All-Solid-State Li-Metal Cell Using Nanocomposite TiO2/Polymer Electrolyte and Self-Standing LiFePO4 Cathode
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Synthesis of Titanium Dioxide Nanotubes
2.3. Preparation of Self-Standing LFP Catholyte
2.4. Preparation of PEO/LiTFSI/TiO2 Electrolytes
2.5. Characterization and Measurements
3. Results and Discussion
3.1. Polymer Electrolytes with LiTFSI
3.2. Polymer Electrolytes with Optimized LiTFSI Concentration and Fillers
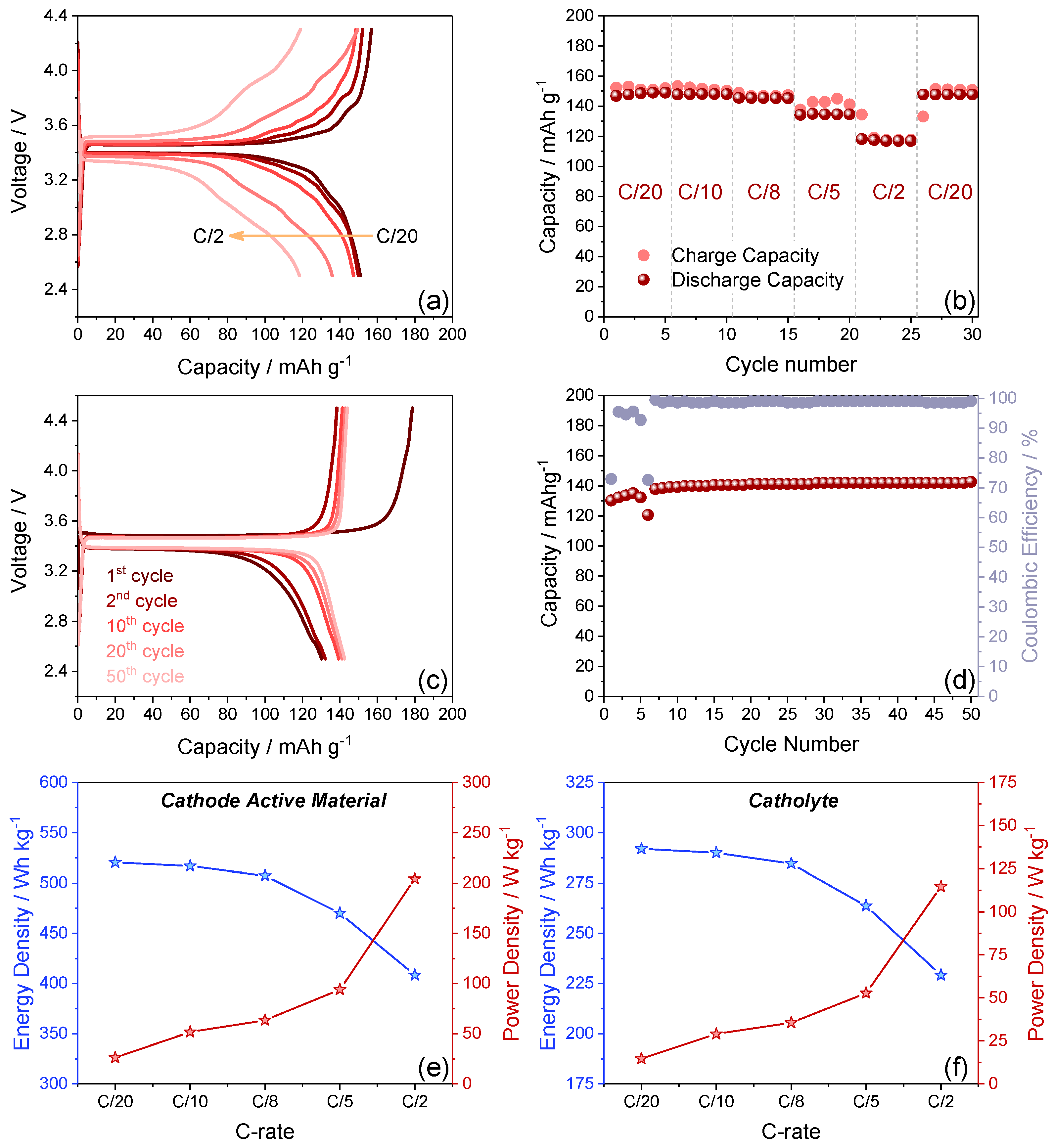
3.3. Full Li-Metal Cell with Self-Standing Catholyte
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Xi, G.; Xiao, M.; Wang, S.; Han, D.; Li, Y.; Meng, Y. Polymer-Based Solid Electrolytes: Material Selection, Design, and Application. Adv. Funct. Mater. 2021, 31, 2007598. [Google Scholar] [CrossRef]
- Aragane, J.; Matsui, K.; Andoh, H.; Suzuki, S.; Fukuda, H.; Ikeya, H.; Kitaba, K.; Ishikawa, R. Development of 10 Wh Class Lithium Secondary Cells in the “New Sunshine Program”. J. Power Sources 1997, 68, 13–18. [Google Scholar] [CrossRef]
- Beaton, S.P.; Bishop, G.A.; Zhang, Y.; Stedman, D.H.; Ashbaugh, L.L.; Lawson, D.R. On-Road Vehicle Emissions: Regulations, Costs, and Benefits. Science 1995, 268, 991–993. [Google Scholar] [CrossRef] [PubMed]
- Scrosati, B. Challenge of Portable Power. Nature 1995, 373, 557–558. [Google Scholar] [CrossRef]
- Zhang, S.; Liang, T.; Wang, D.; Xu, Y.; Cui, Y.; Li, J.; Wang, X.; Xia, X.; Gu, C.; Tu, J. A Stretchable and Safe Polymer Electrolyte with a Protecting-Layer Strategy for Solid-State Lithium Metal Batteries. Adv. Sci. 2021, 8, 2003241. [Google Scholar] [CrossRef]
- Xu, K. Electrolytes and Interphases in Li-Ion Batteries and Beyond. Chem. Rev. 2014, 114, 11503–11618. [Google Scholar] [CrossRef] [PubMed]
- Song, B.; Dhiman, I.; Carothers, J.C.; Veith, G.M.; Liu, J.; Bilheux, H.Z.; Huq, A. Dynamic Lithium Distribution upon Dendrite Growth and Shorting Revealed by Operando Neutron Imaging. ACS Energy Lett. 2019, 4, 2402–2408. [Google Scholar] [CrossRef]
