Synthesis and Characterization of Lithium Phosphate (Li3PO4) as a Solid Electrolyte
Abstract
Highlights
- The single-phase and high-purity β-Li3PO4 was synthesized by a facile co-precipitation method.
- A particle nanoscale sample with a high surface area was successfully prepared.
- A solid electrolyte, lithium phosphate, with high ionic conductivity was achieved.
- Kinetic and thermodynamic studies established lithium phosphate behavior as a function of the temperature.
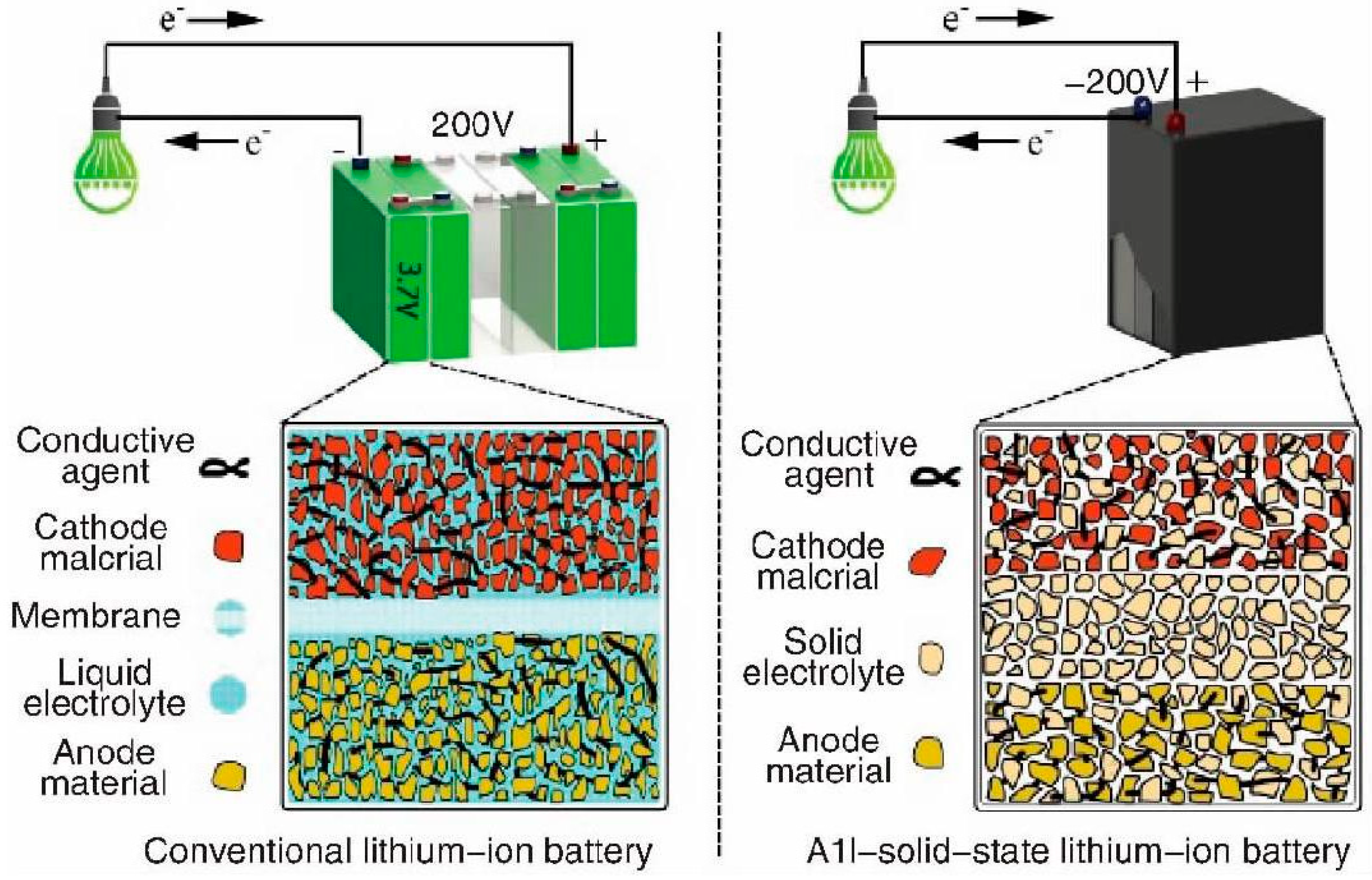
