Abstract
The ongoing transition toward electric vehicles is a major factor in the exponential rise in demand for lithium-ion batteries (LIBs). There is a significant effort to recycle battery materials to support the mining industry in ensuring enough raw materials and avoiding supply disruptions, so that there will be enough raw materials to produce LIBs. Nevertheless, LIBs that have reached the end of their useful lives and are sent for recycling may still have some energy left in them, which could be dangerous during handling and processing. Therefore, it is important to conduct discharge pretreatment of LIBs before dismantling and crushing them, especially in cases where pyrometallurgical recycling is not used. Electrochemical discharge in conducting solutions has been commonly studied and implemented for this purpose, but its effectiveness has yet to be fully validated. Non-electrochemical discharge has also been researched as a potentially cleaner and more efficient discharge technology at the same time. This article presents a non-electrochemical discharge process by completely draining the energy from used batteries before recycling. A comprehensive investigation of the behavior of LIBs during discharge and the amount of energy remaining after fully discharging the battery at different temperatures is analyzed in this work. According to the experimental findings, completely discharging the battery at higher temperatures results in a reduced amount of residual energy in the battery. This outcome holds great importance in terms of safe and environmentally friendly recycling of used LIBs, emphasizing that safety and environmentally friendly recycling must go hand in hand with a cost-effective and sustainable solution.
1. Introduction
In recent years, as the low-carbon economy grows, the new energy industry, including lithium-ion batteries (LIB), has expanded rapidly due to the increasing number of electric vehicles (EV) sold worldwide [1]. In 2019, 2.2 million EVs were sold, and in 2022, more than 10 million EVs were sold, and it is projected to exceed 15 million by 2025, leading to a surge in LIB production [2]. On average, LIBs have a lifespan of 4 to 10 years. Currently, there is a surge of decommissioning of used LIB. In 2030, it is anticipated that more than 11 million tons of LIB will be decommissioned [3]. Spent LIBs contain a significant amount of valuable metals, including cobalt, lithium, nickel, and manganese [4], along with harmful elements like phosphorus, sulfur, and fluorine [5]. If not properly disposed of, these batteries can lead to a loss of valuable resources and heavy-metal pollution of land and water resources [6]. Hence, it is imperative to recycle spent LIBs to protect the environment and conserve valuable resources.
Once removed from the electric vehicle, the LIB pack typically retains 70–80% of its initial capacity. Some of these batteries will be sent for recycling due to various damages and conditions of the battery, posing a significant safety hazard for its recycling [7]. Other used batteries, in good condition, may be sent for second-life applications, such as renewable energy storage [8]. These batteries will eventually retire from their second-life and be sent for recycling, where similar hazards can occur during recycling if their energy is not completely drained. During the charging process of an LIB, energy is stored as lithium ions migrating from the cathode to the anode. Conversely, when the battery is discharged, the ions travel from the anode back to the cathode, thereby releasing energy. If the battery is not completely discharged prior to being recycled, there is a considerable amount of stored energy in the battery that can be hazardous. The recycling of LIBs is typically a multi-step process that involves mechanical, pyrometallurgical [9], and hydrometallurgical or similar treatments [10]. The first step is to mechanically process the batteries to recover valuable metals like copper, aluminum, and steel. In this process, the battery is crushed and shredded to reduce it to small particles, which are then sorted by size and composition. During the shredding process, if the battery is not fully discharged before this process begins, there is a risk of short circuits or other events that can cause the stored energy to be released rapidly, resulting in an explosion or fire [11]. After the mechanical processing, the remaining materials undergo a pyrometallurgical or similar treatment [9], where the materials are exposed to high temperatures to extract valuable metals like cobalt, nickel, and lithium. This process typically involves roasting the materials to remove impurities and then smelting them to extract the metals. The final step in the recycling process is hydrometallurgical or similar processing, where the remaining materials are treated with acids to dissolve and extract metals like cobalt, nickel, and lithium. This process usually involves leaching the materials with an acidic or basic solution to extract the metals. By recycling LIBs, valuable metals can be recovered and environmental hazards can be minimized.
To avoid safety hazards associated with battery disassembly, the state of charge (SoC) should ideally be [12] (corresponding to a voltage below 2 V). Many methods have been suggested, such as deactivating the battery in a liquid nitrogen cooling environment and then disassembling and crushing it [13]. This process requires substantial time and money. The battery can be deactivated using a needling test in a low temperature, inert environment [14]. The standard method of battery discharge involves connecting individual batteries to resistors [15], which is practical for large battery packs used in electric vehicles, but not for smaller cells used in electronic devices due to their varying sizes and shapes. Moreover, the current in the electric circuit must be closely monitored to mitigate the fire risk. The researchers proposed two other discharge pretreatment techniques for used LIBs. One is electrochemical discharge within a conducting solution or a salt-solution immersion discharge [16,17]. The other technique is a conductive-powder buried discharge or a non-electrochemical discharge in a conducting powder [18]. Surprisingly, electrochemical discharge consumes energy via the electrolysis of water in the battery’s anode and cathode poles. Still, it poses challenges in wastewater treatment after the electrochemical discharge [16]. On the other hand, a cleaner form of discharging is non-electrochemical discharge in conductive powder, which produces no harmful substances. But, this discharge method can cause physical damage to the battery, making it difficult to separate and recover valuable materials. Additionally, the use of conductive powder can create additional waste and environmental concerns.
This paper explores one clean discharge or non-electrochemical discharge method to discharge spent Lithium-ion batteries before recycling. This study aims to investigate the effectiveness of battery discharge at different temperatures in removing the remaining energy from the battery. Discharging the battery to a low energy level reduces the risk of thermal runaway during the recycling process, making it safer for workers and reducing the risk of damage to equipment and facilities. A series of experiments were performed under three distinct temperatures (25 °C, 35 °C, and 45 °C) to investigate the remaining energy in the spent LIBs at different temperatures and to find a possible optimum temperature for discharging that would guarantee safe handling of the battery prior to mechanical processing. After discharging it at three different temperatures, we found the optimal temperature to be 35 °C. At this temperature, the amount of remaining energy in a fully discharged LIB is significantly reduced, which reduces the risk of thermal runaway and fire or explosion during the recycling process. The intended contribution of this study is to advance the systematic and scientific understanding in the realm of non-electrochemical discharge before the recycling of LIBs. By offering a detailed exploration of discharge behavior at different temperatures and proposing a cleaner and safer method, this work aims to provide valuable insights and methodologies for improving the sustainability of LIB recycling processes.
Our study primarily focuses on 18,650 cylindrical cells as a representative case within the broader spectrum of LIBs. While our findings provide valuable insights into the discharge behavior of 18,650 cells, we acknowledge that different cell types, such as pouch-type cells and large cylindrical cells, may exhibit unique characteristics due to their diverse designs and chemistries. Although our work directly pertains to 18,650 cells, the principles of non-electrochemical discharge and the importance of optimizing discharge conditions can be extrapolated to other cell formats.
