Assessment of Gene Action and Identification of Heterotic Hybrids for Enhancing Yield in Field Pea
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Site
2.2. Plant Materials and Experimental Design
2.3. Data Collections
2.4. Statistical Analysis
2.4.1. Analysis of Variance for Experimental Design
2.4.2. Estimation of Heterosis
2.4.3. Combining Ability Analysis
2.4.4. Correlation and Principal Component Analysis
3. Results and Discussion
3.1. Analysis of Variance for Experimental Design
3.2. Per se Performance of Parents and Their Crosses
3.3. Heterosis
3.4. Combining Ability Analysis
3.4.1. Analysis of Variance for Combining Ability
3.4.2. Combining Ability Effects
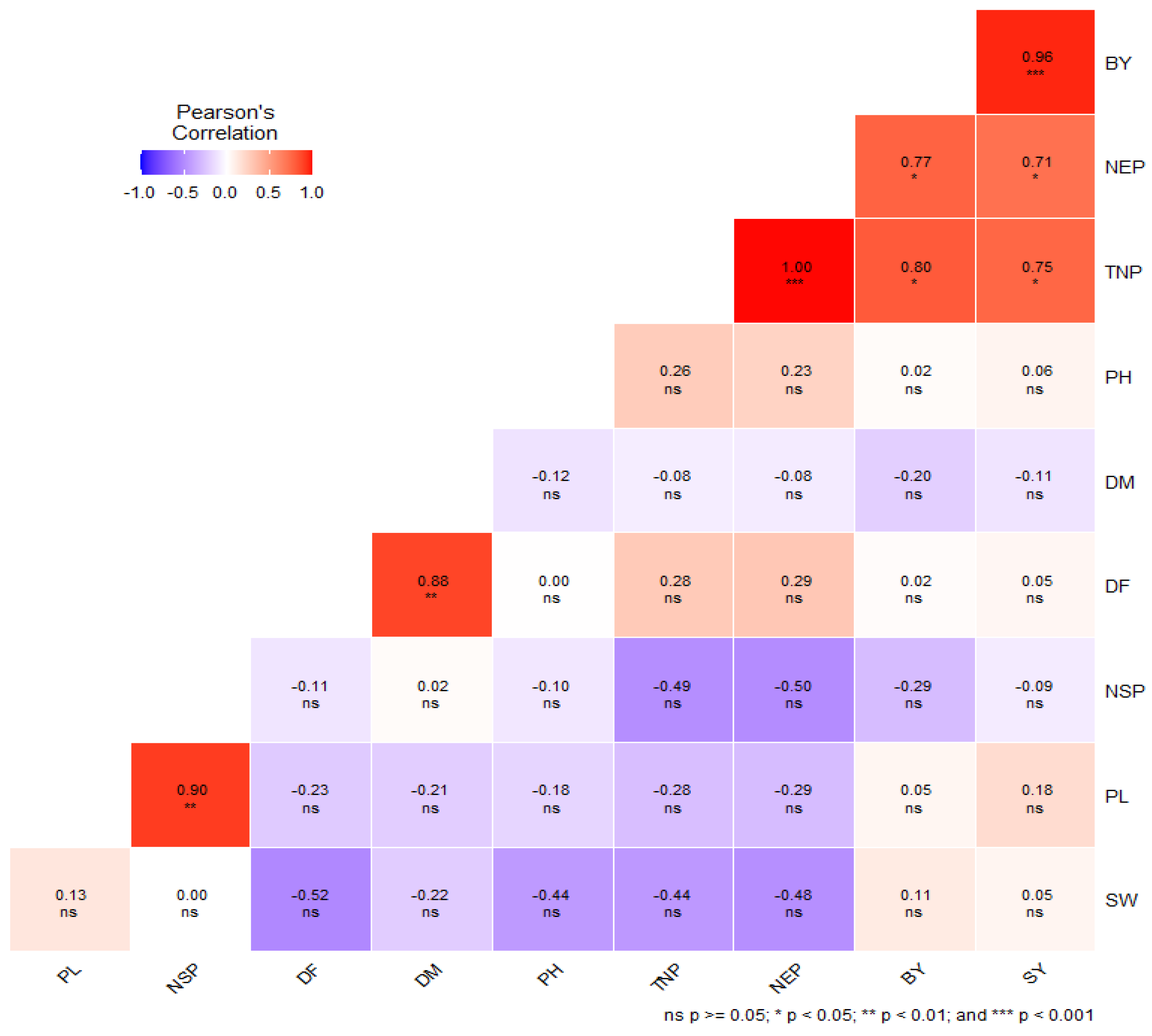
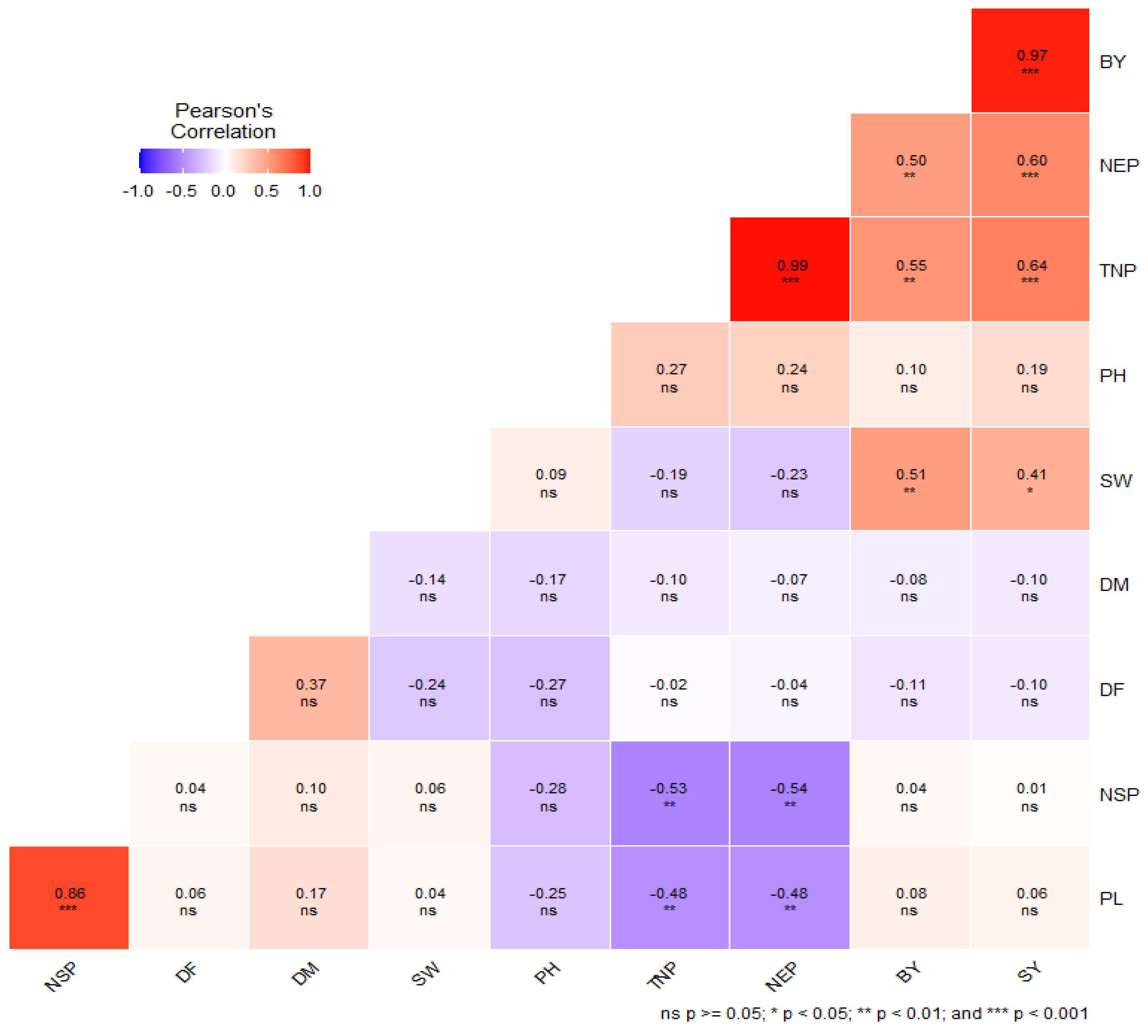
3.5. Correlation Analysis among Combining Abilities of Studied Traits
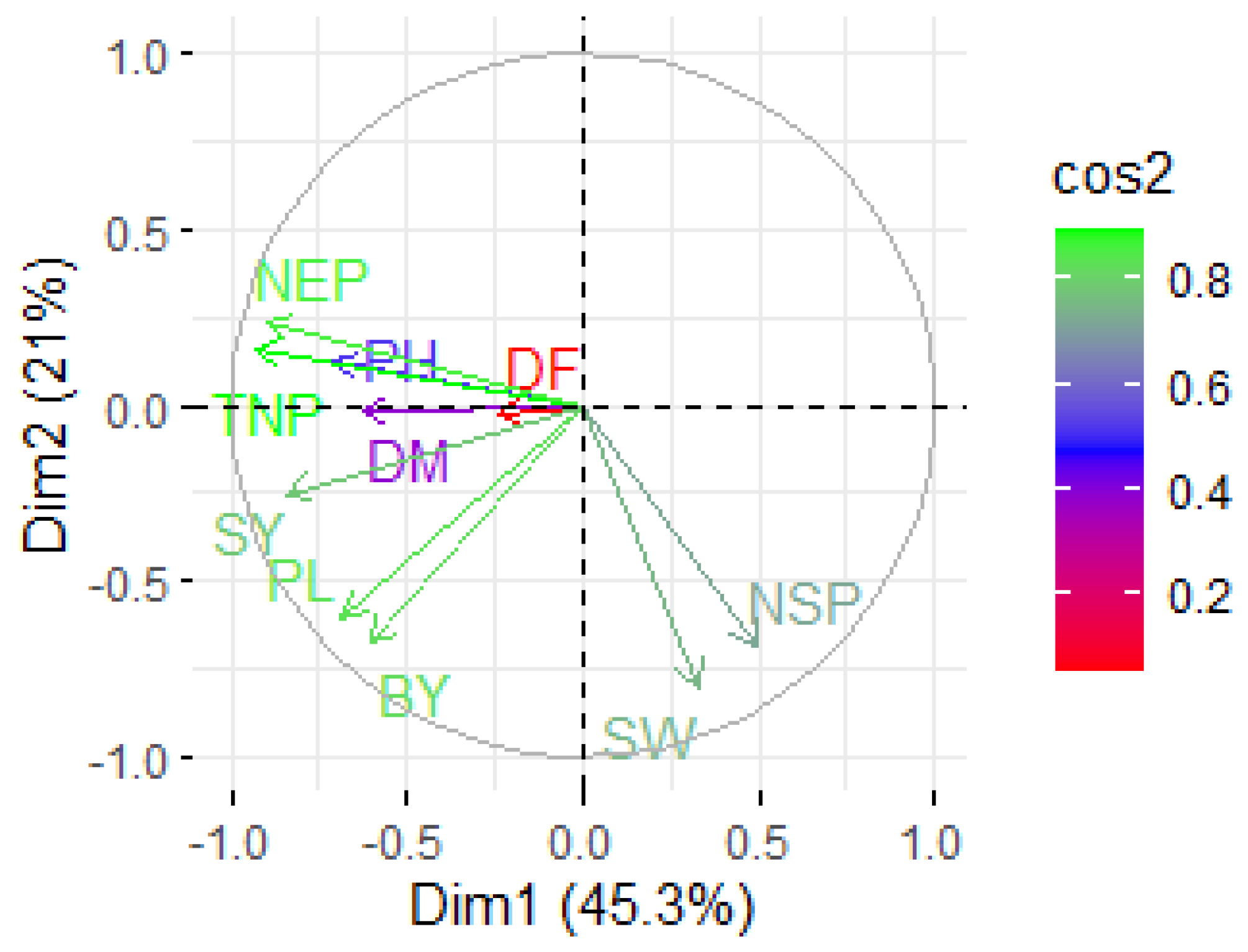
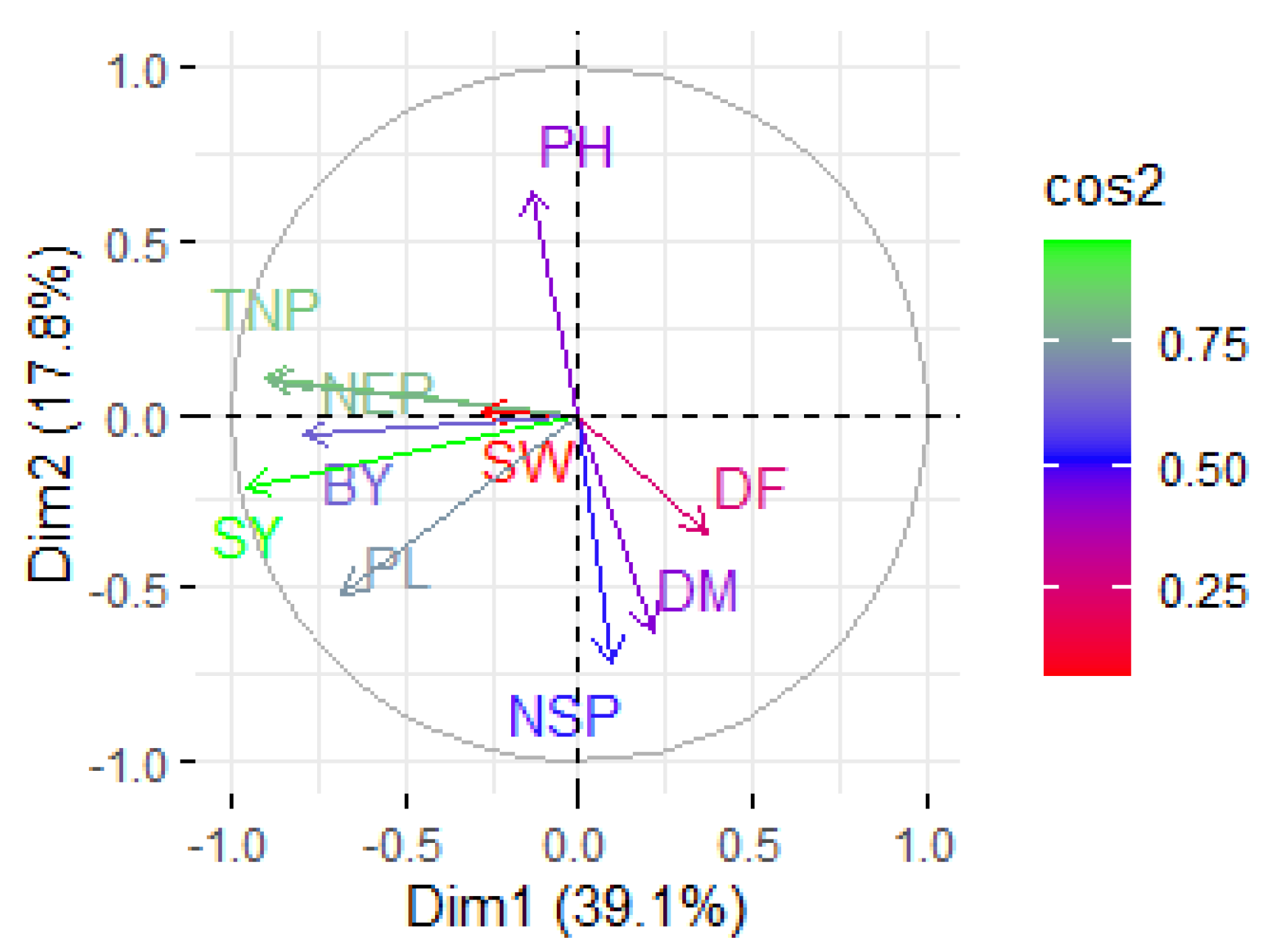
