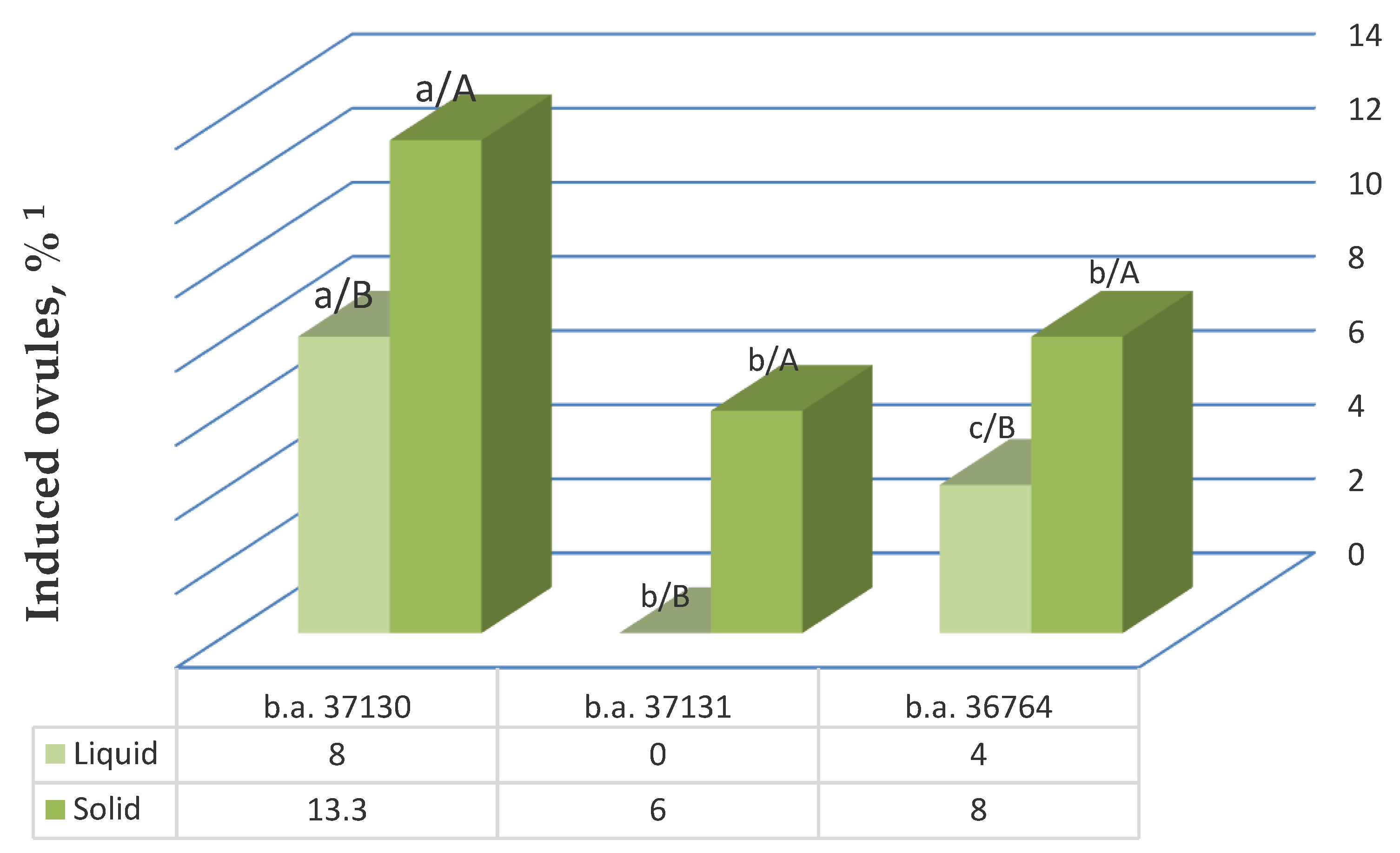
Next, we tested whether addition of the gelling agent to the IMB medium with 0.4 mg/L TDZ affected gynogenesis. We found that the induction activity of sugar beet ovules differed significantly in liquid and solid nutrient medium. In all three genotypes, the best gynogenic development results were achieved on solid nutrient medium and ranged from 6% in b.a. 37131 to 13.3% in b.a. 37130 (Figure 4).
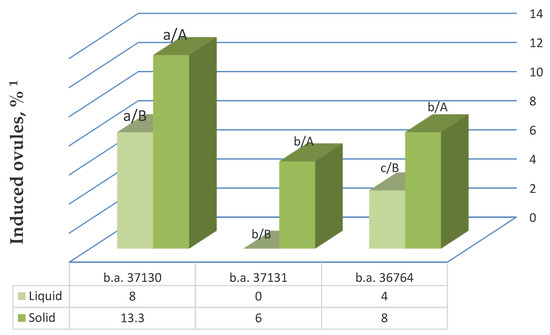
Figure 4.
The effect of addition of gelling agent to the nutrient medium with 0.4 mg/L TDZ on the unpollinated ovule gynogenesis induction in different sugar beet genotypes. Note: 1 The values presented are averages of five replicates; values with the same lowercase letter (comparison of all genotypes in one nutrient medium type) and capital letter (comparison between different nutrient medium options for one genotype) in the chart bars are not significant differences with 95% probability according to the Duncan test (MRT).
The use of liquid induction medium was less effective in all genotypes and resulted in a decrease in the yield of responsive ovules in the most responsive genotypes—b.a. 37130 by a factor of 1.7 and genotype b.a. 36764 by a factor of 2—and it led to the complete absence of induction of gynogenesis in b.a. 37131. The observed differences in the stimulating effect of liquid and solid nutrient media on the yield of responsive ovules were statistically significant in all genotypes. A two-factor ANOVA confirmed a significant effect of the gelling agent addition, as well as genotype on the induction of gynogenesis in vitro. The influence of nutrient medium type and genotype was 34.5% and 52.7%, respectively.
We found that the induction of gynogenic development on liquid nutrient medium happened via direct embryogenesis without the formation of callus structures (Figure 5). The initial period of embryoid growth and development on liquid and solid nutrient media did not differ morphologically. The earliest formation of embryoid structures on liquid medium occurred on the fifth week of cultivation similar to solid nutrient medium. An essential factor determining the success of further development of embryoid structures formed on liquid nutrient medium into viable regenerant plants is the timely transfer of the formed embryoids to solid regeneration nutrient medium under light conditions. Otherwise, the formation of nonviable vitrified embryoids, which lose their ability to grow and develop further and die when transferred to light conditions, was observed.
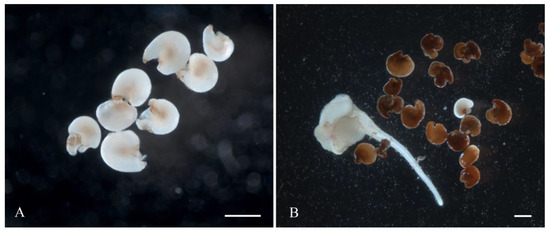
Figure 5.
Induction of gynogenic development of sugar beet through direct embryogenesis on liquid IMB medium with 0.4 mg/L TDZ: (A) isolated ovules on day 3 of cultivation; (B) embryoid on day 35 of cultivation. Scale bars = 1000 µm.
3.2. The Formation of Regenerating Plants
In all genotypes studied, gynogenic development proceeded through direct embryogenesis. The callus structures formed in rare cases from induced ovules, even after successive subcultures on regeneration medium with 1 mg/L BAP and 0.1 mg/L GA3 under light conditions, did not initiate further growth, formation of meristematic regions, or shoot regeneration. Instead, it was followed by degeneration and death. In the case of direct embryogenesis, the embryoids formed by the induction of gynogenesis after placement on regenerating nutrient medium with 1 mg/L BAP and 0.1 mg/L GA3 developed with the formation of microrosettes (Figure 6A). Additional microrosettes, formed by overgrowth of cotyledon and hypocotyl regions, separated by successive subculturing on fresh, hormone-free MS nutrient media with 2% sucrose and 3% phytagel and formed large rosettes with six to eight well-developed leaves (Figure 6B). All leaf rosettes showed development of the root system. Therefore, no additional rooting step was required (Figure 6C,D). All of the genotypes tested were able to initiate regeneration and produce microshoots/microrosettes (Table 4). The highest morphogenetic activity was observed in b.a. 37130, which was able to produce regenerant plants from the majority of induced ovules, with an average of 7.2 microshoots produced per ovule (Table 4). In total, we obtained 31 ex vitro regenerant plants from b.a. 37130.

