Effects of Vanadium/Nickel Contents in Laves Phase-Related Body-Centered-Cubic Solid Solution Metal Hydride Alloys
Abstract
:1. Introduction
2. Experimental Section
3. Results and Discussion
3.1. Alloy Composition
| Alloy | Ti | Zr | V | Cr | Mn | Co | Ni | Al | B/A | |
|---|---|---|---|---|---|---|---|---|---|---|
| P17 | Design | 15.6 | 2.1 | 44.0 | 11.2 | 6.9 | 1.4 | 18.5 | 0.3 | 4.65 |
| ICP | 15.6 | 2 | 44.1 | 11.3 | 6.4 | 1.4 | 18.9 | 0.3 | 4.68 | |
| P22 | Design | 15.6 | 2.1 | 40.0 | 11.2 | 6.9 | 1.4 | 22.5 | 0.3 | 4.65 |
| ICP | 15.5 | 1.8 | 41.0 | 11.2 | 6.5 | 1.4 | 22.2 | 0.4 | 4.78 | |
| P23 | Design | 15.6 | 2.1 | 38.0 | 11.2 | 6.9 | 1.4 | 24.5 | 0.3 | 4.65 |
| ICP | 15.2 | 1.7 | 38.7 | 11.4 | 6.8 | 1.4 | 24.3 | 0.4 | 4.91 | |
| P24 | Design | 15.6 | 2.1 | 36.0 | 11.2 | 6.9 | 1.4 | 26.5 | 0.3 | 4.65 |
| ICP | 15.5 | 1.8 | 36.0 | 11.3 | 6.4 | 1.5 | 27.1 | 0.4 | 4.78 | |
| P25 | Design | 15.6 | 2.1 | 34.0 | 11.2 | 6.9 | 1.4 | 28.5 | 0.3 | 4.65 |
| ICP | 15.5 | 1.8 | 34.4 | 11.4 | 6.6 | 1.4 | 28.4 | 0.4 | 4.77 | |
| P26 | Design | 15.6 | 2.1 | 32.0 | 11.2 | 6.9 | 1.4 | 30.5 | 0.3 | 4.65 |
| ICP | 15.5 | 1.7 | 33.5 | 10.8 | 6.8 | 1.5 | 29.8 | 0.4 | 4.81 | |
| P27 | Design | 15.6 | 2.1 | 30.0 | 11.2 | 6.9 | 1.4 | 32.5 | 0.3 | 4.65 |
| ICP | 15.2 | 1.8 | 30.6 | 11.7 | 6.6 | 1.4 | 32.2 | 0.4 | 4.88 | |
| P28 | Design | 15.6 | 2.1 | 28.0 | 11.2 | 6.9 | 1.4 | 34.5 | 0.3 | 4.65 |
| ICP | 15.3 | 1.9 | 28.1 | 11.4 | 6.6 | 1.4 | 34.9 | 0.4 | 4.81 | |
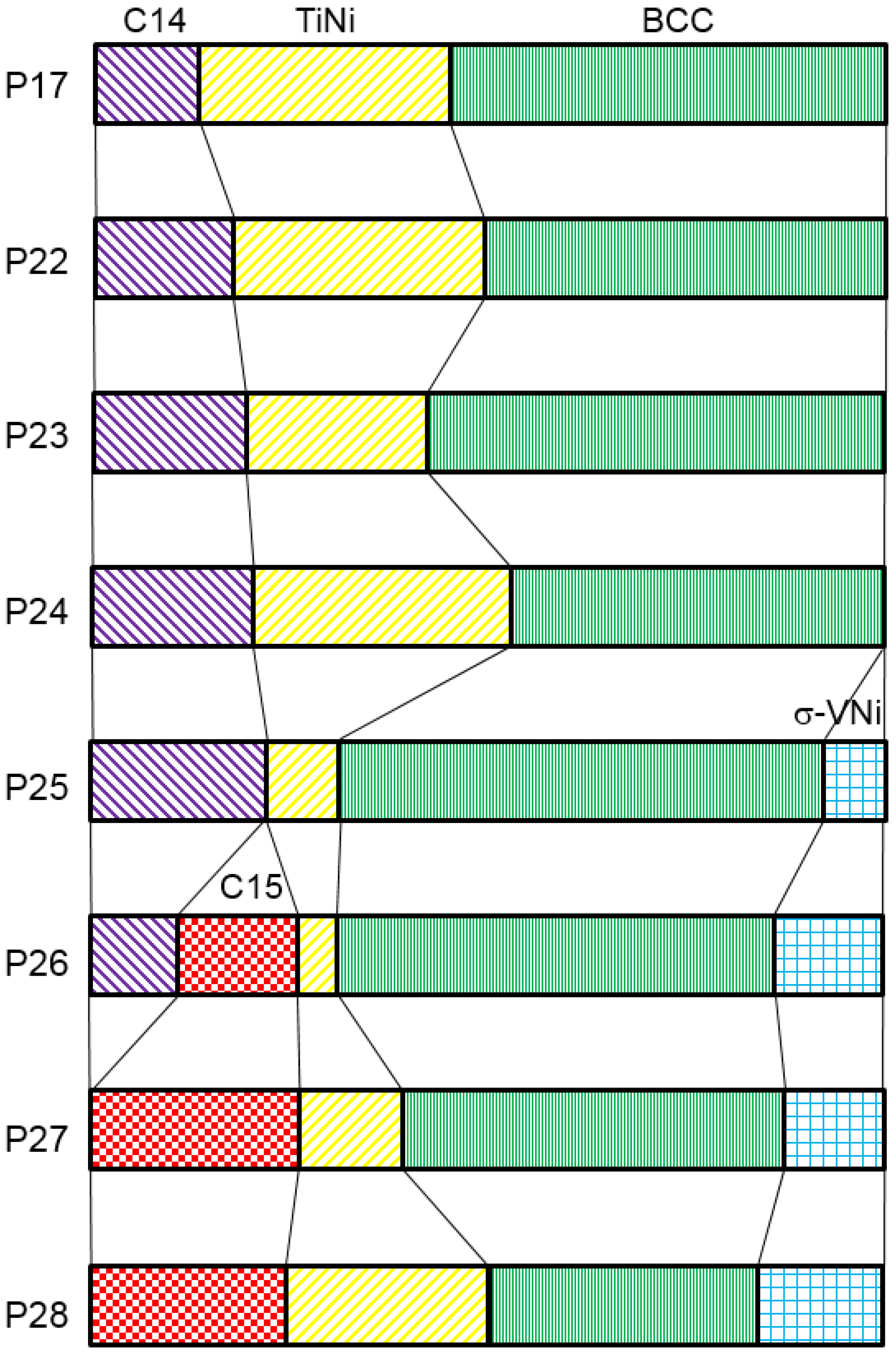
3.2. X-Ray Diffractometer Structure Analysis

| Alloy | BCC | C14 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| a (Å) | XS (Å) | Abundance (wt%) | a (Å) | c (Å) | c/a | Unit cell volume (Å3) | XS (Å) | Abundance (wt%) | |||
| P17 | 2.977 | 171 | 52.8 | 4.912 | 8.010 | 1.631 | 167.3 | 215 | 13.2 | ||
| P22 | 2.974 | 57 | 50.7 | 4.890 | 7.968 | 1.629 | 165.0 | 275 | 17.1 | ||
| P23 | 2.973 | 86 | 57.5 | 4.878 | 7.936 | 1.627 | 163.5 | 311 | 19.4 | ||
| P24 | 2.974 | 72 | 47.0 | 4.865 | 7.915 | 1.627 | 162.2 | 255 | 20.3 | ||
| P25 | 2.954 | 86 | 61.2 | 4.858 | 7.895 | 1.625 | 161.4 | 287 | 22.1 | ||
| P26 | 2.950 | 71 | 55.2 | 4.844 | 7.883 | 1.627 | 160.2 | 272 | 11.1 | ||
| P27 | 2.948 | 68 | 47.8 | - | - | - | - | - | - | ||
| P28 | 2.946 | 73 | 34.2 | - | - | - | - | - | - | ||
| Alloy | C15 | TiNi | VNi | ||||||||
| a (Å) | XS (Å) | Abundance (wt%) | a (Å) | XS (Å) | Abundance (wt%) | a (Å) | c (Å) | XS (Å) | Abundance (wt%) | ||
| P17 | - | - | - | 2.993 | 170 | 34.0 | - | - | - | - | |
| P22 | - | - | - | 2.962 | 175 | 32.4 | - | - | - | - | |
| P23 | - | - | - | 2.959 | 273 | 23.0 | - | - | - | - | |
| P24 | - | - | - | 2.958 | 204 | 32.6 | - | - | - | - | |