3.6. Principal Component Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Olle, M.; Williams, I.H.; Rosa, E.; Tamm, S. Finding best field pea (Pisum sativum L.) cultivars for breeding in Northern climatic conditions. Acta Agric. Scand. B Soil Plant Sci. 2020, 70, 1–7. [Google Scholar] [CrossRef]
- Zohary, D.; Hopf, M. Domestication of Plants in the Old World, 3rd ed.; Oxford University Press: Oxford, UK, 2000; p. 316. [Google Scholar]
- Bhat, T.A.; Gupta, M.; Ganai, M.A.; Ahanger, R.A.; Bhat, H.A. Yield, soil health and nutrient utilization of field pea (Pisum sativum L.) as affected by phosphorus and Biofertilizers under subtropical conditions of Jammu. Int. J. Plant Animal Sci. 2013, 1, 1–8. [Google Scholar]
- Gregory, E.; Shana, F.; Hans, K.; Julie, P.; Michael, W.; Janet, K.; Kenneth, H. Field Pea Production A1166 (Revised); Extension Service; North Dakota State University: Fargo, ND, USA, 2016. [Google Scholar]
- Stone, A.K.; Karalash, A.; Tyler, R.T.; Warkentin, T.D.; Nickerson, M.T. Functional attributes of pea protein isolates prepared using different extraction methods and cultivars. Food Res. Int. 2015, 76, 31–38. [Google Scholar] [CrossRef]
- Makasheva, R.K. The Pea. Russian Translations Series, 16; A. A. Balkema: Rotterdam, The Netherlands, 1984. [Google Scholar]
- Stagnari, F.; Maggio, A.; Galieni, A.; Pisante, M. Multiple benefits of legumes for agriculture sustainability: An overview. Chem. Biol. Technol. Agric. 2017, 4, 2. [Google Scholar] [CrossRef]
- AICRP on MuLLaRP. Project Coordinator’s Report of AICRP-MULLaRP; Indian Institute of Pulse Research: Kanpur, India, 2022. [Google Scholar]
- DES. Annual Report 2021–2022, Directorate of Economics and Statics; Department of Agriculture Cooperation and Farmers Welfare: New Delhi, India, 2022.