2. Experiments
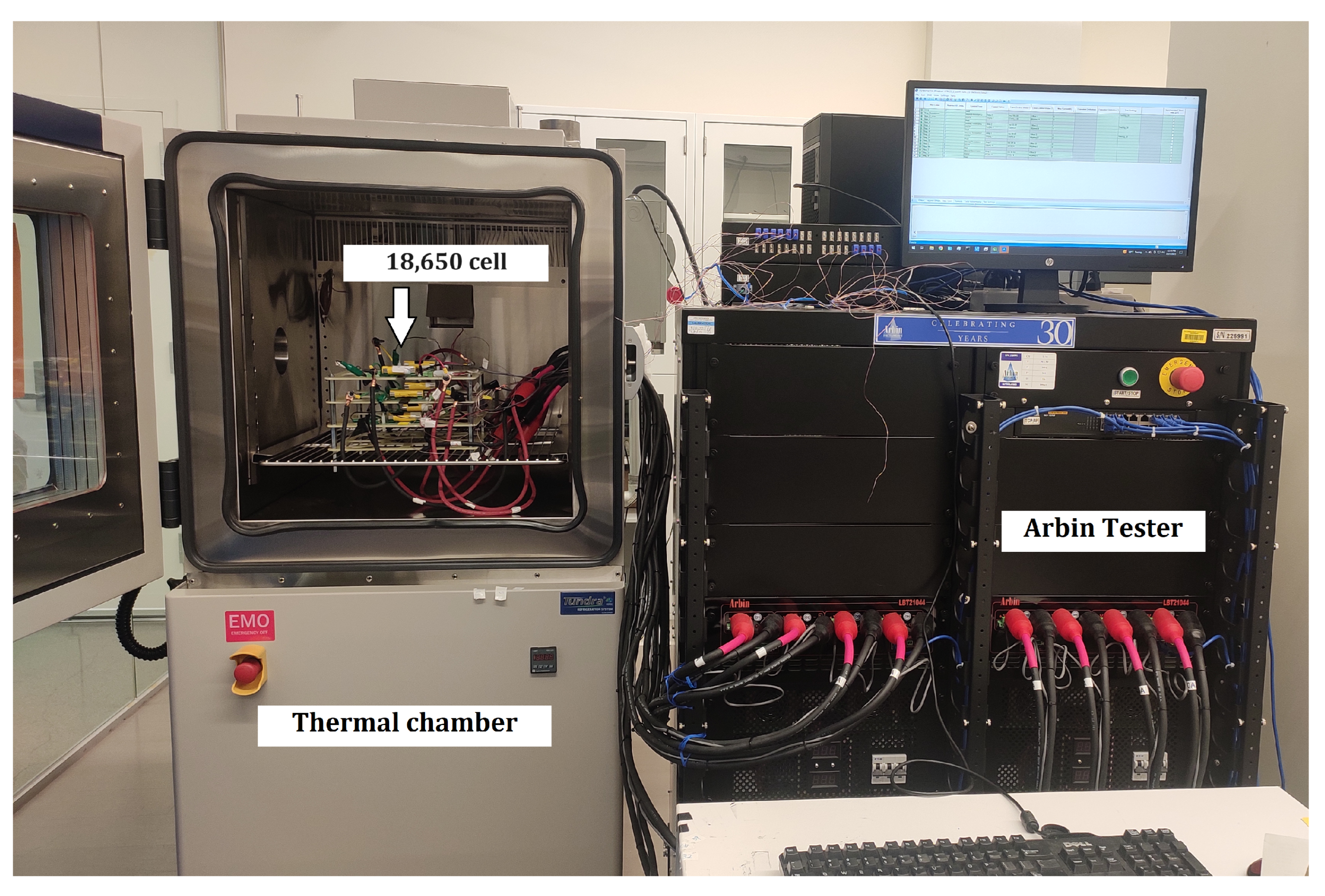
To ensure the safe handling of batteries before engaging in mechanical recycling, it is crucial to have knowledge of the remaining energy contained within them. To investigate the remaining energy of the battery as it undergoes the discharge process, this study employed three distinct temperatures (25 °C, 35 °C, and 45 °C). This work uses six batteries (Cell No:1-6), which are APR18650M1A (Nominal capacity-) cylindrical LIB cells. All the batteries have a nominal voltage of 3.3 V. The maximum voltage is 3.6 V. The lower cut-off voltage is 2 V. Cell 1, Cell 2, and Cell 3 are discharged at a 1 °C rate. Cell 4, Cell 5, and Cell 6 are discharged at a 2 °C rate. Figure 1 shows the experimental setup.

Figure 1.
Experimental setup.
2.1. Discharge Cycle
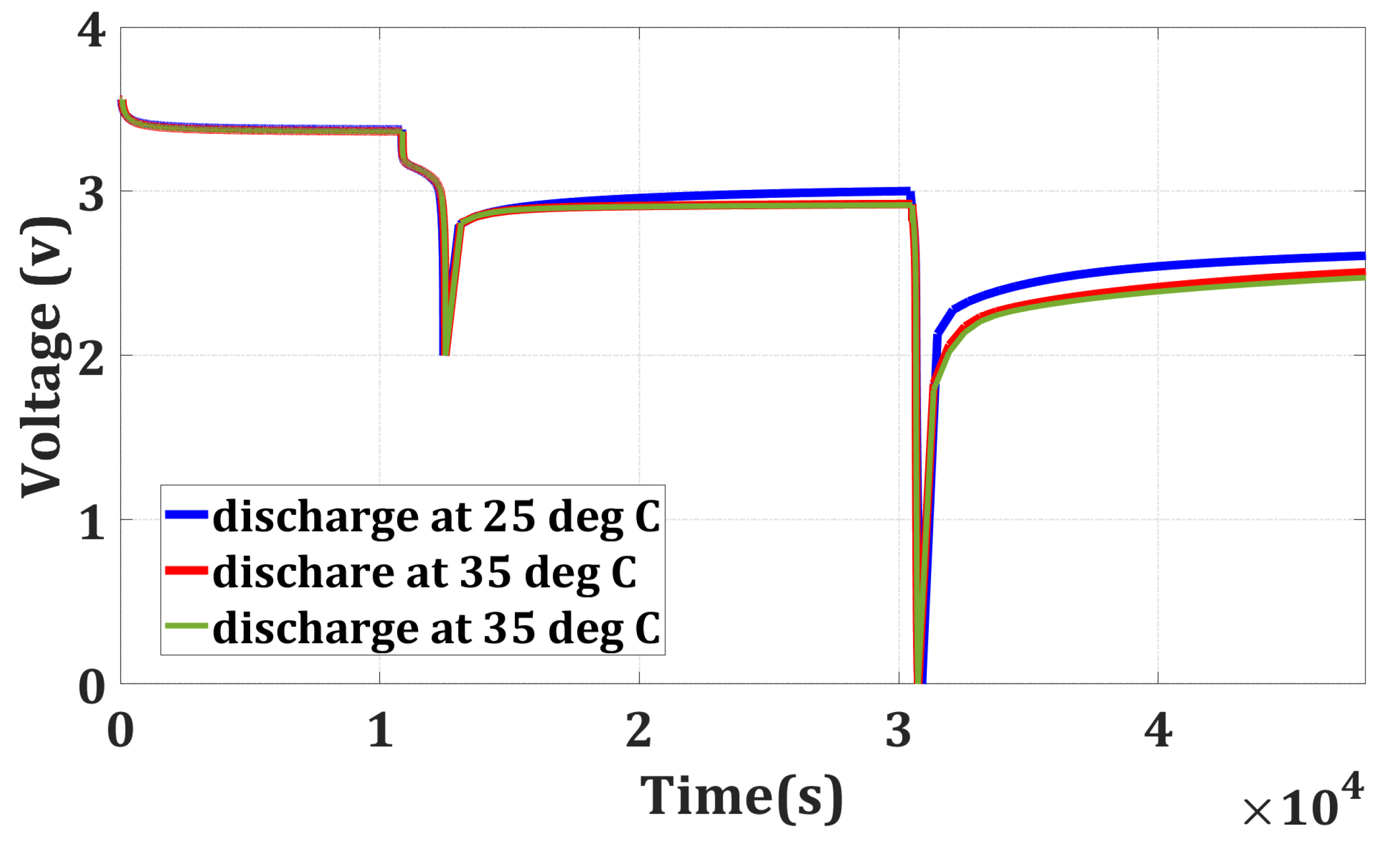
The batteries are fully charged at 25 °C. After keeping all the batteries at rest for 3 h, they are discharged to their cut-off voltage (2 V) at three distinct temperatures (25 °C, 35 °C, and 45 °C). The energy outcome from the batteries for all three temperatures is recorded, which can be seen from Table 1. Then, the batteries are kept at rest for 5 h. During the rest period, the voltage of the batteries underwent a gradual recovery, surpassing its lower cut-off voltage of 2 V. Then, the batteries are further discharged from their recovered voltage to 0 V at 25 °C for evaluating the remaining energy in the battery. In this discharging process, again, the energy outcome is recorded for all four batteries, which is shown in Table 1. Figure 2 shows the voltage of the battery cells during the discharging process at different temperatures, and Table 1 shows that the temperature has a stronger influence on the remaining energy and the recovery voltage. For discharging at higher temperatures, the remaining energy in the battery and the recovery voltage will be less than discharging at a lower temperature. When a battery is discharged at a higher temperature compared