- Yang, X.; Luo, J.; Sun, X. Towards High-Performance Solid-State Li-S Batteries: From Fundamental Understanding to Engineering Design. Chem. Soc. Rev. 2020, 49, 2140–2195. [Google Scholar] [CrossRef]
- Zhou, Q.; Ma, J.; Dong, S.; Li, X.; Cui, G. Intermolecular Chemistry in Solid Polymer Electrolytes for High-Energy-Density Lithium Batteries. Adv. Mater. 2019, 31, e1902029. [Google Scholar] [CrossRef]
- Manthiram, A.; Yu, X.; Wang, S. Lithium Battery Chemistries Enabled by Solid-State Electrolytes. Nat. Rev. Mater. 2017, 2, 16103. [Google Scholar] [CrossRef]
- Zhao, Y.; Zheng, K.; Sun, X. Addressing Interfacial Issues in Liquid-Based and Solid-State Batteries by Atomic and Molecular Layer Deposition. Joule 2018, 2, 2583–2604. [Google Scholar] [CrossRef]
- Umeshbabu, E.; Zheng, B.; Yang, Y. Recent Progress in All-Solid-State Lithium−Sulfur Batteries Using High Li-Ion Conductive Solid Electrolytes. Electrochem. Energy Rev. 2019, 2, 199–230. [Google Scholar] [CrossRef]
- Marangon, V.; Tominaga, Y.; Hassoun, J. An Alternative Composite Polymer Electrolyte for High Performances Lithium Battery. J. Power Sources 2019, 449, 227508. [Google Scholar] [CrossRef]
- Ma, J.; Liu, Z.; Chen, B.; Wang, L.; Yue, L.; Liu, H.; Zhang, J.; Liu, Z.; Cui, G. A Strategy to Make High Voltage LiCoO2 Compatible with Polyethylene Oxide Electrolyte in All-Solid-State Lithium Ion Batteries. J. Electrochem. Soc. 2017, 164, A3454–A3461. [Google Scholar] [CrossRef]
- Lin, Z.; Guo, X.; Yu, H. Amorphous Modified Silyl-Terminated 3D Polymer Electrolyte for High-Performance Lithium Metal Battery. Nano Energy 2017, 41, 646–653. [Google Scholar] [CrossRef]
- Zhang, S.; Xia, X.; Xie, D.; Xu, R.; Xu, Y.; Xia, Y.; Wu, J.; Yao, Z.; Wang, X.; Tu, J. Facile Interfacial Modification via In-Situ Ultraviolet Solidified Gel Polymer Electrolyte for High-Performance Solid-State Lithium Ion Batteries. J. Power Sources 2018, 409, 31–37. [Google Scholar] [CrossRef]
