Analysis of the Aroma Volatile Profile of Muscadine Grape Germplasm by Headspace Solid-Phase Microextraction Coupled with Gas Chromatography-Mass Spectrometry
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials and Sampling
2.2. Sample Preparation for Quality and Volatile Analysis
2.3. Sample Incubation and GC-MS Conditions
2.4. Statistical Analysis
3. Results and Discussion
3.1. Role of Berry Maturity on Volatile Composition
3.2. Diversity of Aroma Volatiles in Muscadine Germplasm
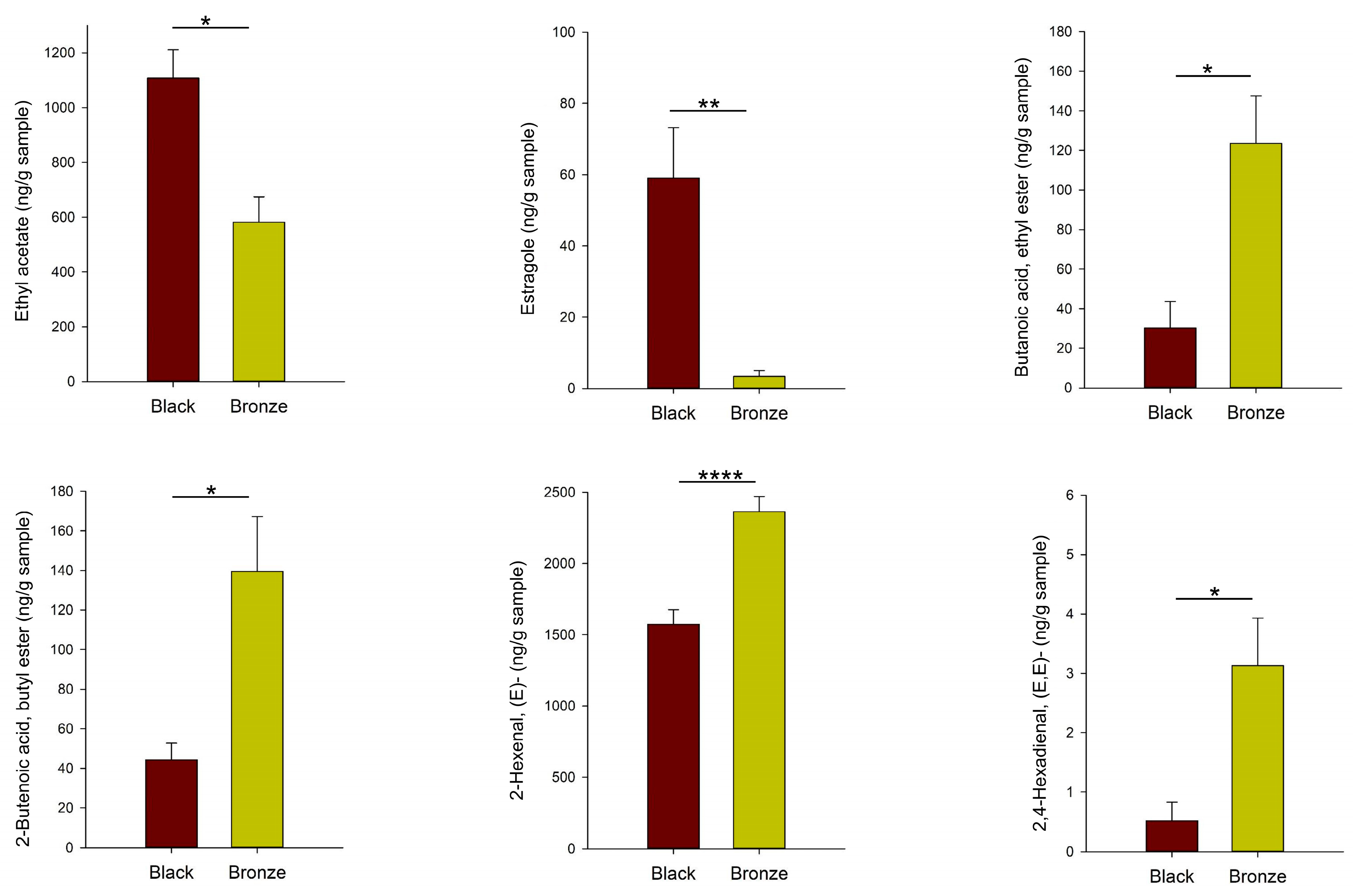
3.3. Differences in the Aroma Volatile Composition between Black and Bronze Muscadines
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hedrick, U.P.; Booth, N.O.; Taylor, O.M.; Wellington, R.; Dorsey, M.J. The Grapes of New York; 15th Annual Report; State of New York, Department of Agriculture: New York, NY, USA, 1908; Volume 3, Part II.