1. Introduction
2. Experimental Procedures and Characterization
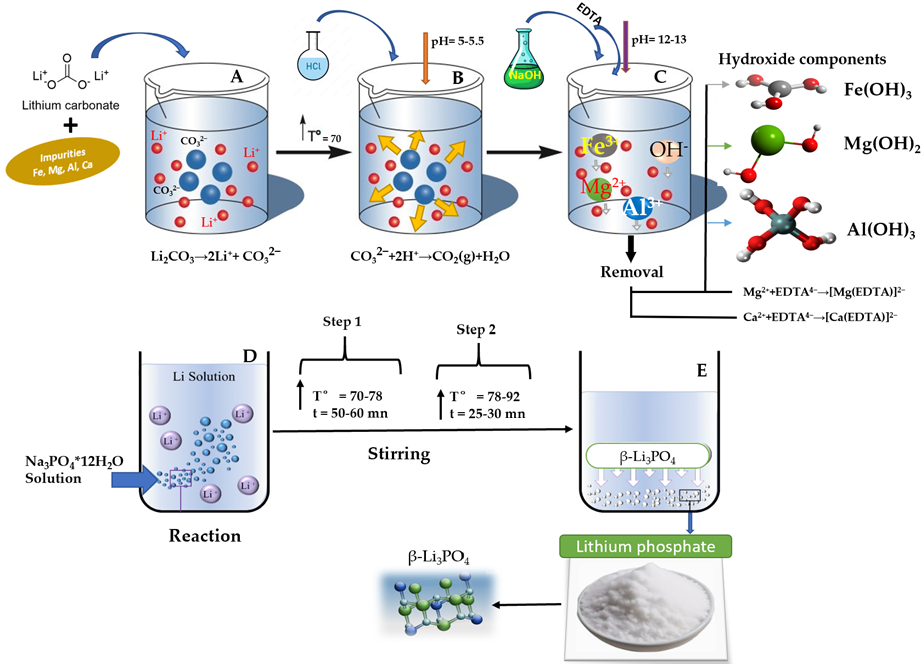
2.1. Titration
2.2. Preparation
2.3. Sample Characterization Methods
2.4. Modeling
3. Results and Discussion
3.1. Lithium Mother Source Study