- Zhou, D.; Shanmukaraj, D.; Tkacheva, A.; Armand, M.; Wang, G. Polymer Electrolytes for Lithium-Based Batteries: Advances and Prospects. Chem 2019, 5, 2326–2352. [Google Scholar] [CrossRef]
- He, K.-Q.; Zha, J.-W.; Du, P.; Cheng, S.H.-S.; Liu, C.; Dang, Z.-M.; Li, R.K.Y. Tailored High Cycling Performance in a Solid Polymer Electrolyte with Perovskite-Type Li0.33La0.557TiO3 Nanofibers for All-Solid-State Lithium Ion Batteries. Dalton Trans. 2019, 48, 3263–3269. [Google Scholar] [CrossRef]
- Shi, C.; Yu, M. Flexible Solid-State Lithium-Sulfur Batteries Based on Structural Designs. Energy Storage Mater. 2023, 57, 429–459. [Google Scholar] [CrossRef]
- Croce, F.; Bonino, F.; Panero, S.; Scrosati, B. Properties of Mixed Polymer and Crystalline Ionic Conductors. Philos. Mag. B 1989, 59, 161–168. [Google Scholar] [CrossRef]
- Shi, C.; Alexander, G.V.; O’neill, J.; Duncan, K.; Godbey, G.; Wachsman, E.D. All-Solid-State Garnet Type Sulfurized Polyacrylonitrile/Lithium-Metal Battery Enabled by an Inorganic Lithium Conductive Salt and a Bilayer Electrolyte Architecture. ACS Energy Lett. 2023, 8, 1803–1810. [Google Scholar] [CrossRef]
- Wetjen, M.; Navarra, M.A.; Panero, S.; Passerini, S.; Scrosati, B.; Hassoun, J. Composite Poly(Ethylene Oxide) Electrolytes Plasticized by N-Alkyl-N-Butylpyrrolidinium Bis(Trifluoromethanesulfonyl)Imide for Lithium Batteries. ChemSusChem 2013, 6, 1037–1043. [Google Scholar] [CrossRef] [PubMed]
- Appetecchi, G.; Croce, F.; Hassoun, J.; Scrosati, B.; Salomon, M.; Cassel, F. Hot-Pressed, Dry, Composite, PEO-Based Electrolyte Membranes: I. Ionic Conductivity Characterization. J. Power Sources 2003, 114, 105–112. [Google Scholar] [CrossRef]
- Cheung, I.; Chin, K.; Greene, E.; Smart, M.; Abbrent, S.; Greenbaum, S.; Prakash, G.; Surampudi, S. Electrochemical and Solid State NMR Characterization of Composite PEO-Based Polymer Electrolytes. Electrochim. Acta 2003, 48, 2149–2156. [Google Scholar] [CrossRef]