| P25 | - | - | - | 2.899 | 105 | 8.9 | 9.009 | 4.686 | 184 | 7.7 | |
| P26 | 6.859 | 637 | 15.1 | 2.905 | 228 | 4.5 | 9.010 | 4.692 | 219 | 14.1 | |
| P27 | 6.860 | 405 | 26.6 | 2.912 | 127 | 13.0 | 9.017 | 4.644 | 250 | 12.6 | |
| P28 | 6.858 | 347 | 24.7 | 2.912 | 115 | 25.5 | 9.012 | 4.642 | 344 | 15.7 | |

3.3. Scanning Electron Microscope/Energy Dispersive Spectroscopy Microstructure Analysis

| Location | Zr | Ti | V | Cr | Mn | Fe | Co | Ni | Al | B/A | e/a | Phase |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Figure 4a-1 | 16.2 | 16.3 | 7.8 | 4.4 | 5.9 | 0.0 | 1.8 | 47.5 | 0.1 | 2.08 | 7.28 | C14 |
| Figure 4a-2 | 4.7 | 36.4 | 7.8 | 0.8 | 3.6 | 0.0 | 2.0 | 44.4 | 0.3 | 1.04 | - | TiNi |
| Figure 4a-3 | 6.2 | 48.5 | 11.7 | 1.2 | 2.3 | 0.0 | 1.8 | 28.3 | 0.1 | 0.51 | - | Ti2Ni |
| Figure 4a-4 | 0.1 | 5.5 | 63.5 | 16.9 | 7.4 | 0.0 | 0.8 | 5.6 | 0.2 | - | - | BCC |
| Figure 4b-1 | 14.3 | 17.3 | 9.4 | 0.6 | 4.8 | 0.0 | 0.8 | 52.5 | 0.3 | 2.16 | 7.44 | C14 |
| Figure 4b-2 | 2.8 | 34.3 | 7.3 | 0.9 | 4.7 | 0.1 | 2.7 | 46.4 | 0.8 | 1.25 | - | TiNi |
| Figure 4b-3 | 0.1 | 3.0 | 60.7 | 17.7 | 11.8 | 0.0 | 0.9 | 5.7 | 0.1 | - | - | BCC |
| Figure 4b-4 | 87.2 | 3.0 | 3.1 | 0.5 | 0.8 | 0.1 | 0.4 | 4.8 | 0.1 | - | - | Zr |
| Figure 4c-1 | 12.0 | 18.6 | 12.4 | 0.7 | 3.5 | 0.0 | 0.9 | 51.6 | 0.3 | 2.27 | 7.38 | C14 |
| Figure 4c-2 | 2.4 | 34.5 | 8.4 | 1.0 | 3.7 | 0.0 | 2.3 | 47.2 | 0.5 | 1.21 | - | TiNi |
| Figure 4c-3 | 0.1 | 4.5 | 58.1 | 18.2 | 8.6 | 0.0 | 1.1 | 9.3 | 0.1 | - | - | BCC |
| Figure 4c-4 | 93.9 | 1.1 | 2.5 | 0.4 | 0.3 | 0.1 | 0.1 | 1.5 | 0.1 | - | - | Zr |
| Figure 4d-1 | 10.6 | 17.9 | 16.5 | 2.5 | 4.7 | 0.0 | 1.4 | 46.2 | 0.2 | 2.51 | 7.20 | C14 |
| Figure 4d-2 | 1.9 | 33.9 | 8.6 | 1.1 | 3.5 | 0.0 | 2.4 | 47.6 | 1.0 | 1.25 | - | TiNi |
| Figure 4d-3 | 0.0 | 2.3 | 60.0 | 21.1 | 8.3 | 0.0 | 1.0 | 7.1 | 0.2 | - | - | BCC |
| Figure 4d-4 | 94.7 | 0.7 | 1.8 | 0.4 | 0.1 | 0.0 | 0.2 | 1.9 | 0.2 | - | - | Zr |
| Figure 4e-1 | 10.8 | 16.0 | 14.2 | 1.8 | 4.2 | 0.0 | 1.0 | 51.7 | 0.3 | 2.73 | 7.45 | C15 |
| Figure 4e-2 | 8.5 | 17.7 | 18.2 | 2.5 | 5.0 | 0.0 | 1.5 | 46.2 | 0.3 | 2.81 | 7.22 | C14 |
| Figure 4e-3 | 1.6 | 33.8 | 8.6 | 1.1 | 4.1 | 0.1 | 2.5 | 47.5 | 0.8 | 1.28 | - | TiNi |
| Figure 4e-4 | 0.2 | 7.0 | 48.2 | 6.6 | 5.2 | 0.1 | 1.3 | 31.3 | 0.1 | 0.81 | - | VNi |
| Figure 4e-5 | 0.0 | 2.4 | 58.7 | 21.8 | 8.5 | 0.0 | 1.0 | 7.4 | 0.1 | - | - | BCC |
| Figure 4e-6 | 89.0 | 3.2 | 2.4 | 0.4 | 0.2 | 0.0 | 0.2 | 4.5 | 0.1 | - | - | Zr |
| Figure 4e-7 | 4.7 | 69.5 | 7.8 | 1.3 | 1.6 | 0.0 | 0.6 | 14.1 | 0.3 | - | - | TiO2 |
| Figure 4f-1 | 10.5 | 16.2 | 13.3 | 1.9 | 4.4 | 0.0 | 1.0 | 52.5 | 0.2 | 2.75 | 7.50 | C15 |