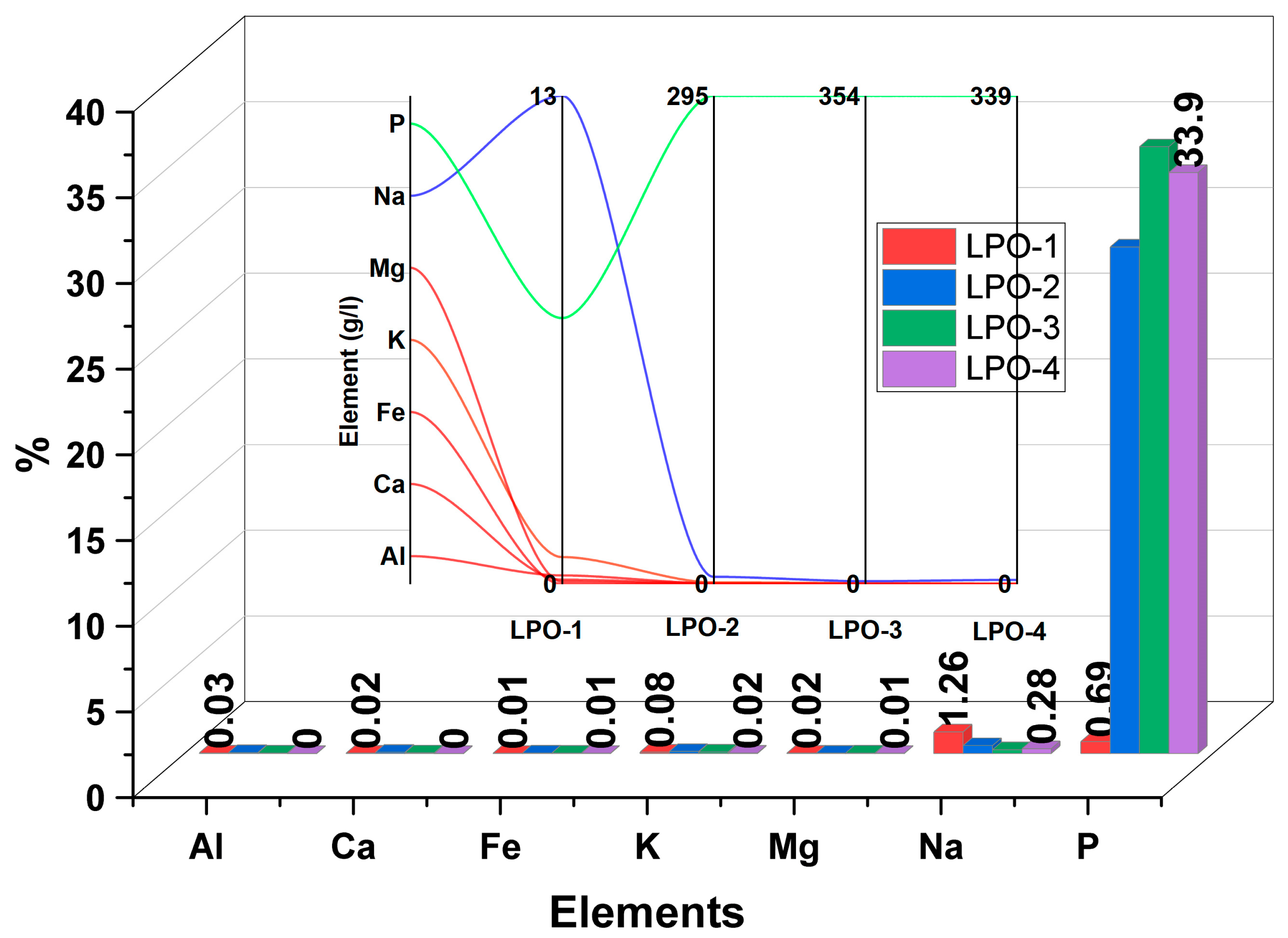
3.2. Impurities Removal Process and Their Distribution in Synthesized Samples
3.3. Surfaces, Pore Distribution, and Isotherm (N2) Analysis
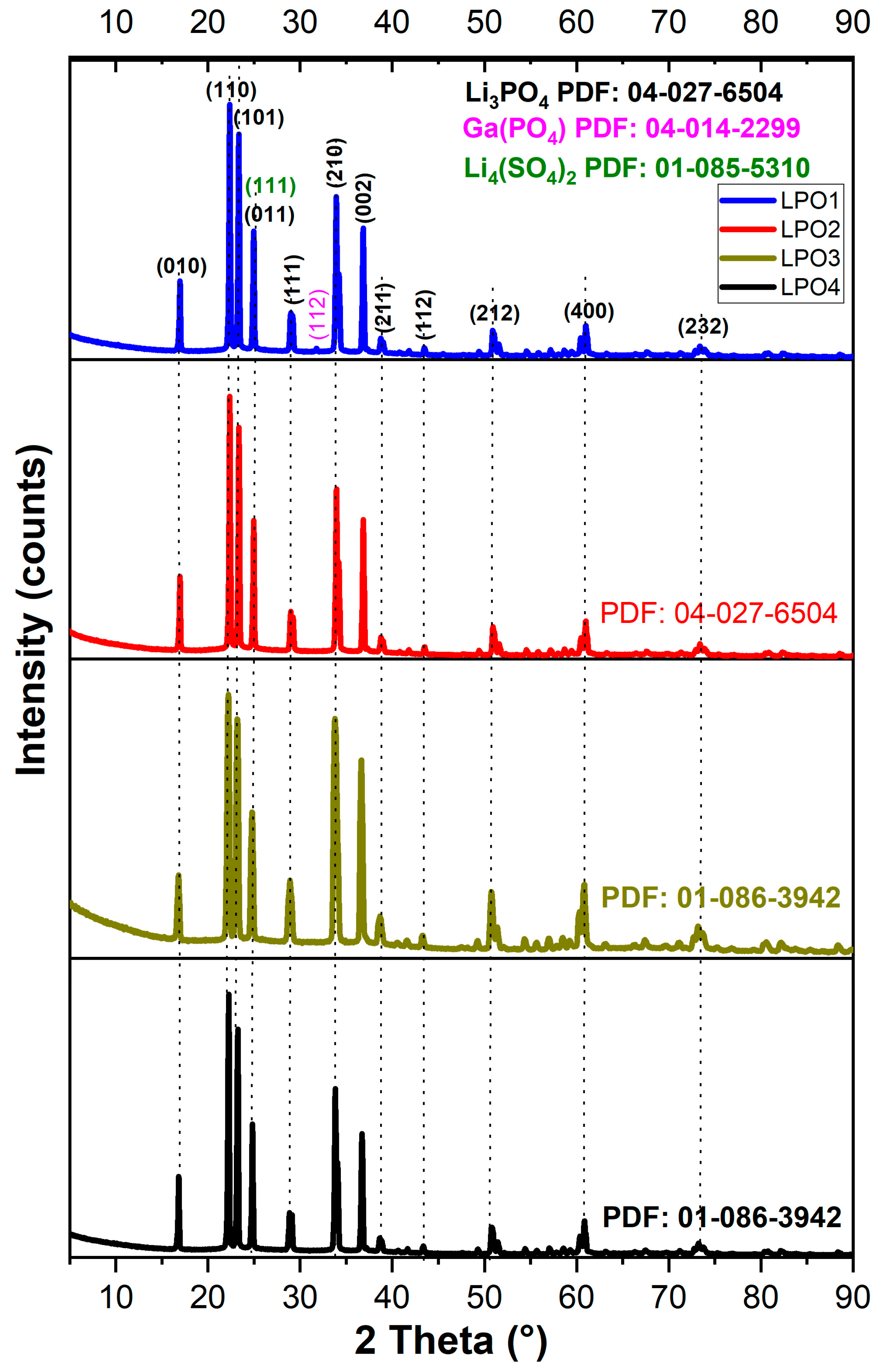
3.4. Crystallography Analysis