- Liu, Y.; Lee, J.; Hong, L. In Situ Preparation of Poly(Ethylene Oxide)-SiO2 Composite Polymer Electrolytes. J. Power Sources 2004, 129, 303–311. [Google Scholar] [CrossRef]
- Krawiec, W.; Scanlon, L.; Fellner, J.; Vaia, R.; Vasudevan, S.; Giannelis, E. Polymer Nanocomposites: A New Strategy for Synthesizing Solid Electrolytes for Rechargeable Lithium Batteries. J. Power Sources 1995, 54, 310–315. [Google Scholar] [CrossRef]
- Croce, F.; Appetecchi, G.B.; Persi, L.; Scrosati, B. Nanocomposite Polymer Electrolytes for Lithium Batteries. Nature 1998, 394, 456–458. [Google Scholar] [CrossRef]
- Golodnitsky, D.; Ardel, G.; Peled, E. Ion-Transport Phenomena in Concentrated PEO-Based Composite Polymer Electrolytes. Solid State Ion. 2002, 147, 141–155. [Google Scholar] [CrossRef]
- Bloise, A.; Tambelli, C.; Franco, R.; Donoso, J.; Magon, C.; Souza, M.; Rosario, A.; Pereira, E. Nuclear Magnetic Resonance Study of PEO-Based Composite Polymer Electrolytes. Electrochim. Acta 2001, 46, 1571–1579. [Google Scholar] [CrossRef]
- Kumar, B.; Rodrigues, S.J.; Scanlon, L.G. Ionic Conductivity of Polymer-Ceramic Composites. J. Electrochem. Soc. 2001, 148, A1191–A1195. [Google Scholar] [CrossRef]
- Feng, J.; Wang, L.; Chen, Y.; Wang, P.; Zhang, H.; He, X. PEO Based Polymer-Ceramic Hybrid Solid Electrolytes: A Review. Nano Converg. 2021, 8, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Sun, K.C.; Qadir, M.B.; Jeong, S.H. Hydrothermal Synthesis of TiO2 Nanotubes and Their Application as an Over-Layer for Dye-Sensitized Solar Cells. RSC Adv. 2014, 4, 23223–23230. [Google Scholar] [CrossRef]
- Sun, K.C.; Yun, S.H.; Yoon, C.H.; Ko, H.H.; Yi, S.; Jeong, S.H. Enhanced Power Conversion Efficiency of Dye-Sensitized Solar Cells Using Nanoparticle/Nanotube Double Layered Film. J. Nanosci. Nanotechnol. 2013, 13, 7938–7943. [Google Scholar] [CrossRef] [PubMed]
