Detection of Internal Browning Disorder in ‘Greensis’ Pears Using a Portable Non-Destructive Instrument
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material and Treatment
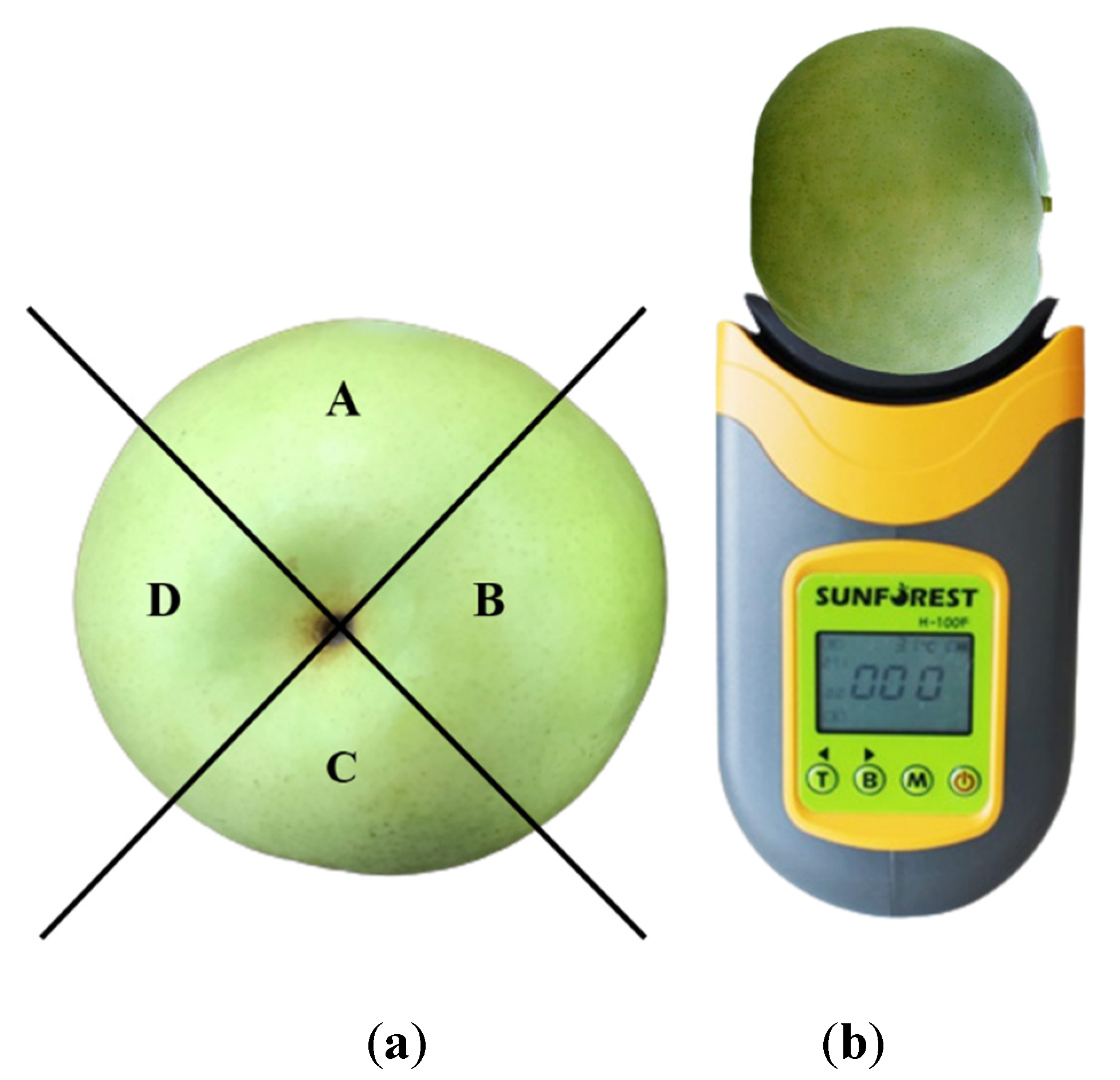
2.2. Designation of Browning Index Value
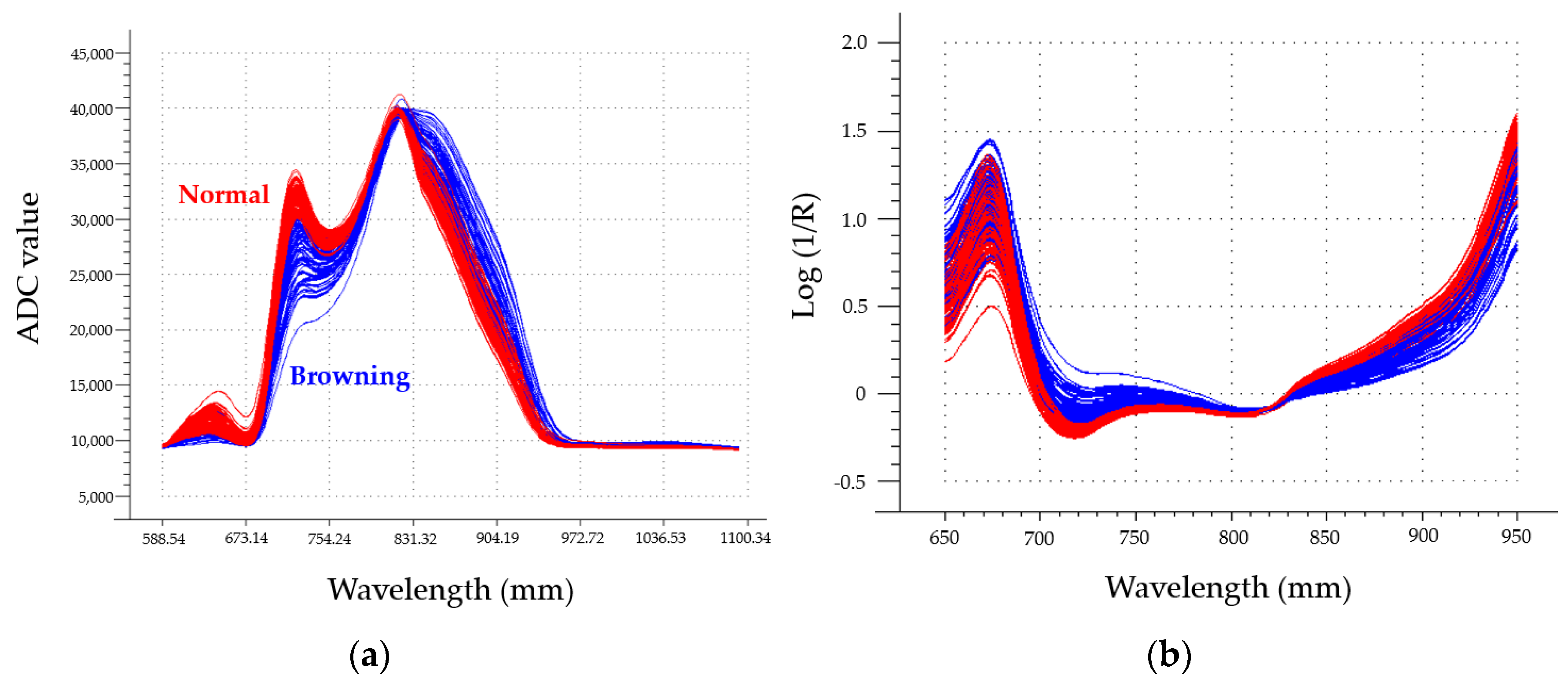
2.3. Spectrum Measurement
2.4. Determination Model for Detecting Internal Browning Disorder in Pears