| Figure 4f-2 | 8.9 | 17.3 | 15.7 | 2.5 | 5.1 | 0.0 | 1.4 | 48.8 | 0.2 | 2.81 | 7.35 | C14 |
| Figure 4f-3 | 1.4 | 30.7 | 10.3 | 1.1 | 5.1 | 0.0 | 2.0 | 48.6 | 0.8 | 1.36 | - | TiNi |
| Figure 4f-4 | 0.2 | 6.8 | 48.4 | 7.1 | 6.8 | 0.0 | 1.5 | 29.0 | 0.1 | 0.80 | - | VNi |
| Figure 4f-5 | 0.0 | 2.1 | 58.3 | 21.6 | 8.7 | 0.0 | 1.1 | 8.0 | 0.1 | - | - | BCC |
| Figure 4f-6 | 6.0 | 79.0 | 8.3 | 0.1 | 0.5 | 0.0 | 0.2 | 6.0 | 0.0 | - | - | TiO2 |
| Figure 4g-1 | 10.4 | 16.5 | 11.9 | 2.1 | 5.2 | 0.1 | 1.2 | 52.4 | 0.3 | 2.72 | 7.53 | C15 |
| Figure 4g-2 | 4.5 | 30.2 | 9.2 | 1.0 | 5.0 | 0.0 | 1.0 | 48.8 | 0.2 | 1.28 | - | TiNi |
| Figure 4g-3 | 0.1 | 6.6 | 46.2 | 9.4 | 6.6 | 0.0 | 1.8 | 29.3 | 0.1 | 0.89 | - | VNi |
| Figure 4g-4 | 0.0 | 1.8 | 55.4 | 25.3 | 8.6 | 0.0 | 1.1 | 7.6 | 0.1 | - | - | BCC |
| Figure 4g-5 | 85.5 | 5.1 | 2.1 | 0.5 | 0.6 | 0.2 | 0.1 | 5.8 | 0.1 | - | - | Zr |
| Figure 4g-6 | 3.5 | 82.8 | 7.6 | 0.1 | 0.6 | 0.0 | 0.1 | 5.2 | 0.1 | - | - | TiO2 |
| Figure 4h-1 | 10.2 | 16.2 | 11.8 | 2.4 | 5.5 | 0.0 | 1.1 | 52.5 | 0.3 | 2.79 | 7.53 | C15 |
| Figure 4h-2 | 1.3 | 30.1 | 9.1 | 1.0 | 6.3 | 0.0 | 2.3 | 49.0 | 0.9 | 1.47 | - | TiNi |
| Figure 4h-3 | 0.1 | 6.6 | 43.5 | 11.6 | 6.8 | 0.2 | 1.8 | 29.2 | 0.2 | 0.99 | - | VNi |
| Figure 4h-4 | 0.0 | 1.7 | 53.0 | 27.4 | 8.3 | 0.0 | 1.2 | 8.3 | 0.1 | - | - | BCC |
| Figure 4h-5 | 86.1 | 3.9 | 2.2 | 0.4 | 0.7 | 0.0 | 0.2 | 6.2 | 0.2 | - | - | Zr |
| Figure 4h-6 | 2.0 | 90.6 | 3.3 | 0.1 | 0.5 | 0.0 | 0.2 | 3.3 | 0.1 | - | - | TiO2 |

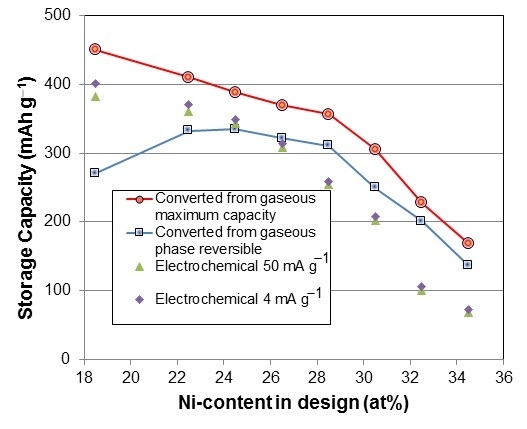
3.4. Gaseous Phase Study

| Alloy No. | Desorption pressure @ 30 °C | Desorption pressure @ 60 °C | Maximum capacity @ 30 °C | Reversible capacity @ 30 °C | PCT hysteresis @ 30 °C | PCT hysteresis @ 60 °C | −ΔH | −ΔS |
|---|---|---|---|---|---|---|---|---|
| (MPa) | (MPa) | (wt%) | (wt%) | (kJ·mol−1) | (J·mol−1·K−1) | |||
| P17 | 0.17 | 0.53 | 1.68 | 1.01 | 1.06 | 1.10 | 30.9 | 107 |
| P22 | 0.21 | 0.60 | 1.53 | 1.24 | 1.14 | 1.13 | 29.9 | 105 |
| P23 | 0.22 | 0.79 | 1.45 | 1.25 | 1.20 | 1.06 | 35.3 | 123 |
| P24 | 0.32 | 1.07 | 1.38 | 1.20 | 1.10 | 0.86 | 34.2 | 122 |
| P25 | 0.45 | 1.42 | 1.33 | 1.16 | 0.77 | 0.82 | 32.1 | 119 |
| P26 | 0.43 | 1.36 | 1.14 | 0.93 | 0.79 | 0.84 | 32.4 | 119 |
| P27 | 1.06 | 3.20 | 0.85 | 0.75 | 0.79 | 0.46 | 31.0 | 122 |
| P28 | 1.90 | - | 0.63 | 0.51 | 0.76 | - | - | - |
3.5. Electrochemical Measurement

| Alloy No. | 4th cycle capacity @ 50 mA·g−1 | 4th cycle capacity @ 4 mA·g−1 | HRD200 | Activation cycle to reach maximum capacity | Diffusion coefficient, D @RT | Exchange current Io @RT | Ms | H1/2 | Open circuit voltage |
|---|---|---|---|---|---|---|---|---|---|
| (mAh·g−1) | (mAh·g−1) | (10−10·cm2·s−1) | (mA·g−1) | (emu·g−1) | (kOe) | (V) | |||