to lower temperatures, the energy outcome is more, and the remaining energy is typically reduced. This is because higher temperatures can accelerate internal chemical reactions, leading to greater energy outcomes. Consequently, when the battery is allowed to rest or recover after discharging, the recovery voltage is also lower at higher temperatures. This lowered recovery voltage reflects the battery’s decreased ability to regain its voltage level after discharge. From Table 1, it can be shown that for discharge at 25 °C, the remaining energy in the four batteries is 0.1051 Wh, 0.0993 Wh, 0.087 Wh, and 0.096 Wh, respectively. Whereas, for discharging at 35 °C, the remaining energy are 0.0588 Wh, 0.0566 Wh, 0.0468 Wh, and 0.051 Wh, respectively. The remaining energy in the battery at 35 °C is almost half of the remaining energy at 25 °C. The remaining energy is reduced further with the increase in the temperature (45 °C); however, it is not significant.

Table 1.
Energy remaining for different temperatures.
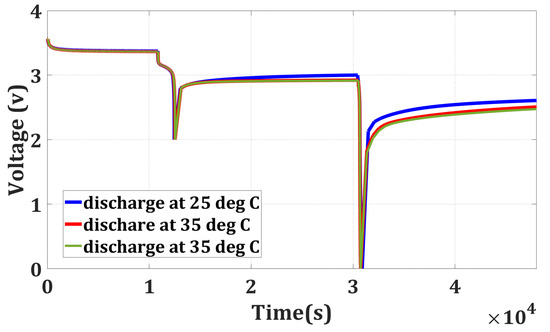
Figure 2.
Testing Schedule for Full Discharge.
2.2. Sequential Discharge Cycle
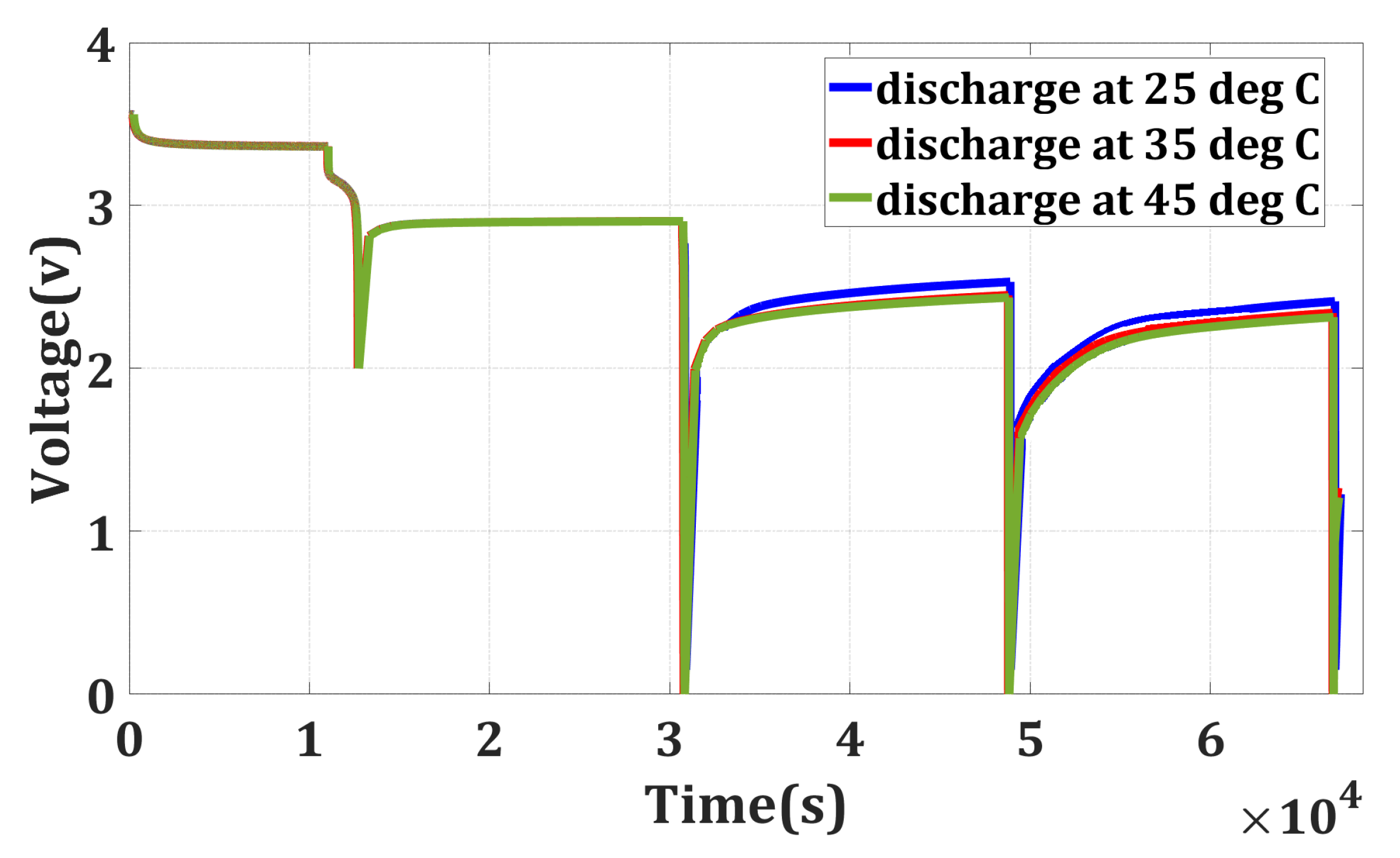
In the previous section, it was noted that when a battery is discharged until it reaches 0 V, it may appear that all the energy has been depleted. However, even at this point, some residual energy remains within the battery. It shows that the battery can regain some of its voltage even after being discharged to 0 V. It is important to highlight that based on these findings, measuring the voltage immediately after discharge could provide a misleading indication that the battery is safe to handle mechanically with minimal risk. To gain a deeper understanding, a sequential discharge was conducted by reintroducing the batteries to room temperature (25 °C) after their voltage had recovered. The discharge process is continued for three cycles. The outcomes of this sequential discharge are illustrated in Figure 3 and in Table 1 and Table 2.
