Physiological and Structural Changes in Apple Tree Branches of Different Varieties during Dormancy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials and Experimental Design
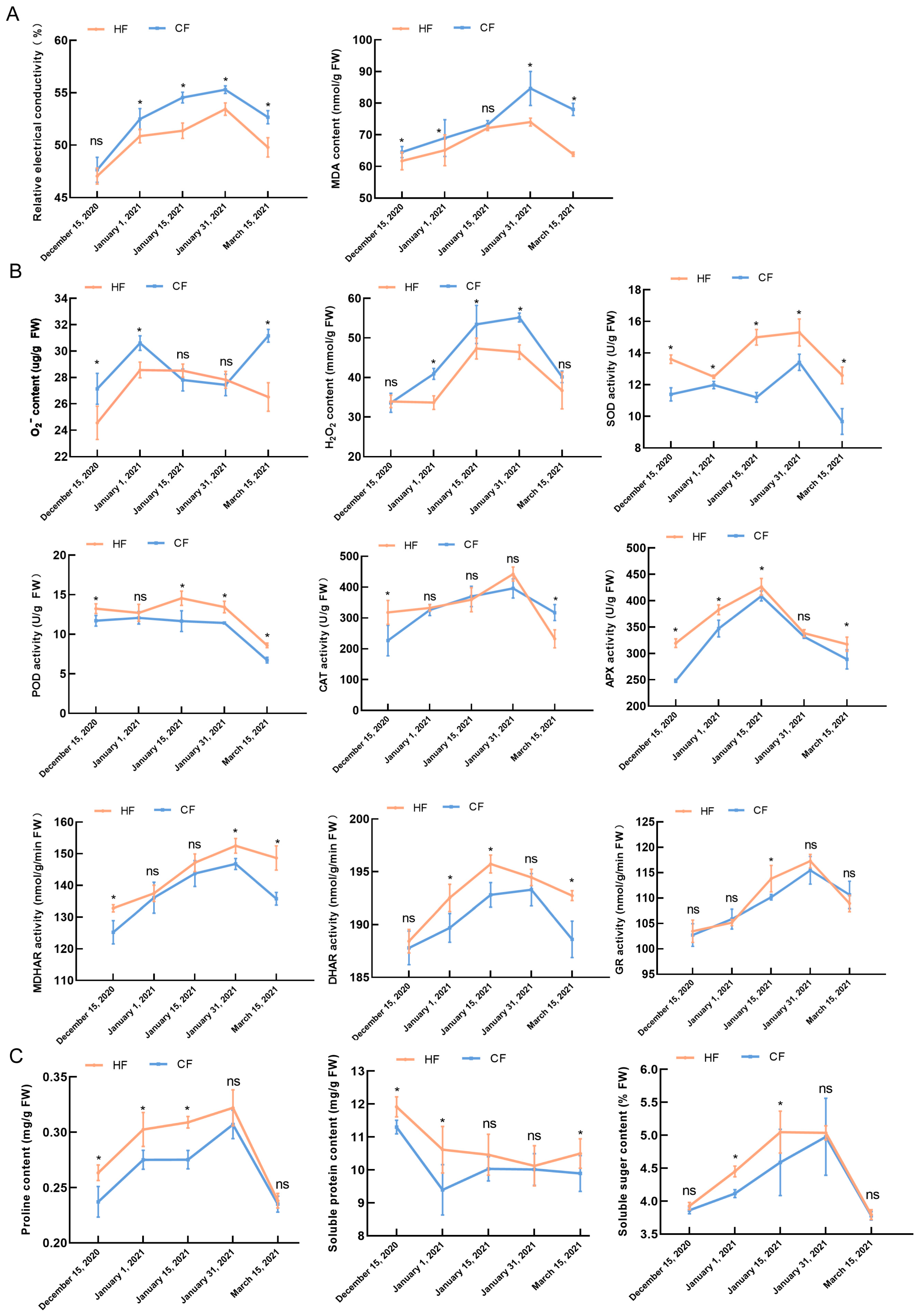
2.2. Determination of Relative Electrical Conductivity
2.3. Determination of MDA, O2−, and H2O2 Contents
2.4. Determination of Antioxidative Enzyme Activities
2.5. Determination of Proline, Soluble Protein, and Soluble Sugar Contents
2.6. Determination of Sucrose, Glucose, Fructose, and Sorbitol Contents
2.7. Anatomical Structure Analysis of One-Year-Old Branches
2.8. Statistical Analyses
3. Results
3.1. Changes in Temperature during Dormancy
3.2. Effects of Natural Low Temperatures on Physiology and Biochemistry of Apple Tree Branches during Dormancy
3.3. Effects of Natural Low Temperatures on the Anatomical Structure of One-Year-Old Apple tree Branches
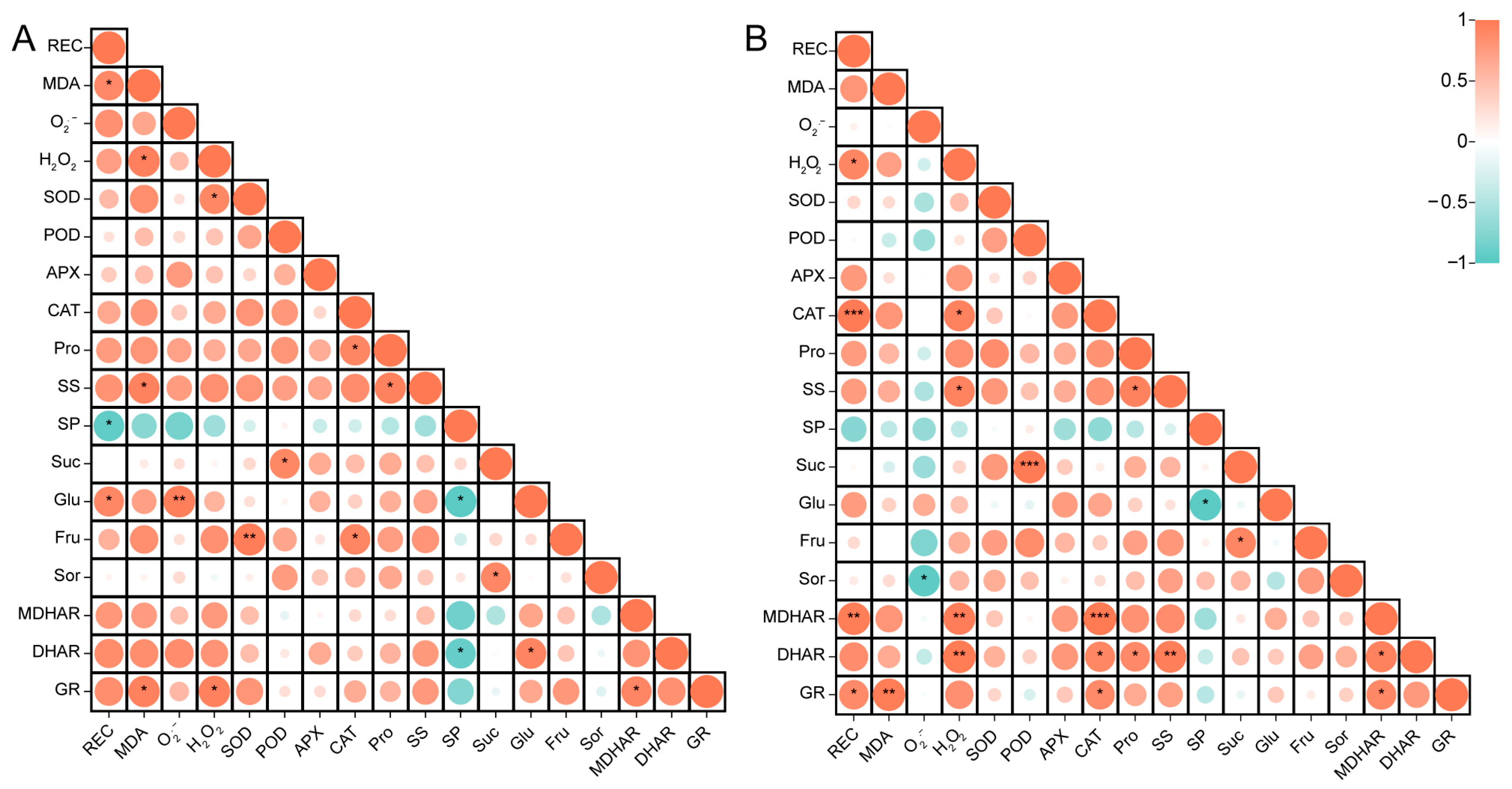
3.4. Correlation Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Xu, G.; Zhou, J.; Lyu, D.; Qin, S. Cold resistance evaluation of four apple varieties. J. Fruit Sci. 2023, 40, 669–679. [Google Scholar]
- Theocharis, A.; Clément, C.; Barka, E.A. Physiological and molecular changes in plants grown at low temperatures. Planta 2012, 235, 1091–1105. [Google Scholar]
- Manasa, S.L.; Panigrahy, M.; Panigrahi, K.C.; Rout, G.R. Overview of cold stress regulation in plants. Bot. Rev. 2022, 88, 359–387. [Google Scholar]
- Wang, H.; Gong, M.; Xin, H.; Tang, L.; Dai, D.; Gao, Y.; Liu, C. Effects of chilling stress on the accumulation of soluble sugars and their key enzymes in Jatropha curcas seedlings. Physiol. Mol. Biol. Plants 2018, 24, 857–865. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Wu, H.; Wang, B.; Zhang, Y.; Wang, J.; Cheng, C.; Huang, Y. Exogenous melatonin enhances cold resistance by improving antioxidant defense and cold-responsive genes’ expression in banana. Horticulturae 2022, 8, 260. [Google Scholar] [CrossRef]
- Mishra, N.; Jiang, C.; Chen, L.; Paul, A.; Chatterjee, A.; Shen, G. Achieving abiotic stress tolerance in plants through antioxidative defense mechanisms. Front. Plant Sci. 2023, 14, 1110622. [Google Scholar] [PubMed]
- Wei, Y.; Chen, H.; Wang, L.; Zhao, Q.; Wang, D.; Zhang, T. Cold acclimation alleviates cold stress-induced PSII inhibition and oxidative damage in tobacco leaves. Plant Signal. Behav. 2022, 17, 2013638. [Google Scholar] [CrossRef] [PubMed]
- Dvořák, P.; Krasylenko, Y.; Zeiner, A.; Šamaj, J.; Takáč, T. Signaling Toward Reactive Oxygen Species-Scavenging Enzymes in Plants. Front. Plant Sci. 2021, 11, 618835. [Google Scholar] [PubMed]