- Evans, J.; Vincent, C.A.; Bruce, P.G. Electrochemical Measurement of Transference Numbers in Polymer Electrolytes. Polymer 1987, 28, 2324–2328. [Google Scholar] [CrossRef]
- Barsoukov, E.; Macdonald, J.R. Impedance Spectroscopy: Theory, Experiment, and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2005. [Google Scholar]
- Boukamp, B.A. A Package for Impedance/Admittance Data Analysis. Solid State Ion. 1986, 18–19, 136–140. [Google Scholar] [CrossRef]
- Boukamp, B.A. A Nonlinear Least Squares Fit Procedure for Analysis of Immittance Data of Electrochemical Systems. Solid State Ion. 1986, 20, 31–44. [Google Scholar] [CrossRef]
- Li, N.; Pan, N.; Li, D.; Lin, S. Natural Dye-Sensitized Solar Cells Based on Highly Ordered TiO2Nanotube Arrays. Int. J. Photoenergy 2013, 2013, 598753. [Google Scholar] [CrossRef]
- Rao, B.M.; Roy, S.C. Anatase TiO2 Nanotube Arrays with High Temperature Stability. RSC Adv. 2014, 4, 38133–38139. [Google Scholar] [CrossRef]
- Shaban, M.; Poostforooshan, J.; Weber, A.P. Surface-Initiated Polymerization on Unmodified Inorganic Semiconductor Nanoparticles: Via Surfactant-Free Aerosol-Based Synthesis toward Core-Shell Nanohybrids with a Tunable Shell Thickness. J. Mater. Chem. A 2017, 5, 18651–18663. [Google Scholar] [CrossRef]
- Xu, H.; Chien, P.-H.; Shi, J.; Li, Y.; Wu, N.; Liu, Y.; Hu, Y.-Y.; Goodenough, J.B. High-Performance All-Solid-State Batteries Enabled by Salt Bonding to Perovskite in Poly(Ethylene Oxide). Proc. Natl. Acad. Sci. USA 2019, 116, 18815–18821. [Google Scholar] [CrossRef]
- Olmedo-Martínez, J.L.; Porcarelli, L.; Guzmán-González, G.; Calafel, I.; Forsyth, M.; Mecerreyes, D.; Müller, A.J. Ternary Poly(Ethylene Oxide)/Poly(l, l -Lactide) PEO/PLA Blends as High-Temperature Solid Polymer Electrolytes for Lithium Batteries. ACS Appl. Polym. Mater. 2021, 3, 6326–6337. [Google Scholar] [CrossRef]