| P17 | 382.7 | 400.7 | 0.815 | 6 | 1.69 | 36.5 | 0.39 | 0.11 | 1.302 |
| P22 | 360.4 | 371.0 | 0.873 | 5 | 1.67 | 25.2 | 1.40 | 0.11 | 1.306 |
| P23 | 342.6 | 348.8 | 0.920 | 5 | 2.95 | 25.5 | 0.62 | 0.11 | 1.326 |
| P24 | 308.1 | 314.1 | 0.939 | 6 | 2.85 | 20.0 | 0.92 | 0.10 | 1.330 |
| P25 | 253.9 | 259.2 | 0.965 | 5 | 1.58 | 18.9 | 1.03 | 0.10 | 1.312 |
| P26 | 201.6 | 207.7 | 0.913 | 5 | 1.22 | 12.8 | 1.81 | 0.11 | 1.288 |
| P27 | 100.1 | 105.6 | - | 6 | 1.39 | 12.3 | 1.42 | 0.14 | 1.264 |
| P28 | 68.0 | 72.4 | - | 6 | 1.46 | 16.3 | 1.40 | 0.12 | 1.332 |



4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Iba, H.; Akiba, E. Hydrogen absorption and microstructure in BCC alloys with C14-type Lave phase. J. Jpn. Inst. Met. Mater. 1994, 58, 1225–1232. [Google Scholar]
- Akiba, E.; Iba, H. Hydrogen absorption by Laves phase related BCC solid solution. Intermetallics 1998, 6, 461–470. [Google Scholar] [CrossRef]
- Enomoto, M. The Cr-Ti-V system. J. Phase Equilibria 1992, 13, 195–200. [Google Scholar] [CrossRef]
- Inoue, H.; Arai, S.; Iwakura, C. Crystallographic and electrochemical characterization of TiV4−xNix alloys for nickel-metal hydride batteries. Electrochim. Acta 1996, 41, 937–939. [Google Scholar] [CrossRef]
- Yu, X.; Wu, Z.; Xia, B.; Xu, N. A Ti-V-based bcc phase alloy for use as metal hydride electrode with high discharge capacity. J. Chem. Phys. 2004, 121, 987–990. [Google Scholar] [CrossRef] [PubMed]
- Young, K.-H.; Ouchi, T.; Huang, B.; Nei, J. Structure, hydrogen storage, and electrochemical properties of body-centered-cubic Ti40V30Cr15Mn13X2 alloys (X = B, Si, Mn, Ni, Zr, Nb, Mo, and La). Batteries 2015, 1, 74–90. [Google Scholar] [CrossRef]
- Chen, N.; Li, R.; Zhu, Y.; Liu, Y.; Pan, H. Electrochemical hydrogenation and dehydrogenation mechanisms of the Ti-V base multiphase hydrogen storage electrode alloy. Acta Metall. Sin. 2004, 40, 1200–1204. [Google Scholar]
- Iba, H.; Akiba, E. The relation between microstructure and hydrogen absorbing property in Laves phase-solid solution multiphase alloys. J. Alloy. Compd. 1995, 231, 508–512. [Google Scholar] [CrossRef]
- Rönnebro, E.; Noréus, D.; Sakai, T.; Tsukahara, M. Structural studies of a new Laves phase alloy (Hf,Ti)(Ni,V)2 and its very stable hydride. J. Alloy. Compd. 1995, 231, 90–94. [Google Scholar] [CrossRef]
- Tsukahara, M.; Takahashi, K.; Mishima, T.; Isomura, A.; Sakai, T. V-based solid solution alloys with Laves phase network: Hydrogen absorption properties and microstructure. J. Alloy. Compd. 1996, 236, 151–155. [Google Scholar] [CrossRef]