- Fujita, M.; Hasanuzzaman, M. Approaches to Enhancing Antioxidant Defense in Plants. Antioxidants 2022, 11, 925. [Google Scholar] [CrossRef]
- Rani, A.; Kiran, A.; Sharma, K.D.; Prasad, P.V.V.; Jha, U.C.; Siddique, K.H.M.; Nayyar, H. Cold Tolerance during the Reproductive Phase in Chickpea (Cicer arietinum L.) Is Associated with Superior Cold Acclimation Ability Involving Antioxidants and Cryoprotective Solutes in Anthers and Ovules. Antioxidants 2021, 10, 1693. [Google Scholar] [CrossRef]
- Yu, D.J.; Hwang, J.Y.; Chung, S.W.; Oh, H.D.; Yun, S.K.; Lee, H.J. Changes in cold hardiness and carbohydrate content in peach (Prunus persica) trunk bark and wood tissues during cold acclimation and deacclimation. Sci. Hortic. 2017, 219, 45–52. [Google Scholar] [CrossRef]
- Kwon, J.H.; Nam, E.Y.; Yun, S.K.; Kim, S.J.; Yu, D.J.; Lee, H.J. Comparative carbohydrate metabolism in the shoots of a cold-hardy and a cold-sensitive peach (Prunus persica) cultivar during cold acclimation and deacclimation. Hortic. Environ. Biotechnol. 2022, 63, 39–53. [Google Scholar] [CrossRef]
- Buchner, O.; Neuner, G. Winter frost resistance of Pinus cembra measured in situ at the alpine timberline as affected by temperature conditions. Tree Physiol. 2011, 31, 1217–1227. [Google Scholar] [CrossRef] [PubMed]
- Serra, O.; Mähönen, A.P.; Hetherington, A.J.; Ragni, L. The making of plant armor: The periderm. Annu. Rev. Plant Biol. 2022, 73, 405–432. [Google Scholar] [CrossRef] [PubMed]
- Zhai, J.T. A Structural and Physiological Study of Winter Hardiness of Different Variance-Typed Hibiscus syriacus. Master’s Thesis, Hebei Normal University of Science & Technology, Qinhuangdao, China, 2015. [Google Scholar]
- Hajihashemi, S.; Noedoost, F.; Geuns, J.M.C.; Djalovic, I.; Siddique, K.H.M. Effect of Cold Stress on Photosynthetic Traits, Carbohydrates, Morphology, and Anatomy in Nine Cultivars of Stevia rebaudiana. Front. Plant Sci. 2018, 9, 1430. [Google Scholar] [CrossRef] [PubMed]
- Pandey, S. Climatic influence on tree wood anatomy: A review. J. Wood Sci. 2021, 67, 24. [Google Scholar] [CrossRef]
- Qin, S.; Xu, G.; He, J.; Li, L.; Ma, H.; Lyu, D. A chromosome-scale genome assembly of Malus domestica, a multi-stress resistant apple variety. Genomics 2023, 115, 110627. [Google Scholar] [CrossRef]
- Xu, G.; Li, L.; Zhou, J.; Lyu, D.; Zhao, D.; Qin, S. Comparison of transcriptome and metabolome analysis revealed differences in cold resistant metabolic pathways in different apple cultivars under low temperature stress. Hortic. Plant J. 2023, 9, 183–198. [Google Scholar] [CrossRef]