- Bailey, L.H. The Standard Cyclopedia of Horticulture; Macmillan: New York, NY, USA, 1908. [Google Scholar]
- Moore, M.O. Classification and systematics of eastern North American Vitis L. (Vitaceae) north of Mexico. SIDA Contrib. Bot. 1991, 14, 339–367. [Google Scholar]
- Radford, A.E.; Ahles, H.E.; Bell, C.R. Manual of the Vascular Flora of the Carolinas; University of North Carolina Press: Chapel Hill, NC, USA, 2010. [Google Scholar]
- Wen, J. Vitaceae. In The Families and Genera of Vascular Plants; Kubitz, K., Ed.; Springer: Berlin/Heidelberg, Germany, 2007; pp. 467–479. [Google Scholar]
- Hoffmann, M.; Conner, P.; Brannen, P.; Burrack, H.; Mitchem, W.; Cline, B.; Perkins-Veazie, P.; Poling, B. Muscadine Grape Production Guide for the Southeast. North Carolina State Ext. AG-94. 29 January 2021. Available online: https://smallfruits.org/files/2020/07/muscadine-grape-production-guide-southeast.pdf (accessed on 5 October 2022).
- Hickey, C.C.; Smith, E.D.; Cao, S.; Conner, P. Muscadine (Vitis rotundifolia Michx., syn. Muscandinia rotundifolia (Michx.) small): The resilient, native grape of the southeastern US. Agriculture 2019, 9, 131. [Google Scholar] [CrossRef]
- Wang, S.Y.; Lin, H.S. Antioxidant activity in fruits and leaves of blackberry, raspberry, and strawberry varies with cultivar and developmental stage. J. Agric. Food Chem. 2000, 48, 140–146. [Google Scholar] [CrossRef] [PubMed]
- Pastrana-Bonilla, E.; Akoh, C.C.; Sellappan, S.; Krewer, G. Phenolic content and antioxidant capacity of muscadine grapes. J. Agric. Food Chem. 2003, 51, 5497–5503. [Google Scholar] [CrossRef] [PubMed]
- Bartoshuk, L.M.; Klee, H.J. Better fruits and vegetables through sensory analysis. Curr. Biol. 2013, 23, R374–R378. [Google Scholar] [CrossRef]
- Colantonio, V.; Ferrão, L.F.V.; Tieman, D.M.; Bliznyuk, N.; Sims, C.; Klee, H.J.; Munoz, P.; Resende, M.F., Jr. Metabolomic selection for enhanced fruit flavor. Proc. Natl. Acad. Sci. USA 2022, 119, e2115865119. [Google Scholar] [CrossRef]
- Rodríguez, A.; Alquézar, B.; Peña, L. Fruit aromas in mature fleshy fruits as signals of readiness for predation and seed dispersal. New Phytol. 2013, 197, 36–48. [Google Scholar] [CrossRef]
- Baek, H.H.; Cadwallader, K.R.; Marroquin, E.; Silva, J.L. Identification of predominant aroma compounds in muscadine grape juice. J. Food Sci. 1997, 62, 249–252. [Google Scholar] [CrossRef]
- Lee, B.; Lin, P.C.; Cha, H.S.; Luo, J.; Chen, F. Characterization of volatile compounds in Cowart muscadine grape (Vitis rotundifolia) during ripening stages using GC-MS combined with principal component analysis. Food Sci. Biotechnol. 2016, 25, 1319–1326. [Google Scholar] [CrossRef]
- Lamikanra, O. Aroma constituents of muscadine wines1. J. Food Qual. 1987, 10, 57–66. [Google Scholar] [CrossRef]
- Deng, H.; He, R.; Long, M.; Li, Y.; Zheng, Y.; Lin, L.; Dong, L.; Zhang, X.; Liao, M.; Lv, X.; et al. Comparison of the Fruit Volatile Profiles of Five Muscadine Grape Cultivars (Vitis rotundifolia Michx.) using HS-SPME-GC/MS Combined with Multivariate Statistical Analysis. Front. Plant Sci. 2021, 12, 2302. [Google Scholar] [CrossRef] [PubMed]