3.5. Morphology and Impurities Distribution Analysis (SEM-EDS and ICP-OES)
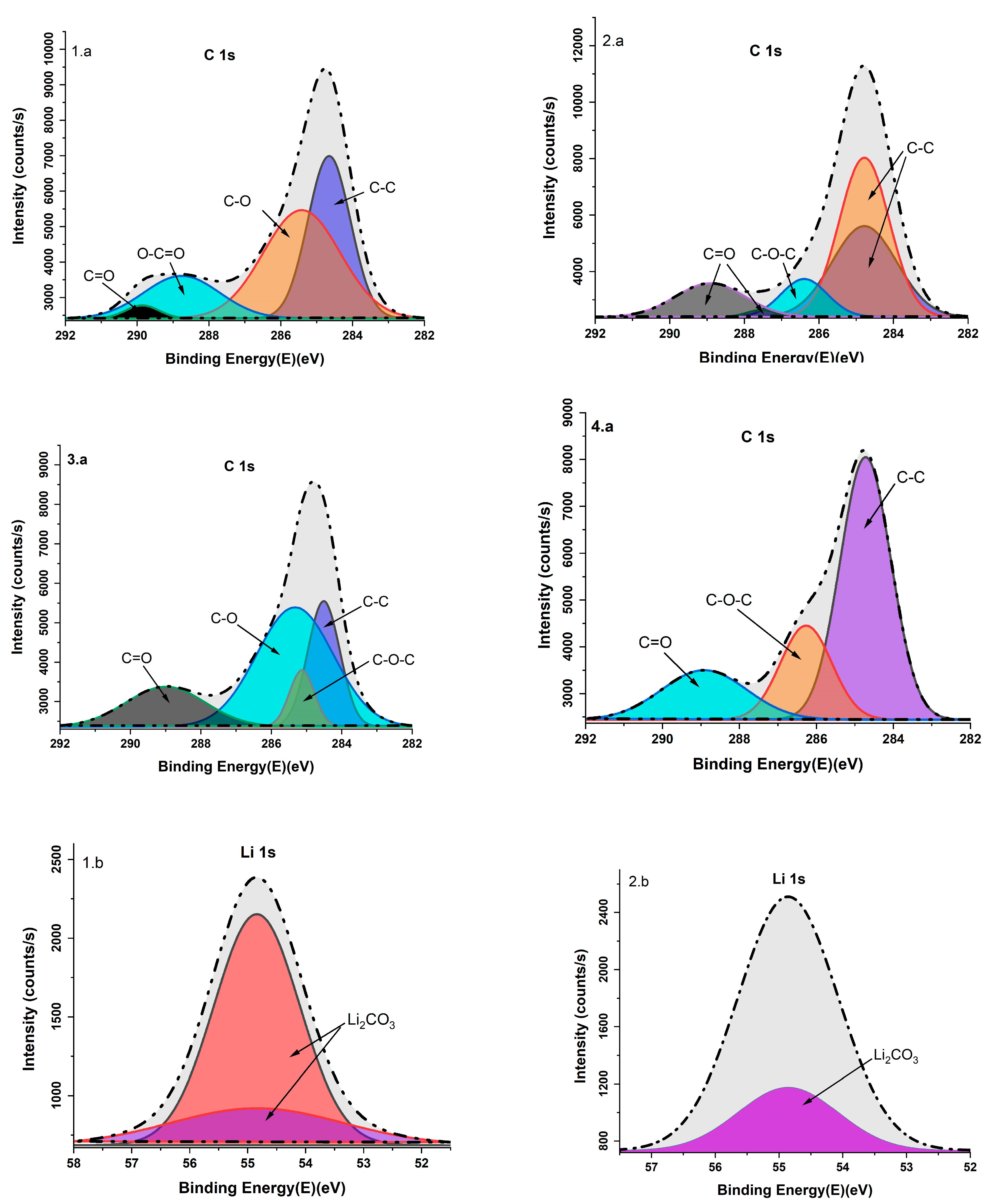
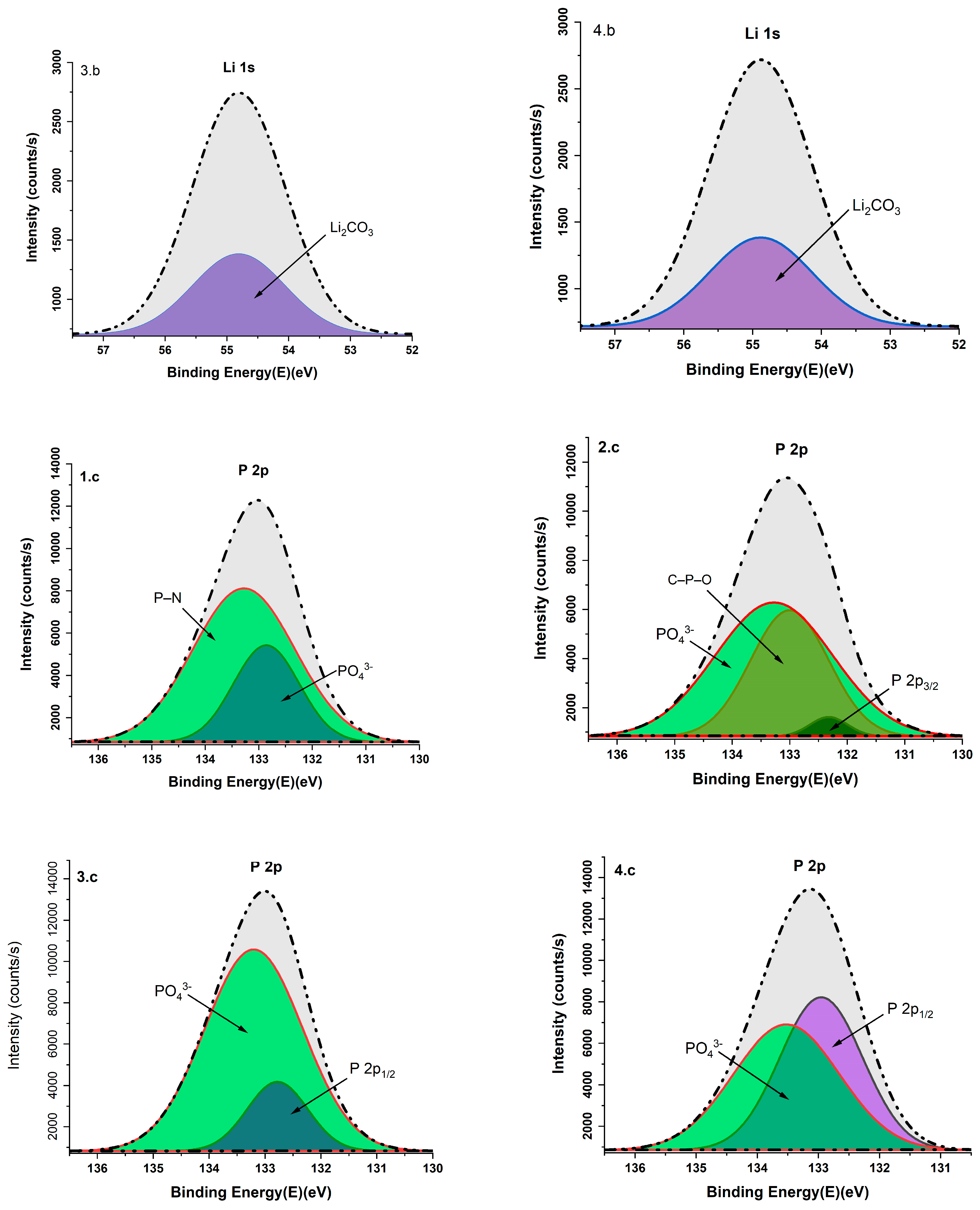
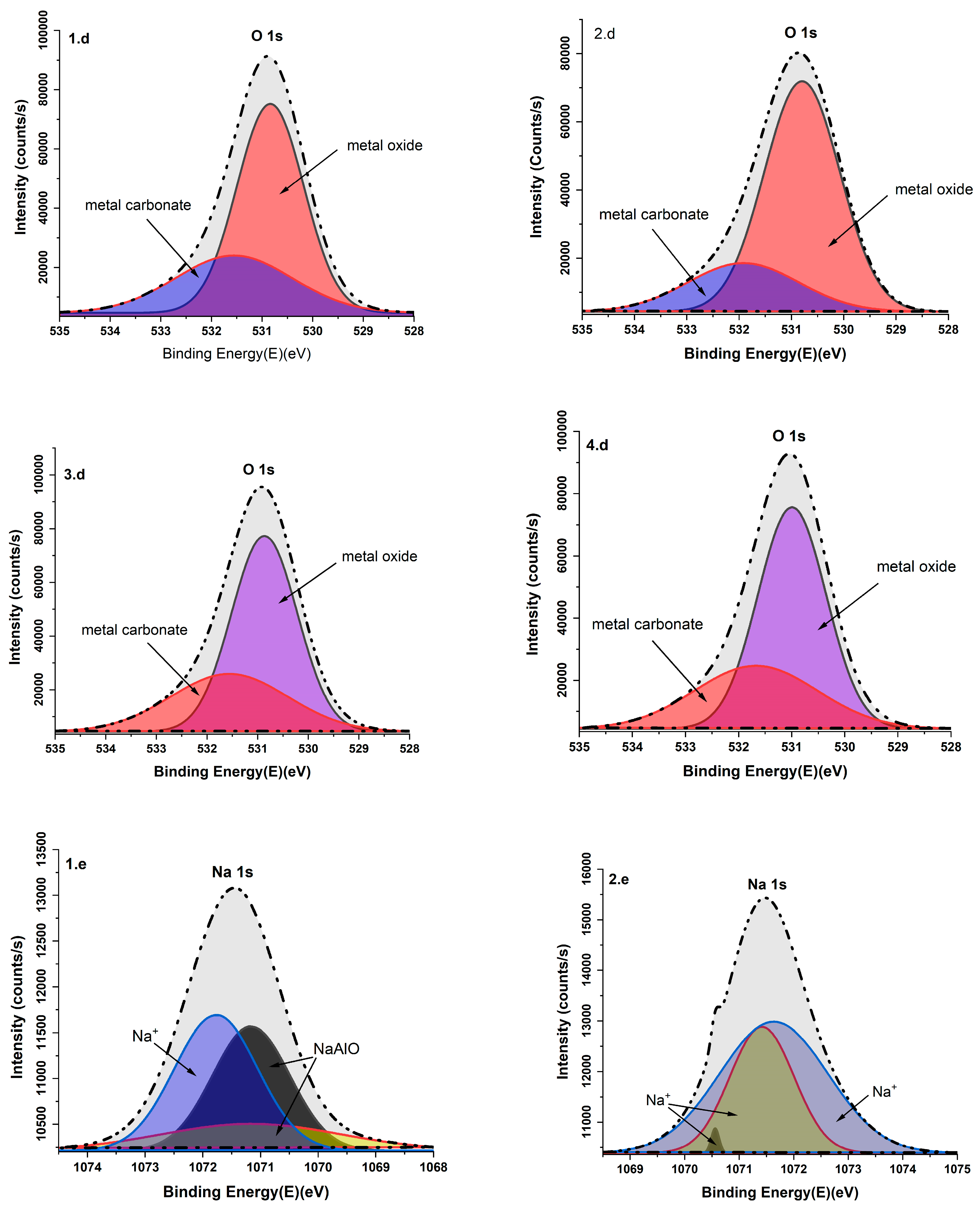
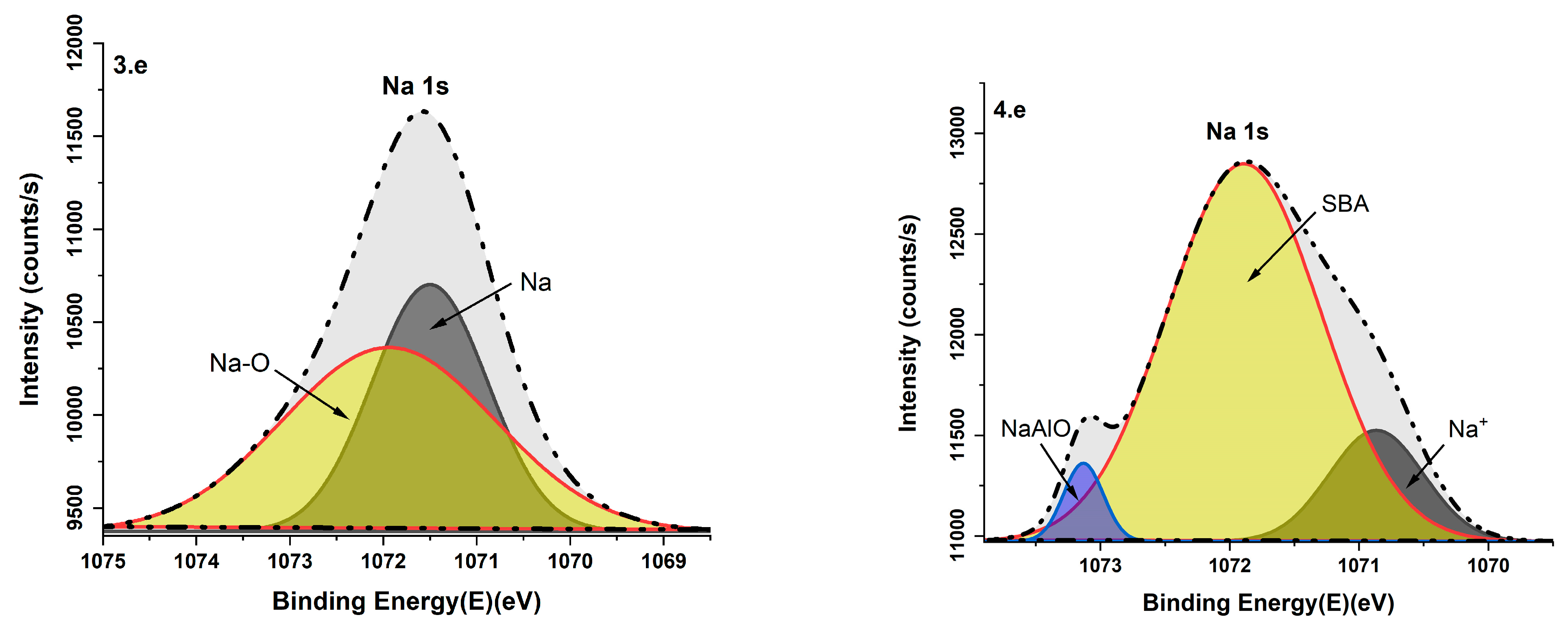
3.6. X-Ray Photoelectron Analysis
