Effect of Heat Stress on Root Architecture, Photosynthesis, and Antioxidant Profile of Water Spinach (Ipomoea aquatica Forsk) Seedlings
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Setup
2.2. Growth Attributes and Root Morphology
2.3. Photosynthesis Related Parameters
2.4. SEM, TEM, and Leaf Paraffin Analysis
2.5. Secondary Metabolites
2.6. Oxidative Stress Biomarkers and Antioxidant Enzymes Analysis
2.7. Leaf H2O2, O2•−, and MDA Staining
2.8. Statistical Analysis
3. Results
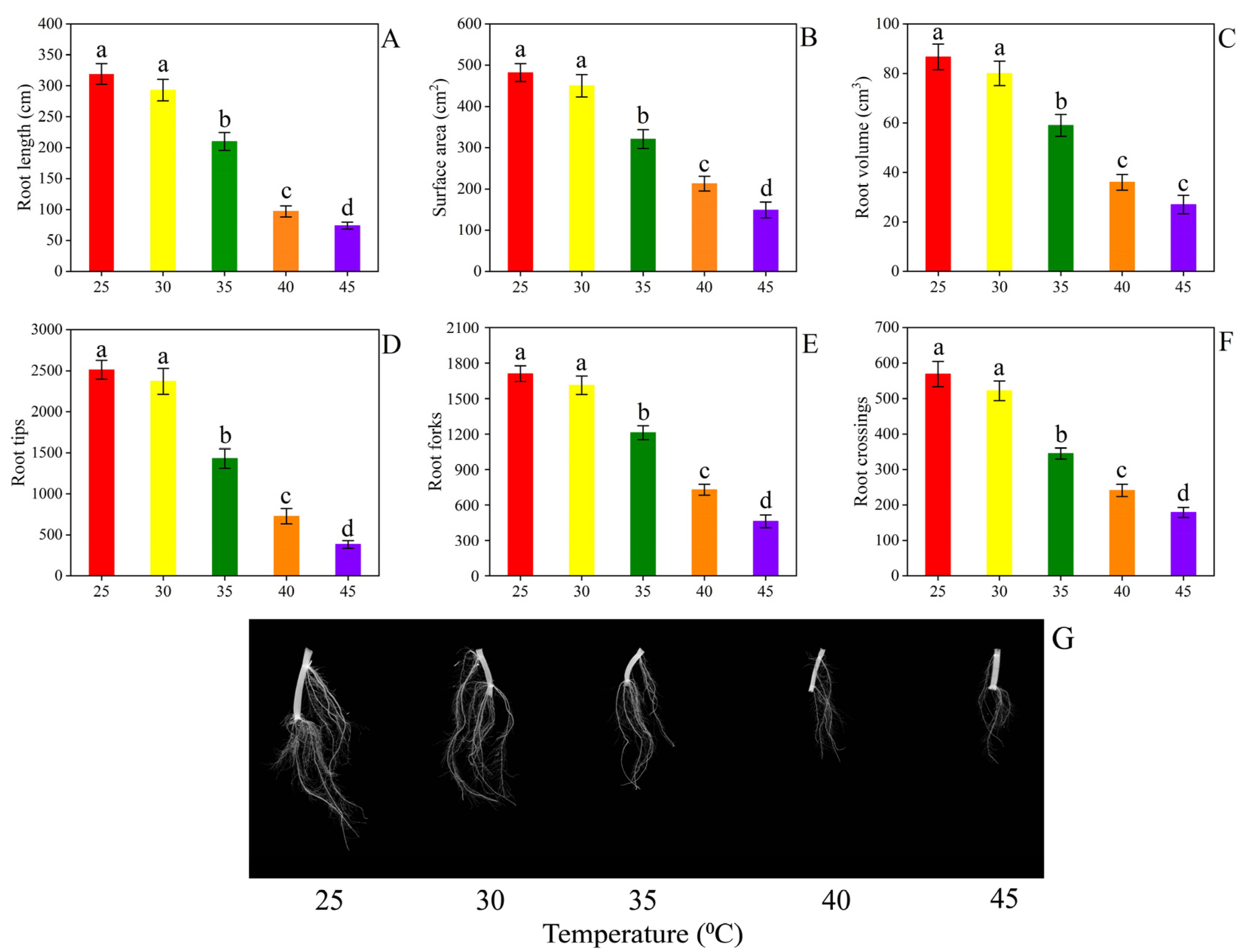
3.1. Plant Growth and Root Morphology
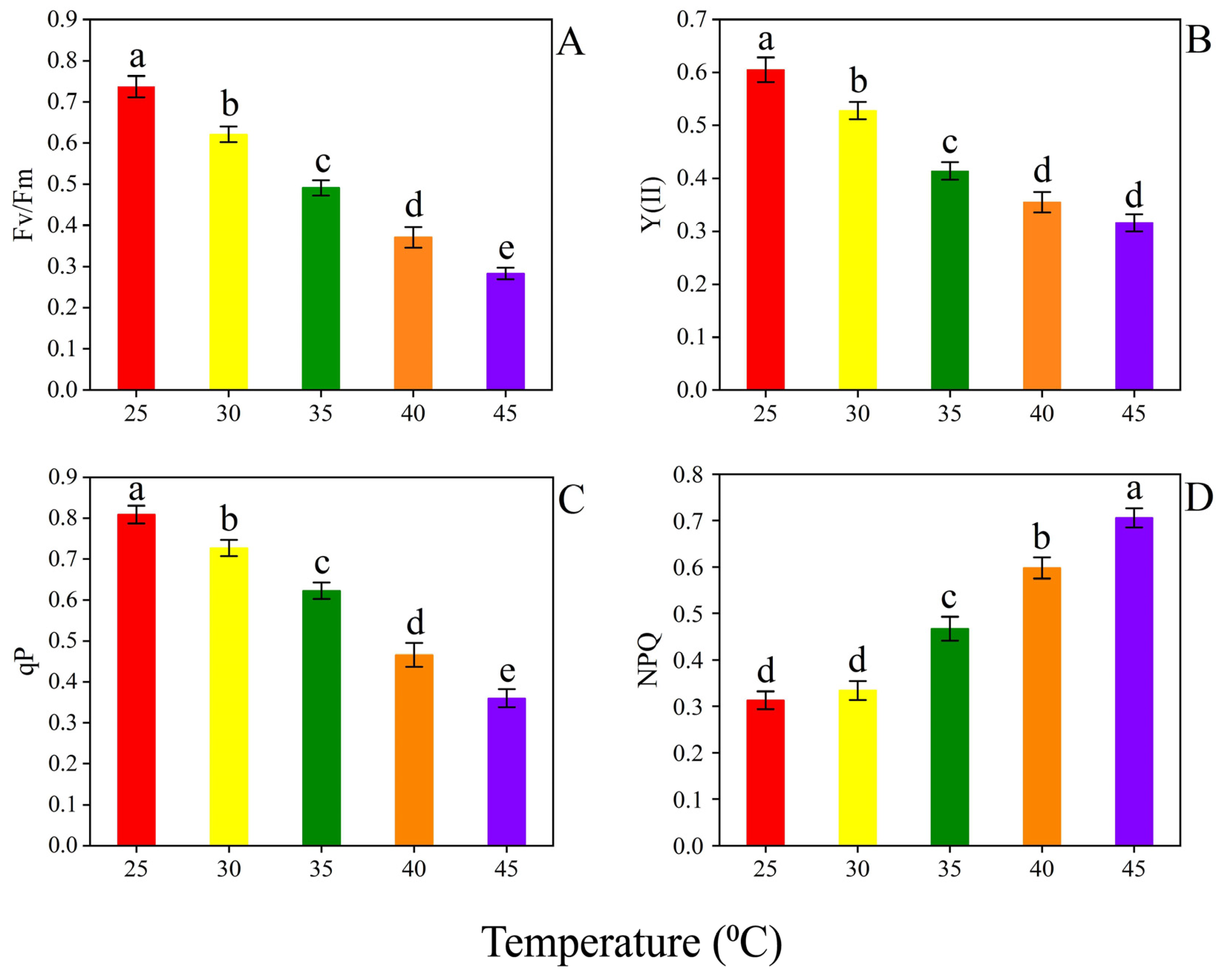
3.2. Photosynthesis Related Parameters
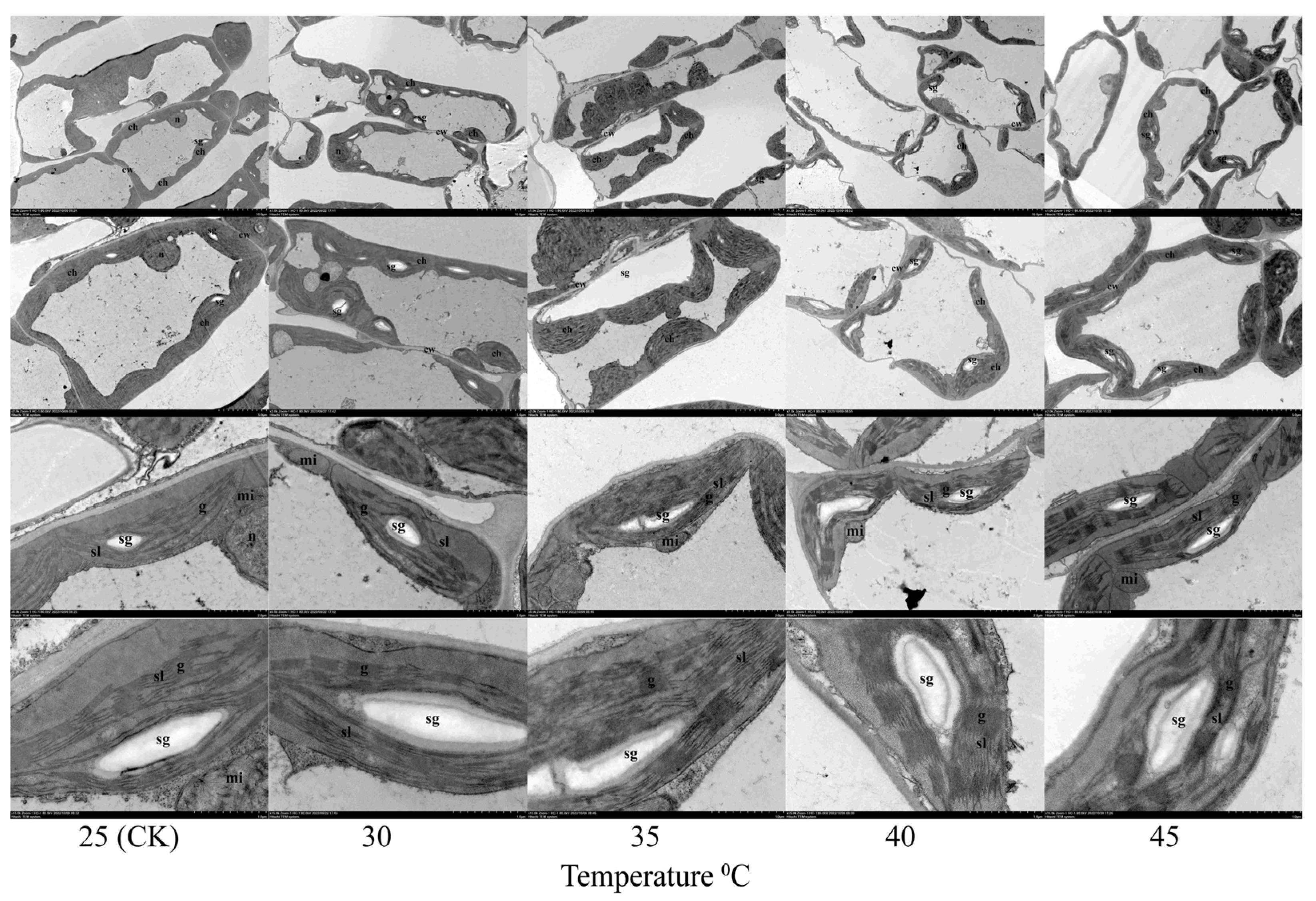
3.3. Effects of Temperature on the Ultrastructure and Paraffin Analysis of Leaves
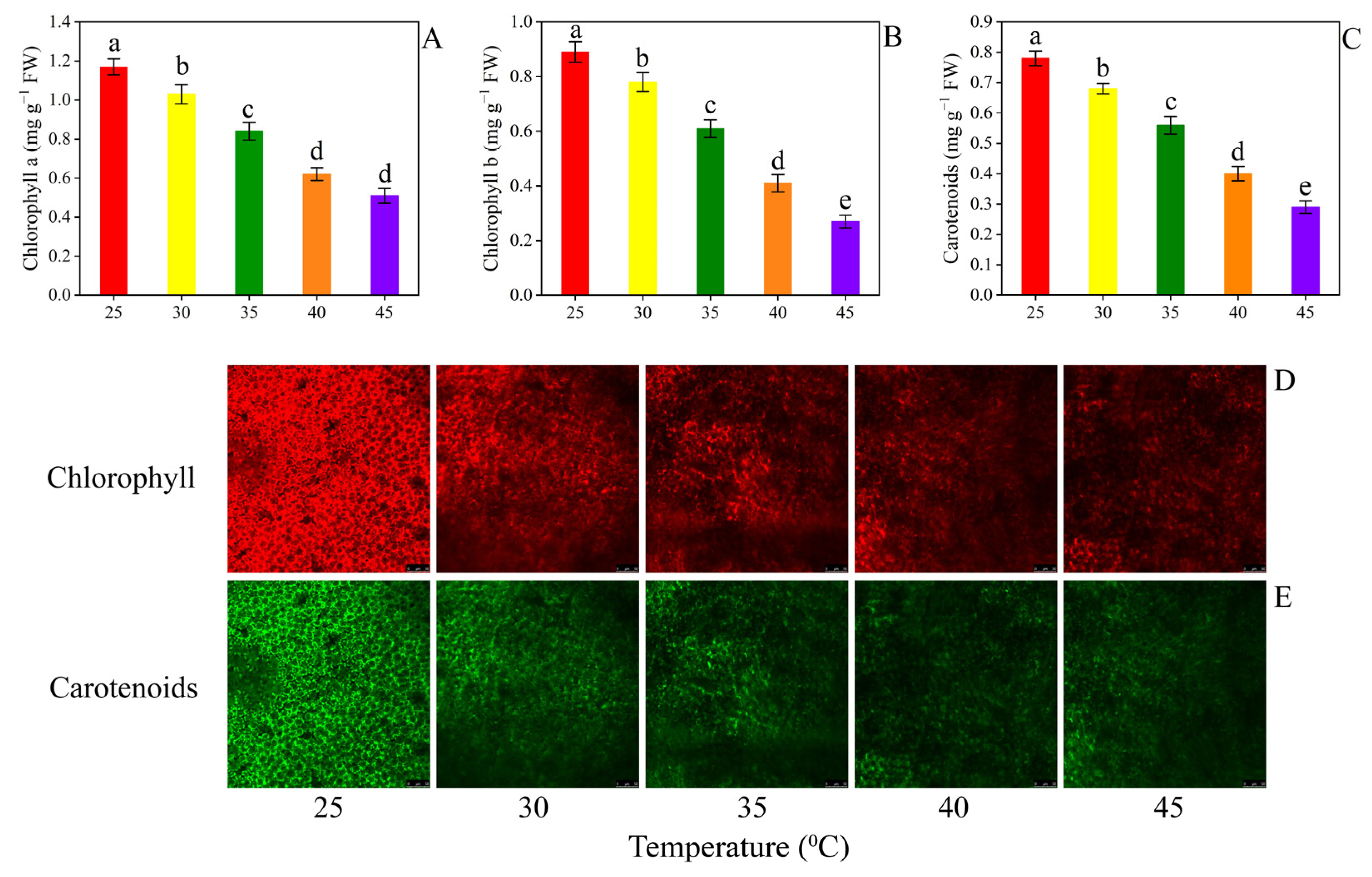
3.4. Secondary Metabolites
3.5. Oxidative Stress Biomarkers, Antioxidant Enzymes, and Leaf Staining Analysis
4. Discussion
4.1. Plant Growth and Root Morphology
4.2. Photosynthesis Related Parameters
4.3. Secondary Metabolites
4.4. Oxidative Stress Biomarkers and Antioxidant Enzymes Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Guo, R.; Wang, X.; Han, X.; Chen, X.; Wang-Pruski, G. Physiological and transcriptomic responses of water spinach (Ipomoea aquatica) to prolonged heat stress. BMC Genom. 2020, 21, 533. [Google Scholar] [CrossRef] [PubMed]
- Altaf, M.A.; Sharma, N.; Singh, J.; Samota, M.K.; Sankhyan, P.; Singh, B.; Kumar, A.; Naz, S.; Lal, M.K.; Tiwari, R.K.; et al. Mechanistic insights on melatonin-mediated plant growth regulation and hormonal cross-talk process in solanaceous vegetables. Sci. Hortic. 2023, 308, 111570. [Google Scholar] [CrossRef]
- Jahan, M.S.; Shu, S.; Wang, Y.; Chen, Z.; He, M.; Tao, M. Melatonin alleviates heat-induced damage of tomato seedlings by balancing redox homeostasis and modulating polyamine and nitric oxide biosynthesis. BMC Plant Bio. 2019, 19, 414. [Google Scholar]
- Hasan, M.M.; Skalicky, M.; Jahan, M.S.; Hossain, M.N.; Anwar, Z.; Nie, Z.F. Spermine: Its emerging role in regulating drought stress responses in plants. Cells 2021, 10, 261. [Google Scholar] [CrossRef] [PubMed]
- Ahmar, S.; Zolkiewicz, K.; Gruszka, D. Analyses of genes encoding the Glycogen Synthase Kinases in rice and Arabidopsis reveal mechanisms which regulate their expression during development and responses to abiotic stresses. Plant Sci. 2023, 332, 111724. [Google Scholar] [CrossRef] [PubMed]
- Raza, A.; Razzaq, A.; Mehmood, S.S.; Zou, X.; Zhang, X.; Lv, Y.; Xu, J. Impact of climate change on crops adaptation and strategies to tackle its outcome: A review. Plants 2019, 8, 34. [Google Scholar] [CrossRef] [PubMed]
- Jahan, M.S.; Guo, S.; Sun, J.; Shu, S.; Wang, Y.; Abou El-Yazied, A. Melatonin-mediated photosynthetic performance of tomato seedlings under high-temperature stress. Plant Physiol. Biochem. 2021, 167, 309–320. [Google Scholar] [CrossRef]
- Aleem, S.; Sharif, I.; Amin, E.; Tahir, M.; Parveen, N.; Aslam, R. Heat tolerance in vegetables in the current genomic era: An overview. Plant Growth Regul. 2020, 92, 497–516. [Google Scholar] [CrossRef]
