Bismuth Nanoparticles Encapsulated in a Porous Carbon Skeleton as Stable Chloride-Storage Electrodes for Seawater Desalination
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials and Chemicals
2.2. Preparation of Materials
2.3. Electrode Fabrication
2.4. Material Characterization
2.5. Electrochemical Testing
3. Results and Discussion
3.1. Optimization of the Preparation Process of MOF-Derived Bismuth Carbon Materials
3.1.1. Optimization of Sintering Temperature
3.1.2. Optimization of Sintering Time
3.2. Characterization of Bismuth Carbon Materials Derived from MOFs
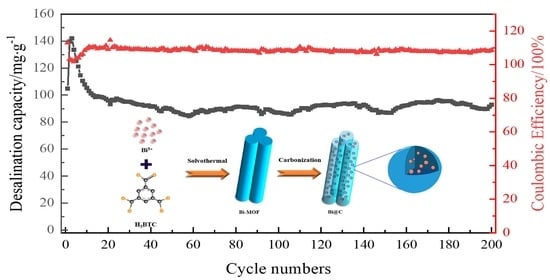
3.3. Electrochemical Performance Test
3.4. Analysis of Desalination Mechanism
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Barnett, T.P.; Adam, J.C.; Lettenmaier, D.P. Potential Impacts of a Warming Climate on Water Availability in Snow-Dominated Regions. Nature 2005, 438, 303–309. [Google Scholar] [CrossRef] [PubMed]
- Dolan, F.; Lamontagne, J.; Link, R.; Hejazi, M.; Reed, P.; Edmonds, J. Evaluating the Economic Impact of Water Scarcity in a Changing World. Nat. Commun. 2021, 12, 1915. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Wang, W.; Zhu, M.; Li, C. Recent Advances in Desalination Battery: An Initial Review. ACS Appl. Mater. Interfaces 2020, 12, 57671–57685. [Google Scholar] [CrossRef]
- Qasim, M.; Badrelzaman, M.; Darwish, N.N.; Darwish, N.A.; Hilal, N. Reverse Osmosis Desalination: A State-of-the-Art Review. Desalination 2019, 459, 59–104. [Google Scholar] [CrossRef]
- Al-Othman, A.; Tawalbeh, M.; El Haj Assad, M.; Alkayyali, T.; Eisa, A. Novel Multi-Stage Flash (MSF) Desalination Plant Driven by Parabolic Trough Collectors and a Solar Pond: A Simulation Study in UAE. Desalination 2018, 443, 237–244. [Google Scholar] [CrossRef]
- Service, R.F. Desalination Freshens Up. Science 2006, 313, 1088–1090. [Google Scholar] [CrossRef]
- Suss, M.E.; Presser, V. Water Desalination with Energy Storage Electrode Materials. Joule 2018, 2, 10–15. [Google Scholar] [CrossRef]
- Porada, S.; Zhao, R.; Van Der Wal, A.; Presser, V.; Biesheuvel, P.M. Review on the Science and Technology of Water Desalination by Capacitive Deionization. Prog. Mater. Sci. 2013, 58, 1388–1442. [Google Scholar] [CrossRef]
- Wang, G.; Yan, T.; Zhang, J.; Shi, L.; Zhang, D. Trace-Fe-Enhanced Capacitive Deionization of Saline Water by Boosting Electron Transfer of Electro-Adsorption Sites. Environ. Sci. Technol. 2020, 54, 8411–8419. [Google Scholar] [CrossRef]
- Wang, H.; Wei, D.; Gang, H.; He, Y.; Deng, H.; Hou, L.; Shi, Y.; Wang, S.; Yang, W.; Zhang, L. Hierarchical Porous Carbon from the Synergistic “Pore-on-Pore” Strategy for Efficient Capacitive Deionization. ACS Sustain. Chem. Eng. 2020, 8, 1129–1136. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, M.; Liu, G.; Jin, W.; Li, X. Fungal Cell Wall-Graphene Oxide Microcomposite Membrane for Organic Solvent Nanofiltration. Adv. Funct. Mater. 2021, 31, 2100110. [Google Scholar] [CrossRef]
- Doornbusch, G.J.; Dykstra, J.E.; Biesheuvel, P.M.; Suss, M.E. Fluidized Bed Electrodes with High Carbon Loading for Water Desalination by Capacitive Deionization. J. Mater. Chem. A 2016, 4, 3642–3647. [Google Scholar] [CrossRef]