- Griffing, B. Concepts of general and specific combining ability in relation to diallel crossing system. Aust. J. Biol. Sci. 1956, 9, 463–493. [Google Scholar] [CrossRef]
- Kumar, M.; Jeberson, M.S.; Singh, N.B.; Sharma, R. Genetic analysis of seed yield and its contributing traits and pattern of their inheritance in field pea (Pisum sativum L.). Int. J. Curr. Microbiol. Appl. Sci. 2017, 6, 172–181. [Google Scholar] [CrossRef]
- Suman, H.; Kumar, B.; Nageshwar; Rathi, M.; Tamatam, D. Heterosis and combining ability for grain yield and yield associated traits in 10 × 10 diallel analysis in pea (Pisum sativum L.). Int. J. Curr. Microbiol. Appl. Sci. 2017, 6, 1574–1585. [Google Scholar] [CrossRef]
- Yadav, V.; Sao, A.; Gauraha, D. Combining ability analysis for yield and attributing traits in field pea (Pisum sativum L.). Int. J. Curr. Microbiol. Appl. Sci. 2019, 8, 1976–1981. [Google Scholar] [CrossRef]
- Bardisi, S.A.; Zyada, H.G. Combining ability and heterosis for yield and quality traits in pea (Pisum sativum L.). Sci. J. Agric. Sci. 2021, 3, 78–86. [Google Scholar] [CrossRef]
- Sharma, V.; Dodiya, N.S.; Dubey, R.B.; Khandagale, S.G.; Khan, R. Combining ability analysis over environments in bread wheat. Electron. J. Plant Breed. 2019, 10, 1397–1404. [Google Scholar] [CrossRef]
- Khan, R.; Ranwah, B.R.; Sharma, V.; Khandagale, S. Combining ability analysis for grain and fodder traits in sorghum [Sorghum bicolor (L.) Moench]. Bangladesh J. Bot. 2020, 49, 487–497. [Google Scholar] [CrossRef]
- Sprague, G.F.; Tatum, L.A. General vs specific combining ability in single crosses of corn. Agronomy 1942, 34, 923–932. [Google Scholar] [CrossRef]
- Cockerham, C.C. Implication of genetic hybrids breeding programme. Crop Sci. 1961, 1, 47–52. [Google Scholar] [CrossRef]
- Shull, G.H. A pure line method of com breeding. Am. Breed. Assoc. Rep. 1909, 5, 51–59. [Google Scholar]
- Gupta, D.K.; Acharya, S.; Patel, J.B. Combining ability and heterosis studies in pigeon pea using A2 cytoplasm from Cajanus scarabaeoides as source of male sterility. J. Food Legumes 2011, 24, 58–64. [Google Scholar]
- Rebika, T. Heterosis Study for Yield and Yield Components in Pea (Pisum sativum L.). Int. J. Curr. Microbiol. Appl. Sci. 2017, 6, 45–50. [Google Scholar] [CrossRef]
- Joshi, D.J.; Ravindrababu, Y.; Patel, A.M. Diallel analysis in field pea [Pisum sativum (L.) var. arvense]. Electron. J. Plant Breed. 2016, 7, 611–619. [Google Scholar] [CrossRef]
- Directorate of Pulse Development (DPD). Department of Agriculture Cooperation and Farmers Wlefare; Government of India: New Delhi, India, 2017.
- Pratap, V.; Sharma, V.; Kumar, H.; Kamaluddin; Shukla, G.; Kumar, M. Multivariate Analysis of Quantitative Traits in Field Pea (Pisum sativum var. arvense). Legume Res. 2021, 1, 6. [Google Scholar] [CrossRef]
- Sharma, V.; Singh, C.M.; Chugh, V.K.; Prajapati, P.K.; Mishra, A.; Kaushik, P.; Dhanda, P.S.; Yadav, A.; Satyendra. Morpho-Physiological and Biochemical Responses of Field Pea Genotypes under Terminal Heat Stress. Plants 2023, 12, 256. [Google Scholar] [CrossRef]
- Software, R. The R Studio for Statistical Computing. 2023. Available online: https://posit.co/download/rstudio-desktop/ (accessed on 25 June 2023).