- He, J.; Li, H.; Ma, C.; Zhang, Y.; Polle, A.; Rennenberg, H.; Cheng, X.; Luo, Z.B. Overexpression of bacterial g-glutamylcysteine synthetase mediates changes in cadmium influx, allocation and detoxification in poplar. New Phytol. 2015, 205, 240–245. [Google Scholar] [CrossRef]
- Zhou, J.; Wan, H.; He, J.; Lyu, D.; Li, H. Integration of cadmium accumulation, subcellular distribution, and physiological responses to understand cadmium tolerance in apple rootstocks. Front. Plant Sci. 2017, 8, 966. [Google Scholar] [CrossRef]
- Li, L.; Yang, B.; Zhao, X.; Wang, P.; Lyu, D.; Qin, S. Auxin Participates in the Regulation of the Antioxidant System in Malus baccata Borkh. Roots under Sub-Low Temperature by Exogenous Sucrose Application. Horticulturae 2023, 9, 297. [Google Scholar] [CrossRef]
- He, J.; Li, H.; Luo, J.; Ma, C.; Li, S.; Qu, L.; Gai, Y.; Jiang, X.; Janz, D.; Polle, A.; et al. A transcriptomic network underlies microstructural and physiological responses to cadmium in Populus × canescens. Plant Physiol. 2013, 162, 424–439. [Google Scholar] [CrossRef] [PubMed]
- Yemm, E.W.; Willis, A.J. The estimation of carbohydrates in plant extracts by anthrone. Biochem. J. 1954, 57, 508–514. [Google Scholar] [CrossRef]
- Huang, Y.; Qin, S.; He, J.; Lyu, D. Integration of cell wall fraction, organic matter content, and membrane to understand crispness changes in apples. Sci. Hortic. 2023, 321, 112309. [Google Scholar] [CrossRef]
- Faust, M.; Erez, A.; Rowland, L.J.; Wang, S.Y.; Norman, H.A. Bud dormancy in perennial fruit trees; physiological basis for dormancy induction maintenance and release. HortScience 1997, 32, 623–629. [Google Scholar] [CrossRef]
- Fadón, E.; Herrero, M.; Rodrigo, J. Dormant flower buds actively accumulate starch over winter in sweet cherry. Front. Plant Sci. 2018, 9, 171. [Google Scholar] [CrossRef] [PubMed]
- Considine, M.J.; Considine, J.A. On the language and physiology of dormancy and quiescence in plants. J. Exp. Bot. 2016, 67, 3189–3203. [Google Scholar] [CrossRef]
- Heide, O.M.; Prestrud, A.K. Low temperature, but not photoperiod, controls growth cessation and dormancy induction and release in apple and pear. Tree Physiol. 2005, 25, 109–114. [Google Scholar] [CrossRef]
- Cooke, J.E.K.; Eriksson, M.E.; Junttila, O. The dynamic nature of bud dormancy in trees: Environmental control and molecular mechanisms. Plant Cell Environ. 2012, 35, 1707–1728. [Google Scholar] [CrossRef]
- Beauvieux, R.; Wenden, B.; Dirlewanger, E. Bud dormancy in perennial fruit tree species: A pivotal role for oxidative cues. Front. Plant Sci. 2018, 9, 657. [Google Scholar] [CrossRef]