- Suss, M.E.; Porada, S.; Sun, X.; Biesheuvel, P.M.; Yoon, J.; Presser, V. Water Desalination via Capacitive Deionization: What Is It and What Can We Expect from It? Energy Environ. Sci. 2015, 8, 2296–2319. [Google Scholar] [CrossRef]
- Liu, X.; Shanbhag, S.; Bartholomew, T.V.; Whitacre, J.F.; Mauter, M.S. Cost Comparison of Capacitive Deionization and Reverse Osmosis for Brackish Water Desalination. ACS EST Eng. 2021, 1, 261–273. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, K.; Xu, X.; Eid, K.; Abdullah, A.M.; Pan, L.; Yamauchi, Y. Recent Advances in Faradic Electrochemical Deionization: System Architectures versus Electrode Materials. ACS Nano 2021, 15, 13924–13942. [Google Scholar] [CrossRef]
- Chen, F.; Huang, Y.; Kong, D.; Ding, M.; Huang, S.; Yang, H.Y. NaTi2(PO4)3-Ag Electrodes Based Desalination Battery and Energy Recovery. FlatChem 2018, 8, 9–16. [Google Scholar] [CrossRef]
- Xiong, Y.; Yu, F.; Ma, J. Research Progress in Chlorine Ion Removal Electrodes for Desalination by Capacitive Deionization. ACTA Phys.-Chim. Sin. 2021, 38, 20–31. [Google Scholar] [CrossRef]
- Ahn, J.; Lee, J.; Kim, S.; Kim, C.; Lee, J.; Biesheuvel, P.M.; Yoon, J. High Performance Electrochemical Saline Water Desalination Using Silver and Silver-Chloride Electrodes. Desalination 2020, 476, 114216. [Google Scholar] [CrossRef]
- He, Y.; Huang, L.; Zhao, Y.; Yang, W.; Hao, T.; Wu, B.; Deng, H.; Wei, D.; Wang, H.; Luo, J. A Newly Synthesized Highly Stable Ag/N-Carbon Electrode for Enhanced Desalination by Capacitive Deionization. Environ. Sci. Nano 2020, 7, 3007–3019. [Google Scholar] [CrossRef]
- Liang, M.; Wang, L.; Presser, V.; Dai, X.; Yu, F.; Ma, J. Combining Battery-Type and Pseudocapacitive Charge Storage in Ag/Ti3C2Tx MXene Electrode for Capturing Chloride Ions with High Capacitance and Fast Ion Transport. Adv. Sci. 2020, 7, 2000621. [Google Scholar] [CrossRef]
- Yue, Z.; Ma, Y.; Zhang, J.; Li, H. Pseudo-Capacitive Behavior Induced Dual-Ion Hybrid Deionization System Based on Ag@rGO‖Na1.1V3O7.9@rGO. J. Mater. Chem. A 2019, 7, 16892–16901. [Google Scholar] [CrossRef]
- Xu, D.; Wang, W.; Zhu, M.; Li, C. Carbon Nanotubes Composite Embedded with Silver Nanoparticles as Chloride Storage Electrode for High-Capacity Desalination Batteries. Sep. Purif. Technol. 2022, 299, 121731. [Google Scholar] [CrossRef]
- Nam, D.-H.; Choi, K.-S. Bismuth as a New Chloride-Storage Electrode Enabling the Construction of a Practical High Capacity Desalination Battery. J. Am. Chem. Soc. 2017, 139, 11055–11063. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Huang, Y.; Guo, L.; Sun, L.; Wang, Y.; Yang, H.Y. Dual-ions electrochemical deionization: A desalination generator. Energ. Environ. Sci. 2017, 10, 2081–2089. [Google Scholar] [CrossRef]
- Chang, J.; Duan, F.; Su, C.; Li, Y.; Cao, H. Removal of Chloride Ions Using a Bismuth Electrode in Capacitive Deionization (CDI). Environ. Sci. Water Res. Technol. 2020, 6, 373–382. [Google Scholar] [CrossRef]
- Nam, D.-H.; Lumley, M.A.; Choi, K.-S. A Desalination Battery Combining Cu3[Fe(CN)6]2 as a Na-Storage Electrode and Bi as a Cl-Storage Electrode Enabling Membrane-Free Desalination. Chem. Mater. 2019, 31, 1460–1468. [Google Scholar] [CrossRef]