- Yang, X.; Zhu, X.; Wei, J.; Li, W.; Wang, H.; Xu, Y.; Yang, Z.; Xu, C.; Li, P. Primary root response to combined drought and heat stress is regulated via salicylic acid metabolism in maize. BMC Plant Bio. 2022, 22, 417. [Google Scholar]
- Wu, W.; Ma, B.L.; Whalen, J.K. Enhancing rapeseed tolerance to heat and drought stresses in a changing climate: Perspectives for stress adaptation from root system architecture. Adv. Agron. 2018, 151, 87–157. [Google Scholar]
- Heckathorn, S.A.; Giri, A.; Mishra, S.; Bista, D. Heat stress and roots. In Climate Change and Plant Abiotic Stress Tolerance; Wiley: Hoboken, NJ, USA, 2013; pp. 109–136. [Google Scholar]
- Tao, M.Q.; Jahan, M.S.; Hou, K.; Shu, S.; Wang, Y.; Sun, J. Bitter melon (Momordica charantia L.) rootstock improves the heat tolerance of cucumber by regulating photosynthetic and antioxidant defense pathways. Plants 2020, 9, 692. [Google Scholar] [CrossRef]
- Wahid, A.; Farooq, M.; Hussain, I.; Rasheed, R.; Galani, S. Responses and management of heat stress in plants. In Environmental Adaptations and Stress Tolerance of Plants in the Era of Climate Change; Springer: New York, NY, USA, 2012; pp. 135–157. [Google Scholar]
- Ahmad, P.; Prasad, M.N.V. Environmental Adaptations and Stress Tolerance of Plants in the Era of Climate Change; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2011. [Google Scholar]
- Jahan, M.S.; Shu, S.; Wang, Y.; Hasan, M.; El-Yazied, A.A.; Alabdallah, N.M. Melatonin pretreatment confers heat tolerance and repression of heat-induced senescence in tomato through the modulation of ABA-and GA-mediated pathways. Front. Plant Sci. 2021, 12, 650955. [Google Scholar] [CrossRef]
- Kattge, J.; Knorr, W. Temperature acclimation in a biochemical model of photosynthesis: A reanalysis of data from 36 species. Plant Cell Environ. 2007, 30, 1176–1190. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Huang, B. Photosynthetic acclimation to high temperatures associated with heat tolerance in creeping bentgrass. J. Plant Physiol. 2008, 165, 1947–1953. [Google Scholar] [CrossRef] [PubMed]
- Ahammed, G.J.; Xu, W.; Liu, A.; Chen, S. COMT1 silencing aggravates heat stress-induced reduction in photosynthesis by decreasing chlorophyll content, photosystem II activity, and electron transport efficiency in tomato. Front. Plant Sci. 2018, 9, 998. [Google Scholar] [CrossRef]
- Hu, S.; Ding, Y.; Zhu, C. Sensitivity and responses of chloroplasts to heat stress in plants. Front. Plant Sci. 2020, 11, 375. [Google Scholar] [CrossRef] [PubMed]
- Sun, A.Z.; Guo, F.Q. Chloroplast retrograde regulation of heat stress responses in plants. Front. Plant Sci. 2016, 7, 398. [Google Scholar] [CrossRef] [PubMed]
- Lal, M.K.; Tiwari, R.K.; Altaf, M.A.; Kumar, A.; Kumar, R. Abiotic and biotic stress in horticultural crops: Insight into recent advances in the underlying tolerance mechanism. Front. Plant Sci. 2023, 14, 1212982. [Google Scholar] [CrossRef]