- Qiu, S.; Chu, H.; Zhang, Y.; Sun, D.; Song, X.; Sun, L.; Fen, X. Electrochemical kinetics and its temperature dependence behaviors of Ti0.17Zr0.08V0.35Cr0.10Ni0.30 alloy electrode. J. Alloy. Compd. 2009, 471, 453–456. [Google Scholar] [CrossRef]
- Iba, H.; Akiba, E. Hydrogen absorption and modulated structure in Ti–V–Mn alloys. J. Alloy. Compd. 1997, 253–254, 21–24. [Google Scholar] [CrossRef]
- Shashikala, K.; Banerjee, S.; Kumar, A.; Pai, M.R.; Pillai, C.G.S. Improvement of hydrogen storage properties of TiCrV alloy by Zr substitution for Ti. Int. J. Hydrog. Energy 2009, 34, 6684–6689. [Google Scholar] [CrossRef]
- Yang, X.; Li, J.; Zhang, T.; Hu, R.; Xue, X.; Fu, H. Role of defect structure on hydrogenation properties of Zr0.9Ti0.1V2 alloy. Int. J. Hydrog. Energy 2011, 36, 9318–9323. [Google Scholar] [CrossRef]
- Huot, J.; Akiba, E.; Ogura, T.; Ishido, Y. Crystal structure, phase abundance and electrode performance of Laves phase compounds (Zr, A)V0.5Ni1.1Mn0.2Fe0.2 (A ≡ Ti, Nb or Hf). J. Alloy. Compd. 1995, 218, 101–109. [Google Scholar] [CrossRef]
- Qiu, S.-J.; Chu, H.-L.; Zhang, J.; Zhang, Y.; Sun, L.-X.; Xu, F.; Sun, D.-L.; Ouyang, L.-Z.; Zhu, M.; Grolier, J.-P.E.; et al. Effect of La partial substitution for Zr on the structural and electrochemical properties of Ti0.17Zr0.08−xLaxV0.35Cr0.1Ni0.3 (x = 0–0.04) electrode alloys. Int. J. Hydrog. Energy 2009, 34, 7246–7252. [Google Scholar] [CrossRef]
- Huang, Z.; Cuevas, F.; Liu, X.; Jiang, L.; Wang, S.; Latroche, M.; Du, J. Effects of Si addition on the microstructure and the hydrogen storage properties of Ti26.5V45Fe8.5Cr20Ce0.5 BCC solid solution alloys. Int. J. Hydrog. Energy 2009, 34, 9385–9392. [Google Scholar] [CrossRef]
- Kamegawa, A.; Shirasaki, K.; Tamura, T.; Kuriiwa, T.; Takamura, H.; Okada, M. Crystal structure and protium absorption properties of Ti–Cr–X alloys. Mater. Trans. 2002, 43, 470–473. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, S.; Li, R.; Gao, M.; Zhong, K.; Miao, H.; Pen, H. Electrochemical performances of the Pd-added Ti-V-based hydrogen storage alloys. Int. J. Hydrog. Energy 2008, 33, 728–734. [Google Scholar] [CrossRef]
- Kuriiwa, T.; Tamura, T.; Amemiya, T.; Fuda, T.; Kamegawa, A.; Takamura, H.; Okada, M. New V-based alloys with high protium absorption and desorption capacity. J. Alloy. Compd. 1999, 293–295, 433–436. [Google Scholar] [CrossRef]
- Yu, J.; Liu, B.; Cho, K.; Lee, J. The effects of partial substitution of Mn by Cr on the electrochemical cycle life of Ti-Zr-V-Mn-Ni alloy electrodes of a Ni/MH battery. J. Alloy. Compd. 1998, 278, 283–290. [Google Scholar] [CrossRef]
- Lee, H.-H.; Lee, K.-Y.; Lee, J.-Y. The Ti-based metal hydride electrode for Ni–MH rechargeable batteries. J. Alloy. Compd. 1996, 239, 63–70. [Google Scholar] [CrossRef]