3. Results and Discussion
3.1. Effect of Storage Period on Internal Browning Disorder Incidence
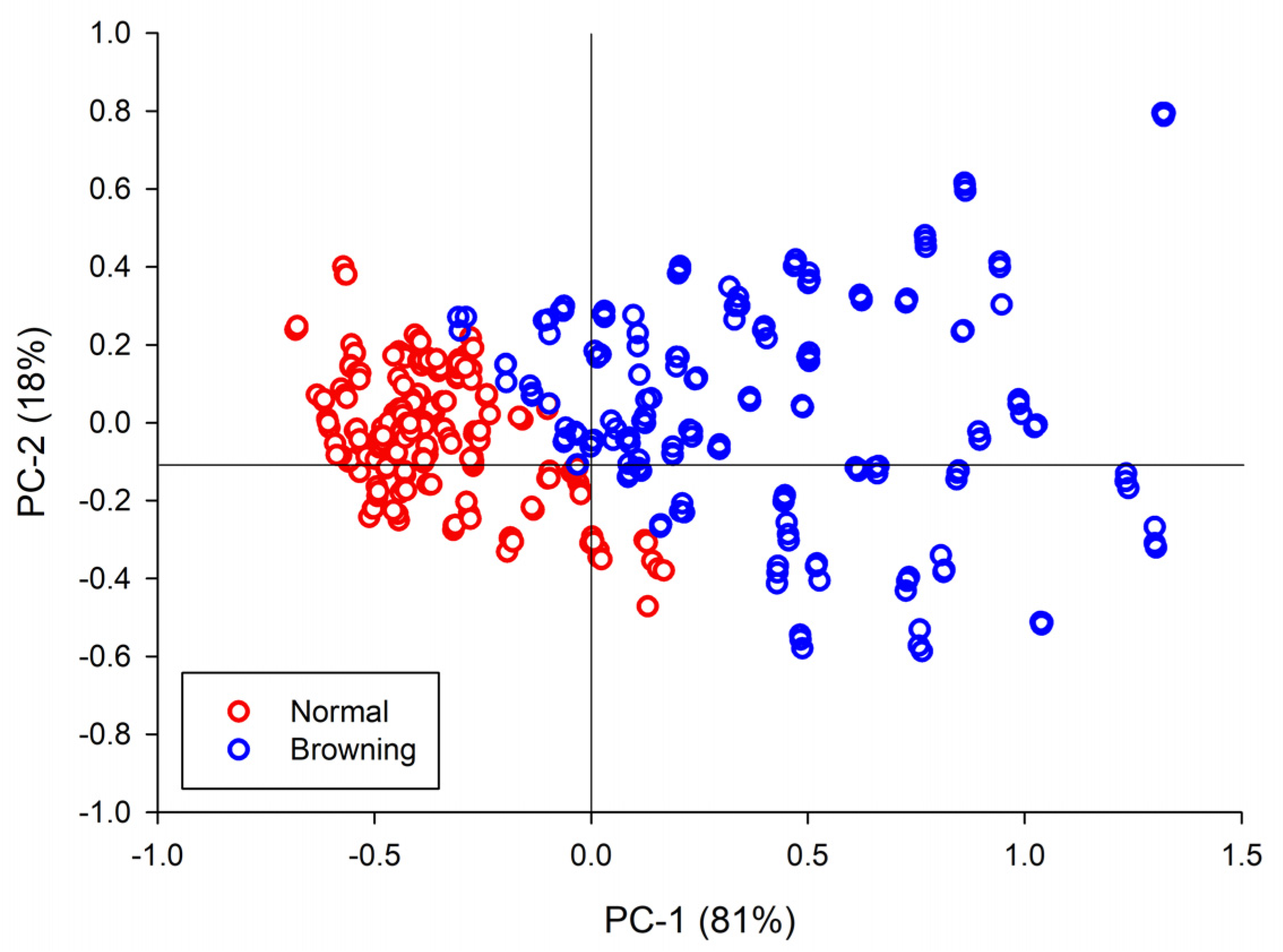
3.2. PCA Results
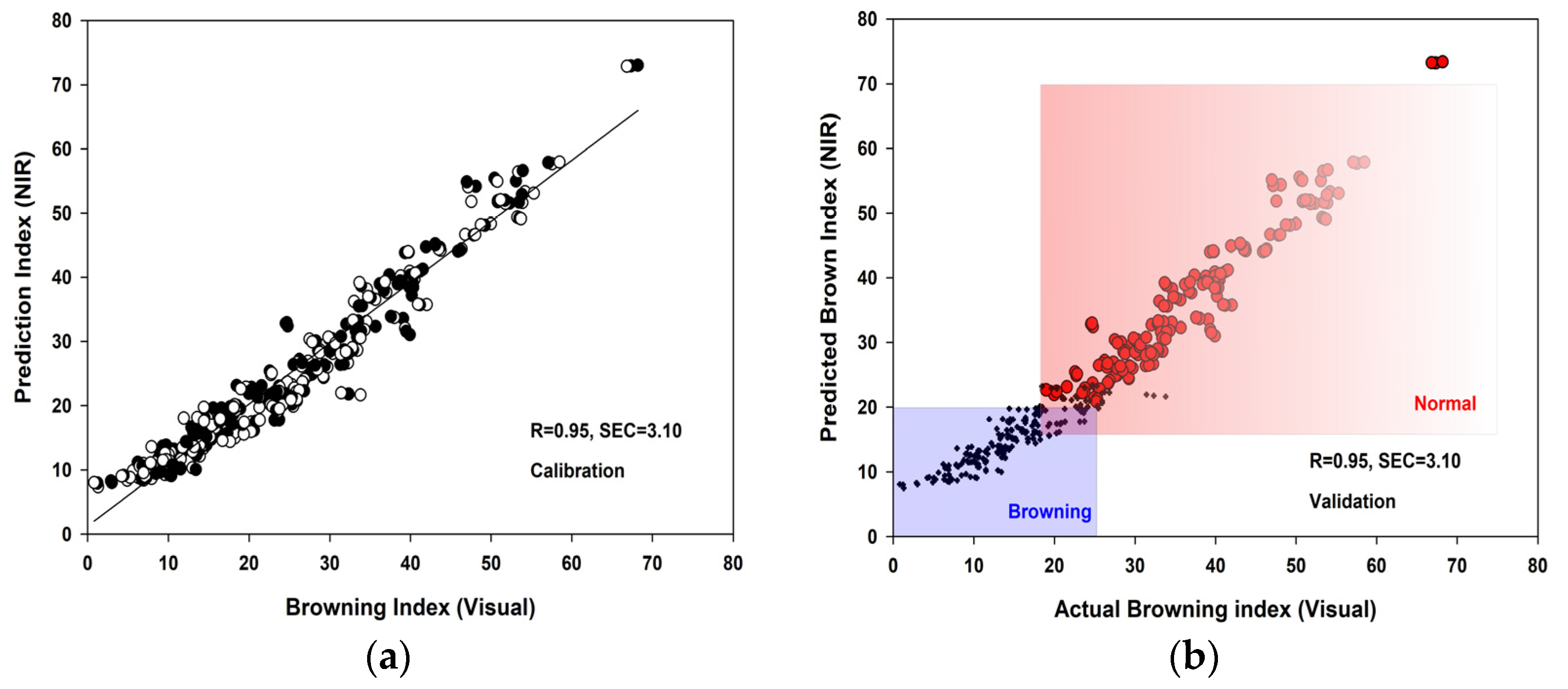
3.3. PLSR Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Korean Statistical Information Service. Available online: http://kosis.kr/search/search.do (accessed on 1 May 2023).