- Panse, V.C.; Sukhatme, P.V. Statistical Methods for Agricultural Workers; Indian Council of Agricultural Research: New Delhi, India, 1985. [Google Scholar]
- Fonseca, S.; Patterson, F.L. Hybrid vigour in seven-parental diallel crosses in common winter wheat (Triticum aestivum L.). Crop Sci. 1968, 8, 85–88. [Google Scholar] [CrossRef]
- Meredith, W.R.; Bridge, R.R. Heterosis and gene action in cotton (Gossypium hirsutam). Crop Sci. 1972, 12, 304–310. [Google Scholar] [CrossRef]
- Tampha, S.; Jeberson, M.S.; Sastry, E.V.D.; Shashidhar, K.S.; Sharma, R.P. Line × tester analysis for yield and its contributing traits in field pea (Pisum sativum L.). Pharma Innov. J. 2018, 7, 104–109. [Google Scholar]
- Pratap, V.; Sharma, V.; Kamaluddin; Shukla, G. Assessment of genetic variability and relationship between different quantitative traits in field pea (Pisum sativum var. arvense) Germplasm. Legume Res. 2021, 1, 6. [Google Scholar] [CrossRef]
- Ceyhan, E.; Avci, M.A.; Karadas, S. Line x Tester analysis in pea (Pisum sativum L.): Identification of superior parents for seed yield and its components. Afr. J. Biotechnol. 2008, 7, 2810–2817. [Google Scholar]
- Kumar, C.S.; Sreelakshami, C.H.; Verma, P.K. Studies on combining ability and heterosis in pigeon pea (Cajanus cajan L.). Legume Res. 2009, 32, 92–97. [Google Scholar]
- Kumar, D.; Malik, S.; Kumar, A.; Singh, S.K.; Kumar, S. Studies on combining ability through line x tester analysis in garden pea (Pisum sativum L.). Ann. Hortic. 2016, 9, 64–72. [Google Scholar] [CrossRef]
- Hasan, M.N.M.; Abd El-Aty, Y.Y.; Zayed, G.A.; Abd El Lah, H.S. Heterosis, correlation and gene action in some genotypes of pea (Pisum sativum L.). Minia 2nd Conf. Agri. Environ. Sci. 2010, 3, 151–160. [Google Scholar]
- Brar, P.S.; Dhall, R.K.; Dinesh. Heterosis and combining ability in garden pea (Pisum sativum L.) for yield and its contributing traits. Veg. Sci. 2012, 39, 51–54. [Google Scholar]
- Galal, R.M.; Mohamed, A.G.; Ismail, E.E.M. Genetic analysis of some crosses for yield and its components and earliness in pea (Pisum sativum L.). Egypt. J. Hortic. 2019, 46, 1–11. [Google Scholar] [CrossRef]
- Hama-Amin, T.N. Half diallel analysis of seven pea cultivars for seed yield and its components for F4 generation under Sulaimani condition. Iraqi J. Agric. Sci. 2020, 51, 600–610. [Google Scholar] [CrossRef]
- Towfiq, S.I.; Hama-Amin, T.N.; Ahmed, D.A.; Aziz, O.K. Half diallel analysis for F3 generation of pea (Pisum sativum L.) under Sulaimani condition, Iraq Kurdistan region. Iraqi J. Agric. Sci. 2020, 51, 848–855. [Google Scholar] [CrossRef]
- Kumar, M.; Gupta, R.K.; Kumar, S.; Samnotra, R.K.; Bhushan, A.; Dey, T. Combining ability studies for various horticultural traits in garden pea (Pisum sativum L.). Int. J. Curr. Microbiol. Appl. Sci. 2020, 9, 841–853. [Google Scholar] [CrossRef]
- Manjunath, B.; Devaraju, V.; Srinivasa, M.; Hanumantappa, D.; Lakshmana; Aghora, T.S. Combining Ability Studies for Yield and Yield Contributing Traits in Garden Pea (Pisum sativum L.). Int. J. Curr. Microbiol. App. Sci. 2020, 9, 3261–3268. [Google Scholar] [CrossRef]
- Kumar, S.; Katoch, V.; Bharti, A.; Sharma, S.; Sharma, A.; Kumari, V. Heterosis, inbreeding depression and combining ability studies in garden pea (Pisum sativum L.). Legume Res. 2021, 44, 268–274. [Google Scholar] [CrossRef]
- Boraiah, K.M.; Byregowda, M.; Keerthi, C.M.; Vijayakumar, H.P.; Ramesh, S.; Reena, M. Frequency of heterotic hybrids in relation to parental genetic divergence and general combining ability in blackgram [Vigna mungo (L.) Hepper]. Legume Res. 2019, 42, 595–602. [Google Scholar] [CrossRef]
- Enrique, L.C.; María, A.E.; Lenna, G.; Fernando, S.L.A. Combining abilities and heterotic groups in Pisum sativum L. Aust. J. Crop Sci. 2013, 7, 1634–1641. [Google Scholar]
- Kosev, V. A brief study on the combining abilities of quantitative traits in field pea (Pisum sativum L.). Pinnacle Biol. Sci. 2013, 1, 5. [Google Scholar]