Figure 6.
Plant regeneration in sugar beet unfertilized ovule culture and adaptation to ex vitro conditions: (A) regeneration of shoots from embryoids; (B–D) development of sugar beet regenerant plants under in vitro conditions; (E,F) the haploid sugar beet plant adapted to ex vitro conditions; (G) sugar beet taproot of haploid sugar beet plant ready for germination; (H) germination of sugar beet taproots at 4–5 °C.

Table 4.
Regeneration capacity of different sugar beet genotypes from induced ovules.
A two-factor ANOVA confirmed a significant effect of genotype on the regeneration ability of sugar beet cultivars with a factor influence of 92.9%. Low regenerative capacity in all the genotypes was due to the formation of vitrified, deformed leaf rosettes after a number of successive subcultivations. The adaptation period of the regenerant plants from the b.a. 37130 and b.a. 36764 genotypes with a well-formed leaf rosette and developed root system, planted in peat mixture, to the ex vitro conditions was two to four weeks (Figure 6E,F). After a further 2–3 months of rearing, 24 plants of the two genotypes b.a. 37130 and b.a. 36764 grew fully formed sugar beet taproots weighing from 50 to 115 g (Figure 6G). They were placed in the root storage facility for germination at 4–5 °C for four months (Figure 6H).
3.3. Ploidy-Level Analysis of Sugar Beet Regenerant Plants Obtained in an Unpollinated Ovule Culture In Vitro
To determine the ploidy level of sugar beet regenerant plants obtained in the culture of unpollinated ovules in vitro, three methods were used: flow cytometry of cell nuclei, direct counting of chromosomes in apical meristems, and counting the number of chloroplasts in the stomata closing cells.
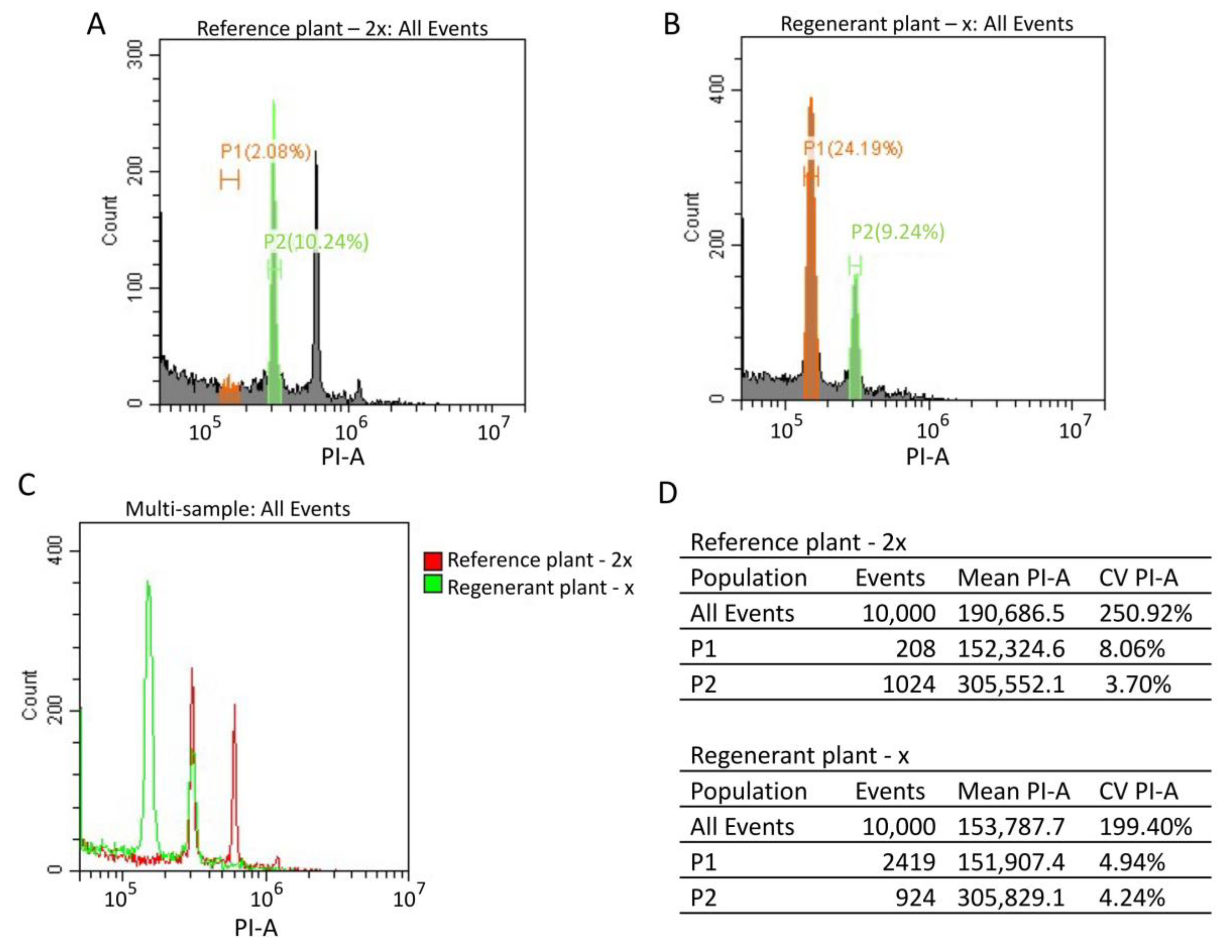
For cytometric ploidy evaluation, we chopped reference and regenerant plants in lysis buffer with a razor blade. Then, we filtered nuclei and stained them with PI. We generated reference standard and regenerant histograms (Figure 7A,B). The overlay of the reference standard and regenerant histograms showed that the regenerant G2 peak overlays with the reference G0/G1 peak (Figure 7C). The regenerant G0/G1 peak mean value is two times smaller than the control G0/G1 peak mean value (mean (P2control)/mean (P1regenerant) = 2.01) (Figure 7D). These results demonstrate that the regenerant is a haploid plant (2n = x = 9). Similar results were observed for all regenerant plants.
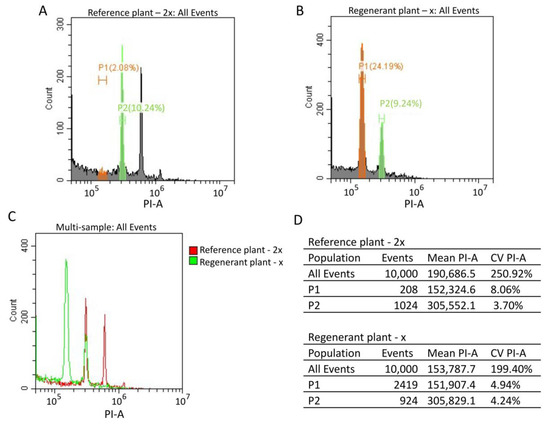
Figure 7.
Cytometric analysis of the reference standard (2n = 2x = 18) sugar beet plant and regenerant plants obtained from an in vitro culture of unpollinated ovules. (A,B) Isolated nuclei from reference and regenerant plants were stained with PI. The reference standard (A) and regenerant (B) histograms were generated by flow cytometry. G0/G1 populations were selected for the reference standard and regenerant plants (green P2 and orange P1 populations, respectively) (A,B). (C) The reference standard (red) and regenerant (green) histogram overlay. (D) The reference standard and regenerant histogram statistics tables, including the number of events, mean and CV values for all events, P1 and P2 populations.