- Kim, J.; Paik, C.; Cho, W.; Cho, B.; Yun, K.; Kim, S. Corrosion behaviour of Zr1−xTixV0.6Ni1.2M0.2 (M = Ni, Cr, Mn) AB2-type metal hydride alloys in alkaline solution. J. Power Sources 1998, 75, 1–8. [Google Scholar] [CrossRef]
- Young, K.; Wong, D.; Wang, L. Effect of Ti/Cr content on the microstructures and hydrogen storage properties of Laves phase-related body-centered-cubic solid solution alloys. J. Alloy. Compd. 2015, 622, 885–893. [Google Scholar] [CrossRef]
- Young, K.; Ouchi, T.; Nei, J.; Wang, L. Annealing effects on Laves phase-related body-centered-cubic solid solution metal hydride alloys. J. Alloy. Compd. 2016, 654, 216–225. [Google Scholar] [CrossRef]
- Young, K.; Ouchi, T.; Nei, J.; Meng, T. Effects of Cr, Zr, V, Mn, Fe, and Co to the hydride properties of Laves phase-related body-centered-cubic solid solution alloys. J. Power Sources 2015, 281, 164–172. [Google Scholar] [CrossRef]
- Young, K.; Ouchi, T.; Reichman, B.; Koch, J.; Fetcenko, M.A. Effects of Mo additive on the structure and electrochemical properties of low-temperature AB5 metal hydride alloys. J. Alloy. Compd. 2011, 509, 3995–4001. [Google Scholar] [CrossRef]
- Chai, Y.; Zhao, M. Structure and electrochemical properties of Ti0.25V0.35Cr0.40−xNix (x = 0.05–0.40) solid solution alloys. Int. J. Hydrog. Energy 2005, 30, 279–283. [Google Scholar] [CrossRef]
- Young, K.; Fetcenko, M.A.; Li, F.; Ouchi, T. Structural, thermodynamic, and electrochemical properties of TixZr1−x(VNiCrMnCoAl)2 C14 Laves phase alloys. J. Alloy. Compd. 2008, 464, 238–247. [Google Scholar] [CrossRef]
- Young, K.; Ouchi, T.; Fetcenko, M.A. Roles of Ni, Cr, Mn, Sn, Co, and Al in C14 Laves phase alloys for NiMH battery application. J. Alloy. Compd. 2009, 476, 774–781. [Google Scholar] [CrossRef]
- Li, R.; Pan, H.; Gao, M.; Zhu, Y.; Liu, Y.; Jin, Q.; Lei, Y. Structural and electrochemical properties of hydrogen storage alloys Ti0.8Zr0.2V2.7Mn0.5Cr0.8Nix (x = 1.50–2.25). J. Alloy. Compd. 2004, 373, 223–230. [Google Scholar] [CrossRef]
- Pan, H.; Li, R.; Gao, M.; Liu, Y.; Lei, Y.; Wang, Q. Effects of Ni on the structural and electrochemical properties of Ti-V-based hydrogen storage alloys. Int. J. Hydrog. Energy 2006, 31, 1188–1195. [Google Scholar] [CrossRef]
- Pearson, W.; Christian, J. The structure of the σ phase in vanadium-nickel alloys. Acta Crystallogr. 1952, 5, 157–162. [Google Scholar] [CrossRef]
- Massalski, T.; Okamoto, H.; Subramanian, P.; Kacprzak, L. Binary Alloy Phase Diagram, 2nd ed.; ASM International: Materials Park: Ohio, USA, 1990; p. 2880. [Google Scholar]
- Kodentzov, A.A.; Dunaev, S.F.; Slusarenko, E.M. Determination of the phase diagram of the V-Ni-Cr system using diffusion couples and equilibrated alloys. J. Less Common Met. 1987, 135, 15–24. [Google Scholar] [CrossRef]