- Sharma, B.B.; Sharma, V.K.; Dhar, S. Combining ability studies for yield and other horticultural traits in garden pea (Pisum sativum var. hortense L.). Indian J. Hortic. 2015, 72, 500–505. [Google Scholar] [CrossRef]
- Katoch, V.; Bharti, A.; Sharma, A.; Rathore, N.; Kumari, V. Heterosis and combining ability studies for economic traits in garden pea (Pisum sativum L.). Legume Res. 2019, 42, 153–161. [Google Scholar] [CrossRef]
- Sharma, V.; Dodiya, N.S.; Dubey, R.B.; Khan, R. Combining ability analysis in bread wheat (Triticum aestivum (L.) Em. Thell) under different environmental conditions. Bangladesh J. Bot. 2019, 48, 85–93. [Google Scholar] [CrossRef]
- Khandagale, S.G.; Sharma, V.; Lone, R.P.; Khandagale, V.G.; Kumara-Swamy, R.V. Combining ability analysis and gene action in pearl millet [Pennisetum glaucum (L.) R. Br.]. Electron. J. Plant Breed. 2014, 5, 445–450. [Google Scholar]
- Singh, S.K.; Mazeed, A.; Singh, V.P.; Srivastava, S.; Dwivedi, A.K.; Yadav, S.K.; Srivastava, R.K. Evaluation of Seed Yield and Genetic Divergence in the Germplasms of field pea. J. Pharmacogn. Phytochem. 2017, 1, 1016–1021. [Google Scholar]
- Kumawat, P.K.; Singh, P.; Singh, D.; Mukherjee, S.; Kumawat, M. Study of correlation and path analysis for green pod yield and its contributing traits in vegetable pea (Pisum sativum L.). Int. J. Curr. Microbiol. Appl. Sci. 2018, 7, 3497–3502. [Google Scholar] [CrossRef]
- Prasad, D.; Verma, O.P.; Lal, K.; Verma, H.; Jaiswal, A.; Yadav, M.K. Identification of elite genotypes for certain quantitative traits in field pea (Pisum sativum L. var. arvense). J. Pharmacogn. Phytochem. 2019, 8, 498–505. [Google Scholar]
- Parihar, A.K.; Dixit, G.P.; Pathak, V.; Singh, D. Assessment of the genetic components and trait associations in diverse set of Field pea (Pisum sativum L.) genotypes. Bangladesh J. Bot. 2014, 43, 323–330. [Google Scholar] [CrossRef]
- Mohapatra, C.; Chand, R.; Tiwari, J.K.; Singh, A.K. Effect of heat stress during flowering and pod formation in pea (Pisum sativum L.). Physiol. Mol. Biol. Plants 2019, 26, 1119–1125. [Google Scholar] [CrossRef]
- Hanci, F.; Cebeci, E. Determination of morphological variability of different Pisum genotypes using principal component analysis. Legume Res. 2018, 42, 162–167. [Google Scholar] [CrossRef]
- Umar, H.M.I.; Rehman, S.U.; Bilal, M.; Naqvi, A.H.; Manzoor, S.A.; Ghafoor, A.; Khalid, M.; Iqbal, M.T.; Qayyum, A.; Ahmad, F.; et al. Evaluation of genetic diversity in pea (Pisum sativum L.) based on morpho-agronomic characteristics for yield and yield associated traits. J. Biodiver. Environ. Sci. 2014, 4, 321–328. [Google Scholar]
| S.N. | Genotype | Source |
|---|---|---|
| 1. | P-1541-16 | IIPR, India |
| 2. | P-1297-97 | IIPR, India |
| 3. | HFP-1314 | IIPR, India |
| 4. | Aman | IIPR, India |
| 5. | EC-564817 | IIPR, India |
| 6. | Vikas | IIPR, India |
| 7. | P-92-97-11 | IIPR, India |
| 8. | P-1426 | IIPR, India |
| S.N. | Cross Combination |
|---|---|
| 1. | P-1541-16 × P-1297-97 |
| 2. | P-1541-16 × HFP-1314 |
| 3. | P-1541-16 × Aman |
| 4. | P-1541-16 × EC-564817 |
| 5. | P-1541-16 × Vikas |
| 6. | P-1541-16 × P-92-97-11 |
| 7. | P-1541-16 × P-1426 |
| 8. | P-1297-97 × HFP-1314 |
| 9. | P-1297-97 × Aman |
| 10. | P-1297-97 × EC-564817 |
| 11. | P-1297-97 × Vikas |
| 12. | P-1297-97 × P-92-97-11 |
| 13. | P-1297-97 × P-1426 |
| 14. | HFP-1314 × Aman |
| 15. | HFP-1314 × EC-564817 |
| 16. | HFP-1314 × Vikas |
| 17. | HFP-1314 × P-92-97-11 |
| 18. | HFP-1314 × P-1426 |
| 19. | Aman × EC-564817 |
| 20. | Aman × Vikas |
| 21. | Aman × P-92-97-11 |
| 22. | Aman × P-1426 |
| 23. | EC-564817 × Vikas |
| 24. | EC-564817 × P-92-97-11 |
| 25. | EC-564817 × P-1426 |
| 26. | Vikas × P-92-97-11 |
| 27. | Vikas × P-1426 |
| 28. | P-92-97-11 × P-1426 |
| S.N. | Traits | Source | |||||
|---|---|---|---|---|---|---|---|
| Rep | Genotype | Parent | F1 | P vs. F1 | Error | ||
| df = 1 | df = 35 | df = 7 | df = 27 | df = 1 | df = 35 | ||
| 1 | DF | 0.4 ns | 112.2 * | 48.6 * | 23.4 * | 11.2 ns | 10.9 |
| 2 | DM | 6.1 ns | 28.3 * | 2.5 ns | 7.1 ns | 37.3 ns | 25.2 |