- Fadón, E.; Fernandez, E.; Behn, H.; Luedeling, E. A conceptual framework for winter dormancy in deciduous trees. Agronomy 2020, 10, 241. [Google Scholar] [CrossRef]
- Liu, H.; Lu, C.; Wang, S.; Ren, F.; Wang, H. Climate warming extends growing season but not reproductive phase of terrestrial plants. Global Ecol. Biogeogr. 2021, 30, 950–960. [Google Scholar] [CrossRef]
- Pagter, M.; Hausman, J.F.; Arora, R. Deacclimation kinetics and carbohydrate changes in stem tissues of Hydrangea in response to an experimental warm spell. Plant Sci. 2011, 180, 140–148. [Google Scholar] [CrossRef]
- Takahashi, D.; Li, B.; Nakayama, T.; Kawamura, Y.; Uemura, M. Plant plasma membrane proteomics for improving cold tolerance. Front. Plant Sci. 2013, 4, 90. [Google Scholar] [CrossRef] [PubMed]
- Hassan, M.A.; Xiang, C.; Farooq, M.; Muhammad, N.; Yan, Z.; Hui, X.; Yuanyuan, K.; Bruno, A.K.; Lele, Z.; Jincai, L. Cold Stress in Wheat: Plant Acclimation Responses and Management Strategies. Front. Plant Sci. 2021, 12, 676884. [Google Scholar] [CrossRef]
- Chen, L.J.; Xiang, H.Z.; Miao, Y.; Zhang, L.; Guo, Z.F.; Zhao, X.H.; Lin, J.W.; Li, T.L. An overview of cold resistance in plants. J. Agron. Crop Sci. 2014, 200, 237–245. [Google Scholar] [CrossRef]
- Morin, X.; Améglio, T.; Ahas, R.; Kurz-Besson, C.; Lanta, V.; Lebourgeois, F.; Miglietta, F.; Chuine, I. Variation in cold hardiness and carbohydrate concentration from dormancy induction to bud burst among provenances of three European oak species. Tree Physiol. 2007, 27, 817–825. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.X.; Ya, H.U.; Chen, B.H.; Zhu, Y.F.; Dawuda, M.M.; Svetla, S. Physiological mechanisms of resistance to cold stress associated with 10 elite apple rootstocks. J. Integr. Agric. 2018, 17, 857–866. [Google Scholar] [CrossRef]
- Devireddy, A.R.; Tschaplinski, T.J.; Tuskan, G.A.; Muchero, W.; Chen, J.G. Role of Reactive Oxygen Species and Hormones in Plant Responses to Temperature Changes. Int. J. Mol. Sci. 2021, 22, 8843. [Google Scholar] [CrossRef]
- Sedaghat, S.; Gaaliche, B.; Rahemi, M.; Zare, H.; Jafari, M. Enzymatic activity and physico-chemical changes of terminal bud in rain-fed fig (Ficus carica L.‘Sabz’) during dormant season. Hortic. Plant J. 2022, 8, 195–204. [Google Scholar] [CrossRef]
- Meng, A.; Wen, D.; Zhang, C. Maize Seed Germination Under Low-Temperature Stress Impacts Seedling Growth Under Normal Temperature by Modulating Photosynthesis and Antioxidant Metabolism. Front. Plant Sci. 2022, 13, 843033. [Google Scholar] [CrossRef] [PubMed]
- Jing, J.; Liu, M.; Yin, B.; Liang, B.; Li, Z.; Zhang, X.; Xu, J.; Zhou, S. Effects of 10 Dwarfing Interstocks on Cold Resistance of ‘Tianhong 2’ Apple. Horticulturae 2023, 9, 827. [Google Scholar] [CrossRef]