- Yan, Y.; Chen, Y.; Liang, H.; Zhou, X.; Wu, C.; Tao, M.; Pang, L. Hydrogen storage properties of V–Ti–Cr–Fe alloys. J. Alloy. Compd. 2008, 454, 427–431. [Google Scholar] [CrossRef]
- Young, K.; Nei, J.; Wong, D.; Wang, L. Structural, hydrogen storage, and electrochemical properties of Laves-phase related body-centered-cubic solid solution metal hydride alloys. Int. J. Hydrog. Energy 2014, 39, 21489–21499. [Google Scholar] [CrossRef]
- Johnston, R.L.; Hoffmann, R. Structure-bonding relationships in the Laves phases. Z. Anorg. Allg. Chem. 1992, 616, 105–120. [Google Scholar] [CrossRef]
- Nei, J.; Young, K.; Salley, S.O.; Ng, K. Determination of C14/C15 phase abundance in Laves phase alloys. Mater. Chem. Phys. 2012, 136, 520–527. [Google Scholar] [CrossRef]
- Young, K.; Fetcenko, M.A.; Li, F.; Ouchi, T.; Koch, J. Effect of vanadium substitution in C14 Laves phase alloys for NiMH battery application. J. Alloy. Compd. 2009, 468, 482–492. [Google Scholar] [CrossRef]
- Young, K.; Ouchi, T.; Huang, B.; Reichman, B.; Fetcenko, M.A. Effect of molybdenum content on structure, gaseous storage, and electrochemical properties of C14-predominant AB2 metal hydride alloys. J. Power Sources 2011, 196, 8815–8821. [Google Scholar] [CrossRef]
- Young, K. Metal Hydrides. In Elsevier Reference Module in Chemistry, Molecular Sciences and Chemical Engineering; Elsevier B.V.: Waltham, MA, USA, 2013. [Google Scholar]
- Griessen, R.; Riesterer, T. Heat of Formation Models. In Hydrogen in Intermetallic Compounds I; Schlapbach, L., Ed.; Springer-Verlag: Berlin, Germany, 1988; p. 273. [Google Scholar]
- Balasubramaniam, R. Hysteresis in metal-hydrogen systems. J. Alloy. Compd. 1997, 253–254, 203–206. [Google Scholar] [CrossRef]
- Wong, D.; Young, K.; Nei, J.; Wang, L.; Ng, K. Effects of Nd-addition on the structural, hydrogen storage, and electrochemical properties of C14 metal hydride alloys. J. Alloy. Compd. 2015, 647, 507–518. [Google Scholar] [CrossRef]
- Nakano, H.; Wakao, S. Substitution effect of elements in Zr-based alloys with Laves phase for nickel-hydride battery. J. Alloy. Compd. 1995, 231, 587–593. [Google Scholar] [CrossRef]
- Nei, J.; Young, K.; Sally, S.O.; Ng, K. Effects of annealing on Zr8Ni19X2 (X = Ni, Mg, Al, Sc, V, Mn, Co, Sn, La, and Hf): Hydrogen storage and electrochemical properties. Int. J. Hydrog. Energy 2012, 37, 8418–8427. [Google Scholar] [CrossRef]
- Sun, D.; Jiang, J.; Lei, Y.; Liu, W.; Wu, J.; Wang, Q.; Yang, G. Effects of measurement factor on electrochemical capacity of some hydrogen storage alloys. Mater. Sci. Eng. B 1995, 30, 19–22. [Google Scholar] [CrossRef]