- St-Onge, V.; Cui, M.; Rochon, S.; Daigle, J.-C.; Claverie, J.P. Reducing Crystallinity in Solid Polymer Electrolytes for Lithium-Metal Batteries via Statistical Copolymerization. Commun. Mater. 2021, 2, 83. [Google Scholar] [CrossRef]
- Croce, F.; Persi, L.; Ronci, F.; Scrosati, B. Nanocomposite Polymer Electrolytes and Their Impact on the Lithium Battery Technology. Solid State Ion. 2000, 135, 47–52. [Google Scholar] [CrossRef]
- Wieczorek, W.; Florjanczyk, Z.; Stevens, J. Composite Polyether Based Solid Electrolytes. Electrochim. Acta 1995, 40, 2251–2258. [Google Scholar] [CrossRef]
- Vignarooban, K.; Dissanayake, M.; Albinsson, I.; Mellander, B.-E. Effect of TiO2 Nano-Filler and EC Plasticizer on Electrical and Thermal Properties of Poly(Ethylene Oxide) (PEO) Based Solid Polymer Electrolytes. Solid State Ion. 2014, 266, 25–28. [Google Scholar] [CrossRef]
- Panero, S.; Scrosati, B.; Sumathipala, H.; Wieczorek, W. Dual-Composite Polymer Electrolytes with Enhanced Transport Properties. J. Power Sources 2007, 167, 510–514. [Google Scholar] [CrossRef]
- Mao, G.; Saboungi, M.-L.; Price, D.L.; Armand, M.B.; Howells, W.S. Structure of Liquid PEO-LiTFSI Electrolyte. Phys. Rev. Lett. 2000, 84, 5536–5539. [Google Scholar] [CrossRef]
- Maurel, A.; Armand, M.; Grugeon, S.; Fleutot, B.; Davoisne, C.; Tortajada, H.; Courty, M.; Panier, S.; Dupont, L. Poly(Ethylene Oxide)−LiTFSI Solid Polymer Electrolyte Filaments for Fused Deposition Modeling Three-Dimensional Printing. J. Electrochem. Soc. 2020, 167, 070536. [Google Scholar] [CrossRef]
- Besenhard, J.O. Handbook of Battery Materials; John Wiley & Sons: Hoboken, NJ, USA, 2007. [Google Scholar]
- Marangon, V.; Minnetti, L.; Barcaro, E.; Hassoun, J. Room-Temperature Solid-State Polymer Electrolyte in Li-LiFePO4, Li-S and Li-O2 Batteries. Chem.–A Eur. J. 2023, 29, e202301345. [Google Scholar] [CrossRef]
- Carbone, L.; Gobet, M.; Peng, J.; Devany, M.; Scrosati, B.; Greenbaum, S.; Hassoun, J. Comparative Study of Ether-Based Electrolytes for Application in Lithium–Sulfur Battery. ACS Appl. Mater. Interfaces 2015, 7, 13859–13865. [Google Scholar] [CrossRef]