- Liu, Y.; Gao, X.; Wang, Z.; Wang, K.; Dou, X.; Zhu, H.; Yuan, X.; Pan, L. Controlled Synthesis of Bismuth Oxychloride-Carbon Nanofiber Hybrid Materials as Highly Efficient Electrodes for Rocking-Chair Capacitive Deionization. Chem. Eng. J. 2021, 403, 126326. [Google Scholar] [CrossRef]
- Nam, D.-H.; Lumley, M.A.; Choi, K.-S. A Seawater Battery with Desalination Capabilities Enabling a Dual-Purpose Aqueous Energy Storage System. Energy Storage Mater. 2021, 37, 556–566. [Google Scholar] [CrossRef]
- Wang, M.; Xu, X.T.; Liu, Y.; Li, Y.J.; Lu, T.; Pan, L.K. From metal-organic frameworks to porous carbons: A promising strategy to prepare high-performance electrode materials for capacitive deionization. Carbon 2016, 108, 433–439. [Google Scholar] [CrossRef]
- Kim, T.; Yoon, J. CDI ragone plot as a functional tool to evaluate desalination performance in capacitive deionization. RSC advances. RSC Adv. 2015, 5, 1456–1461. [Google Scholar] [CrossRef]
- Kim, S.; Lee, J.; Kim, C.; Yoon, J. Na2FeP2O7 as a novel material for hybrid capacitive deionization. Electrochim. Acta 2016, 203, 265–271. [Google Scholar] [CrossRef]
- Cao, J.; Wang, Y.; Wang, L.; Yu, F.; Ma, J. Na3V2(PO4)3@C as faradaic electrodes in capacitive deionization for high-performance desalination. Nano Lett. 2019, 19, 823–828. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Ding, Z.; Li, J.; Li, J.; Lu, T.; Pan, L. Highly efficient and stable desalination via novel hybrid capacitive deionization with redox-active polyimide cathode. Desalination 2019, 469, 114098. [Google Scholar] [CrossRef]
- Guo, Z.; Ma, Y.; Dong, X.; Hou, M.; Wang, Y.; Xia, Y. Integrating Desalination and Energy Storage using a Saltwater-based Hybrid Sodium-ion Supercapacitor. ChemSusChem 2018, 11, 1741–1745. [Google Scholar] [CrossRef]
- Lee, J.; Jo, K.; Lee, J.; Hong, S.P.; Kim, S.; Yoon, J. Rocking-chair capacitive deionization for continuous brackish water desalination. ACS. Sustain. Chem. Eng. 2018, 6, 10815–10822. [Google Scholar] [CrossRef]
- Huang, Y.; Chen, F.; Guo, L.; Zhang, J.; Chen, T.; Yang, H.Y. Low energy consumption dual-ion electrochemical deionization system using NaTi2(PO4)3-AgNPs electrodes. Desalination 2018, 451, 241–247. [Google Scholar] [CrossRef]
- Zhao, W.; Guo, L.; Ding, M.; Huang, Y.; Yang, H.Y. Ultrahigh-desalination-capacity dual-ion electrochemical deionization device based on Na3V2(PO4)3@C–AgCl electrodes. ACS Appl. Mater. Inter. 2018, 10, 40540–40548. [Google Scholar] [CrossRef]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dong, X.; Wang, Y.; Zou, Q.; Li, C. Bismuth Nanoparticles Encapsulated in a Porous Carbon Skeleton as Stable Chloride-Storage Electrodes for Seawater Desalination. Batteries 2024, 10, 35. https://doi.org/10.3390/batteries10010035
Dong X, Wang Y, Zou Q, Li C. Bismuth Nanoparticles Encapsulated in a Porous Carbon Skeleton as Stable Chloride-Storage Electrodes for Seawater Desalination. Batteries. 2024; 10(1):35. https://doi.org/10.3390/batteries10010035
Chicago/Turabian StyleDong, Xiaoqing, Ying Wang, Qian Zou, and Chaolin Li. 2024. "Bismuth Nanoparticles Encapsulated in a Porous Carbon Skeleton as Stable Chloride-Storage Electrodes for Seawater Desalination" Batteries 10, no. 1: 35. https://doi.org/10.3390/batteries10010035