- Li, S. Novel insight into functions of ascorbate peroxidase in higher plants: More than a simple antioxidant enzyme. Redox Biol. 2023, 64, 102789. [Google Scholar] [CrossRef]
- Hernandez, J.A.; Díaz-Vivancos, P.; Martínez-Sánchez, G.; Alburquerque, N.; Martínez, D.; Barba-Espín, G.; Acosta-Motos, J.R.; Carrera, E.; García-Bruntón, J. Physiological and biochemical characterization of bud dormancy: Evolution of carbohydrate and antioxidant metabolisms and hormonal profile in a low chill peach variety. Sci. Hortic. 2021, 281, 109957. [Google Scholar] [CrossRef]
- Li, Y.; Liu, Y.; Zhang, J. Advances in the research on the AsA-GSH cycle in horticultural crops. Front. Agric. China 2010, 4, 84–90. [Google Scholar] [CrossRef]
- Wang, J.; Fang, R.; Yuan, L.; Yuan, G.; Zhao, M.; Zhu, S.; Hou, J.; Chen, G.; Wang, C. Response of photosynthetic capacity and antioxidative system of chloroplast in two wucai (Brassica campestris L.) genotypes against chilling stress. Physiol. Mol. Biol. Plants 2020, 26, 219–232. [Google Scholar] [CrossRef]
- Sang, Y.; Yang, W.; Liu, Y.; Zhang, W.; Guo, T.; Shen, P.; Tang, Y.; Guo, M.; Chen, G. Influences of low temperature on the postharvest quality and antioxidant capacity of winter jujube (Zizyphus jujuba Mill. cv. Dongzao). LWT 2022, 154, 112876. [Google Scholar] [CrossRef]
- Raza, A.; Charagh, S.; Abbas, S.; Hassan, M.U.; Saeed, F.; Haider, S.; Sharif, R.; Anand, A.; Corpas, F.J.; Jin, W.; et al. Assessment of proline function in higher plants under extreme temperatures. Plant Biol. 2023, 25, 379–395. [Google Scholar] [CrossRef]
- Mohammadrezakhani, S.; Rezanejad, F.; Hajilou, J. Effect of putrescine and proline on proflies of GABA, antioxidant activities in leaves of three Citrus species in response to low temperature stress. J. Plant Biochem. Biot. 2021, 30, 545–553. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhou, M.; Xu, K.; Li, J.; Li, S.; Zhang, S.; Yang, X. Integrated transcriptomics and metabolomics analyses provide insights into cold stress response in wheat. Crop J. 2019, 7, 857–866. [Google Scholar] [CrossRef]
- Qi, W.; Wang, F.; Ma, L.; Qi, Z.; Liu, S.; Chen, C.; Wu, J.; Wang, P.; Yang, C.; Wu, Y.; et al. Physiological and Biochemical Mechanisms and Cytology of Cold Tolerance in Brassica napus. Front. Plant Sci. 2020, 11, 1241. [Google Scholar] [CrossRef]
- Karimi, R.; Ershadi, A.; Esna-Ashari, M.; Boojar, M.M.A. Seasonal changes in soluble proteins, total phenol and malondialdehyde content and their relationship with cold hardiness of some grapevine cultivars. J. Crop. Improv. 2014, 16, 999–1013. [Google Scholar]