- Gu, X.; Zhang, X.; Wang, K.; Lv, X.; Li, R.; Ma, W. GC–MS Untargeted Analysis of Volatile Compounds in Four Red Grape Varieties (Vitis vinifera L. cv) at Different Maturity Stages near Harvest. Foods 2022, 11, 2804. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.N.; Zheng, F.P.; Yu, A.N.; Sun, B.G. Changes of the free and bound volatile compounds in Rubus corchorifolius L. f. fruit during ripening. Food Chem. 2019, 287, 232–240. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Li, Q.; Li, J.; Luo, J.; Chen, W.; Li, X. Comparative study of volatile compounds in the fruit of two banana cultivars at different ripening stages. Molecules 2018, 23, 2456. [Google Scholar] [CrossRef] [PubMed]
- Corpas Iguarán, E.; Taborda Ocampo, G.; Tapasco Alzate, O. Identification of volatile compound markers during the ripening and senescence of lulo (Solanum quitoense Lam.). J. Food Sci. Technol. 2018, 55, 437–442. [Google Scholar] [CrossRef] [PubMed]
- Zidi, K.; Kati, D.E.; Bachir-Bey, M.; Genva, M.; Fauconnier, M.L. Comparative study of fig volatile compounds using headspace solid-phase microextraction-gas chromatography/mass spectrometry: Effects of cultivars and ripening stages. Front. Plant Sci. 2021, 12, 1312. [Google Scholar] [CrossRef]
- Senesi, E.; Di Cesare, L.F.; Prinzivalli, C.; Scalzo, R.L. Influence of ripening stage on volatiles composition, physicochemical indexes and sensory evaluation in two varieties of muskmelon (Cucumis melo L. var reticulatus Naud). J. Sci. Food Agric. 2005, 85, 1241–1251. [Google Scholar] [CrossRef]
- Poling, E.B.; Mainland, C.M.; Bland, W.T.; Cline, B.; Sorensen, K.A. Muscadine Grape Production Guide for North Carolina; AG-94; NC State Extension: Raleigh, NC, USA, 2003; Available online: https://content.ces.ncsu.edu/muscadine-grape-production-guide (accessed on 2 March 2023).
- Conner, P.J.; Worthington, M.L. Muscadine Grape Breeding. In Plant Breeding Reviews; Goldman, I., Ed.; John Wiley & Sons: Hoboken, NJ, USA, 2022. [Google Scholar] [CrossRef]
- Mortensen, J.A.; Harris, J.W.; Hopkins, D.L.; Andersen, P.C. ‘Southern Home’: An interspecific hybrid grape with ornamental value. HortScience 1994, 29, 1371–1372. [Google Scholar] [CrossRef]
- Bloodworth, P.J. Grapevine Plant Named ‘JB06-43-6-22‘. US Plant Patent 31,010, 11 February 2019. [Google Scholar]
- Lanier, M.R.; Morris, J.R. Evaluation of Density Separation for Defining Fruit Maturities and Maturation Rates of Once-over Harvested Muscadine Grapes. J. Am. Soc. Hortic. Sci. 1979, 104, 249–252. [Google Scholar] [CrossRef]
- R Core Team, R. A Language and Environment for Statistical Computing. Available online: https://www.r-project.org/ (accessed on 11 February 2022).
- Kolde, R. Pheatmap: Pretty Heatmaps. R Package Version 1.0.12. 2019. Available online: https://CRAN.R-project.org/package=pheatmap (accessed on 11 February 2022).