- Iqbal, Z.; Sarkhosh, A.; Balal, R.M.; Rauf, S.; Khan, N.; Altaf, M.A.; Camara-Zapata, J.M.; Garcia-Sanchez, F.; Shahid, M.A. Silicon nanoparticles mitigate hypoxia-induced oxidative damage by improving antioxidants activities and concentration of osmolytes in southern highbush blueberry plants. Agronomy 2021, 11, 2143. [Google Scholar] [CrossRef]
- Narayanan, S.; Tamura, P.J.; Roth, M.R.; Prasad, P.V.; Welti, R. Wheat leaf lipids during heat stress: I. High day and night temperatures result in major lipid alterations. Plant Cell Environ. 2016, 39, 787–803. [Google Scholar] [CrossRef]
- Mittler, R.; Vanderauwera, S.; Suzuki, N.; Miller, G.A.D.; Tognetti, V.B.; Vandepoele, K. ROS signaling: The new wave? Trends Plant Sci. 2011, 16, 300–309. [Google Scholar] [CrossRef]
- Allakhverdiev, S.I.; Kreslavski, V.D.; Klimov, V.V.; Los, D.A.; Carpentier, R. Heat stress: An overview of molecular responses in photosynthesis. Photosynth Res. 2008, 98, 541–550. [Google Scholar] [CrossRef] [PubMed]
- Tang, T.; Liu, X.; Wang, L.; Zuh, A.A.; Qiao, W.; Huang, J. Uptake, translocation and toxicity of chlorinated polyfluoroalkyl ether potassium sulfonate (F53B) and chromium co-contamination in water spinach (Ipomoea aquatica Forsk). Environ. Poll. 2020, 266, 115385. [Google Scholar] [CrossRef]
- Fu, H.; Xie, B.; Ma, S.; Zhu, X.; Fan, G.; Pan, S. Evaluation of antioxidant activities of principal carotenoids available in water spinach (Ipomoea aquatica). J. Food Comp. Anal. 2011, 24, 288–297. [Google Scholar] [CrossRef]
- Nawaz, M.A.; Jiao, Y.; Chen, C.; Shireen, F.; Zheng, Z.; Imtiaz, M. Melatonin pretreatment improves vanadium stress tolerance of watermelon seedlings by reducing vanadium concentration in the leaves and regulating melatonin biosynthesis and antioxidant-related gene expression. J. Plant Physiol. 2018, 220, 115–127. [Google Scholar] [CrossRef]
- Arnon, D.I. Copper enzymes in isolated chloroplasts. Polyphenol oxidase in Beta vulgaris. Plant Physiol. 1949, 24, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Mumtaz, M.A.; Hao, Y.; Mehmood, S.; Shu, H.; Zhou, H.; Jin, W. Physiological and Transcriptomic analysis provide molecular Insight into 24- epibrassinolide mediated Cr (VI)-Toxicity tolerance in pepper plants. Environ. Pollut. 2022, 306, 119375. [Google Scholar] [CrossRef]
- Altaf, M.A.; Shu, H.; Hao, Y.; Mumtaz, M.A.; Lu, X.; Wang, Z. Melatonin Affects the Photosynthetic Performance of Pepper (Capsicum annuum L.) Seedlings under Cold Stress. Antioxidants 2022, 11, 2414. [Google Scholar] [CrossRef]
- Pathan, A.K.; Bond, J.; Gaskin, R.E. Sample preparation for scanning electron microscopy of plant surfaces–horses for courses. Micron 2008, 39, 1049–1061. [Google Scholar] [CrossRef]
- Kuo, J. Processing plant tissues for ultrastructural study. Methods Mol. Biol. 2014, 1117, 39–55. [Google Scholar]
- Hewitson, T.D.; Wigg, B.; Becker, G.J. Tissue preparation for histochemistry: Fixation, embedding, and antigen retrieval for light microscopy. Methods Mol. Biol. 2010, 611, 3–18. [Google Scholar]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.T.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350e356. [Google Scholar] [CrossRef]