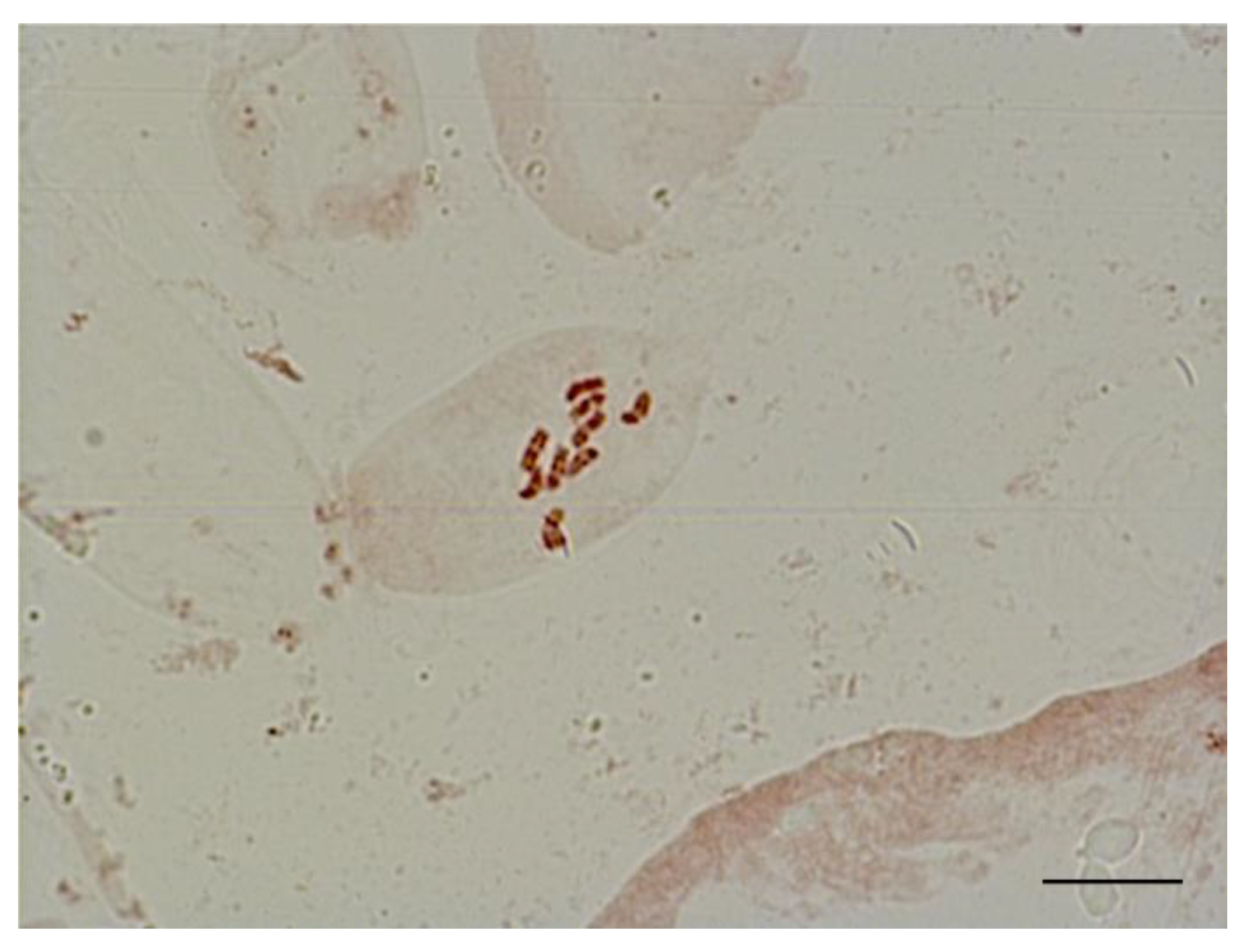
For karyological analysis, we isolated apical meristem cells of sugar beet plants obtained in an unpollinated ovule culture. We stained chromosomes using the propion-lacmoid method and counted the number of chromosomes in metaphase plates. We found that all regenerant plants had a single set of chromosomes (2n = x = 9) (Figure 8).
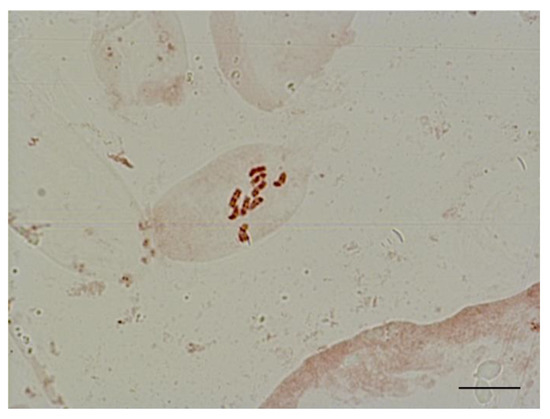
Figure 8.
Metaphase in shoot apical meristem of haploid sugar beet regenerant plants (2n = x = 9), obtained in an unpollinated ovule culture in vitro. Scale bar = 10 µm.
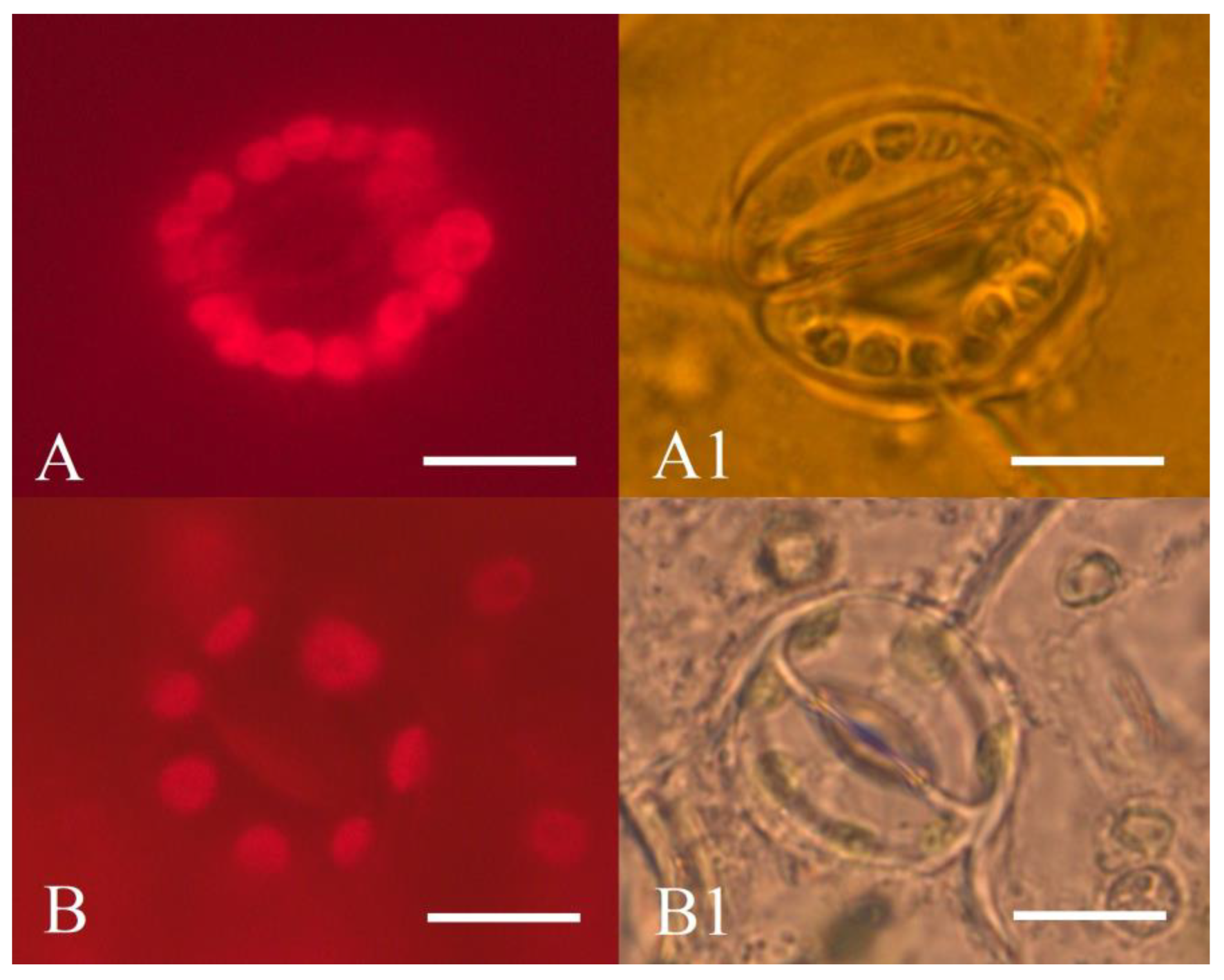
It was reported that haploid plants have a reduced number of chloroplasts compared to diploid plants. For instance, diploid sugar beets have 12–17 chloroplasts in stomata guard cells, but the chloroplast number is reduced to 6–8 in haploid plants [68]. We imaged stomata guard cells of abaxial leaf surface epidermis and found that diploid donor plants had 12–20 chloroplasts, while all regenerant plants contained from 7 to 11 chloroplasts (Figure 9).