- Marangon, V.; Di Lecce, D.; Minnetti, L.; Hassoun, J. Novel Lithium-Sulfur Polymer Battery Operating at Moderate Temperature. ChemElectroChem 2021, 8, 3971–3981. [Google Scholar] [CrossRef]
- Minnetti, L.; Marangon, V.; Hassoun, J. Synthesis and Characterization of a LiFe0.6Mn0.4PO4 Olivine Cathode for Application in a New Lithium Polymer Battery. Adv. Sustain. Syst. 2022, 6, 202100464. [Google Scholar] [CrossRef]
- Kim, G.-T.; Appetecchi, G.B.; Alessandrini, F.; Passerini, S. Solvent-Free, PYR1ATFSI Ionic Liquid-Based Ternary Polymer Electrolyte Systems. I. Electrochemical Characterization. J. Power Sources 2007, 171, 861–869. [Google Scholar] [CrossRef]
- Kim, K.; Kuhn, L.; Alabugin, I.V.; Hallinan, D.T. Lithium Salt Dissociation in Diblock Copolymer Electrolyte Using Fourier Transform Infrared Spectroscopy. Front. Energy Res. 2020, 8, 569442. [Google Scholar] [CrossRef]
- Khalid, A.; Ahmad, P.; Alharthi, A.I.; Muhammad, S.; Khandaker, M.U.; Faruque, M.R.I.; Din, I.U.; Alotaibi, M.A. Unmodified Titanium Dioxide Nanoparticles as a Potential Contrast Agent in Photon Emission Computed Tomography. Crystals 2021, 11, 171. [Google Scholar] [CrossRef]
- Chen, C.-H.; Chiu, J.-M.; Shown, I.; Wang, C.-H. Simple Way of Making Free-Standing Cathode Electrodes for Flexible Lithium-Ion Batteries. RSC Adv. 2022, 12, 9249–9255. [Google Scholar] [CrossRef]
- Streltsov, V.A.; Belokoneva, E.L.; Tsirelson, V.G.; Hansen, N.K. Multipole Analysis of the Electron Density in Triphylite, LiFePO4, Using X-ray Diffraction Data. Acta Crystallogr. Sect. B Struct. Sci. 1993, 49, 147–153. [Google Scholar] [CrossRef]
- Padhi, A.K.; Nanjundaswamy, K.S.; Goodenough, J.B. Phospho-olivines as Positive-Electrode Materials for Rechargeable Lithium Batteries. J. Electrochem. Soc. 1997, 144, 1188–1194. [Google Scholar] [CrossRef]