- Jahan, M.S.; Guo, S.; Baloch, A.R.; Sun, J.; Shu, S.; Wang, Y. Melatonin alleviates nickel phytotoxicity by improving photosynthesis, secondary metabolism and oxidative stress tolerance in tomato seedlings. Ecotoxicol. Environ. Saf. 2020, 197, 110593. [Google Scholar] [CrossRef]
- Altaf, M.A.; Hao, Y.; He, C.; Mumtaz, M.A.; Shu, H.; Fu, H. Physiological and biochemical responses of pepper (Capsicum annuum L.) seedlings to nickel toxicity. Front. Plant Sci. 2022, 13, 950932. [Google Scholar] [CrossRef]
- Pompella, A.; Maellaro, E.; Casini, A.F.; Comporti, M. Histochemical detection of lipid peroxidation in the liver of bromobenzene–poisoned mice. Am. J. Pathol. 1987, 129, 295–301. [Google Scholar]
- Masouleh, S.S.S.; Sassine, Y.N. Molecular and biochemical responses of horticultural plants and crops to heat stress. Ornam. Hortic. 2020, 26, 148–158. [Google Scholar] [CrossRef]
- Utami, D.; Aryanti, E. Impact of heat stress on germination and seedling growth of chili pepper (Capsicum annuum L.). In IOP Conference Series: Earth and Environmental Science; IOP Publishing: Bristol, UK, 2021; Volume 637, p. 012032. [Google Scholar]
- Gulen, H.; Eris, A. Some physiological changes in strawberry (Fragaria× ananassa ‘Camarosa’) plants under heat stress. J. Hortic. Sci. Biotech. 2003, 78, 894–898. [Google Scholar] [CrossRef]
- Camejo, D.; Rodríguez, P.; Morales, M.A.; Dell’Amico, J.M.; Torrecillas, A.; Alarcón, J.J. High temperature effects on photosynthetic activity of two tomato cultivars with different heat susceptibility. J. Plant Physiol. 2005, 162, 281–289. [Google Scholar] [CrossRef] [PubMed]
- Pagamas, P.; Nawata, E. Sensitive stages of fruit and seed development of chili pepper (Capsicum annuum L. var. Shishito) exposed to high-temperature stress. Sci. Hortic. 2008, 117, 21–25. [Google Scholar] [CrossRef]
- Tang, R.; Niu, S.; Zhang, G.; Chen, G.; Haroon, M.; Yang, Q. Physiological and growth responses of potato cultivars to heat stress. Botany 2018, 96, 897–912. [Google Scholar] [CrossRef]
- Benlloch-Gonzalez, M.; Bochicchio, R.; Berger, J.; Bramley, H.; Palta, J.A. High temperature reduces the positive effect of elevated CO2 on wheat root system growth. Field Crops Res. 2014, 165, 71–79. [Google Scholar] [CrossRef]
- Kadir, S.; Sidhu, G.; Al-Khatib, K. Strawberry (Fragaria× ananassa Duch.) growth and productivity as affected by temperature. Hort. Sci. 2006, 41, 1423–1430. [Google Scholar] [CrossRef]
- Arai-Sanoh, Y.; Ishimaru, T.; Ohsumi, A.; Kondo, M. Effects of soil temperature on growth and root function in rice. Plant Prod. Sci. 2010, 13, 235–242. [Google Scholar] [CrossRef]
- Gladish, D.K.; Rost, T.L. The effects of temperature on primary root growth dynamics and lateral root distribution in garden pea (Pisum sativum L., cv.“Alaska”). Environ. Exp. Bot. 1993, 33, 243–258. [Google Scholar] [CrossRef]