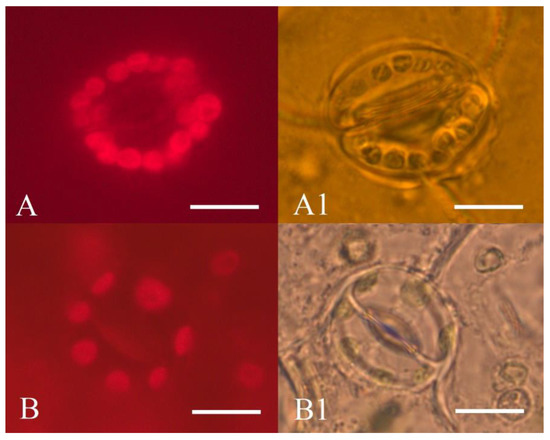
Figure 9.
Epidermis of abaxial leaf surface of sugar beet plants: (A,A1)—diploid plant (2n = 2x = 18); (B,B1)—haploid regenerant plant (2n = 1x = 9), obtained in an unpollinated ovule culture in vitro; (A,B)—chloroplast autofluorescence of sugar beet stomata guard cells (680 nm); (A1,B1)—sugar beet stomata guard cells. Scale bars = 10 µm.
In conclusion, we demonstrated by three different methods that all 24 sugar beet regenerant plants that successfully passed adaptation to ex vitro conditions were haploids.
The ploidy of the obtained regenerant plants determined by various experimental methods manifested phenotypically. The haploid sugar beet regenerants obtained in gynogenesis culture were characterized by a larger number of leaves, narrower leaf plates and underdeveloped root system.
4. Discussion
The great economic importance of sugar beet necessitates the attention of researchers from a number of international scientific institutions to study this crop. Over the past few decades, significant scientific advances have been made in optimizing in vitro protocols for direct and callus-mediated regeneration, obtaining homozygous lines, somatic hybridization and genetic transformation of sugar beet [69], creating doubled haploids (DH) of this crop, and accelerating sugar beet breeding processes [59]. Since the discovery of the first haploid sugar beet plant [70], the development and improvement in obtaining doubled haploids of sugar beet from unfertilized ovules in vitro has been pursued worldwide [41,51,52,54,56,58,59,71]. Despite the advances in in vitro cultivation of sugar beet cells and tissues and the development of protocols for producing doubled haploids, there is no universal technique for producing DH-plants of this culture. The protocols are not productive and are not suitable for routine haploid induction [44].
The main factors limiting the use of doubled sugar beet haploids are the negligible gynogenesis efficiency of 1% to 15%, the low yield of regenerant plants of 40% [44,51] and high genotype dependence [72]. Furthermore, successful induction of gynogenesis in all cultures critically depends on the determination of the necessary stage of female gametophyte development. It is known that ovules containing almost mature or fully mature seven- to eight-kernel germ sacs are most responsive in in vitro culture and more easily pass from the gametophytic to sporophytic development [45,47]. According to the literature, neither the size of the ovaries nor the size of the buds predict the efficiency of the ovary induction [46]. Sugar beet buds collected 1–3 days before flowering already contain fully mature female gametophytes and are suitable for introduction into gynogenesis culture in vitro. [73]. In contrast to androgenesis in vitro, ovules can be responsive in gynogenesis culture in a wide range of developmental stages [60]. Dubois et al. (1990) [74] confirmed by cytological analysis that complete maturation of the ovules is not necessary for gynogenesis induction. In a study by Van Geyt J. et al. [47], only ovules with a typical comma shape were responsive, but young spherical ovules degenerated after a few days of cultivation. In our research, we used the ovule shape to detect the stage suitable for gynogenesis. For this purpose, a certain section of the flower stalk with a group of buds in which they would be contained was isolated. It was found experimentally that this fragment of the inflorescence is convenient to choose, focusing on the first opened bud. In our studies with all genotypes, we used only comma-shaped ovules isolated from the buds collected from the 2–5 cm-long section of the spike-like peduncle immediately above the opened bud.
After introduction of explants onto nutrient media, we observed a change in size and color of the ovules from white to brown, consistent with the literature [47]. Formation of gynogenic plants started from an embryo-like structure developing from the micropylar part of the ovule [47,75]. According to the literature, most haploid sugar beet plants in unpollinated ovule culture in vitro are formed by direct embryogenesis [44] directly from the ovule [73]. In other species, in addition to oocytes, synergids and antipodes are also capable of embryogenesis or callus formation [76]. There are reports of gynogenic development of sugar beet in vitro through indirect organogenesis [77] and callus growth without shoot regeneration [47]. Galatowitsch and Smith (1990) [55] reported regeneration of well-formed sugar beet shoots from abnormal shoots developing on the surface of organogenic callus, with a callus formation rate of only 1%. In our study, formation of embryoids was predominantly observed, whereas in the case of table beet, we observed formation of both embryoids and callus [64].
Optimization of the composition and concentration of nutrient media components plays an important role in increasing the efficiency of induction and regeneration processes in uninoculated sugar beet ovule culture in vitro. There are a number of factors that have been successfully used to stimulate the gynogenic response of some crops, but the possibility of using them in in vitro cultivation of unpollinated sugar beet ovules has not yet been studied and published in the literature. This applies to the use of TDZ in sugar beet gynogenesis culture in vitro. To our knowledge, TDZ has not yet been applied to sugar beet ovule culture either as a pretreatment or in culture medium; only somatic embryogenesis data exist [63]. According to the literature, solid MS nutrient medium [65] [47,49,58,59] supplemented with cytokinin BAP [44] was most commonly used for induction of sugar beet gynogenesis. Thus, Pazuki et al. [41] reported an excess of double the yield of responsive ovules on nutrient medium with 2 mg/L BAP compared to medium without growth regulators and achieved an induction of 10.75%. In Gürel et al. (2000) [42] increasing the BAP concentration from 1 mg/L to 2 mg/L resulted in an increase in sugar beet induction from 7.2% to 9.6%. W.E. Wremerth and Levall M. W. (2003) [51] in their protocol for effective induction of sugar beet embryogenesis recommended a combination of 0.3 mg/L BAP and 0.05 mg/L 2.4-D growth regulators. Lux et al. [49] used a combination of 2 mg/L BAP and 2 mg/L IBA with a haploid embryogenesis efficiency of 7.5%. Sohrabi et al. (2021) [58] used a combination of 0.2 mg/L BAP and 0.5 mg/L NAA (naphthalene acetic acid) in their studies. Cultivation of beet ovules showed the highest responsiveness in nutrient medium N6 [78] with 0.5 mg/L IAA (indole-3-acetic acid) and 0.2 mg/L BAP as growth regulators [79]. There are also data on the use of 0.05 mg/L and 0.5 mg/L kinetin with sugar beet gynogenesis efficiencies of 7.58% and 10.05%, respectively [52]. In a recently published protocol for obtaining gynogenic table beet plants, nutrient medium B5 containing 0.2 mg/L BAP and 0.5 mg/L NAA was used at the induction stage [80].
The successful use of TDZ, as a cytokinin, in tobacco cell culture and in cotton callus culture has been reported since its initial discovery as a defoliant for cotton [81]. TDZ is a phenylurea herbicide with strong cytokinin-like activity exceeding that of zeatin [82] and is used to induce organogenesis in clonal micropropagation in woody plants, grain legumes and peanuts [83,84,85]. The increased efficacy of TDZ compared with BAP in the organogenesis system may be related to the greater stability of TDZ, to the indirect accumulation of purine cytokinins [86,87], and to the inhibition of cytokinin oxidase [88]. However, TDZ can also cause developmental abnormalities, including vitrification in some species [84,85]. TDZ is also another widely used growth regulator in induction and regeneration media to improve gynogenic response [35]. For example, TDZ has been successfully used in the culture of unpollinated cucumber ovules to enhance ovule induction activity [61,62,89]. In carrot ovule cultivation, addition of 9.08 µM TDZ resulted in the highest embryoid yield of 2.14% at the same time as a high callus formation rate of 1.69% [90]. It is known that in sugar beet somatic cell culture, the use of TDZ at 2.3 ± 4.6 mM was more effective than BAP in stimulating the formation of adventitious shoots [63].