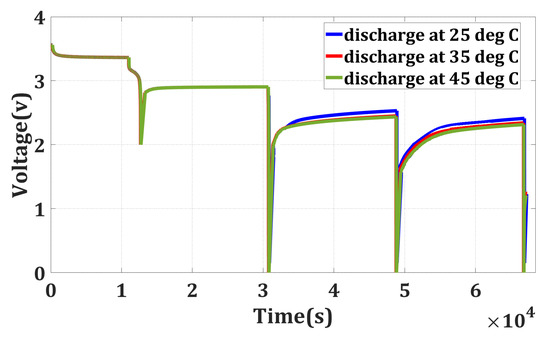
Figure 3.
Sequential discharge.

Table 2.
Energy remaining for different temperatures at higher °C rate.
Before every discharge cycle, a 5 h rest period is given. Monitoring the energy output during each discharge cycle makes it possible to determine the extent of the residual energy in the battery and analyze its behavior. This experiment helps us to understand the discharge characteristics of the battery more comprehensively and provides insights into its energy storage and energy release capabilities.
2.3. Discharge at Higher °C Rate
The experiment is carried out for two different °C-rates. For the first experiment, the batteries are discharged at a 1 °C rate, and the results are given in Table 1. For the second experiment, the batteries are discharged at 2 °C rate, and the results are given in Table 2. After discharging the batteries at two different °C-rates, it can be seen that when a battery is discharged at a higher discharge rate and a higher temperature before recycling, the energy outcome for the complete discharging cycle tends to be higher compared to discharging at a lower rate or lower temperature. This is because higher discharge rates and temperatures facilitate faster chemical reactions within the battery, allowing for a more efficient extraction of stored energy. By discharging the battery more extensively, the amount of energy remaining in the battery after the process will be significantly reduced. This is beneficial for initiating the recycling process of the battery. Maximizing the discharge and reducing the remaining energy reduces the potential risks associated with handling and processing the battery, such as thermal runaway or releasing hazardous substances. Additionally, the recycling process becomes more efficient as there is less energy to be managed or discharged later on.
3. Mechanism of the Discharge at High Temperature
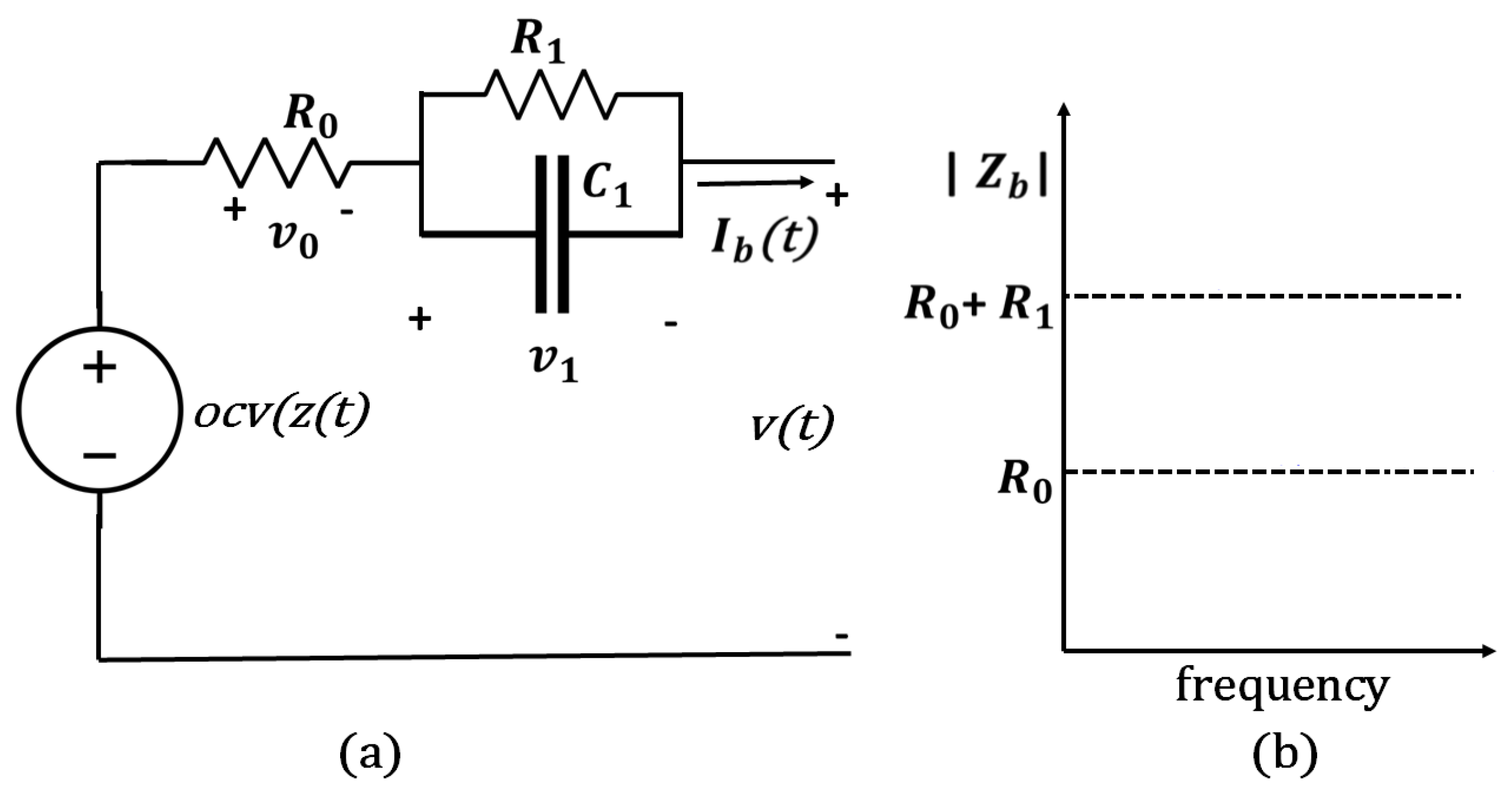
When a lithium-ion battery is discharged at a higher temperature before recycling, the energy remaining in the battery is less than discharging at a lower temperature, while the energy outcome is higher. This can be described using an equivalent circuit model (ECM), as in Figure 4, where the open-circuit voltage (OCV) of the battery is a function of its SoC (). The battery’s current, , is positive during discharge and negative during charge. The OCV is greater than the terminal voltage () during discharge and less than v during recharge of the cell. In this model, is included to signify the battery’s internal resistance, which is responsible for energy inefficiency in the cell since dissipates power. is the resistance of the electrode and electrolyte materials. The resistance–capacitance (RC) networks represent the diffusion process in the battery’s electrolyte. With a higher number of RC elements, it increases the accuracy of the ECM but also increases the circuit’s complexity. and are the equivalent polarization resistance and capacitance, respectively. The terminal voltage can be expressed as follows:
where is the voltage drop across the internal resistance. can be represented by the following equation:
where can be defined as,
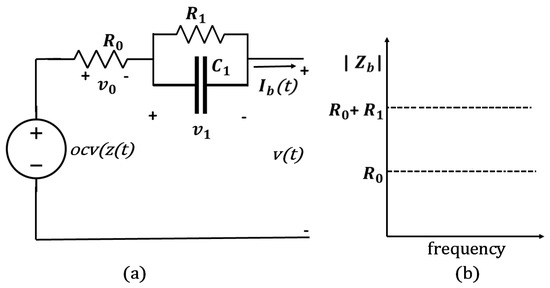
Figure 4.