- Mathur, S.; Agrawal, D.; Jajoo, A. Photosynthesis: Response to high temperature stress. J. Photochem. Photobiol. B 2014, 137, 116–126. [Google Scholar] [CrossRef]
- Berry, J.; Bjorkman, O. Photosynthetic response and adaptation to temperature in higher plants. Annual Rev. Plant Physiol. 1980, 31, 491–543. [Google Scholar] [CrossRef]
- Wang, L.J.; Fan, L.; Loescher, W.; Duan, W.; Liu, G.J.; Cheng, J.S. Salicylic acid alleviates decreases in photosynthesis under heat stress and accelerates recovery in grapevine leaves. BMC Plant Bio. 2010, 10, 34. [Google Scholar]
- Haldimann, P.; Feller, U.R.S. Growth at moderately elevated temperature alters the physiological response of the photosynthetic apparatus to heat stress in pea (Pisum sativum L.) leaves. Plant Cell Environ. 2005, 28, 302–317. [Google Scholar] [CrossRef]
- Camejo, D.; Jiménez, A.; Alarcón, J.J.; Torres, W.; Gómez, J.M.; Sevilla, F. Changes in photosynthetic parameters and antioxidant activities following heat-shock treatment in tomato plants. Funct. Plant Bio. 2006, 33, 177–187. [Google Scholar] [CrossRef] [PubMed]
- Ding, X.; Jiang, Y.; Hao, T.; Jin, H.; Zhang, H.; He, L. Effects of heat shock on photosynthetic properties, antioxidant enzyme activity, and downy mildew of cucumber (Cucumis sativus L.). PLoS ONE 2016, 11, e0152429. [Google Scholar] [CrossRef]
- Tan, W.; Meng, Q.W.; Brestic, M.; Olsovska, K.; Yang, X. Photosynthesis is improved by exogenous calcium in heat-stressed tobacco plants. J. Plant Physiol. 2011, 168, 2063–2071. [Google Scholar] [CrossRef]
- Georgieva, K.; Lichtenthaler, H.K. Photosynthetic response of different pea cultivars to low and high temperature treatments. Photosynthetica 2006, 44, 569–578. [Google Scholar] [CrossRef]
- Feng, B.; Liu, P.; Li, G.; Dong, S.T.; Wang, F.H.; Kong, L.A. Effect of heat stress on the photosynthetic characteristics in flag leaves at the grain-filling stage of different heat-resistant winter wheat varieties. J. Agro. Crop Sci. 2014, 200, 143–155. [Google Scholar] [CrossRef]
- Yüzbaşıoğlu, E.; Dalyan, E.; Akpınar, I. Changes in photosynthetic pigments, anthocyanin content and antioxidant enzyme activities of maize (Zea mays L.) seedlings under high temperature stress conditions. Trak. Univ. J. Nat. Sci. 2017, 18, 97–104. [Google Scholar]
- Chary, N.S.; Kamala, C.; Raj, D.S.S. Assessing risk of heavy metals from consuming food grown on sewage irrigated soils and food chain transfer. Ecotoxicol. Environ. Saf. 2008, 69, 513–524. [Google Scholar] [CrossRef] [PubMed]
- Khandaker, M.M.; Fazil, R.; Saifuddin, M.A.A.M.; Zakaria, A.J. Effects of temperature treatment on seed germination, root development and seedling growth of Citrullus lanatus (watermelon). Bulg. J. Agri. Sci. 2020, 26, 558–566. [Google Scholar]
- Ibrahim, M.A.; Nissinen, A.; Prozherina, N.; Oksanen, E.J.; Holopainen, J.K. The influence of exogenous monoterpene treatment and elevated temperature on growth, physiology, chemical content and headspace volatiles of two carrot cultivars (Daucus carota L.). Environ. Exp. Bot. 2016, 56, 95–107. [Google Scholar] [CrossRef]