- Lee, U.Y.; Oh, K.Y.; Shim, H.K.; Lee, H.J.; Hwang, Y.S.; Chen, J.P. Comparison of fruit quality among fruits set on various position within cluster in ‘Niitaka’ Pears. Korean J. Agric. Sci. 2010, 37, 13–18. [Google Scholar] [CrossRef]
- Lwin, H.P.; Lee, J. Preharvest 1-methylcyclopropene treatment effects on fruit quality attributes and targeted metabolites in ‘Wonhwang’ pears stored at room temperature after cold storage. Sci. Hortic. 2021, 289, 110480. [Google Scholar] [CrossRef]
- Kim, Y.K.; Kang, S.S.; Won, K.H.; Shin, I.S.; Cho, K.S.; Ma, K.B.; Kim, M.S.; Choi, J.J.; Choi, J.H. Breeding of the Scab-Resistant Pear Cultivar ‘Greensis’. Hortic. Sci. Technol. 2016, 34, 655–661. [Google Scholar] [CrossRef]
- Latt, T.T.; Lwin, H.P.; Seo, H.-J.; Lee, J. 1-Methylcyclopropene delays degradation of peel greenness but induces internal physiological disorders in cold-stored fruit of interspecific pears. Sci. Hortic. 2023, 312, 111852. [Google Scholar] [CrossRef]
- Arzani, K.; Khoshghalb, H.; Malakouti, M.J.; Barzegar, M. Polyphenoloxidase activity, polyphenol and ascorbic acid concentrations and internal browning in Asian pear (Pyrus serotina Rehd.) fruit during storage in relation to time of harvest. Eur. J. Hortic. Sci. 2009, 74, 61–65. [Google Scholar]
- Choi, J.H.; Lee, U.Y.; Lee, J.H.; Choi, J.J.; Chun, J.P. Effect of 1-methylcyclopropene on quality of new mid-season Asian pear ‘Changjo’ during simulated marketing. Korean J. Agric. Sci. 2017, 44, 332–338. [Google Scholar] [CrossRef]
- Kim, J.G.; Oh, K.Y.; Lee, U.Y.; Ma, K.B.; Hwang, Y.S.; Choi, J.M.; Chun, J.P. Changes of the fruit quality according to temperature environment and marketing period during simulated exportation in ‘Whasan’ pears. J. Bio-Environ. Control 2011, 20, 399–405. [Google Scholar]
- Hayama, H.; Mitani, N.; Yamane, T.; Kusaba, S. Alleviation of Vascular Bundle Browning in the Japanese Pear ‘Rinka’ by Preharvest Application of Ethephon. Hortic. J. 2022, 91, 329–336. [Google Scholar] [CrossRef]
- Oh, K.Y.; Lee, U.Y.; Moon, S.J.; Kim, Y.; Yook, H.; Hwang, Y.; Chun, J. Transportation and distribution temperatures affect fruit quality and physiological disorders in ‘Wonhwang’ pears. Korean J. Hortic. Sci. Technol. 2010, 28, 434–441. [Google Scholar]
- Seo, H.-J.; Chen, P.-A.; Lin, S.-Y.; Choi, J.-H.; Kim, W.-S.; Haung, T.-B.; Roan, S.-F.; Chen, I.-Z. The Potassium to Magnesium Ratio Enables the Prediction of Internal Browning Disorder during Cold Storage of Asian Pears. Korean J. Hortic. Sci. Technol. 2015, 33, 535–541. [Google Scholar] [CrossRef]
- Seo, H.-J.; Wang, Y.-S.; Lwin, H.P.; Choi, J.-H.; Chun, J.-P.; Roan, S.-F.; Chen, I.-Z.; Lee, J. Early season ‘Wonhwang’ pear fruit quality following international transport and storage is negatively impacted by fruitlet stage gibberellic acid4+7 (GA4+7) application but improved by postharvest 1-methylcyclopropene (1-MCP). Sci. Hortic. 2019, 256, 108549. [Google Scholar] [CrossRef]
- Hasanzadeh, B.; Abbaspour-Gilandeh, Y.; Soltani-Nazarloo, A.; De La Cruz-Gámez, E.; Hernández-Hernández, J.L.; Martínez-Arroyo, M. Non-Destructive Measurement of Quality Parameters of Apple Fruit by Using Visible/Near-Infrared Spectroscopy and Multivariate Regression Analysis. Sustainability 2022, 14, 14918. [Google Scholar] [CrossRef]