(a) Illustration of the LIB’s discharge through equivalent circuit; (b) equivalent internal resistance.
is the initial resistance, is the temperature coefficient of the material, and are the changes in the temperature. By substituting the into (2), we obtain:
At higher temperatures, the resistance of its electrode and electrolyte materials decreases, which means the internal voltage drop across the battery is reduced as in (4). This results in a higher output power for the battery. Since the voltage drop across the internal resistance is reduced at higher temperatures, the overall voltage (v) output as in (1) increases. Now, let us consider the energy delivered by the battery during discharge is E. It can be calculated by integrating the product of the voltage (v) and current () over time (t):
In (5), for higher output voltage v, the energy output (E) of the battery is higher at higher temperatures. Additionally, the polarization capacitance of the battery also decreases, leading to a faster discharge rate. However, the diffusion coefficient of lithium ions in the electrolyte decreases with the increasing temperature, which reduces the amount of stored energy in the battery. At high temperatures, the lithium ions are more mobile and can move faster, leading to a higher discharge current and a lower energy capacity. In this paper, the temperature coefficient is considered as 0.005 ohms per degree Celsius for the change in internal impedance. At 25 °C, the internal impedance for cells 1–3 are measured as 0.083 ohms, 0.1396 ohms, and 0.161 ohms, respectively, and at 35 °C measured as 0.071 ohms, 0.1205 ohms, and 0.118 ohms, respectively. Based on these impedance values, the energy outcome is calculated using (5). Table 3 presents the difference in energy outcome between 25 °C and 35 °C for three cells for both the calculated and the measured value. The error between the calculated and the experimentally measured value are , , and , respectively.

Table 3.
Difference in energy outcome between 25 °C and 35 °C.
In summary, the changes in the circuit parameters at high temperatures cause the energy outcome to be higher but the remaining energy in the battery to be less.
4. Scaling up the Study for a Larger Battery Pack
When recycling a battery pack, it is important to discharge it as much as possible to minimize the remaining energy. This is because the remaining energy can be hazardous and pose a safety risk during recycling. Discharging the battery pack at a slightly higher temperature than room temperature can be beneficial for battery recycling because it ensures that the battery pack is fully discharged and less energy remains in the battery pack. On the other hand, if the battery pack is discharged at a lower temperature, the internal resistance of the battery increases, causing the battery voltage to drop more slowly. This slower discharge rate means that there will be more energy remaining in the battery pack, which can be very dangerous during the recycling process. In this section, a thorough study is carried out on different battery packs of different electric vehicles (EVs), which are shown in Table 4. Based on the table, it is evident that different battery packs retain significant energy even after a full discharge. For instance, in the case of Tesla, Lucid Motors, Jaguar EV, etc., discharging the battery at room temperature (25 °C) results in significant remaining energy levels of 2.16 kWh, 3.26 kWh, and2.60 kWh, respectively. This poses a risk during the battery recycling process. However, discharging the battery at a slightly higher temperature (35 °C) reduces the remaining energy to 0.99 kWh, 1.49 kWh, and 1.18 kWh, respectively, which is approximately half the energy level at room temperature.

Table 4.
Energy remaining for different temperatures.
5. Proposed Heating Method
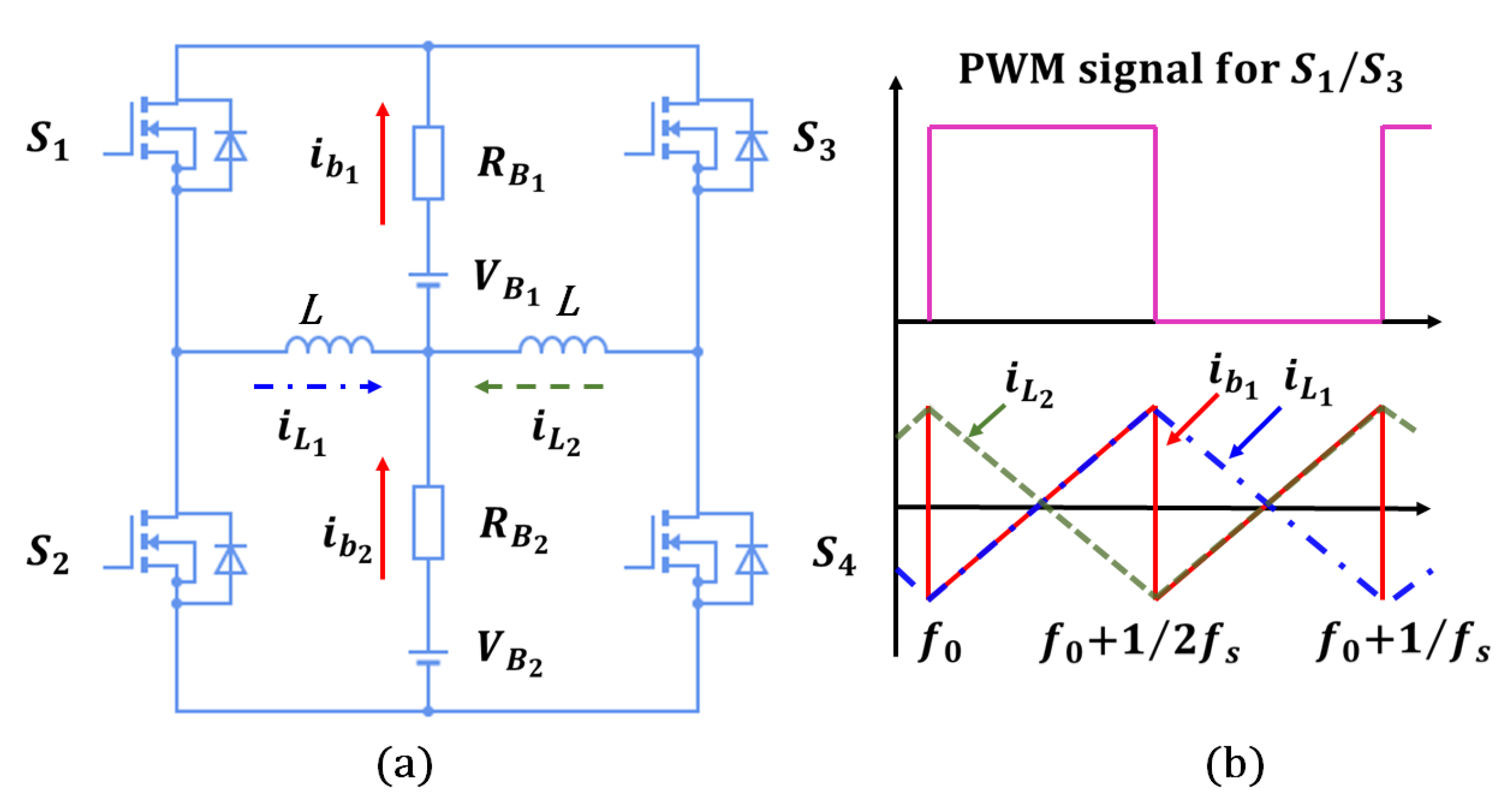