- Wu, J.; Zhang, X.; Ju, Z.; Wang, L.; Hui, Z.; Mayilvahanan, K.; Takeuchi, K.J.; Marschilok, A.C.; West, A.C.; Takeuchi, E.S.; et al. From Fundamental Understanding to Engineering Design of High-Performance Thick Electrodes for Scalable Energy-Storage Systems. Adv. Mater. 2021, 33, 2101275. [Google Scholar] [CrossRef]
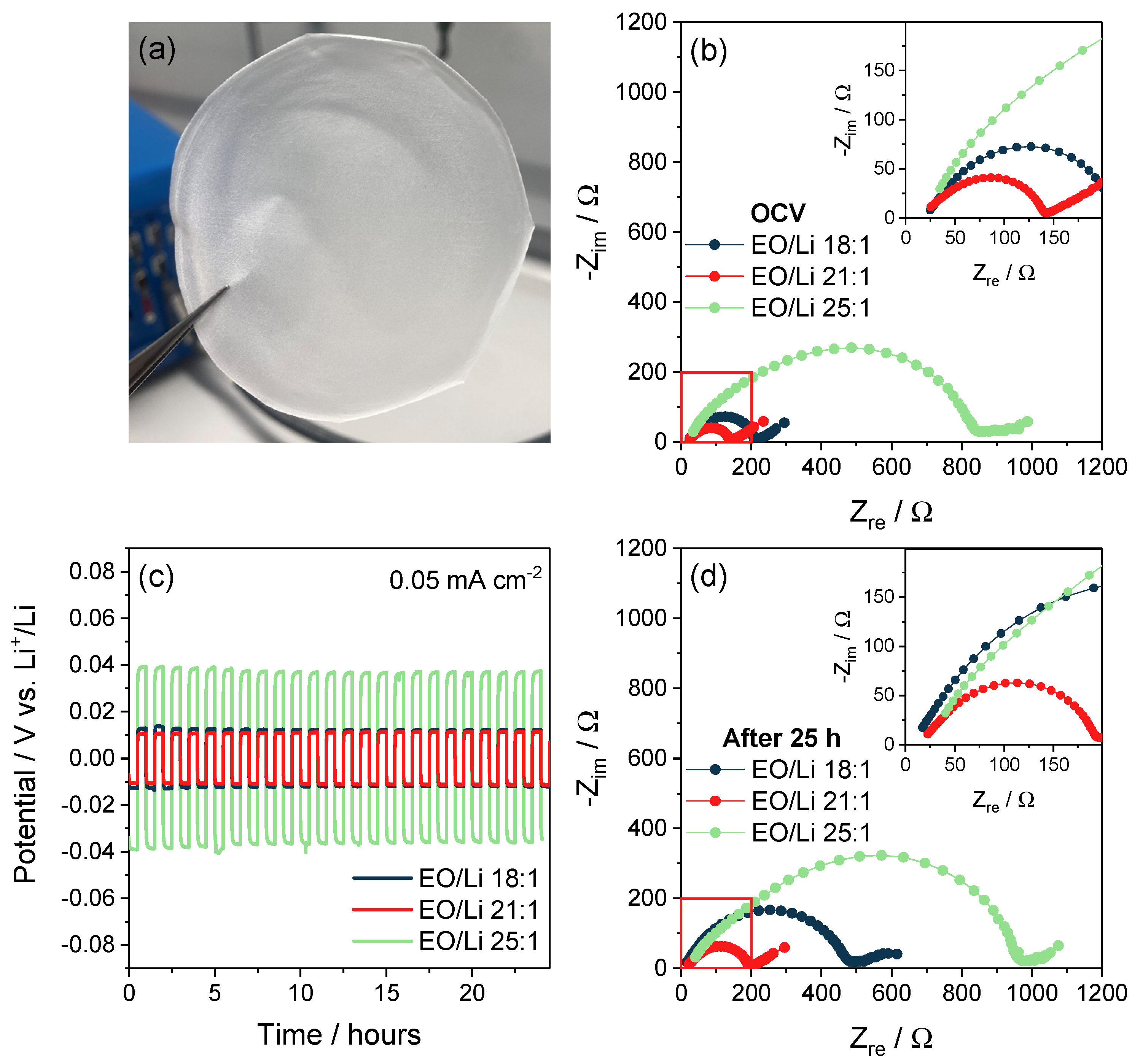

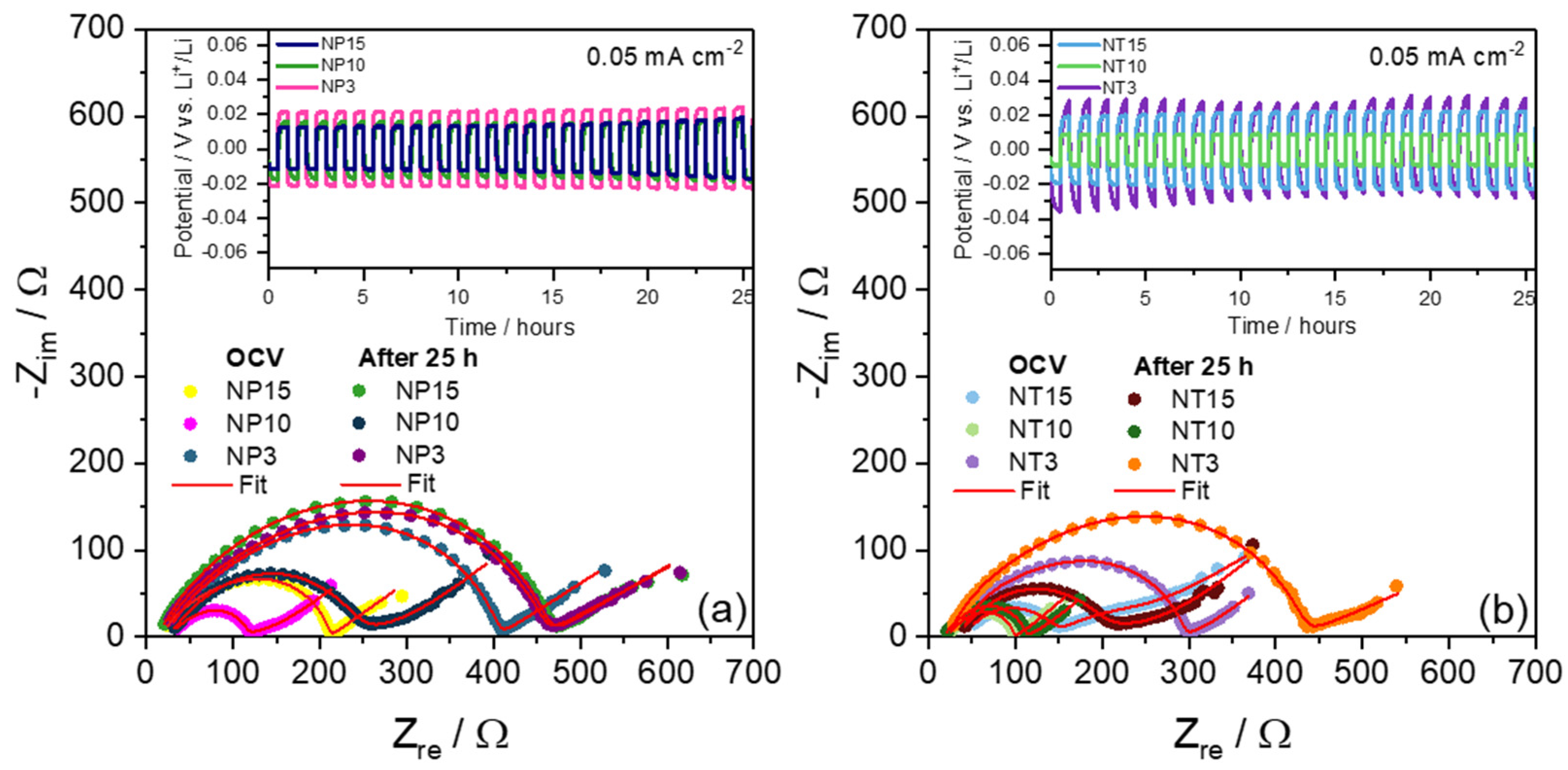
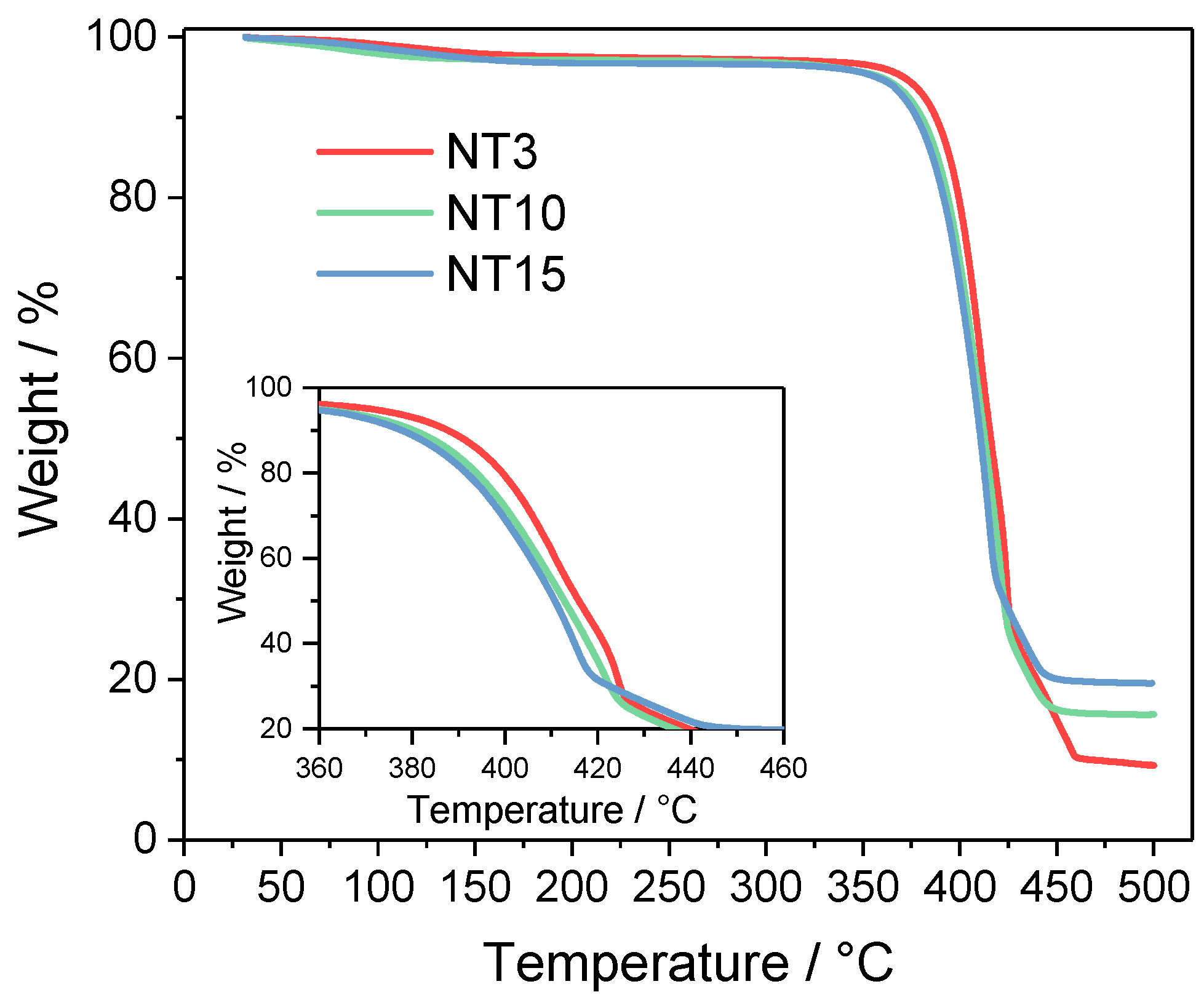

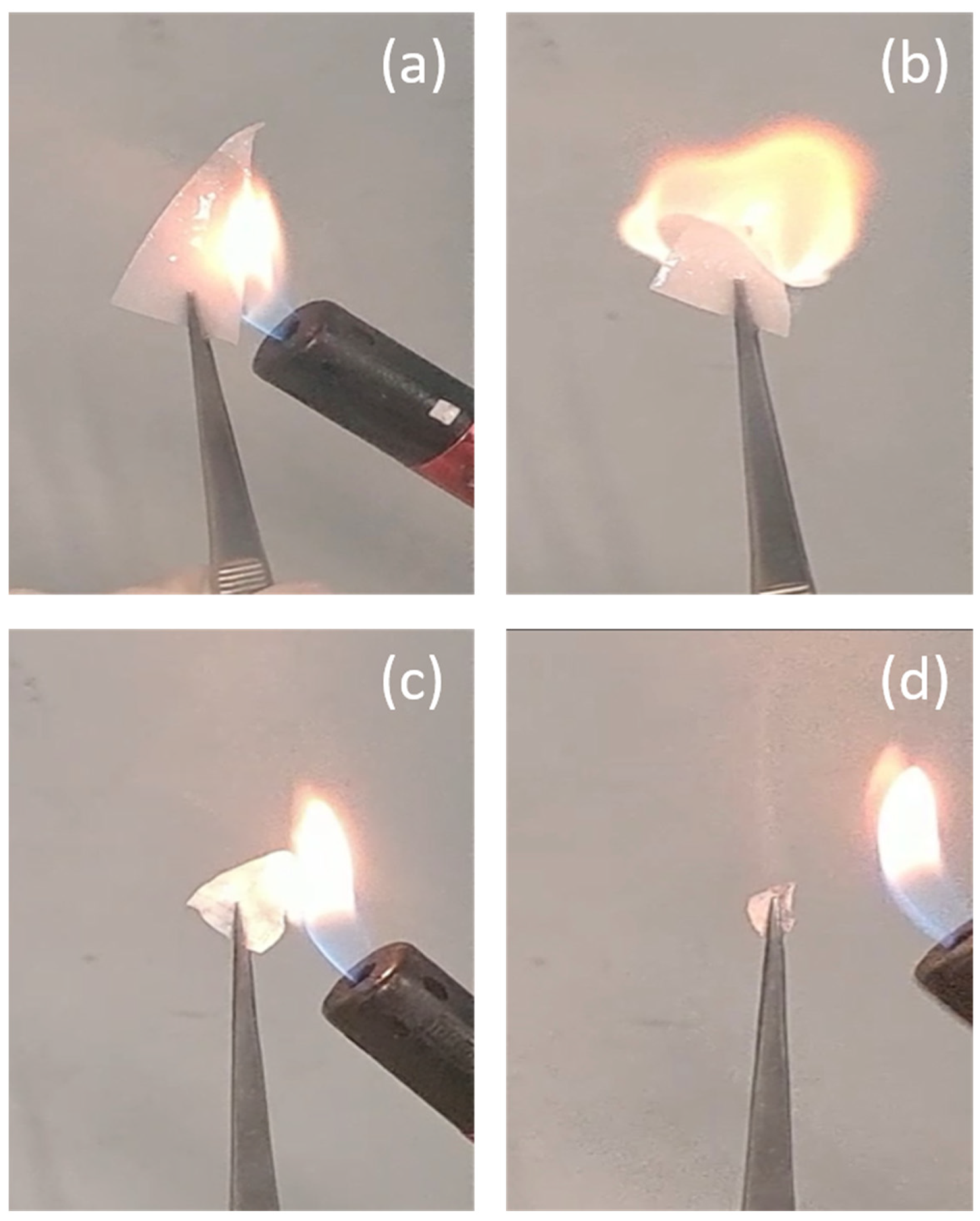


| Sample | Tg (°C) |
|---|---|
| Pristine membrane:PEO/LiTFSI 21:1 | −44.7 |
| NT3 | −41.8 |
| NT10 | −45.2 |
| NT15 | −46.8 |
| PEO | −54.3 |
| Temperature (°C) | Initial Current (i0) (A) | Steady-State Current (iSS) (A) | Initial Resistance (R0) (Ω) | Steady-State Resistance (RSS) (Ω) | Li+ Transference Number (τ+) |
|---|---|---|---|---|---|
| 45 | 3.25 × 10−5 | 1.23 × 10−5 | 322.61 | 321.19 | 0.27 |
| 55 | 9.94 × 10−5 | 3.86 × 10−5 | 134.16 | 148.12 | 0.28 |
| 65 | 1.57 × 10−4 | 8.60 × 10−5 | 129.81 | 134.39 | 0.29 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Patriarchi, A.; Darjazi, H.; Minnetti, L.; Sbrascini, L.; Elia, G.A.; Castorani, V.; Muñoz-Márquez, M.Á.; Nobili, F. All-Solid-State Li-Metal Cell Using Nanocomposite TiO2/Polymer Electrolyte and Self-Standing LiFePO4 Cathode. Batteries 2024, 10, 11. https://doi.org/10.3390/batteries10010011
Patriarchi A, Darjazi H, Minnetti L, Sbrascini L, Elia GA, Castorani V, Muñoz-Márquez MÁ, Nobili F. All-Solid-State Li-Metal Cell Using Nanocomposite TiO2/Polymer Electrolyte and Self-Standing LiFePO4 Cathode. Batteries. 2024; 10(1):11. https://doi.org/10.3390/batteries10010011
Chicago/Turabian StylePatriarchi, Asia, Hamideh Darjazi, Luca Minnetti, Leonardo Sbrascini, Giuseppe Antonio Elia, Vincenzo Castorani, Miguel Ángel Muñoz-Márquez, and Francesco Nobili. 2024. "All-Solid-State Li-Metal Cell Using Nanocomposite TiO2/Polymer Electrolyte and Self-Standing LiFePO4 Cathode" Batteries 10, no. 1: 11. https://doi.org/10.3390/batteries10010011