Our study was initiated in 2019 on selectively valuable sugar beet genotypes and aimed to investigate factors of gynogenesis induction and regeneration of sugar beet plants through unpollinated ovule culture in vitro. Based on literature data, in the unpollinated sugar beet ovule culture in vitro, solid nutrient media were most commonly used. Tomaszewska-Sowa et al. (2022) [54] reported the use of an innovative two-step method involving culturing ovules on liquid MS induction medium containing 4.4 lmol/L (BAP) followed by transfer of regenerating structures to solid nutrient medium with 4.4 lmol/L BAP. Ovule induction capacity of all three sugar beet genotypes studied in our work was significantly different when TDZ at concentrations of 0.4 mg/L and 0.8 mg/L was used as part of IMB solid induction nutrient medium. In experiments with 0.4 mg/L TDZ, induction of gynogenesis was greatest in all genotypes and ranged from 8% to 16.7%. The use of TDZ at a concentration of 0.8 mg/L resulted in inhibition of gynogenesis induction, or a decrease in induction by a factor of 1.5 to 1.9 depending on the genotype. The use of liquid induction medium IMB with 0.4 mg/L TDZ in our studies was less effective in all genotypes and resulted in up to 8% of responsive ovules, which is half as much as in solid medium.
Genotype plays a decisive role in the production of doubled haploids of sugar beet in an in vitro culture of unpollinated ovules [35,41,44,49,52]. The experiments confirmed a significant effect of genotype on gynogenesis and regeneration induction in an in vitro culture of unpollinated ovules. The highest induction (16.7%) and regeneration responsiveness (up to 7.2 microshoots per ovule) were observed in the genotype b. a. 37130. So far, there is only speculation as to how the genotype determines the gynogenesis-sensitive phenotype. For example, it has been suggested that genotypic dependence of this trait is related to allogamy in sugar beet [52]. The effects of genotypic variability not only limit the intensity of gynogenesis induction but also affect regenerative ability [32]. Thus, the percentage of plants grown from induced embryoids according to different study groups was 25% [49], 10% [48], 10.9% [45], 36.9% [75], 43.8% [60], and 36.1% [53].
In in vitro culture of plant cells and tissues, carbohydrates, which have important functions in growth and development, are key substrates for respiration or secondary metabolism in a number of biochemical processes as sources of carbon as building materials and storage carbohydrates [91]. They can also protect against osmotic stress as osmoprotectors [92]. The choice of the appropriate type of carbohydrate depends on the plant species and the type of explant, sucrose being the most commonly used. Sucrose in in vitro cell and tissue cultures is usually used in a concentration of 2–3% [35]. In unpollinated ovule cultures in vitro, there is a general tendency to use nutrient media with a higher carbohydrate content for the induction of embryogenesis and a lower one for regeneration [93]. In gynogenesis culture of plants of the family Graminaceae, e.g., barley, sucrose is used in the highest concentrations (12% or 14%) [94]. Thus, in a recently published protocol for obtaining doubled haploids in unpollinated ovule culture and in earlier studies in in vitro ovary culture, the use of sucrose at a concentration of 3% was noted [47,59,95]. In sugar beet gynogenesis culture, higher concentrations of this carbohydrate are possible: 6% [32,96], 8% [51], or 10% [42,49,72]. Hosemans and Bossoutrot (1983) [45] reported an embryonic development rate of 4.4% when using 8% sucrose. Some authors believe that the most optimal sucrose concentration required to achieve maximum induction of sugar beet gynogenesis is 60 g/L [58,72]. In several studies, increasing sucrose content to 9–10% had no stimulating effect on the number of responsive ovules in sugar beet gynogenesis culture [46,50]. In Sohrabi (2021) [58], a concentration of 90 g/L was found to be toxic to ovules, inhibiting embryo and shoot formation. The authors noted that with increasing carbohydrate concentration, the potential for ovule development in gynogenesis culture decreases [58]. For table beet, the use of nutrient media with 60 g/L sucrose [79] and 55 g/L sucrose was successful [64]. The most optimal sucrose concentration during the shoot regeneration stage in gynogenesis culture is 20 g/L [49,51]. In our studies, the highest efficiency in gynogenesis culture was achieved at a sucrose concentration of 5%. A reduction to 2% or an increase to 8% was not effective.
Several physical environmental factors, including high and low temperatures and high osmotic pressure applied to either donor plants or cultivated explants, can have a significant effect on the induction of embryogenesis [35]. The application of cold stress has been successfully used to induce gynogenesis in sugar beet buds [32,42,44]. Lux et al. (1990) [49] reported that pretreatment of sugar beet buds with cold at 4 °C for 4 or 5 days in the dark resulted in the highest embryo yield; increasing the treatment duration to 7 days was less successful. The highest level of gynogenesis in a study by Pazuki (2017) [41] was achieved when ovules that were treated for one week in the cold were cultured on medium containing 2 mg/L BAP. More recently, cold pretreatment for one week in combination with kinetin at a concentration of 0.05 mg/L or 0.5 mg/L was shown to be effective by these authors [52]. In our study, cold pretreatment of buds had no stimulating effect on the induction ability of ovules of any genotypes, and the highest efficiency of gynogenesis induction in all genotypes was achieved in the control variant without pretreatment.
One of the important steps in the technology of sugar beet doubling haploids is to obtain seed progeny from gynogenic plants. The process of formation of plants in the second year of vegetation and seed setting can be influenced by the biological characteristics of sugar beet, associated with the two-year cycle of development of this crop, inbred depression and the degree of ploidy of plants. In sugar beet, the majority of plants obtained in an unpollinated ovule culture in vitro are haploids [53]; only 2–10% of gynogenic plants may have spontaneously doubled chromosome sets [51]. All regenerant plants obtained in our study possessed a haploid set of chromosomes, which was confirmed using flow cytometry and direct counting of chromosomes and chloroplasts in stomatal guard cells of epidermal stomata of leaves of gynogenic sugar beet plants. In the case of fertility, all gynogenic lines, both on their own and as a component of hybrids, will be a valuable source material for sugar beet breeding.
5. Conclusions
In this study, the possibility of using TDZ in solid and liquid nutrient media IMB as an agent stimulating mainly direct embryogenesis in sugar beet unfertilized ovule culture in vitro was shown. The incorporation of TDZ at a concentration of 0.4 mg/L into solid IMB induction medium with 50 g/L sucrose resulted in the highest yield of responsive sugar beet ovules up to 16.7%. The highest efficiency of gynogenesis induction in all studied sugar beet genotypes was achieved in the variant without the use of pretreatment of buds by cold. It was found that sugar beet embryo development was successful, with formation of microrosettes on MS regeneration nutrient medium with 1 mg/L BAP and 0.1 mg/L GA3. For successful formation and development of root systems in forming sugar beet microshoots, sequential subcultivation on MS hormone-free nutrient medium is necessary. The established protocol for obtaining sugar beet haploids by induction of gynogenesis in in vitro cultures from unfertilized ovules is promising, as all regenerated plants are haploid.
Author Contributions
Conceptualization, T.Z. and K.A.; methodology, E.D.; chromosome analysis, L.K.; investigation, T.V., O.R., Y.T., A.M., V.Z., A.E. and M.F.; data curation, T.Z. and K.A.; writing—original draft preparation T.Z. and K.A.; writing—review and editing, E.D.; visualization, A.E., L.K. and M.F. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Not applicable.