- Nägele, T.; Heyer, A.G. Approximating subcellular organisation of carbohydrate metabolism during cold acclimation in different natural accessions of Arabidopsis thaliana. New Phytol. 2013, 198, 777–787. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, E.; Cuneo, I.F.; Luedeling, E.; Alvarado, L.; Farias, D.; Saa, S. Starch and hexoses concentrations as physiological markers in dormancy progression of sweet cherry twigs. Trees 2019, 33, 1187–1201. [Google Scholar] [CrossRef]
- Zhang, Y.; Ma, K.; Li, Q. Research on Chilling Requirements and Physiological Mechanisms of Prunus mume. Horticulturae 2023, 9, 603. [Google Scholar] [CrossRef]
- Rady, M.M.; Seif El-Yazal, M.A. Response of “Anna” apple dormant buds and carbohydrate metabolism during floral bud break to onion extract. Sci. Hortic. 2013, 155, 78–84. [Google Scholar] [CrossRef]
- Gholizadeh, J.; Sadeghipour, H.; Abdolzadeh, A.; Hemmati, K.; Hassani, D.; Vahdati, K. Redox rather than carbohydrate metabolism differentiates endodormant lateral buds in walnut cultivars with contrasting chilling requirements. Sci. Hortic. 2017, 225, 29–37. [Google Scholar] [CrossRef]
- Citadin, I.; Pertille, R.H.; Loss, E.M.S.; Oldoni, T.L.C.; Danner, M.A.; Júnior, A.W.; Lauri, P.É. Do low chill peach cultivars in mild winter regions undergo endodormancy? Trees 2022, 36, 1273–1284. [Google Scholar] [CrossRef]
- Quamme, H.; Weiser, C.J.; Stushnoff, C. The mechanism of freezing injury in xylem of winter apple twigs. Plant Physiol. 1973, 51, 273–277. [Google Scholar] [CrossRef] [PubMed]
- Pramsohler, M.; Hacker, J.; Neuner, G. Freezing pattern and frost killing temperature of apple (Malus domestica) wood under controlled conditions and in nature. Tree Physiol. 2012, 32, 819–828. [Google Scholar] [CrossRef]
- Gordon, D.; Damiano, C.; DeJong, T.M. Preformation in vegetative buds of Prunus persica: Factors influencing number of leaf primordia in overwintering buds. Tree Physiol. 2006, 26, 537–544. [Google Scholar] [CrossRef] [PubMed]
- Ray, D.M.; Savage, J.A. Seasonal changes in temperate woody plant phloem anatomy and physiology: Implications for long-distance transport. AoB Plants 2021, 13, plab028. [Google Scholar] [CrossRef] [PubMed]
- Chang, F.; Zhang, L.; Dong, Q.; Luan, H.; Jia, P.; Qi, G.; Guo, S.; Zhang, X. The anatomical structure character of raspberry stems is a key factor affecting its cold resistance. Flora 2023, 298, 152196. [Google Scholar] [CrossRef]
- Begum, S.; Nakaba, S.; Yamagishi, Y.; Oribe, Y.; Funada, R. Regulation of cambial activity in relation to environmental conditions: Understanding the role of temperature in wood formation of trees. Physiol. Plant. 2013, 147, 46–54. [Google Scholar] [CrossRef] [PubMed]