- Chen, Y.; Sidisky, L.M. Quantification of 4-hydroxy-2, 5-dimethyl-3-furanone in fruit samples using solid phase microextraction coupled with gas chromatography–mass spectrometry. J. Chromatogr. A 2011, 1218, 6817–6822. [Google Scholar] [CrossRef]
- Dutra, M.D.C.P.; de Souza, J.F.; Viana, A.C.; de Oliveira, D.; Pereira, G.E.; dos Santos Lima, M. Rapid determination of the aromatic compounds methyl-anthranilate, 2′-aminoacetophenone and furaneol by GC-MS: Method validation and characterization of grape derivatives. Food Res. Int. 2018, 107, 613–618. [Google Scholar] [CrossRef] [PubMed]
- Kambiranda, D.; Basha, S.M.; Singh, R.K.; He, H.; Calvin, K.; Mercer, R. In depth proteome analysis of ripening muscadine grape berry cv. Carlos reveals proteins associated with flavor and aroma compounds. J. Proteome Res. 2016, 15, 2910–2923. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Wang, Y.; Wu, B.; Fang, S.; Li, S. Volatile evolution of three table grapes with different flavour during and after maturation. Food Chem. 2011, 128, 823–830. [Google Scholar] [CrossRef]
- Rahman, F.U.; Nawaz, M.A.; Liu, R.; Sun, L.; Jiang, J.; Fan, X.; Liu, C.; Zhang, Y. Evaluation of volatile aroma compounds from Chinese wild grape berries by headspace-SPME with GC-MS. Food Sci. Technol. 2022, 42, e54320. [Google Scholar] [CrossRef]
- Yang, C.; Wang, Y.; Liang, Z.; Fan, P.; Wu, B.; Yang, L.; Wang, Y.; Li, S. Volatiles of grape berries evaluated at the germplasm level by headspace-SPME with GC-MS. Food Chem. 2009, 114, 1106–1114. [Google Scholar] [CrossRef]
- Liu, X.; Fan, P.; Jiang, J.; Gao, Y.; Liu, C.; Li, S.; Liang, Z. Evolution of volatile compounds composition during grape berry development at the germplasm level. Sci. Hort. 2022, 293, 110669. [Google Scholar] [CrossRef]
- Fennell, J.L. Two new North American species of Vitis. J. Wash. Acad. Sci. 1940, 30, 15–19. Available online: https://www.jstor.org/stable/24530082 (accessed on 11 February 2022).
- Cao, S.; Stringer, S.; Gunawan, G.; McGregor, C.; Conner, P.J. Genetic diversity and pedigree analysis of muscadine grape using SSR markers. J. Am. Soc. Hortic. Sci. 2020, 145, 143–151. [Google Scholar] [CrossRef]
- Basiouny, F.M.; Himelrick, D.G. Muscadine Grapes; ASHS Press: Alexandria, VA, USA, 2001. [Google Scholar]
- Maoz, I.; Kaplunov, T.; Raban, E.; Dynkin, I.; Degani, O.; Lewinsohn, E.; Lichter, A. Insights into the chemosensory basis of flavor in table grapes. J. Sci. Food Agric. 2020, 100, 1405–1417. [Google Scholar] [CrossRef]
- Ismail, A.; Gajjar, P.; Park, M.; Mahboob, A.; Tsolova, V.; Subramanian, J.; Darwish, A.G.; El-Sharkawy, I. A recessive mutation in muscadine grapes causes berry color-loss without influencing anthocyanin pathway. Commun. Biol. 2022, 5, 1012. [Google Scholar] [CrossRef]
- Varanasi, A.; Worthington, M.; Nelson, L.; Brown, A.; Chizk, T.M.; Threlfall, R.; Howard, L.; Conner, P.; Figueroa-Balderas, R.; Massonnet, M.; et al. Glutathione S-transferase: A candidate gene for berry color in muscadine grapes (Vitis rotundifolia). G3 Genes|Genomes|Genet. 2022, 12, jkac060. [Google Scholar] [CrossRef] [PubMed]






| Genotype | Female Parent | Male Parent | Berry Color z | Reference | Notes |
|---|---|---|---|---|---|
| AM-195 | AM-19 | AM-1 | Black | M.L.W. breeding records | Fresh-market selection. |
| AM-77 | Carlos | NC. 67A015-26 | Black | M.L.W. breeding records | Red wine and juice breeding selection. |
| Carlos | Howard | NC. 11-173 | Bronze | [24] | Leading white wine muscadine cultivar. |
| Cowart | Higgins | Ga. 28 | Black | [24] | Fresh-market cultivar with a pronounced aroma. |