Acknowledgments
The authors express special gratitude to Aleksey V. Logvinov and Vladimir N. Mishchenko for the initial sugar beet breeding material provided.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Letschert, J.P.W.; Lange, W.; Frese, L.; Van Der Berg, D. Taxonomy of the section Beta. J. Sugar Beet Res. 1993, 31, 69–85. [Google Scholar] [CrossRef]
- Lange, W.; Brandenburg, W.A.; De Bock, T.S.M. Taxonomy and cultonomy of beet (Beta vulgaris L.). Bot. J. Linnean Soc. 1999, 130, 81–96. [Google Scholar] [CrossRef]
- Hassani, M.; Heidari, B.; Dadkhodaie, A.; Stevanato, P. Genotype by environment interaction components underlying variations in root, sugar and white sugar yield in sugar beet (Beta vulgaris L.). Euphytica. 2018, 79, 214. [Google Scholar] [CrossRef]
- Valli, V.; Gomez-Caravaca, A.M.; Di Nunzio, M.; Danesi, F.; Caboni, M.F.; Bordoni, A. Sugar cane and sugar beet molasses, antioxidant-rich alternatives to refined sugar. J. Agric. Food Chem. 2012, 60, 12508–12515. [Google Scholar] [CrossRef] [PubMed]
- Koga, N. An energy balance under a conven tional crop rotation system in northern Japan: Perspectives on fuel ethanol production from sugar beet. Agric. Ecosyst. Environm. 2008, 125, 101–110. [Google Scholar] [CrossRef]
- Gerbens-Leenesa, W.; Hoekstraa, A.Y.; Van der Meerb, T.H. The water footprint of bioenergy. Proc. Nat. Acad. Sci. USA 2009, 106, 10219–10223. [Google Scholar] [CrossRef]
- Asadi, M. Beet Sugar Handbook; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2007; pp. 435–450. [Google Scholar]
- OECD. Section 8—Sugar Beet (Beta vulgaris L.). In Safety Assessment of Transgenic Organisms; OECD Consensus Documents; OECD Publishing: Paris, France, 2006; Volume 1, pp. 174–196. [Google Scholar] [CrossRef]
- Ford-Lloyd, B.V. Sources of genetic variation, Genus Beta. In Genetics and Breeding of Sugar Beet; Biancardi, E., Campbell, L.G., Skaracis, G.N., De Biaggi, M., Eds.; Science Publishers Inc.: Enfield, NH, USA, 2005; pp. 25–33. [Google Scholar]
- De Bock, T.S.M. The genus Beta: Domestication, taxonomy and interspecific hybridization for plant breeding. Acta Hortic. 1986, 182, 335–343. [Google Scholar] [CrossRef]
- Knapp, E. Beta Rüben. In Handbuch der Pflanzenzüchtung; Roemer, T., Rudorf, W., Eds.; Paul Parey: Berlin, Germany, 1958; Volume 3, pp. 196–284. [Google Scholar]
- Fischer, H.E. Origin of the “Weisse schlesische Rübe” (White Silesian beet) and resyntesis of sugar beet. Euphytica 1989, 41, 75–80. [Google Scholar] [CrossRef]
- Shilov, I.A.; Aniskina, Y.V.; Shalaeva, T.V.; Kolobova, O.S.; Velishaeva, N.S.; Mischenko, V.N.; Logvinov, A.V. Creation of modern sugar beet hybrids using microsatellite analysis. Sugar 2020, 8, 27–31. [Google Scholar] [CrossRef]
- Biancardi, E.; McGrath, J.M.; Panella, L.W.; Lewellen, R.T.; Stevanato, P. Sugar Beet. In Root and Tuber Crops, Handbook of Plant Breeding; Bradshaw, J.E., Ed.; Springer: New York, NY, USA, 2010; Volume 7, pp. 173–219. [Google Scholar]
- Smith, G.A. Sugar beet. In Principles of Cultivar Development; Fehr, W.R., Ed.; Macmillan: New York, NY, USA, 1987; Volume 2, pp. 577–625. [Google Scholar]
- Oltmann, W. Verfahren und Erfolge der Zuckerrübenzüchtung seit Mitte des 19. Jahrhunderts bis zur Gegenwart. In Geschichte der Zuckerrübe: 200 Jahre Anbau und Züchtung; Bartens, A., Ed.; Verlag Dr.: Berlin, Germany, 1984; pp. 48–66. [Google Scholar]
- Elliott, M.C.; Weston, G.D. Biology and physiology of the sugar-beet plant. In The Sugar Beet Crop; World Crop Series; Cooke, D.A., Scott, R.K., Eds.; Springer: Dordrecht, The Netherlands, 1993; pp. 37–66. [Google Scholar] [CrossRef]
- Finkenstadt, V.L. A Review on the complete utilization of the sugarbeet. Sugar Technol. 2013, 4, 339–346. [Google Scholar] [CrossRef]
- Mordenti, A.L.; Giaretta, E.; Campidonico, L.; Parazza, P.; Formigoni, A. A Review Regarding the Use of Molasses in Animal Nutrition. Animals 2021, 11, 115. [Google Scholar] [CrossRef] [PubMed]
- Burenin, V.I.; Pivovarov, V.F. Beet; VNIISSOK: St. Petersburg, Russia, 1998; p. 215. [Google Scholar]
- Heikkila, H.O.; Melaja, J.A.; Millner, D.E.D.; Virtanen, J.J. Betaine Recovery Process. U.S. Patent 4,359,430, 16 November 1982. [Google Scholar]
- Kotsiopoulou, N.G.; Liakos, T.I.; Lazaridis, N.K. Melanoidin chromophores and betaine osmoprotectant separation from aqueous solutions. J. Mol. Liq. 2016, 216, 496–502. [Google Scholar] [CrossRef]
- Escudero, I.; Ruiz, M.O. Extraction of betaine from beet molasses using membrane contactors. J. Membr. Sci. 2011, 372, 258–268. [Google Scholar] [CrossRef]
- FAOSTAT. Available online: http://www.fao.org/faostat/ru (accessed on 25 November 2022).
- Lima, I.M.; White, P.M.J. Sugarcane bagasse and leaf residue biochars as soil amendment for increased sugar and cane yields. Int. Sugar J. 2017, 119, 1421. [Google Scholar]
- Patel, H. Environmental valorisation of bagasse fly ash: A review. R. Soc. Chem. Adv. 2020, 10, 31611–31621. [Google Scholar] [CrossRef]
- Giacobello, S.; Storti, G.; Tola, G. Design of a simulated moving bed for sucrose-betaine separations. J. Chromatogr. 2000, 872, 23–35. [Google Scholar] [CrossRef]
- McFarlane, J.S. Variety development. In Advances in Sugar Beet Production; Jonhson, R.T., Alexander, J.T., Bush, G.E., Hawkes, G.R., Eds.; Iowa State University Press: Ames, IA, USA, 1971; pp. 402–435. [Google Scholar]
- Vasilchenko, E.N.; Zhuzhalova, T.P.; Kolesnikova, E.O. Accelerated production of new homozygous sugar beet lines (B. vulgaris L.). Sugar 2020, 2, 30–32. [Google Scholar]
- Abbasi, Z.; Arzani, A.; Majidi, M.M. Evaluation of genetic diversity of sugar beet (Beta vulgaris L.) crossing parents using agromorphological traits and molecular markers. J. Agric. Sci. Technol. 2014, 16, 1397–1411. [Google Scholar]
- Larsen, K. Self incompatibility in Beta vulgaris L. Four gametophytic complementary S-loci in sugar beet. Hereditas 1977, 85, 227–248. [Google Scholar] [CrossRef]
- Svirshchevskaya, A.; Dolezel, J. Production and Performance of Gynogenetic Sugar beet Lines. J. Sugar Beet Res. 2000, 37, 117–133. [Google Scholar] [CrossRef][Green Version]
- De La Fuente, G.N.; Frei, U.K.; Lübberstedt, T. Accelerating plant breeding. Trends Plant Sci. 2013, 18, 667–672. [Google Scholar] [CrossRef] [PubMed]
- Klimek-Chodacka, M.; Baranski, R. Comparison of haploid and doubled haploid sugar beet clones in their ability to micropropagate and regenerate. Electron. J. Biotechnol. 2013, 16, fulltext-3. [Google Scholar] [CrossRef]
- Chen, J.; Li, C.; Malik, A.; Mbira, K.G. In vitro haploid and dihaploid production via unfertilized ovule culture. Plant Cell Tissue Organ Cult. (PCTOC) 2011, 104, 311–319. [Google Scholar] [CrossRef]