- Hao, Y.; Li, X.; Zhang, C.; Lei, Z. Online Inspection of Browning in Yali Pears Using Visible-Near Infrared Spectroscopy and Interpretable Spectrogram-Based CNN Modeling. Biosensors 2023, 13, 203. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.; Shim, J.; Kim, B.; Lee, H.; Lim, J. Non-destructive prediction of soluble solid contents in Fuji apples using visible near-infrared spectroscopy and various statistical methods. J. Food Eng. 2022, 321, 110945. [Google Scholar] [CrossRef]
- Ma, T.; Zhao, J.; Inagaki, T.; Su, Y.; Tsuchikawa, S. Rapid and nondestructive prediction of firmness, soluble solids content, and pH in kiwifruit using Vis–NIR spatially resolved spectroscopy. Postharvest Biol. Technol. 2022, 186, 111841. [Google Scholar] [CrossRef]
- Torres, C.A.; Mogollon, R. Characterization of sun-injury and prediction of sunscald on ‘Packham’s Triumph’ pears using Vis-NIR spectroscopy. Postharvest Biol. Technol. 2022, 184, 111776. [Google Scholar] [CrossRef]
- Li, J.L.; Sun, D.W.; Cheng, J.H. Recent Advances in Nondestructive Analytical Techniques for Determining the Total Soluble Solids in Fruits: A Review. Compr. Rev. Food Sci. Food Saf. 2016, 15, 897–911. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Peng, J.; Xie, C.; Bao, Y.; He, Y. Fruit Quality Evaluation Using Spectroscopy Technology: A Review. Sensors 2015, 15, 11889–11927. [Google Scholar] [CrossRef] [PubMed]
- Jang, K.E.; Kim, G.; Shin, M.H.; Cho, J.G.; Jeong, J.H.; Lee, S.K.; Kang, D.; Kim, J.G. Field Application of a Vis/NIR Hyperspectral Imaging System for Nondestructive Evaluation of Physicochemical Properties in ‘Madoka’ Peaches. Plants 2022, 11, 2327. [Google Scholar] [CrossRef] [PubMed]
- Zeb, A.; Qureshi, W.S.; Ghafoor, A.; Malik, A.; Imran, M.; Mirza, A.; Tiwana, M.I.; Alanazi, E. Towards sweetness classification of orange cultivars using short-wave NIR spectroscopy. Sci. Rep. 2023, 13, 325. [Google Scholar] [CrossRef] [PubMed]
- Mogollon, M.; Jara, A.; Contreras, C.; Zoffoli, J. Quantitative and qualitative VIS-NIR models for early determination of internal browning in ‘Cripps Pink’ apples during cold storage. Postharvest Biol. Technol. 2020, 161, 111060. [Google Scholar] [CrossRef]
- Huang, Y.; Lu, R.; Chen, K. Detection of internal defect of apples by a multichannel Vis/NIR spectroscopic system. Postharvest Biol. Technol. 2020, 161, 111065. [Google Scholar] [CrossRef]
- Kim, B.G.; Lim, J.G. Discrimination of internally browned apples utilizing near-infrared non-destructive fruit sorting system. J. Korea Acad. Ind. Coop. Soc. 2021, 22, 208–213. [Google Scholar] [CrossRef]
- Fu, X.; Ying, Y.; Lu, H.; Xu, H. Comparison of diffuse reflectance and transmission mode of visible-near infrared spectroscopy for detecting brown heart of pear. J. Food Eng. 2007, 83, 317–323. [Google Scholar] [CrossRef]
- Han, D.; Tu, R.; Lu, C.; Liu, X.; Wen, Z. Nondestructive detection of brown core in the Chinese pear ‘Yali’ by transmission visible–NIR spectroscopy. Food Control 2006, 17, 604–608. [Google Scholar] [CrossRef]
- Sun, X.; Liu, Y.; Li, Y.; Wu, M.; Zhu, D. Simultaneous measurement of brown core and soluble solids content in pear by on-line visible and near infrared spectroscopy. Postharvest Biol. Technol. 2016, 116, 80–87. [Google Scholar] [CrossRef]