| Fennel’s 3-way hybrid | Fennell’s 2-way hybrid | V. popenoei | Black | [25] | Fennel’s 2-way hybrid is ‘Scuppernong’ × V. rotundifolia var. munsoniana. |
| Fry | Ga. 19-13 | Ga. 19-11 | Bronze | [24] | Leading bronze fresh-market cultivar. |
| Ga. 13-3-36 | Ga. 6-9-91 | Ga. 6-1-217 | Bronze | P.J.C. breeding records | Ga. 18-5 is a grandparent, Ga. 13-3-36 10.9% V. vinifera and 89.1% V. rotundifolia. |
| Ga. 1-6-14 | Scarlet | Tara | Bronze | P.J.C. breeding records | Fresh-market muscadine selection with a “honey” flavor. |
| Ga. 18-5 | Ga. 14-32 | Ga. 12-2-1 | Black | P.J.C. breeding records | Interspecific hybrid that is 43.75% V. vinifera and 56.25% V. rotundifolia. |
| Golden Isles | Fry | Ga. 19-6 | Bronze | [24] | White wine muscadine cultivar released for more neutral flavored wine. |
| Hall | Fry | Tara | Bronze | [24] | Fresh-market muscadine cultivar. |
| Lane | Supreme | Tara | Black | [24] | Fresh-market muscadine cultivar. |
| Magnolia | Unnamed seedling | Topsail × Tarheel | Bronze | [24] | White wine muscadine cultivar. |
| Magoon | Thomas | Burgaw | Black | [24] | Black fresh-market muscadine cultivar. |
| Noble | Thomas | Tarheel | Black | [24] | Leading red wine muscadine cultivar. |
| Oh My! | JB99-1-4-15 | JB03-20-1-21 | Bronze | [24,26] | Stenospermocarpic seedless quasi-BC2 hybrid with an 86.9% V. rotundifolia background. |
| Paulk | Supreme | Tara | Black | [24] | Fresh-market muscadine cultivar. |
| Pineapple | Fry | Senoia | Bronze | [24] | Fresh-market muscadine cultivar with a “pineapple” flavor. |
| Ruby Crisp | Supreme | Tara | Red | [24] | Fresh-market muscadine cultivar with a red color and mild flavor. |
| Scuppernong | Unknown | Unknown | Bronze | [24] | Bronze colored native selection. |
| Southern Home | Summit | Fla. P9-15 | Black | [25] | Interspecific hybrid of V. rotundifolia, V. popenoei, and V. vinifera. |
| Supreme | Black Fry | Dixieland | Black | [24] | Leading black fresh-market muscadine cultivar. |
| Tarheel | Luola | V68 R14 B2 | Black | [24] | Older red wine muscadine cultivar. |
| V. popenoei DVIT 2970 | Unknown | Unknown | Purple | Native selection of V. popenoei. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bhattarai, G.; Giannopoulos, O.; Corn, R.N.; McAvoy, C.E.E.; Deltsidis, A.; Worthington, M.L.; Conner, P.J. Analysis of the Aroma Volatile Profile of Muscadine Grape Germplasm by Headspace Solid-Phase Microextraction Coupled with Gas Chromatography-Mass Spectrometry. Horticulturae 2023, 9, 1054. https://doi.org/10.3390/horticulturae9091054
Bhattarai G, Giannopoulos O, Corn RN, McAvoy CEE, Deltsidis A, Worthington ML, Conner PJ. Analysis of the Aroma Volatile Profile of Muscadine Grape Germplasm by Headspace Solid-Phase Microextraction Coupled with Gas Chromatography-Mass Spectrometry. Horticulturae. 2023; 9(9):1054. https://doi.org/10.3390/horticulturae9091054
Chicago/Turabian StyleBhattarai, Gaurab, Orestis Giannopoulos, Ramsey Nathanal Corn, Camille E. E. McAvoy, Angelos Deltsidis, Margaret L. Worthington, and Patrick J. Conner. 2023. "Analysis of the Aroma Volatile Profile of Muscadine Grape Germplasm by Headspace Solid-Phase Microextraction Coupled with Gas Chromatography-Mass Spectrometry" Horticulturae 9, no. 9: 1054. https://doi.org/10.3390/horticulturae9091054
APA StyleBhattarai, G., Giannopoulos, O., Corn, R. N., McAvoy, C. E. E., Deltsidis, A., Worthington, M. L., & Conner, P. J. (2023). Analysis of the Aroma Volatile Profile of Muscadine Grape Germplasm by Headspace Solid-Phase Microextraction Coupled with Gas Chromatography-Mass Spectrometry. Horticulturae, 9(9), 1054. https://doi.org/10.3390/horticulturae9091054