- Yang, X.; Han, Y.; Hao, J.; Qin, X.; Liu, C.; Fan, S. Exogenous spermidine enhances the photosynthesis and ultrastructure of lettuce seedlings under high-temperature stress. Sci. Hortic. 2022, 291, 110570. [Google Scholar] [CrossRef]
- Aien, A.; Khetarpal, S.; Pal, M. Photosynthetic characteristics of potato cultivars grown under high temperature. Am. Eurasian J. Agric. Environ. Sci. 2011, 11, 633–639. [Google Scholar]
- Naz, N.; Durrani, F.; Shah, Z.; Khan, N.A.; Ullah, I. Influence of heat stress on growth and physiological activities of potato (Solanum tuberosum L.). Phyton 2018, 87, 225. [Google Scholar]
- Wang, P.; Sun, X.; Chang, C.; Feng, F.J.; Liang, D.; Cheng, L.L. Delay in leaf senescence of Malus hupehensis by long-term melatonin application is associated with its regulation of metabolic status and protein degradation. J. Pineal Res. 2013, 55, 424–434. [Google Scholar] [CrossRef]
- Balal, R.M.; Shahid, M.A.; Javaid, M.M.; Iqbal, Z.; Anjum, M.A.; Garcia-Sanchez, F. The role of selenium in amelioration of heat-induced oxidative damage in cucumber under high temperature stress. Acta Physiol. Plantar. 2016, 38, 158. [Google Scholar] [CrossRef]
- Bhusal, N.; Sharma, P.; Sareen, S.; Sarial, A.K. Mapping QTLs for chlorophyll content and chlorophyll fluorescence in wheat under heat stress. Bio. Plantar. 2018, 62, 721–731. [Google Scholar] [CrossRef]
- Tang, L.; Hamid, Y.; Zehra, A.; Sahito, Z.A.; He, Z.; Khan, M.B. Mechanisms of water regime effects on uptake of cadmium and nitrate by two ecotypes of water spinach (Ipomoea aquatica Forsk.) in contaminated soil. Chemosphere 2020, 246, 125798. [Google Scholar] [CrossRef]
- Bukhari, S.A.H.; Zheng, W.; Xie, L.; Zhang, G.; Shang, S.; Wu, F. Cr-induced changes in leaf protein profile, ultrastructure and photosynthetic traits in the two contrasting tobacco genotypes. Plant Growth Regul. 2015, 79, 147.e156. [Google Scholar] [CrossRef]
- Alhaithloul, H.A.; Soliman, M.H.; Ameta, K.L.; El-Esawi, M.A.; Elkelish, A. Changes in ecophysiology, osmolytes, and secondary metabolites of the medicinal plants of Mentha piperita and Catharanthus roseus subjected to drought and heat stress. Biomolecules 2019, 10, 43. [Google Scholar] [CrossRef] [PubMed]
- Shahid, M.; Saleem, M.F.; Anjum, S.A.; Shahid, M.; Afzal, I. Effect of terminal heat stress on proline, secondary metabolites and yield components of wheat (Triticum aestivum L.) genotypes. Philipp. Agric. Sci. 2017, 100, 278–286. [Google Scholar]
- Paupière, M.J.; Müller, F.; Li, H.; Rieu, I.; Tikunov, Y.M.; Visser, R.G.; Bovy, A.G. Untargeted metabolomic analysis of tomato pollen development and heat stress response. Plant Reprod. 2017, 30, 81–94. [Google Scholar] [CrossRef]
- Li, J.; Xie, J.; Yu, J.; Lyv, J.; Zhang, J.; Ding, D.; Li, N.; Zhang, J.; Bakpa, E.P.; Yang, Y.; et al. Melatonin enhanced low-temperature combined with low-light tolerance of pepper (Capsicum annuum L.) seedlings by regulating root growth, antioxidant defense system, and osmotic adjustment. Front. Plant Sci. 2022, 13, 998293. [Google Scholar] [CrossRef]