| 3 | PH | 329.8 * | 1623.4 * | 583.4 * | 356.5 * | 494.2 * | 87.4 |
| 4 | TNP | 153.1 * | 370.0 * | 114.0 * | 133.4 * | 100.1 | 26.5 |
| 5 | NEP | 181.3 * | 358.7 * | 113.1 * | 131.9 * | 79.2 ns | 23.1 |
| 6 | PL | 0.2 * | 0.7 * | 0.2 ns | 0.6 * | 0.1 ns | 0.1 |
| 7 | NSP | 0.1 ns | 0.9 * | 0.2 * | 0.2 * | 0.1 ns | 0.1 |
| 8 | SW | 15.2 * | 11.0 * | 2.3 * | 2.3 * | 16.4 * | 0.8 |
| 9 | BY | 117.1 * | 655.4 * | 157.6 * | 144.2 * | 738.0 * | 60.3 |
| 10 | SY | 10.8 ns | 185.6 * | 80.9 * | 94.0 * | 195.2 * | 23.5 |
| S.N. | Traits | Parents (n = 8) | Cross (n = 28) | ||
|---|---|---|---|---|---|
| Mean | Range | Mean | Range | ||
| 1 | DF | 65.9 | 56.0–85.0 | 67.8 | 55.5–79.5 |
| 2 | DM | 117.9 | 113.0–120.5 | 114.4 | 109.0–119.0 |
| 3 | PH | 116.3 | 62.7–159.9 | 128.9 | 68.1–168.3 |
| 4 | TNP | 54.6 | 27.9–70.7 | 56.6 | 23.6–91.7 |
| 5 | NEP | 51.1 | 25.4–68.5 | 53.0 | 21.6–89.8 |
| 6 | PL | 5.1 | 4.3–5.9 | 4.9 | 2.4–6.3 |
| 7 | NSP | 5.1 | 4.2–6.1 | 5.0 | 3.9–6.7 |
| 8 | SW | 15.7 | 11.7–18.5 | 17.9 | 13.2–21.9 |
| 9 | BY | 82.4 | 56.7–105.9 | 97.8 | 58.7–125.0 |
| 10 | SY | 35.3 | 15.2–46.2 | 44.4 | 15.1–59.6 |
| S. N-. | Traits | Source | |||
|---|---|---|---|---|---|
| GCA | SCA | Error | |||
| [7] | [28] | [35] | |||
| 1 | DF | 133.1 * | 36.8 * | 5.4 | 0.41 |
| 2 | DM | 7.3 ns | 15.9 * | 12.6 | 0.16 |
| 3 | PH | 1720.8 * | 584.4 * | 43.7 | 0.31 |
| 4 | TNP | 301.3 * | 155.9 * | 13.2 | 0.20 |
| 5 | NEP | 282.4 * | 153.6 * | 12.0 | 0.19 |
| 6 | PL | 0.7 * | 0.3 * | 0.1 | 0.27 |
| 7 | NSP | 0.7 * | 0.4 * | 0.1 | 0.17 |
| 8 | SW | 9.2 * | 4.6 * | 0.4 | 0.21 |
| 9 | BY | 290.4 * | 337.1 * | 30.2 | 0.09 |
| 10 | SY | 74.0 * | 97.5 * | 11.8 | 0.07 |
| S.N. | Parents | DF | DM | PH | TNP | NEP | PL | NSP | SW | BY | SY |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. | P-1541-16 | 1.84 * | −0.01 ns | −1.05 ns | 5.86 ** | 5.65 ** | −0.29 ** | −0.47 ** | 0.50 * | 6.88 ** | 1.91 ns |
| 2. | P-1297-97 | −4.71 ** | −0.66 ns | −14.95 ** | 0.71 ns | 0.43 ns | 0.05 ns | −0.00 ns | 1.25 ** | 4.72 * | 2.86 * |
| 3. | HFP-1314 | −3.21 ** | −0.36 ns | 20.53 ** | −3.35 * | −4.14 ** | −0.06 ns | 0.00 ns | 0.67 ** | −2.01 ns | −0.82 ns |
| 4. | Aman | 4.44 ** | 0.59 ns | 5.17 * | 8.15 ** | 7.60 ** | 0.21 * | 0.17 * | −1.12 ** | 6.91 ** | 4.27 ** |
| 5. | EC-564817 | 3.49 ** | 1.19 ns | 1.94 ns | −5.02 ** | −4.89 ** | 0.09 ns | 0.23 ** | −0.18 ns | −4.35 * | −1.29 ns |
| 6. | Vikas | 3.39 ** | 0.99 ns | −8.23 ** | −1.71 ns | −1.12 ns | −0.39 ** | −0.23 ** | −0.62 ** | −6.71 ** | −3.58 ** |
| 7. | P-92-97-11 | −3.01 ** | −1.26 ns | 13.36 ** | 3.15 * | 3.69 ** | −0.02 ns | −0.03 ns | −1.34 ** | −1.47 ns | −0.98 ns |
| 8. | P-1426 | −2.21 * | −0.46 ns | −16.78 ** | −7.78 ** | −7.21 ** | 0.40 ** | 0.34 ** | 0.83 ** | −3.97 * | −2.37 ns |
| S.N. | Crosses | DF | DM | PH | TNP | NEP | PL | NSP | SW | BY | SY |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. | P-1541-16 × P-1297-97 | 0.03 ns | 3.49 ns | 25.90 ** | 12.68 ** | 11.51 ** | −0.26 ns | −0.29 * | 1.15 * | 2.64 ns | 7.40 * |
| 2. | P-1541-16 × HFP-1314 | 5.03 * | −5.81 * | −19.83 ** | 12.15 ** | 10.75 ** | −0.53 ** | −0.4 ** | −1.77 ** | −1.99 ns | 1.55 ns |
| 3. | P-1541-16 × Aman | −7.62 ** | −3.76 ns | 13.49 * | 17.27 ** | 19.71 ** | −0.70 ** | −0.48 ** | −0.22 ns | 3.64 ns | 1.67 ns |
| 4. | P-1541-16 × EC-564817 | 4.83 * | −0.86 ns | 16.77 ** | 0.26 ns | −0.6 ns | −0.02 ns | −0.26 ns | 2.88 ** | 19.10 ** | 8.35 ** |
| 5. | P-1541-16 × Vikas | −1.07 ns | −5.16 ns | 6.54 ns | 1.59 ns | −0.25 ns | −0.73 ** | −0.42 ** | −0.23 ns | −10.09 * | −6.82 * |
| 6. | P-1541-16 × P-92-97-11 | 11.83 ** | 3.09 ns | −31.26 ** | 1.81 ns | −0.42 ns | −0.1 ns | 0.20 ns | 2.40 ** | 25.22 ** | 10.05 ** |
| 7. | P-1541-16 × P-1426 | −1.97 ns | −1.71 ns | 31.09 ** | 8.06 ** | 7.16 * | 0.06 ns | −0.63 ** | −0.33 ns | 5.98 ns | 2.21 ns |
| 8. | P-1297-97 × HFP-1314 | −2.42 ns | −5.16 ns | 36.07 ** | −9.27 ** | −10.44 ** | −0.15 ns | −0.18 ns | 2.53 ** | −8.27 ns | −4.50 ns |
| 9. | P-1297-97 × Aman | −1.57 ns | 1.39 ns | 48.24 ** | 0.54 ns | −0.93 ns | 0.07 ns | −0.08 ns | −0.77 ns | −3.34 ns | −1.58 ns |