The reason for discharging a battery at a higher temperature before recycling is to increase the energy output while reducing the amount of energy left in the battery. Heating the battery before discharging enhances the electrochemical reaction rate, leading to higher energy extraction. A self-heating method using the LIB’s internal resistance to produce the Joules heat for preheating the battery is proposed in the previous paper [19], rather than using external power supplies for heating up the batteries. According to the electrical model in Figure 4a, the equivalent impedance can be defined as,
As shown in Figure 4b, during high-frequency excitations, the battery impedance is nearly equal to in accordance with (6). Due to the self-heating current typically operating at a high frequency of 10 kHz or more, it is precise enough to allow to determine the generation of Joules heat ;. The proposed self-heater, illustrated in Figure 5a, utilizes an inductor ladder structure. The inductors are connected to the center of the series cell string, and the power switches are connected to the battery pack terminals. In the interleaved design, energy can be transferred between battery strings. Thus, the self-heater may generate an ac heating current without external power. As illustrated in Figure 5b, power switches and have complementary pulse-width modulation (PWM) signals and duty ratios without dead time. Assuming equal voltage and resistance of battery cells, , and , the battery heating currents and and the inductor currents and are symmetrical. For instance, when and are turned ON, increases. For a switching frequency , and for the time period , is
where represents the overall resistance of the power switches, inductors’ parasitic resistance, etc. The symmetrical topology makes equal to , and 180 lagged to when and are ON. The heating current frequency is twice because and are complementary. The RMS heating current will be
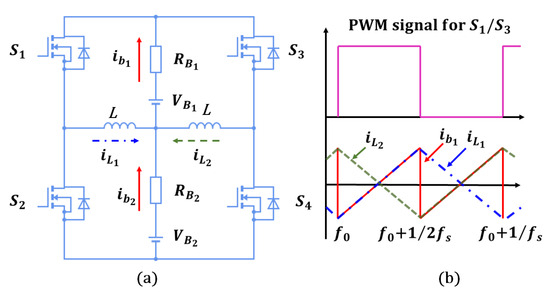
Figure 5.
Self-Heating Circuit [19]: (a) Circuit topology; (b) Waveform.
According to (8), it can be concluded that by reducing the switching frequency, the temperature of the battery can be increased and vice versa. During the self-heating process, the surface temperature needs to be monitored, and the cell voltage and current data need to be recorded. A core temperature estimation method utilizes an Extended State Observer (ESO) to estimate the core temperature based on the recorded voltage, current, and surface temperature data [19].
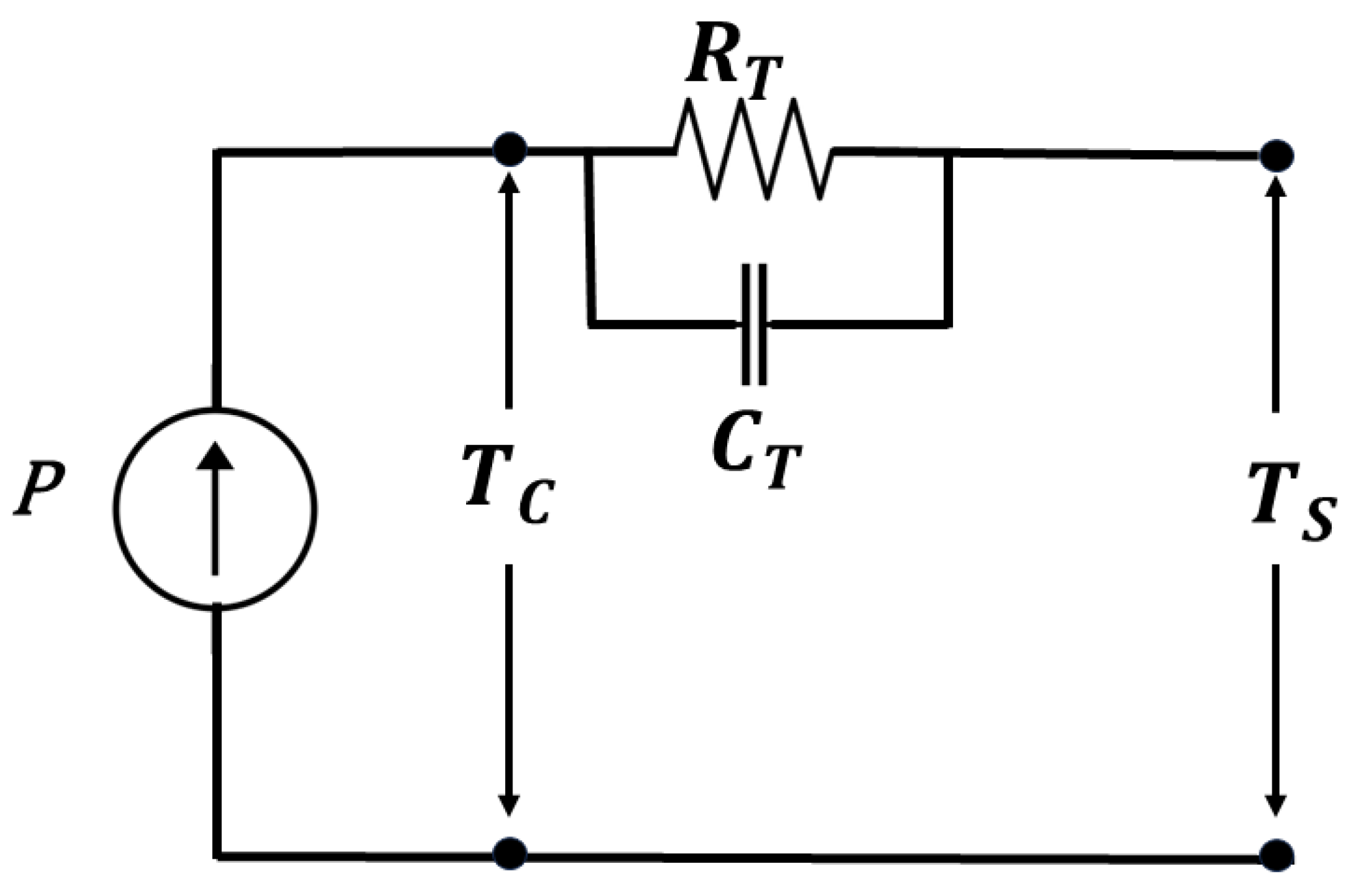
This self-heating process can be presented by a thermal model, as shown in Figure 6. In this model, the battery is represented as a thermal resistance () connected in parallel with a thermal capacitance (). represents the heat transfer resistance within the battery, including factors such as thermal conductivity and contact resistance. represents the thermal inertia or ability of the battery to store heat energy. is the core temperature, and is the surface temperature or the ambient temperature. Initially, both the surface and core temperatures are at ambient temperature. During the self-heating process, it is required to monitor the estimated core temperature provided by the core temperature estimation algorithm. The heat transfer equation for this thermal circuit model can be derived by considering the flow of heat through the resistances and capacitances. Assuming steady-state conditions, the heat transfer equation can be written as
where Q is the heat generated within the battery. Then, from this thermal model, we can know how much heating power is required to reach the core temperature at 35 °C from the ambient temperature. By controlling the power and adjusting the RMS heating current according to that, we can also change the switching frequency. In this way, we can control the temperature of the battery. When the core temperature of the battery reaches the desired 35 °C, proceed with the discharging of the battery while monitoring the core temperature. During this discharging process, the core temperature at 35 °C can be maintained by continuously monitoring the core temperature and adjusting the frequency accordingly. Once the battery has been discharged completely, stop the discharge process and disconnect the heating circuit.