- Neocleous, D.; Vasilakakis, M. Antioxidant Response of Salt-Treated Strawberry Plants to Heat Stress. In Workshop on Berry Production in Changing Climate Conditions and Cultivation Systems. COST-Action 863: Euroberry Research: From Genomics to Sustainable Production, Quality and Health; ISHS: Leuven, Belgium, 2008; Volume 838, pp. 217–222. [Google Scholar]
- Wu, X.; Zhang, S.; Liu, X.; Shang, J.; Zhang, A.; Zhu, Z. Chalcone synthase (CHS) family members analysis from eggplant (Solanum melongena L.) in the flavonoid biosynthetic pathway and expression patterns in response to heat stress. PLoS ONE 2020, 15, e0226537. [Google Scholar] [CrossRef]
- Liu, B.; Kong, L.; Zhang, Y.; Liao, Y. Gene and metabolite integration analysis through transcriptome and metabolome brings new insight into heat stress tolerance in potato (Solanum tuberosum L.). Plants 2021, 10, 103. [Google Scholar] [CrossRef]
- Uz Zaman, Q.; Abbasi, A.; Tabassum, S.; Ashraf, K.; Ahmad, Z.; Siddiqui, M.H. Calcium induced growth, physio-biochemical, antioxidants, osmolytes adjustments and phytoconstituents status in spinach under heat stress. S. Afr. J. Bot. 2022, 149, 701–711. [Google Scholar] [CrossRef]
- Huang, B.; Chen, Y.E.; Zhao, Y.Q.; Ding, C.B.; Liao, J.Q.; Hu, C. Exogenous melatonin alleviates oxidative damages and protects photosystem II in maize seedlings under drought stress. Front. Plant Sci. 2019, 10, 677. [Google Scholar] [CrossRef] [PubMed]
- Sharkey, T.D. Effects of moderate heat stress on photosynthesis: Importance of thylakoid reactions, rubisco deactivation, reactive oxygen species, and thermotolerance provided by isoprene. Plant Cell Environ. 2015, 28, 269–277. [Google Scholar] [CrossRef]
- Fukuoka, N.; Hamada, T. Effects of heat stress on the biological maillard reaction, oxidative stress, and occurrence of internal browning in Japanese radish (Raphanus sativus L.). J. Plant Physiol. 2021, 256, 153326. [Google Scholar] [CrossRef]
- Xin, Q.; Liu, B.; Sun, J.; Fan, X.; Li, X.; Jiang, L. Heat shock treatment promoted callus formation on postharvest sweet potato by adjusting active oxygen and phenylpropanoid metabolism. Agriculture 2022, 12, 1351. [Google Scholar] [CrossRef]
- Sharma, V.; Singh, C.M.; Chugh, V.; Prajapati, P.K.; Mishra, A.; Kaushik, P. Morpho-Physiological and Biochemical Responses of Field Pea Genotypes under Terminal Heat Stress. Plants 2023, 12, 256. [Google Scholar] [CrossRef]
- Zhou, R.; Kong, L.; Yu, X.; Ottosen, C.O.; Zhao, T.; Jiang, F. Oxidative damage and antioxidant mechanism in tomatoes responding to drought and heat stress. Acta Physiol. Plantar. 2019, 41, 20. [Google Scholar] [CrossRef]
- Commisso, M.; Toffali, K.; Strazzer, P.; Stocchero, M.; Ceoldo, S.; Baldan, B. Impact of phenylpropanoid compounds on heat stress tolerance in carrot cell cultures. Front. Plant Sci. 2016, 7, 1439. [Google Scholar] [CrossRef]
- Anjos Neto, A.P.D.; Oliveira, G.R.F.; Mello, S.D.C.; Silva, M.S.D.; Gomes-Junior, F.G.; Novembre, A.D.D.L.C. Seed priming with seaweed extract mitigate heat stress in spinach: Effect on germination, seedling growth and antioxidant capacity. Bragantia 2020, 79, 502–511. [Google Scholar] [CrossRef]