- Begum, S.; Kudo, K.; Rahman, M.H.; Nakaba, S.; Yamagishi, Y.; Nabeshima, E.; Nugroho, W.D.; Oribe, Y.; Kitin, P.; Jin, H.; et al. Climate change and the regulation of wood formation in trees by temperature. Trees 2018, 32, 3–15. [Google Scholar] [CrossRef]
- Amrina, S.; Vivek, D.; Tejpal, G.; Singh, A.P.; Yelam, S.; Pandey, G.K. Simultaneous over-expression of PaSOD and RaAPX in transgenic Arabidopsis thaliana confers cold stress tolerance through increase in vascular lignifications. PLoS ONE 2014, 9, e110302. [Google Scholar]
- Ito, A.; Sugiura, T.; Sakamoto, D.; Moriguchi, T. Effects of dormancy progression and low-temperature response on changes in the sorbitol concentration in xylem sap of Japanese pear during winter season. Tree Physiol. 2013, 33, 398–408. [Google Scholar] [CrossRef]
- Ketchie, D.O.; Kammereck, R. Seasonal variation of cold resistance in Malus woody tissue as determined by differential thermal analysis and viability tests. Can. J. Bot. 1987, 65, 2640–2645. [Google Scholar] [CrossRef]
- Guo, X.; Xiao, X.; Xu, X.; Dong, F.; Zhang, L. Observation on the vessel elements of secondary xylem in late-ripening peach trees. J. Fruit Sci. 2008, 25, 22–26. [Google Scholar]
- Schweingruber, F.H.; Ríha, P.; Doležal, J. Variation in stem anatomical characteristics of Campanuloideae species in relation to evolutionary history and ecological preferences. PLoS ONE 2014, 9, e88199. [Google Scholar] [CrossRef]
- García-Cervigón, A.I.; Fajardo, A.; Caetano-Sánchez, C.; Camarero, J.J.; Olano, J.M. Xylem anatomy needs to change, so that conductivity can stay the same: Xylem adjustments across elevation and latitude in Nothofagus pumilio. Ann. Bot. 2020, 125, 1101–1112. [Google Scholar] [CrossRef] [PubMed]
- Vicente, E.; Didion-Gency, M.; Morcillo, L.; Morin, X.; Vilagrosa, A.; Grossiord, C. Aridity and cold temperatures drive divergent adjustments of European beech xylem anatomy, hydraulics and leaf physiological traits. Tree Physiol. 2022, 42, 1720–1735. [Google Scholar] [CrossRef] [PubMed]
- Arnič, D.; Gričar, J.; Jevšenak, J.; Božič, G.; von Arx, G.; Prislan, P. Different Wood Anatomical and Growth Responses in European Beech (Fagus sylvatica L.) at Three Forest Sites in Slovenia. Front. Plant Sci. 2021, 12, 669229. [Google Scholar] [CrossRef] [PubMed]



| Date | 15 November 2020 | 15 January 2021 | 15 March 2021 | |||
|---|---|---|---|---|---|---|
| Species | CF | HF | CF | HF | CF | HF |
| Radius of branch (μm) | 3311.24 a ± 253.03 | 3468.56 a ± 330.71 | 3143.46 a ± 176.79 | 2963.72 a ± 208.08 | 3059.78 a ± 185.54 | 3202.48 a ± 268.12 |