Figure 6.
Thermal Model.
Self-heating and simultaneous discharging of the battery at a higher temperature before starting the recycling process have several advantages. Heating the battery increases the reaction rates within the cells, allowing for a more efficient energy extraction during the discharging process. Using the battery’s own self-heating circuit eliminates the need for external and expensive equipment dedicated solely to heating the battery. This simplifies the overall process and reduces the associated costs. Traditional methods of heating batteries often involve the use of specialized heating chambers or equipment, which can be costly to purchase, operate, and maintain. By leveraging the self-heating capability of the battery, one can eliminate these expenses and streamline the process. The self-heating circuit within the battery provides a controlled and stable temperature environment during the discharging process. This allows for precise temperature regulation and ensures that the battery operates within safe temperature limits. This helps us to optimize the discharging process.
6. Investigation on the Effectiveness of the Discharge Method
Investigating the effectiveness of the discharging method before the recycling process is essential to ensure efficient and sustainable battery recycling. By exploring the effectiveness, we can optimize battery recycling practices and promote a sustainable and resource-efficient approach to managing battery waste. The section aims to evaluate the discharge efficiency and environmental impact of the proposed clean discharge at controlled temperature conditions. By examining these factors, we can gain insights into the advantages of the proposed clean discharge method as a potential pre-recycling approach.
6.1. Discharging Efficiency
Discharging is a crucial step in the recycling of spent LIBs due to the significant safety risks posed by the remaining energy within them. However, there is currently a lack of specific studies that directly examine the remaining energy in the LIBs before the recycling process. Reference [18] calculated the residual electric quantity () after complete discharge, and, based on that, the discharge efficiency of different discharge media is evaluated. In [20], the researchers considered the recovery voltage phenomenon after discharging to calculate the efficiency of different discharge methods. In this paper, we considered the remaining energy for calculating the efficiency of the discharging methods as shown in (10). Calculating the discharging efficiency based on remaining energy provides a more comprehensive measure of the battery’s performance. It considers the overall energy extracted from the battery, considering both the capacity and the voltage. Recovery voltage refers to the temporary increase in voltage that occurs after completing the discharging process. This voltage rebound does not necessarily reflect the actual energy consumed by the discharging methods and the energy remaining in the battery. Also, different battery chemistries can exhibit varying rebound voltage characteristics, making it difficult to compare discharging efficiencies based solely on rebound voltage. Calculating efficiency based on remaining energy provides a consistent metric for different battery types, allowing for easier comparison and analysis. By calculating the discharge efficiency based on remaining energy, we can account for the actual energy delivered, providing a more accurate representation of the battery’s present capacity. After the complete discharge, the remaining energy is used to further compare the discharge effects of different discharge temperatures. From Table 1, it can be shown that the discharge efficiency for Cell 1, at 25 °C, 35 °C, and 45 °C are , , and , respectively.
As shown in Table 1, the remaining energy after discharging at 45 °C is the least, followed by that of the discharge at 35 °C and 25 °C. However, only a increase is observed from 35 °C to 45 °C discharge. Hence, 35 °C is proposed for the optimal temperature for pre-treating the batteries before recycling.
6.2. Assessment of Environmental Protection and Effectiveness of Discharging Technique
In discharge pretreatment, it is crucial to consider not only discharge efficiency but also environmental protection. Using electrochemical discharge and conductive powder discharge methods before the recycling process has several disadvantages compared to clean discharge at a higher temperature than room temperature. Firstly, these methods are often more complex and require specialized equipment, adding to the overall complexity and cost of the process. They may involve using additional chemicals or powders, requiring careful handling and disposal procedures. Secondly, safety considerations arise due to the involvement of hazardous materials such as corrosive electrolytes or conductive powders, necessitating proper safety measures and precautions. Additionally, these methods may introduce the risk of contamination as the additional substances can interact with battery materials, potentially leading to impurities that can affect the recycling process or downstream applications. Moreover, the effectiveness and compatibility of these methods can vary across different battery chemistries, requiring specific adaptations for optimal performance. In contrast, the proposed clean discharge at a specific temperature offers better control and stability during the process, producing no or minimum environmental hazards. The proposed self-heating and discharge combined approach will eliminate the use of expensive equipment needed for the pretreatment of spent LIBs.
7. Conclusions
To reduce the environmental impact of LIBs, it is crucial to implement an efficient recycling process that recovers their raw materials. This process should prioritize speed, cost effectiveness, and safety to minimize the potential risks of fire and explosion in recycling facilities. While the literature mentions various pre-discharge techniques like electrochemical discharge and conductive powder discharge for LIBs, none of these methods have taken into account the evaluation of remaining energy in the battery after discharging to assess the effectiveness of different discharging approaches. However, the remaining energy in a battery is a crucial factor to consider before recycling for several reasons. First and foremost, batteries contain stored electrical energy, which can pose significant safety risks during recycling operations. If a battery with a high energy level is mishandled or subjected to mechanical stress, it can lead to thermal runaway, fire, or even explosions, endangering the safety of personnel and the recycling facility. Unlike other studies, this research focused on the energy remaining in the battery during the discharge of the battery. This study focuses on pre-discharging the battery at various temperatures to analyze the remaining energy in spent LIBs at different temperature conditions. The aim is to identify an optimal discharge temperature that ensures safe handling of the battery before mechanical processing. Our findings reveal that higher temperature discharges result in significantly lower remaining energy, indicating improved safety. However, beyond a certain temperature, there is minimal variation in the remaining energy. Our investigation identifies 35 °C as the optimal temperature for the discharging process. Furthermore, this paper proposes a combined self-heating and discharge approach to discharge the battery packs so as to eliminate the need for expensive equipment for the pretreatment of used EV battery packs.