- Vjurtts, T.S.; Shmykova, N.A.; Fedorova, M.I.; Zayachkovskaya, T.V.; Domblides, E.A. Creation of doubled haploid lines of carrot (Daucus carota L.) using biotechnological methods. Plant Prot. Bull. 2016, 3, 43–44. [Google Scholar]
- Mezei, S.; Kovacev, L.; Nagl, N. Sugar beet micropropagation. Biotechnol. Biotechnol. Equip. 2006, 20, 9–14. [Google Scholar] [CrossRef][Green Version]
- Van Geyt, J.; D’Halluin, K.; Jacobs, M. Induction of nuclear and cell divisions in microspores of sugar beet (Beta vulgaris L.). Z. Pflanzenzuecht. 1985, 95, 325–335. [Google Scholar]
- Speckmann, G.J.; Van Geyt, J.P.C.; Jacobs, M. The induction of haploids of sugar beet (Beta vulgaris L.) using anther and free pollen culture or ovule and ovary culture. In Genetic Manipulation in Plant Breeding; Horn, W., Jensen, C.J., Odenbach, W., Schieder, O., Eds.; De Gruyter: Berlin, Germany, 1986; pp. 351–353. [Google Scholar]
- Rogozinska, J.H.; Goska, M.; Kuzdowicz, A. Induction of plants from anthers of Beta vulgaris cultured in vitro. Acta Soc. Bot. Pol. 1977, 46, 471–479. [Google Scholar] [CrossRef]
- Pazuki, A.; Aflaki, F.; Gürel, E.; Ergül, A.; Gürel, S. Gynogenesis Induction in Sugar Beet (Beta vulgaris) Improved by 6- Benzylaminopurine (BAP) and Synergized with Cold Pretreatment. Sugar Tech. 2017, 20, 69–77. [Google Scholar] [CrossRef]
- Gurel, S.; Gurel, E.; Kaya, Z. Doubled haploid plant production from unpollinated ovules of sugar beet (Beta vulgaris L.). Plant Cell Rep. 2000, 19, 1155–1159. [Google Scholar] [CrossRef]
- Nagl, N.; Mezei, S.; Kovacev, L.; Vasic, D.; Cacic, N. Induction and micropropagation potential of sugar beet haploids. Genetika 2004, 36, 187–194. [Google Scholar] [CrossRef]
- Aflaki, F.; Pazuki, A.; Gurel, S.; Stevanato, S.; Biancardi, E.; Gurel, E. Doubled haploid sugar beet: An integrated view of factors influencing the processes of gynogenesis and chromosome doubling. Int. Sugar J. 2017, 119, 884–895. [Google Scholar]
- Hosemans, D.; Bossoutrot, D. Induction of haploid plant from in vitro culture of unpollinated beet ovules (Beta vulgaris L.). J. Plant Breed. 1983, 91, 74–77. [Google Scholar]
- D’Halluin, K.; Keimer, B. Production of haploid sugar beets (Beta vulgaris L.) by ovule culture. In Genetic Manipulation in Plant Breeding; Horn, W., Jensen, C.J., Odenbach, W., Schieder, O., Eds.; De Gruyter: Berlin, Germany, 1986; pp. 307–309. [Google Scholar]
- Van Geyt, J.; Speckmann, G.J.; D’Halluin, K.; Jacobs, M. In vitro induction of haploid plants from unpollinated ovules and ovaries of the sugarbeet (Beta vulgaris L.). Theor. Appl. Genet. 1987, 73, 920–925. [Google Scholar] [CrossRef]
- Doctrinal, M.; Sangwan, R.S.; Sangwan-Norreel, B.S. In vitro gynogenesis in Beta vulgaris L.: Effects of plant growth regulators, temperature, genotypes and season. Plant Cell Tissue Organ Cult. 1989, 17, 1–12. [Google Scholar] [CrossRef]
- Lux, H.; Herrmann, L.; Wetzel, C. Production on haploid sugar beet (Beta vulgaris L.) by culturing unpollinated ovules. Plant Breed. 1990, 104, 177–183. [Google Scholar] [CrossRef]
- Goska, M. Monographs and Scientific Dissertations. In Haploids and Sub-Haploids of Sugar Beet (Beta vulgaris L.) and Possibilities of Their Use in Breeding; Institute of Plant Breeding and Acclimatization: Radzików, Poland, 1997. [Google Scholar]
- Weich, E.W.; Levall, M.W. Doubled haploid production of sugar beet (Beta vulgaris L.): Published protocols for other crop plant species. In Doubled Haploid Production in Crop Plants: A Manual; Maluszynski, M., Kasha, K.J., Forster, B.P., Szareiko, J., Eds.; Springer: Dordrecht, The Netherlands, 2003; pp. 255–263. [Google Scholar] [CrossRef]
- Pazuki, A.; Aflaki, F.; Gürel, S.; Ergül, A.; Gürel, E. Production of doubled haploids in sugar beet (Beta vulgaris): An efficient method by a multivariate experiment. Plant Cell Tissue Organ Cult. 2018, 132, 85–97. [Google Scholar] [CrossRef]
- Tomaszewska-Sowa, M. Effect of growth regulators and other components of culture medium on morphogenesis of sugar beet (Beta vulgaris L.) in unfertilised ovule in vitro cultures. Acta Agrobot. 2012, 65, 91–100. [Google Scholar] [CrossRef]
- Tomaszewska-Sowa, M.; Keutgen, A.J. Plant Regeneration from Unpollinated Ovules of Sugar Beet (Beta vulgaris L.) on Growing Media with Different Carbohydrates. Sugar Tech. 2022, 24, 542–550. [Google Scholar] [CrossRef]
- Galatowitsch, M.W.; Smith, G.A. Regeneration from unfertilized ovule callus of sugarbeet (Beta vulgaris L.). Can. J. Plant Sci. 1990, 70, 83–89. [Google Scholar] [CrossRef]
- Svirshchevskaya, A.M.; Kozyrevich, T.P.; Bormotov, V.E. Haploids in the culture of unfertilized ovules in beet. Dokl. Akad. Nauk Belar. 1993, 37, 74–76. [Google Scholar]
- Hansen, A.L.; Gertz, A.; Joersbo, M.; Andersen, S.B. Short-duration colchicine treatment for in vitro chromosome doubling during ovule culture of Beta vulgaris L. Plant Breed. 1995, 114, 515–519. [Google Scholar] [CrossRef]
- Sohrabi, S.; Abdollahi, M.R.; Mirzaie-Asl, A.; Koulaei, H.E.; Aghaeezadeh, M.; Seguí-Simarro, J.M. A refined method for ovule culture in sugar beet (Beta vulgaris L.). Plant Cell Tissue Organ Cult. 2021, 146, 259–267. [Google Scholar] [CrossRef]
- Gurel, S.; Pazuki, A.; Aflaki, F.; Gurel, E. Production of Doubled Haploid Sugar Beet (Beta vulgaris L.) Plants through Gynogenesis. In Doubled Haploid Technology: Emerging Tools, Cucurbits, Trees, Other Species; Methods in Molecular Biology; Segui-Simarro, J.M., Ed.; Humana: New York, NY, USA, 2021; Volume 3, pp. 313–323. [Google Scholar] [CrossRef]
- Pedersen, H.C.; Keimer, B. Haploidy in sugar beet (Beta vulgaris L.). In In Vitro Haploid Production in Higher Plants; Important Selected Plants; Jain, S.M., Sopory, S.K., Veilleux, R.E., Eds.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 1996; Volume 3, pp. 17–36. [Google Scholar] [CrossRef]
- Gémes-Juhász, A.; Balogh, P.; Ferenczy, A.; Kristóf, Z. Effect of optimal stage of female gametophyte and heat treatment on in vitro gynogenesis induction in cucumber (Cucumis sativus L.). Plant Cell Rep. 2002, 21, 105–111. [Google Scholar]
- Diao, W.-P.; Jia, Y.-Y.; Song, H.; Zhang, X.-Q.; Lou, Q.-F.; Chen, J.-F. Efficient embryo induction in cucumber ovary culture and homozygous identification of the regenetants using SSR markers. Sci. Hortic. 2009, 3, 246–251. [Google Scholar] [CrossRef]
- Zhang, C.L.; Chen, D.F.; Elliott, M.C.; Slater, A. Thidiazuron-induced organogenesis and somatic embryogenesis in sugar beet (Beta vulgaris L.). In Vitro Cell. Dev. Biol.-Plant 2001, 37, 305–310. [Google Scholar] [CrossRef]