| Radius of pith (μm) | 517.08 a ± 62.27 | 517.96 a ± 41.94 | 516.24 a ± 66.11 | 513.84 a ± 49.81 | 528.48 b ± 22.79 | 700.54 a ± 69.04 |
| Thickness of xylem (μm) | 2054.72 b ± 120.29 | 2291.66 a ± 150.65 | 1942.50 b ± 35.03 | 2047.14 a ± 88.29 | 1964.36 b ± 126.46 | 2151.14 a ± 125.19 |
| Thickness of cambium (μm) | 27.94 a ± 1.51 | 23.34 b ± 1.72 | 38.34 a ± 4.66 | 38.20 a ± 4.63 | 27.80 a ± 2.26 | 28.20 a ± 2.01 |
| Thickness of phloem (μm) | 343.04 b ± 37.66 | 419.14 a ± 52.86 | 299.68 b ± 9.22 | 317.82 a ± 10.27 | 302.64 b ± 23.85 | 390.98 a ± 33.90 |
| Thickness of periderm (μm) | 108.98 b ± 8.85 | 131.82 a ± 13.74 | 120.12 a ± 8.33 | 126.04 a ± 10.12 | 123.60 a ± 24.74 | 124.36 a ± 7.48 |
| Number of vessels | 16.60 b ± 1.95 | 21.00 a ± 1.58 | 17.00 b ± 1.00 | 25.60 a ± 1.34 | 18.00 b ± 1.58 | 23.40 a ± 2.07 |
| Vessel area (μm2) | 603.40 a ± 40.17 | 524.28 b ± 53.22 | 654.48 a ± 39.12 | 584.72 b ± 21.65 | 636.32 a ± 54.93 | 582.66 a ± 22.99 |
| Proportion of pith (%) | 15.58 a ± 0.72 | 14.97 a ± 0.85 | 16.38 a ± 1.37 | 17.55 a ± 0.95 | 17.29 b ± 0.47 | 21.85 a ± 0.41 |
| Proportion of xylem (%) | 62.14 b ± 2.01 | 66.24 a ± 2.51 | 61.93 b ± 3.27 | 70.04 a ± 0.56 | 64.20 a ± 1.44 | 67.33 a ± 3.16 |
| Proportion of phloem (%) | 10.34 b ± 0.41 | 12.08 a ± 0.85 | 9.55 b ± 0.26 | 10.88 a ± 0.26 | 9.89 b ± 0.45 | 12.21 a ± 0.26 |
| Proportion of periderm (%) | 3.29 b ± 0.12 | 3.80 a ± 0.14 | 3.82 b ± 0.14 | 4.31 a ± 0.15 | 4.05 a ± 0.55 | 3.90 a ± 0.25 |
| Proportion of cambium (%) | 0.85 a ± 0.03 | 0.67 b ± 0.04 | 1.22 a ± 0.08 | 1.30 a ± 0.10 | 0.91 a ± 0.02 | 0.87 a ± 0.04 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, G.; He, M.; Zhao, D.; Lyu, D.; Qin, S. Physiological and Structural Changes in Apple Tree Branches of Different Varieties during Dormancy. Horticulturae 2023, 9, 947. https://doi.org/10.3390/horticulturae9080947
Xu G, He M, Zhao D, Lyu D, Qin S. Physiological and Structural Changes in Apple Tree Branches of Different Varieties during Dormancy. Horticulturae. 2023; 9(8):947. https://doi.org/10.3390/horticulturae9080947
Chicago/Turabian StyleXu, Gongxun, Meiqi He, Deying Zhao, Deguo Lyu, and Sijun Qin. 2023. "Physiological and Structural Changes in Apple Tree Branches of Different Varieties during Dormancy" Horticulturae 9, no. 8: 947. https://doi.org/10.3390/horticulturae9080947
APA StyleXu, G., He, M., Zhao, D., Lyu, D., & Qin, S. (2023). Physiological and Structural Changes in Apple Tree Branches of Different Varieties during Dormancy. Horticulturae, 9(8), 947. https://doi.org/10.3390/horticulturae9080947