- Cruz, S.; Guerra, R.; Brazio, A.; Cavaco, A.M.; Antunes, D.; Passos, D. Nondestructive simultaneous prediction of internal browning disorder and quality attributes in ‘Rocha’ pear (Pyrus communis L.) using VIS-NIR spectroscopy. Postharvest Biol. Technol. 2021, 179, 111562. [Google Scholar] [CrossRef]
- Choi, J.-H.; Chen, P.-A.; Lee, B.; Yim, S.-H.; Kim, M.-S.; Bae, Y.-S.; Lim, D.-C.; Seo, H.-J. Portable, non-destructive tester integrating VIS/NIR reflectance spectroscopy for the detection of sugar content in Asian pears. Sci. Hortic. 2017, 220, 147–153. [Google Scholar] [CrossRef]
- Kawano, S.; Abe, H.; Iwamoto, M. Development of a Calibration Equation with Temperature Compensation for Determining the Brix Value in Intact Peaches. J. Near Infrared Spectrosc. 1995, 3, 211–218. [Google Scholar] [CrossRef]
- Peinado, A.; van den Berg, F.; Blanco, M.; Bro, R. Temperature-induced variation for NIR tensor-based calibration. Chemom. Intell. Lab. Syst. 2006, 83, 75–82. [Google Scholar] [CrossRef]
- Li, X.; He, Y.; Fang, H. Non-destructive discrimination of Chinese bayberry varieties using Vis/NIR spectroscopy. J. Food Eng. 2007, 81, 357–363. [Google Scholar] [CrossRef]
- Jaillais, B.; Pinto, R.; Barros, A.; Rutledge, D. Outer-product analysis (OPA) using PCA to study the influence of temperature on NIR spectra of water. Vib. Spectrosc. 2005, 39, 50–58. [Google Scholar] [CrossRef]
- Suh, J.-Y.; Woo, Y.-A.; Lim, H.-R.; Kim, H.-J.; Moon, D.-G.; Choi, Y.-H. Determination of the water content in citrus leaves by portable near infrared (NIR) system. Anal. Sci. Technol. 2003, 16, 277–282. [Google Scholar]
- Ryu, D.S.; Noh, S.H.; Hwang, H. Nondestructive internal defects evaluation for pear using NIR/VIS Transmittance spectroscopy. Agric. Biosyst. Eng. 2003, 4, 1–7. [Google Scholar]


| Parameter | Specification |
|---|---|
| Sensor type | Enhanced CMOS Spectrometer (600–1100 nm) |
| Light source | T-Halogen Mini Lamp |
| Measuring time | 2.0 s max |
| Data interface | USB 2.0 (PC) |
| Operating temperature | 5 °C–35 °C |
| Display | TN LCD (45 mm × 30 mm) |
| Dimension and weight (instrument only) | 110 mm × 60 mm ×169 mm (420–450 g) |
| Preprocessing Method | Number of Factors | Slope (Cal/Val) | Calibration | Validation | ||
|---|---|---|---|---|---|---|
| R | SEC | R | SEP | |||
| Smoothing y | 2 | 0.95/0.95 | 0.95 | 3.10 | 0.95 | 3.14 |
| SNV x | 3 | 0.92/0.92 | 0.92 | 3.81 | 0.92 | 3.92 |
| 2nd Derivative w | 5 | 0.96/0.95 | 0.96 | 2.64 | 0.95 | 2.85 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seo, H.-J.; Song, J. Detection of Internal Browning Disorder in ‘Greensis’ Pears Using a Portable Non-Destructive Instrument. Horticulturae 2023, 9, 944. https://doi.org/10.3390/horticulturae9080944
Seo H-J, Song J. Detection of Internal Browning Disorder in ‘Greensis’ Pears Using a Portable Non-Destructive Instrument. Horticulturae. 2023; 9(8):944. https://doi.org/10.3390/horticulturae9080944
Chicago/Turabian StyleSeo, Ho-Jin, and Janghoon Song. 2023. "Detection of Internal Browning Disorder in ‘Greensis’ Pears Using a Portable Non-Destructive Instrument" Horticulturae 9, no. 8: 944. https://doi.org/10.3390/horticulturae9080944
APA StyleSeo, H.-J., & Song, J. (2023). Detection of Internal Browning Disorder in ‘Greensis’ Pears Using a Portable Non-Destructive Instrument. Horticulturae, 9(8), 944. https://doi.org/10.3390/horticulturae9080944