- Chaitanya, K.V.; Sundar, D.; Masilamani, S.; Ramachandra Reddy, A. Variation in heat stress-induced antioxidant enzyme activities among three mulberry cultivars. Plant Growth Regul. 2002, 36, 175–180. [Google Scholar] [CrossRef]
- Mazorra, L.M.; Nunez, M.; Hechavarria, M.; Coll, F.; Sánchez-Blanco, M.J. Influence of brassinosteroids on antioxidant enzymes activity in tomato under different temperatures. Bio. Plantar. 2002, 45, 593–596. [Google Scholar] [CrossRef]
- Hu, W.H.; Xiao, Y.A.; Zeng, J.J.; Hu, X.H. Photosynthesis, respiration and antioxidant enzymes in pepper leaves under drought and heat stresses. Bio. Plantar. 2010, 54, 761–765. [Google Scholar] [CrossRef]
- Gulen, H.; Eris, A. Effect of heat stress on peroxidase activity and total protein content in strawberry plants. Plant Sci. 2004, 166, 739–744. [Google Scholar] [CrossRef]
- Zhao, X.; Sui, X.; Zhao, L.; Gao, X.; Wang, J.; Wen, X. Morphological and physiological response mechanism of lettuce (Lactuca sativa L.) to consecutive heat stress. Sci. Hortic. 2002, 301, 111112. [Google Scholar] [CrossRef]


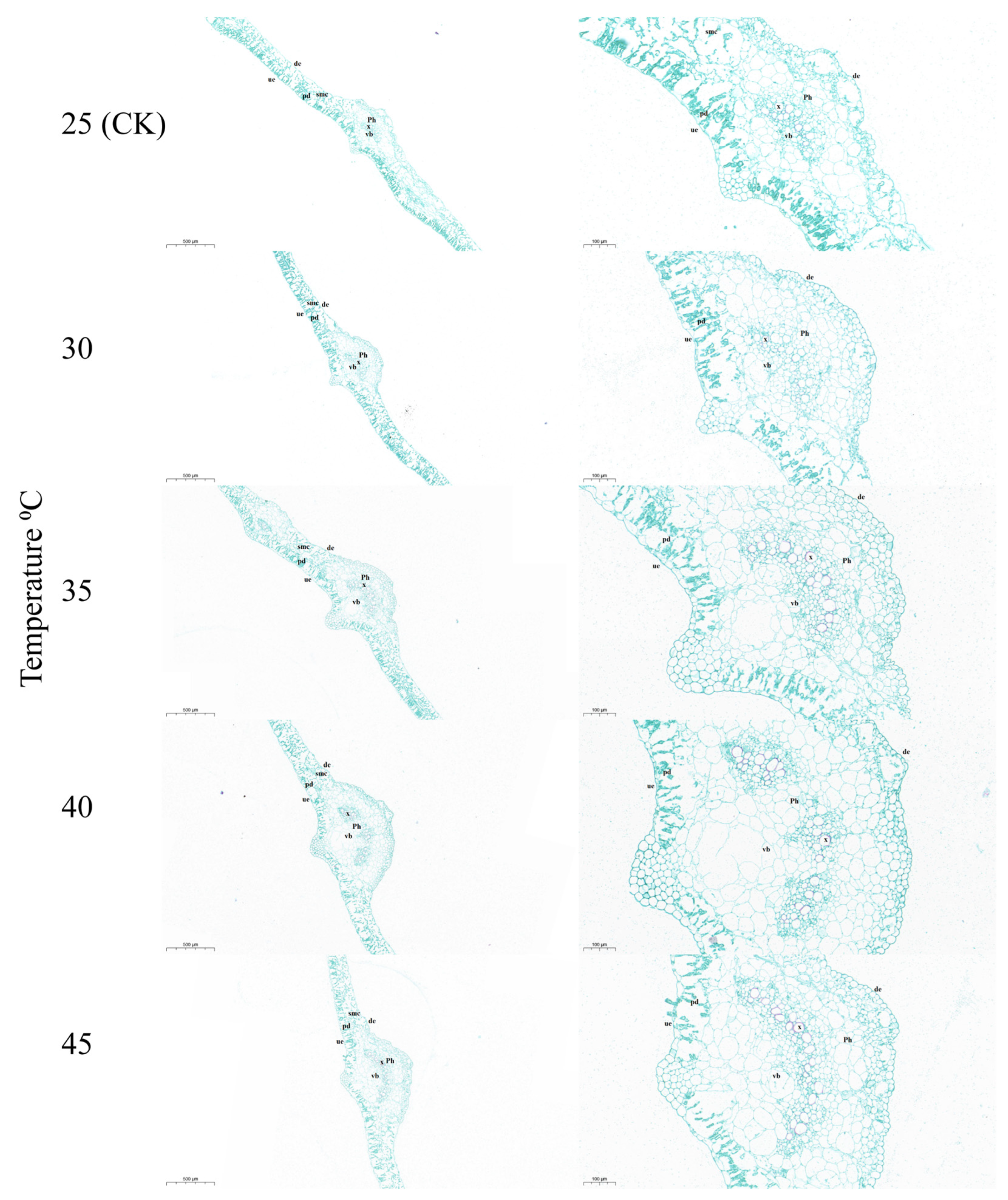
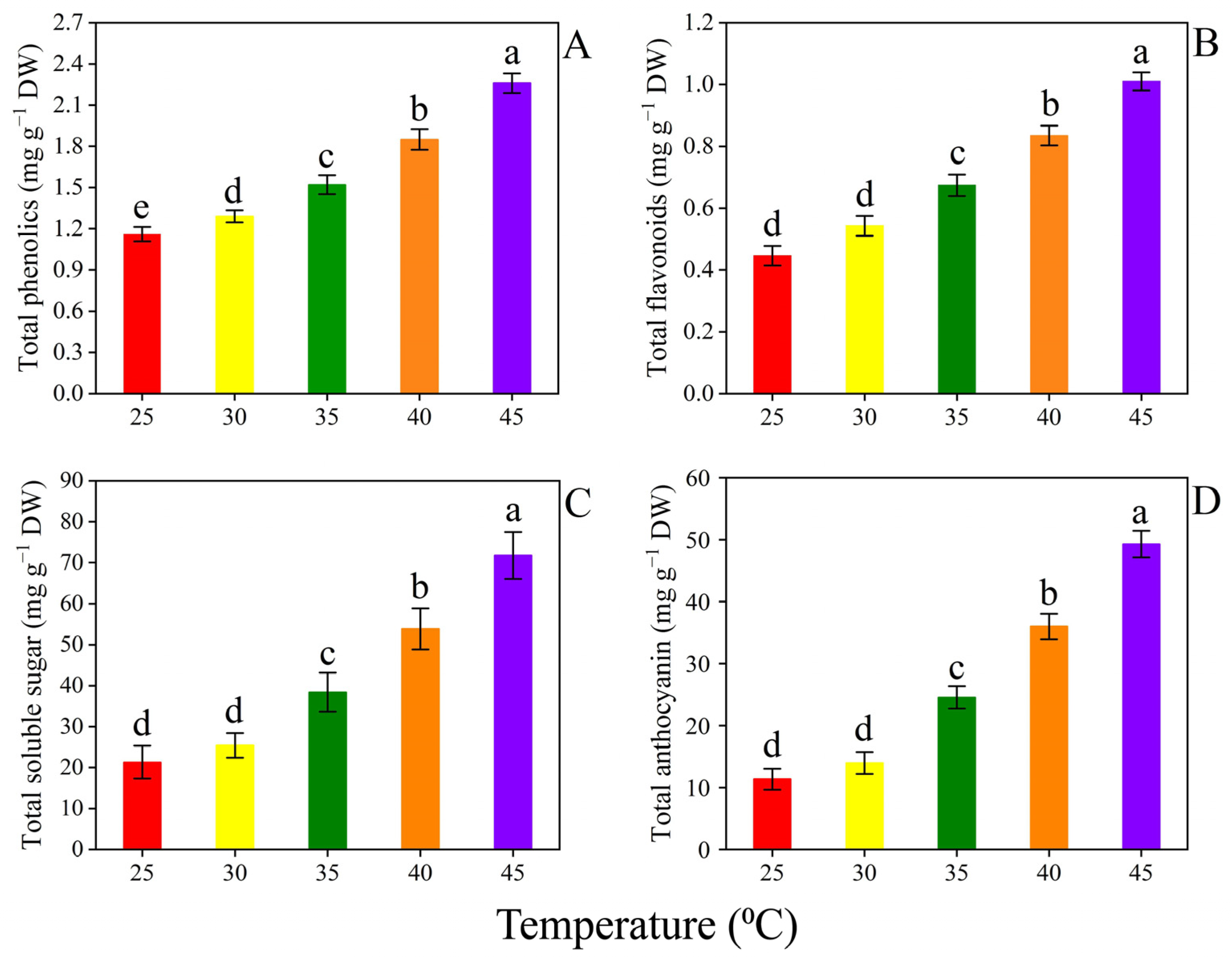
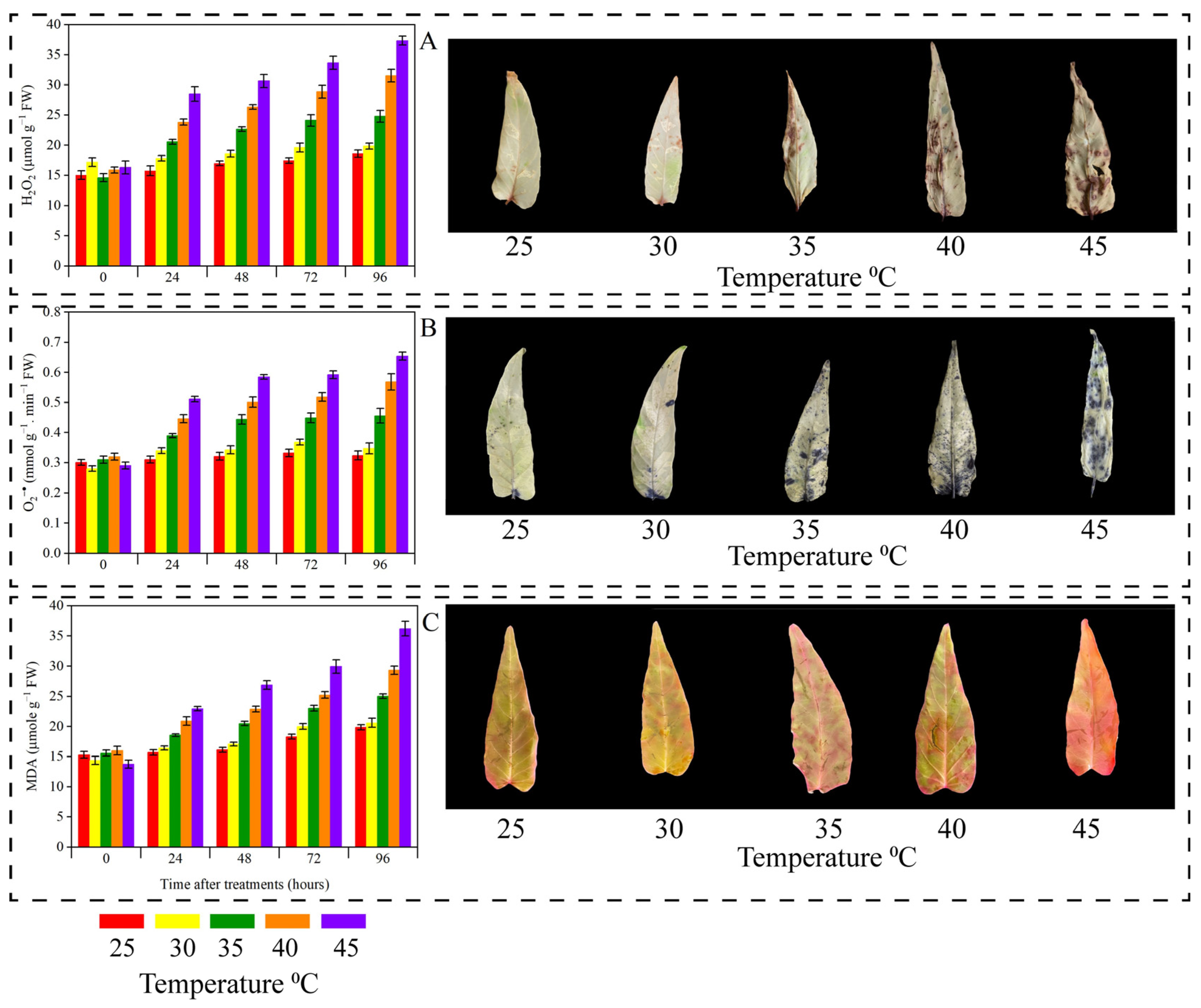
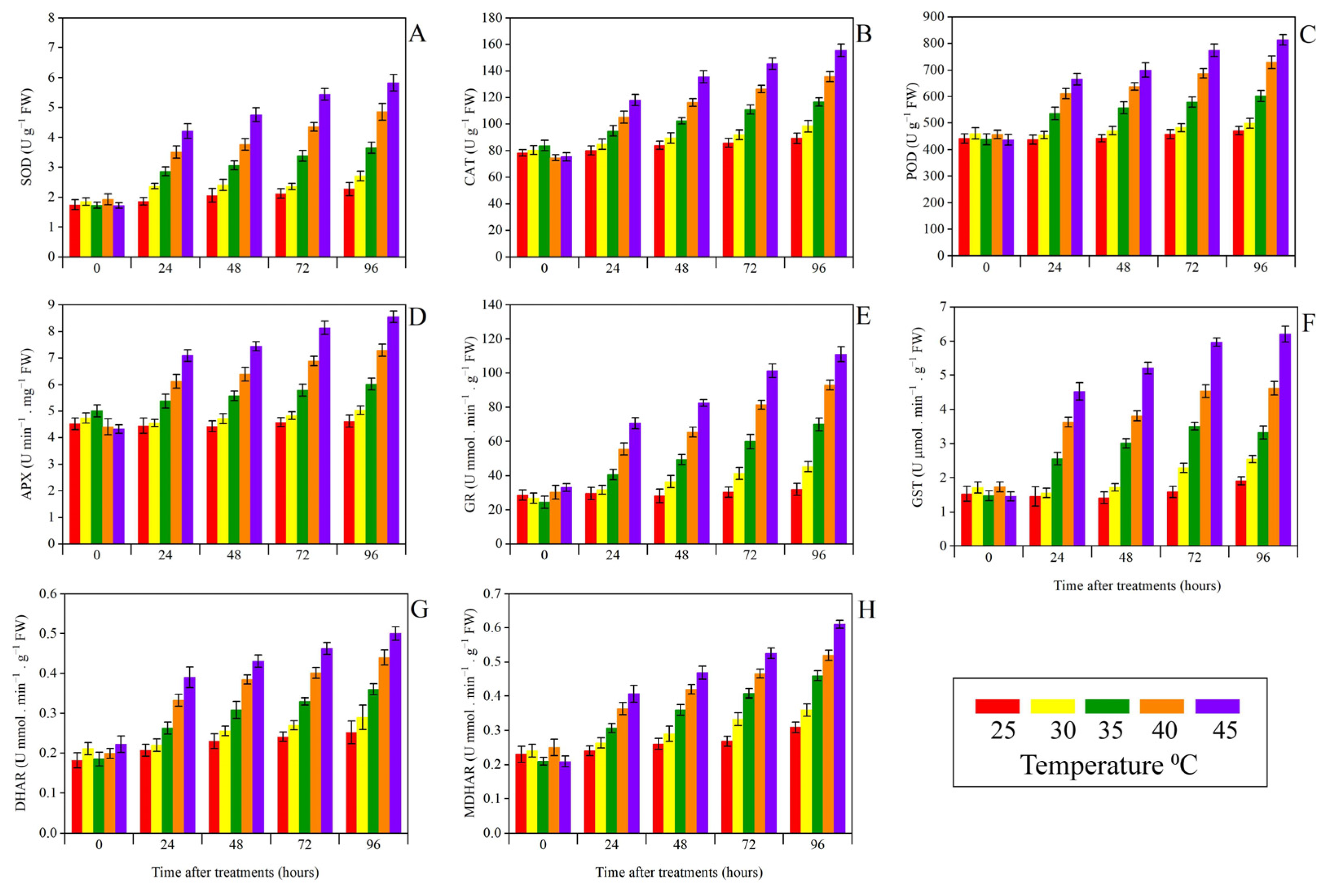
| Temperature (°C) | Biomass Weight/Plant (g) | |||
|---|---|---|---|---|
| Fresh | Dry | |||
| Shoot | Root | Shoot | Root | |
| 25 (CK) | 7.71 ± 0.27 a | 1.483 ± 0.301 a | 1.48 ± 0.05 b | 0.106 ± 0.001 b |
| 30 | 7.61 ± 0.25 a | 1.432 ± 0.035 a | 1.72 ± 0.06 a | 0.114 ± 0.001 a |
| 35 | 6.64 ± 0.20 b | 1.126 ± 0.021 b | 1.26 ± 0.05 c | 0.077 ± 0.003 c |
| 40 | 5.15 ± 0.24 c | 0.823 ± 0.013 c | 0.84 ± 0.05 d | 0.051 ± 0.002 d |
| 45 | 3.82 ± 0.15 d | 0.596 ± 0.018 d | 0.51 ± 0.04 e | 0.036 ± 0.001 e |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Altaf, M.A.; Hao, Y.; Wang, Z.; Zhu, G. Effect of Heat Stress on Root Architecture, Photosynthesis, and Antioxidant Profile of Water Spinach (Ipomoea aquatica Forsk) Seedlings. Horticulturae 2023, 9, 923. https://doi.org/10.3390/horticulturae9080923
Wang X, Altaf MA, Hao Y, Wang Z, Zhu G. Effect of Heat Stress on Root Architecture, Photosynthesis, and Antioxidant Profile of Water Spinach (Ipomoea aquatica Forsk) Seedlings. Horticulturae. 2023; 9(8):923. https://doi.org/10.3390/horticulturae9080923
Chicago/Turabian StyleWang, Xin, Muhammad Ahsan Altaf, Yuanyuan Hao, Zhiwei Wang, and Guopeng Zhu. 2023. "Effect of Heat Stress on Root Architecture, Photosynthesis, and Antioxidant Profile of Water Spinach (Ipomoea aquatica Forsk) Seedlings" Horticulturae 9, no. 8: 923. https://doi.org/10.3390/horticulturae9080923
APA StyleWang, X., Altaf, M. A., Hao, Y., Wang, Z., & Zhu, G. (2023). Effect of Heat Stress on Root Architecture, Photosynthesis, and Antioxidant Profile of Water Spinach (Ipomoea aquatica Forsk) Seedlings. Horticulturae, 9(8), 923. https://doi.org/10.3390/horticulturae9080923