- Zayachkovskaya, T.; Domblides, E.; Zayachkovsky, V.; Kan, L.; Domblides, A.; Soldatenko, A. Production of Gynogenic Plants of Red Beet (Beta vulgaris L.) in Unpollinated Ovule Culture In Vitro. Plants 2021, 10, 2703. [Google Scholar] [CrossRef]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol. Plant 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Skaptsov, M.V.; Smirnov, S.V.; Kutsev, M.G.; Shmakov, A.I. Problems of a standardization in plant flow cytometry. Turczaninowia 2016, 19, 120–122. [Google Scholar]
- Domblides, E.; Ermolaev, A.; Belov, S.; Kan, L.; Skaptsov, M.; Domblides, A. Efficient Methods for Evaluation on Ploidy Level of Cucurbita pepo L. Regenerant Plants Obtained in Unpollinated Ovule Culture In Vitro. Horticulturae 2022, 8, 1083. [Google Scholar] [CrossRef]
- Yudanova, S.S.; Maletskaya, E.I.; Maletsky, S.I. Variability in the number of chloroplasts in populations of stomata guard cells in sugar beet (Beta vulgaris L.). Genetics 2002, 38, 72–78. [Google Scholar]
- Subrahmanyeswari, T.; Gantait, S. Advancements and prospectives of sugar beet (Beta vulgaris L.) biotechnology. Appl. Microbiol. Biotechnol. 2022, 106, 7417–7430. [Google Scholar] [CrossRef] [PubMed]
- Levan, A. A haploid sugar beet after colchicine treatment. Hereditas 1945, 31, 399–410. [Google Scholar] [CrossRef] [PubMed]
- Vasilchenko, E.N.; Zhuzhzhalova, T.P. Biotechnology methods in the genus Beta. In Collection of Articles of the Thirty-Fifth International Scientific Practical Conference, Russian, Belgorod, 1 February 2021; Science and Education and Foreign Experience GK LLC, EDN FNHHTW: Belgorod, Russia, 2021; pp. 65–69. [Google Scholar]
- Pazuki, A.; Aflaki, F.; Gurel, S.; Ergul, A.; Gurel, E. The effects of proline on in vitro proliferation and propagation of doubled haploid sugar beet (Beta vulgaris). Turk. J. Bot. 2018, 42, 280–288. [Google Scholar] [CrossRef]
- Ferrant, V.; Bouharmont, J. Origin of gynogenetic embryos of Beta vulgaris L. Sex. Plant Reprod. 1994, 7, 12–16. [Google Scholar] [CrossRef]
- Dubois, F.; Lenee, P.; Sangwan, R.S.; Sangwan-Norreel, B.S. Do developmental stages of ovules influence in vitro induction of gynogenetic embryos in sugar beet. In Seeds: Genesis of Natural and Artificial Forms; Le Biopole Vegetal: Amiens, France, 1990; Volume 249. [Google Scholar]
- Bossoutrot, D.; Hosemans, D. Gynogenesis in Beta vulgaris L.: From in vitro culture of unpollinated ovules to the production of doubled haploid plants in soil. Plant Cell Rep. 1985, 4, 300–303. [Google Scholar] [CrossRef]
- Mukhambetzhanov, S.K. Culture of nonfertilized female gametophytes in vitro. Plant Cell Tissue Organ Cult. 1997, 48, 111–119. [Google Scholar] [CrossRef]
- Tomaszewska Sowa, M.; Olszewska, D. Evaluation of genetic stability of sugar beet (Beta vulgaris L.) plants obtained from unfertilized ovules using RAPD markers. J. Cent. Eur. Agric. 2019, 20, 928–937. [Google Scholar] [CrossRef]
- Chu, C.C.; Wang, C.C.; Sun, C.S.; Hsu, C.; Yin, K.C.; Chu, C.Y.; Bi, F.Y. Anther culture of cereal. Sci. Sin. 1975, 18, 659–668. [Google Scholar]
- Baranski, R. In vitro gynogenesis in red beet (Beta vulgaris L.): Effects of ovule culture conditions. Acta Soc. Bot. Pol. Tow. Bot. 1996, 65, 57–60. [Google Scholar] [CrossRef]
- Kiszczak, W.; Burian, M.; Kowalska, U.; Gorecka, K.; Podwyszynska, M. Production of Homozygous Red Beet (Beta vulgaris L. subsp. vulgaris) Plants by Ovule Culture. In Doubled Haploid Technology: Emerging Tools, Cucurbits, Trees, Other Species, Methods in Molecular Biology; Segui-Simarro, J.M., Ed.; Humana: New York, NY, USA, 2021; Volume 3, pp. 301–312. [Google Scholar] [CrossRef]
- Arndt, F.; Rusxh, R.; Stilfried, H.V.S.N. 49537 A new cotton defoliant. Plant Physiol. 1976, 57, 99. [Google Scholar]
- Mok, M.C.; Mok, D.W.S.; Armstrong, D.J. Cytokinin activity of N-phenyl-N’-l,2,3-thiadiazol-5-yl urea (Thidiazuron). Phytochemistry 1982, 21, 1509–1511. [Google Scholar] [CrossRef]
- Malik, K.A.; Saxena, P.K. Regeneration in Phaseolus vulgaris L.: High-frequency induction of direct shoot formation in intact seedlings by Nt-benzylaminopurine and thidiazuron. Planta 1992, 186, 384–389. [Google Scholar] [CrossRef]
- Huetteman, C.; Preece, J. Thidiazuron: A potent cytokinin for woody plant tissue culture. Plant Cell Tissue. Organ Cult. 1993, 33, 105–119. [Google Scholar] [CrossRef]
- Li, Z.; Jarret, R.L.; Pittman, R.N.; Demski, J.W. Shoot organogenesis from cultured seed explants of peanut (Arachis hypogaea L.) using thidiazuron. In Vitro Cell. Dev. Biol.–Plant 1994, 30, 187–191. [Google Scholar] [CrossRef]
- Capelle, S.C.; Mok, D.W.S.; Kirchner, S.C.; Mok, M.C. Effects of thidiazuron on cytokinin autonomy and the metabolism of Nt-(/x 2-isopentenyl) [8-14C]adenosine in callus tissues of Phaseolus lunatus L. Plant Physiol. 1983, 73, 796–802. [Google Scholar] [CrossRef] [PubMed]
- Thomas, J.C.; Katterman, F.R. Cytokinin activity induced by thidiazuron. Plant Physiol. 1986, 81, 681–683. [Google Scholar] [CrossRef]
- Kaminek, M.; Armstrong, D.J. Genotypic variation in cytokinin oxidase from Phaseolus callus cultures. Plant Physiol. 1990, 93, 1530–1538. [Google Scholar] [CrossRef]
- Domblides, E.; Belov, S.; Soldatenko, A.; Pivovarov, V. Obtaining doubled haploids of cucumber (Cucumis sativus L.). Veg. Crop Russ. 2019, 5, 3–14. [Google Scholar]
- Kiełkowska, A.; Adamus, A.; Baranski, R. An improved protocol for carrot haploid and doubled haploid plant production using induced parthenogenesis and ovule excision in vitro. In Vitro Cell. Dev. Biol.-Plant. 2014, 50, 376–383. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Yaseen, M.; Ahmad, T.; Sablok, G.; Standardi, A.; Hafiz, I.A. Review: Role of carbon sources for in vitro plant growth and development. Mol. Biol. Rep. 2013, 40, 2837–2849. [Google Scholar] [CrossRef] [PubMed]
- Ciereszko, I. Sucrose metabolism control in plants as response to changes of environmental conditions. Kosmos 2006, 55, 229–241. Available online: http://kosmos.icm.edu.pl/PDF/2006/229.pdf (accessed on 4 August 2023).
- Grigolava, T.R.; Vishnyakova, A.V.; Sinitsyna, A.A.; Voronina, A.V.; Zubko, O.N.; Zudova, O.V.; Monakhos, S.G. Methodological approaches for producing doubled haploids in sugar beet and red beet (Beta vulgaris L.). Vavilovskii Zhurnal Genet Selektsii 2021, 25, 276–283. [Google Scholar] [CrossRef] [PubMed]
- San Noeum, L.H. Haploides d’Hordeum vulgare L. par culture in vitro non fe´conde´s. Annales de l’ame´lioration des plantes 1976, 26, 751–754. [Google Scholar]
- Gos´ka, M.; Jassem, B. Histological observations of sugar—Beet ovules in in vitro cultures. Bull. Pol. Acad. Sci. Biol. 1988, 36, 171–175. [Google Scholar]
- Yu, M.H. Growth and reproduction performance of ovule—Induced sugar beet plants. Sabrao J. Breed. Genet. 1992, 24, 47–55. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).