Author Contributions
Conceptualization, C.C.M.; methodology, C.C.M. and Y.F.; Validation, Y.F.; Formal analysis, A.M.; Investigation, A.M. and W.G.; Resources, Y.F. and W.G.; writing—original draft preparation, A.M., Y.F. and W.G.; writing—review and editing, C.C.M. and W.G.; supervision, C.C.M.; funding acquisition, C.C.M. All authors have read and agreed to the published version of the manuscript.
Funding
California Energy Commission Grant number EPC-19-053.
Data Availability Statement
The data are contained in the article and are available from the corresponding authors on reasonable request.
Acknowledgments
The Authors would like to acknowledge the support of the California Energy Commission under grant number EPC-19-053.
Conflicts of Interest
: The authors declare no conflicts of interest.
References
- Kim, S.; Bang, J.; Yoo, J.; Shin, Y.; Bae, J.; Jeong, J.; Kim, K.; Dong, P.; Kwon, K. A comprehensive review on the pretreatment process in lithium-ion battery recycling. J. Clean. Prod. 2021, 294, 126329. [Google Scholar] [CrossRef]
- Münzel, C.; Plötz, P.; Sprei, F.; Gnann, T. How large is the effect of financial incentives on electric vehicle sales?—A global review and european analysis. Energy Econ. 2019, 84, 104493. [Google Scholar] [CrossRef]
- Garole, D.J.; Hossain, R.; Garole, V.J.; Sahajwalla, V.; Nerkar, J.; Dubal, D.P. Recycle, recover and repurpose strategy of spent li-ion batteries and catalysts: Current status and future opportunities. ChemSusChem 2020, 13, 3079–3100. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; He, Y.; Ge, Z.; Li, H.; Xie, W.; Wang, S. A promising physical method for recovery of licoo2 and graphite from spent lithium-ion batteries: Grinding flotation. Sep. Purif. Technol. 2018, 190, 45–52. [Google Scholar] [CrossRef]
- Guo, Y.; Li, F.; Zhu, H.; Li, G.; Huang, J.; He, W. Leaching lithium from the anode electrode materials of spent lithium-ion batteries by hydrochloric acid (hcl). Waste Manag. 2016, 51, 227–233. [Google Scholar] [CrossRef] [PubMed]
- Dalini, E.A.; Karimi, G.; Zandevakili, S. Treatment of valuable metals from leaching solution of spent lithium-ion batteries. Miner. Eng. 2021, 173, 107226. [Google Scholar] [CrossRef]
- Muhammad, M.; Ahmeid, M.; Attidekou, P.; Milojevic, Z.; Lambert, S.; Das, P. Assessment of spent ev batteries for second-life application. In Proceedings of the 2019 IEEE 4th International Future Energy Electronics Conference (IFEEC), Singapore, 25–28 November 2019; IEEE: Piscataway, NJ, USA; pp. 1–5. [Google Scholar]
- Tao, Y.; Rahn, C.D.; Archer, L.A.; You, F. Second life and recycling: Energy and environmental sustainability perspectives for high-performance lithium-ion batteries. Sci. Adv. 2021, 7, eabi7633. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; He, Y.; Wang, H.; Feng, Y.; Xie, W.; Zhu, X. Application of mechanical crushing combined with pyrolysis-enhanced flotation technology to recover graphite and licoo2 from spent lithium-ion batteries. J. Clean. Prod. 2019, 231, 1418–1427. [Google Scholar] [CrossRef]
- Fan, E.; Li, L.; Wang, Z.; Lin, J.; Huang, Y.; Yao, Y.; Chen, R.; Wu, F. Sustainable recycling technology for li-ion batteries and beyond: Challenges and future prospects. Chem. Rev. 2020, 120, 7020–7063. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Mao, B.; Stoliarov, S.I.; Sun, J. A review of lithium ion battery failure mechanisms and fire prevention strategies. Prog. Energy Combust. Sci. 2019, 73, 95–131. [Google Scholar] [CrossRef]
- Wuschke, L.; Jäckel, H.-G.; Leißner, T.; Peuker, U.A. Crushing of large li-ion battery cells. Waste Manag. 2019, 85, 317–326. [Google Scholar] [CrossRef] [PubMed]
- Cardarelli, F.; Dube, J. Method for Recycling Spent Lithium Metal Polymer Rechargeable Batteries and Related Materials. US Patent 7,192,564, 20 March 2007. [Google Scholar]
- Hua, Y.; Zhou, S.; Huang, Y.; Liu, X.; Ling, H.; Zhou, X.; Zhang, C.; Yang, S. Sustainable value chain of retired lithium-ion batteries for electric vehicles. J. Power Sources 2020, 478, 228753. [Google Scholar] [CrossRef]
- Ojanen, S.; Lundström, M.; Santasalo-Aarnio, A.; Serna-Guerrero, R. Challenging the concept of electrochemical discharge using salt solutions for lithium-ion batteries recycling. Waste Manag. 2018, 76, 242–249. [Google Scholar] [CrossRef]
- Shaw-Stewart, J.; Alvarez-Reguera, A.; Greszta, A.; Marco, J.; Masood, M.; Sommerville, R.; Kendrick, E. Aqueous solution discharge of cylindrical lithium-ion cells. Sustain. Mater. Technol. 2019, 22, e00110. [Google Scholar] [CrossRef]
- Zhang, T.; He, Y.; Ge, L.; Fu, R.; Zhang, X.; Huang, Y. Characteristics of wet and dry crushing methods in the recycling process of spent lithium-ion batteries. J. Power Sources 2013, 240, 766–771. [Google Scholar] [CrossRef]
- Wang, H.; Qu, G.; Yang, J.; Zhou, S.; Li, B.; Wei, Y. An effective and cleaner discharge method of spent lithium batteries. J. Energy Storage 2022, 54, 105383. [Google Scholar] [CrossRef]
- Zhu, C.; Shang, Y.; Lu, F.; Jiang, Y.; Cheng, C.; Mi, C. Core temperature estimation for self-heating automotive lithium-ion batteries in cold climates. IEEE Trans. Ind. Inform. 2019, 16, 3366–3375. [Google Scholar] [CrossRef]
- Rouhi, H.; Karola, E.; Serna-Guerrero, R.; Santasalo-Aarnio, A. Voltage behavior in lithium-ion batteries after electrochemical discharge and its implications on the safety of recycling processes. J. Energy Storage 2021, 35, 102323. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).