| 10. | P-1297-97 × EC-564817 | 3.88 * | 0.79 ns | −36.43 ** | −26.14 ns | −24.23 ** | 0.61 ** | 0.91 ** | −0.01 ns | −32.00 ** | −19.14 ** |
| 11. | P-1297-97 × Vikas | −1.52 ns | 1.99 ns | −17.26 ** | 9.19 ** | 10.8 ** | 0.48 * | 0.31 * | −1.68 ** | 16.22 ** | 9.26 ** |
| 12. | P-1297-97 × P-92-97-11 | −3.12 ns | −12.76 ** | 12.19 * | 2.29 ns | 1.01 ns | −0.03 ns | −0.07 ns | 2.45 ** | 15.86 ** | 9.25 ** |
| 13. | P-1297-97 × P-1426 | 8.58 ** | 3.94 ns | −22.61 ** | 1.56 ns | 0.15 ns | 0.14 ns | −0.20 ns | 1.22 * | 4.58 ns | −0.41 ns |
| 14. | HFP-1314 × Aman | −1.57 ns | 0.06 ns | −29.04 ** | 0.31 ns | 0.93 ns | 0.27 ns | −0.08 ns | 1.03 * | 11.83 * | 5.93 * |
| 15. | HFP-1314 × EC-564817 | −5.62 ** | −0.51 ns | −7.71 ns | 0.12 ns | −1.66 ns | −0.48 * | −0.41 ** | −1.84 ** | −14.51 ** | −10.45 ** |
| 16. | HFP-1314 × Vikas | 1.98 ns | 3.19 ns | 3.56 ns | −11.7 ** | −10.57 ** | −0.57 ** | −0.71 ** | 1.60 ** | −14.80 ** | −10.47 ** |
| 17. | HFP-1314 × P-92-97-11 | 0.88 ns | 2.44 ns | 8.26 ns | −8.55 ** | −6.63 * | 0.12 ns | 0.37 * | −0.17 ns | −9.14 * | −3.87 ns |
| 18. | HFP-1314 × P-1426 | −0.42 ns | −2.36 ns | 23.21 ** | 8.24 ** | 7.65 ** | 0.21 ns | 0.22 ns | −0.80 ns | 12.17 ** | 7.87 * |
| 19. | Aman × EC-564817 | −15.27 ** | −2.96 ns | −12.45 * | −9.93 ns | −9.26 ** | 0.46 * | 0.45 ** | 3.51 ** | 12.56 ** | 5.67 * |
| 20. | Aman × Vikas | 4.33 * | −1.76 ns | −4.28 ns | 14.65 ** | 14.9 ** | −0.24 ns | −0.63 ** | 1.30 * | 19.13 ** | 10.04 ** |
| 21. | Aman × P-92-97-11 | 2.23 ns | −0.51 ns | −1.37 ns | −0.70 ns | 1.58 ns | −0.03 ns | 0.02 ns | −1.68 ** | −5.22 ns | −1.96 ns |
| 22. | Aman × P-1426 | 1.93 ns | 2.19 ns | 14.47 ** | 10.47 ** | 11.75 ** | −0.00 ns | 0.09 ns | −1.75 ** | 6.75 ns | 4.40 ns |
| 23. | EC-564817 × Vikas | 4.28 * | −0.36 ns | 4.00 ns | −4.08 ns | −6.72 * | 1.14 ** | 1.74 ** | 1.25 * | 24.42 ** | 13.20 ** |
| 24. | EC-564817 × P-92-97-11 | 10.18 ** | −0.11 ns | 6.16 ns | −7.56 * | −7.03 * | 0.35 ns | −0.00 ns | −2.67 ** | −29.84 ** | −11.71 ** |
| 25. | EC-564817 × P-1426 | 3.38 ns | 1.09 ns | 18.00 ** | 17.78 ** | 18.30 ** | −0.90 ** | −1.01 ** | 1.05 * | 18.42 ** | 12.24 ** |
| 26. | Vikas × P-92-97-11 | −4.72 * | −0.91 ns | −2.77 ns | 8.9 ** | 8.5 ** | 0.12 ns | −0.15 ns | 1.02 * | 15.01 ** | 8.69 ** |
| 27. | Vikas × P-1426 | 1.98 ns | 0.29 ns | −33.03 ** | −10.47 ** | −8.95 ** | 0.34 ns | 0.47 ** | 0.14 ns | −8.21 ns | −6.27 ns |
| 28. | P-92-97-11 × P-1426 | −6.62 ** | −0.96 ns | 28.53 ** | −4.18 ns | −5.60 * | −0.01 ns | 0.21 ns | 4.67 ** | 19.67 ** | 9.19 ** |
| Principal Components | Parents | Crosses | ||||
|---|---|---|---|---|---|---|
| Eigen Value | Variability (%) | Cumulative Variability (%) | Eigen Value | Variability (%) | Cumulative Variability (%) | |
| PC1 | 2.13 | 45.31 | 45.31 | 1.98 | 39.14 | 39.14 |
| PC2 | 1.45 | 21.01 | 66.32 | 1.34 | 17.84 | 56.99 |
| PC3 | 1.24 | 15.36 | 81.68 | 1.26 | 15.93 | 72.91 |
| PC4 | 0.94 | 8.83 | 90.52 | 1.02 | 10.43 | 83.34 |
| PC5 | 0.75 | 5.63 | 96.14 | 0.81 | 6.62 | 89.96 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yadav, I.; Sharma, V.; Kumar, M.; Yadav, L.P.; Mishra, A.; Singh, V.; Singh Dhanda, P.; Yadav, A.; Yadav, M.; Singh, S.K.; et al. Assessment of Gene Action and Identification of Heterotic Hybrids for Enhancing Yield in Field Pea. Horticulturae 2023, 9, 997. https://doi.org/10.3390/horticulturae9090997
Yadav I, Sharma V, Kumar M, Yadav LP, Mishra A, Singh V, Singh Dhanda P, Yadav A, Yadav M, Singh SK, et al. Assessment of Gene Action and Identification of Heterotic Hybrids for Enhancing Yield in Field Pea. Horticulturae. 2023; 9(9):997. https://doi.org/10.3390/horticulturae9090997
Chicago/Turabian StyleYadav, Indu, Vijay Sharma, Mukul Kumar, Lalu Prasad Yadav, Anuj Mishra, Vivek Singh, Parmdeep Singh Dhanda, Alpa Yadav, Manisha Yadav, Shalesh Kumar Singh, and et al. 2023. "Assessment of Gene Action and Identification of Heterotic Hybrids for Enhancing Yield in Field Pea" Horticulturae 9, no. 9: 997. https://doi.org/10.3390/horticulturae9090997