- Young, K.; Nei, J.; Huang, B.; Ouchi, T.; Fetcenko, M.A. Studies of Ti1.5Zr5.5V0.5(MxNi1−x)9.5 (M = Cr, Mn, Fe, Co, Cu, Al): Part 2. Hydrogen storage and electrochemical properties. J. Alloy. Compd. 2010, 501, 245–254. [Google Scholar] [CrossRef]
- Young, K.; Chao, B.; Huang, B.; Nei, J. Studies on the hydrogen storage characteristic of La1−xCex(NiCoMnAlCuSiZr)5.7 with a B2 secondary phase. J. Alloy. Compd. 2014, 585, 760–770. [Google Scholar] [CrossRef]
- Li, F.; Young, K.; Ouchi, T.; Fetcenko, M.A. Annealing effects on structural and electrochemical properties of (LaPrNdZr)0.83Mg0.17(NiCoAlMn)3.3 alloy. J. Alloy. Compd. 2009, 471, 371–377. [Google Scholar] [CrossRef]
- Young, K.; Ouchi, T.; Huang, B.; Chao, B.; Fetcenko, M.A.; Bendersky, L.A.; Wang, K.; Chiu, C. The correlation of C14/C15 phase abundance and electrochemical properties in the AB2 alloys. J. Alloy. Compd. 2010, 506, 841–848. [Google Scholar] [CrossRef]
- Shim, J.H.; Ko, W.S.; Kim, K.H.; Lee, H.S.; Lee, Y.S.; Suh, J.Y.; Choa, Y.W.; Lee, B.-J. Prediction of hydrogen permeability in V-Al and V-Ni alloys. J. Membr. Sci. 2014, 430, 234–241. [Google Scholar] [CrossRef]
- Young, K.; Nei, J. The current status of hydrogen storage alloy development for electrochemical applications. Materials 2013, 6, 4574–4608. [Google Scholar] [CrossRef]
- Young, K.; Nei, J.; Ouchi, T.; Fetcenko, M.A. Phase abundances in AB2 metal hydride alloys and their correlations to various properties. J. Alloy. Compd. 2011, 509, 2277–2284. [Google Scholar] [CrossRef]
- Young, K.; Wong, D.; Wang, L.; Nei, J.; Ouchi, T.; Yasuoka, S. Mn in misch-metal based superlattice metal hydride alloy—Part 1 structural, hydrogen storage and electrochemical properties. J. Power Sources 2015, 277, 426–432. [Google Scholar] [CrossRef]
- Young, K.; Huang, B.; Regmi, R.K.; Lawes, G.; Liu, Y. Comparisons of metallic clusters imbedded in the surface oxide of AB2, AB5, and A2B7 alloys. J. Alloy. Compd. 2010, 506, 831–840. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Young, K.-h.; Wong, D.F.; Nei, J. Effects of Vanadium/Nickel Contents in Laves Phase-Related Body-Centered-Cubic Solid Solution Metal Hydride Alloys. Batteries 2015, 1, 34-53. https://doi.org/10.3390/batteries1010034
Young K-h, Wong DF, Nei J. Effects of Vanadium/Nickel Contents in Laves Phase-Related Body-Centered-Cubic Solid Solution Metal Hydride Alloys. Batteries. 2015; 1(1):34-53. https://doi.org/10.3390/batteries1010034
Chicago/Turabian StyleYoung, Kwo-hsiung, Diana F. Wong, and Jean Nei. 2015. "Effects of Vanadium/Nickel Contents in Laves Phase-Related Body-Centered-Cubic Solid Solution Metal Hydride Alloys" Batteries 1, no. 1: 34-53. https://doi.org/10.3390/batteries1010034