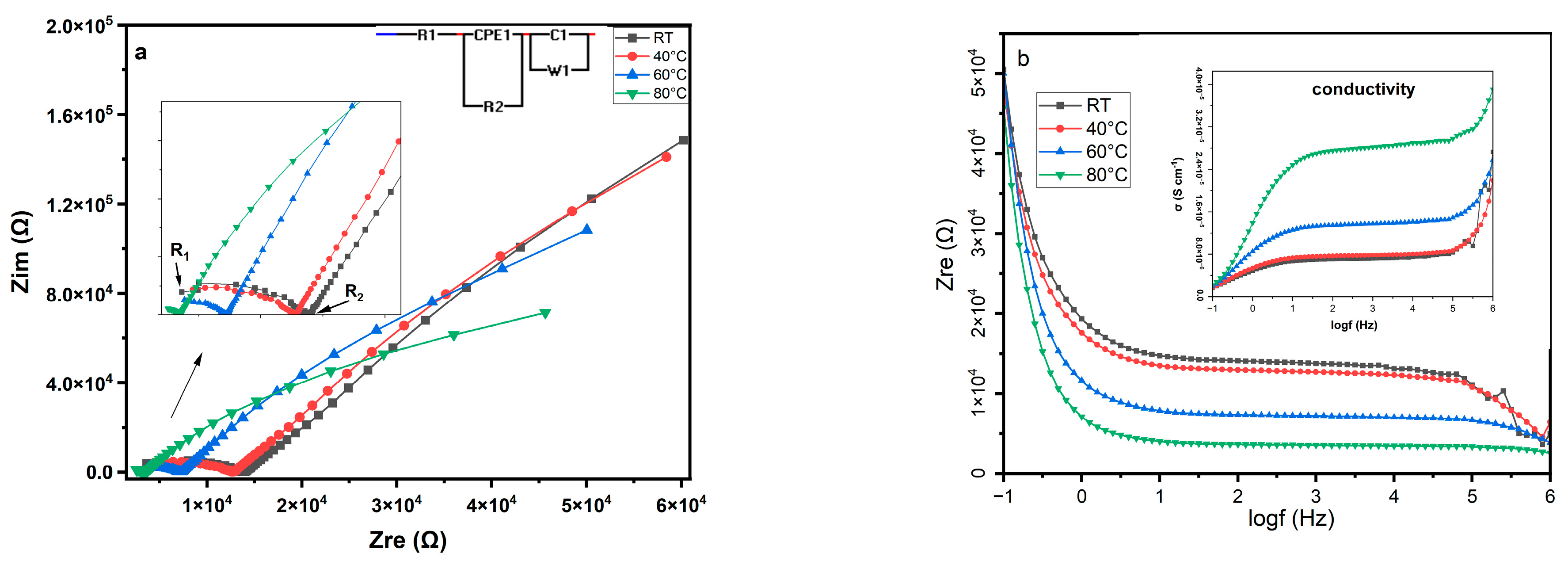
3.7. Electrical Properties
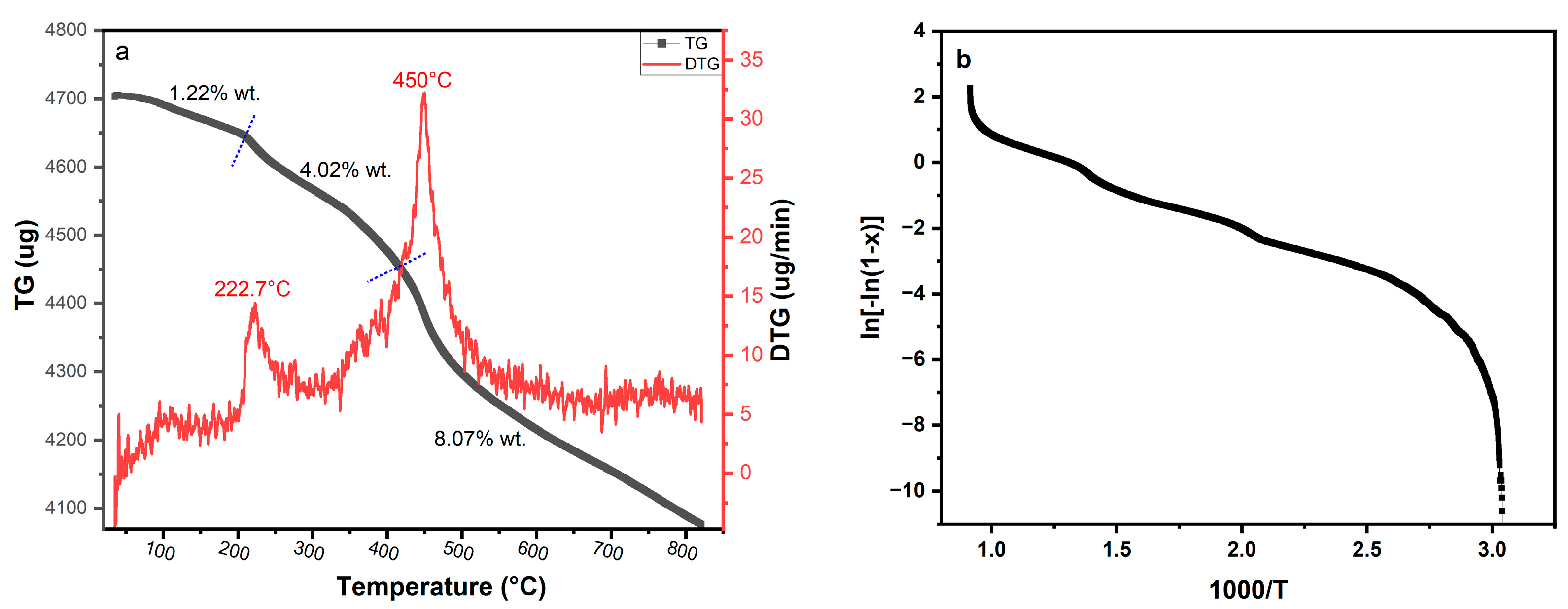
3.8. Kinetic and Thermodynamic Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kim, T.Y.; Song, C.K.; Yun, Y.S.; Yun, D.; Han, J.W.; Yi, J. Active site structure of a lithium phosphate catalyst for the isomerization of 2,3-epoxybutane to 3-buten-2-ol. Mol. Catal. 2018, 445, 133–141. [Google Scholar] [CrossRef]
- Sun, Y.Q.; Luo, X.T.; Zhu, Y.S.; Liao, X.J.; Li, C.J. Li3PO4 electrolyte of high conductivity for all-solid-state lithium battery prepared by plasma spray. J. Eur. Ceram. Soc. 2022, 42, 4239–4247. [Google Scholar] [CrossRef]
- Prayogi, L.D.; Faisal, M.; Kartini, E.; Honggowiranto, W. Supardi, Morphology and conductivity study of solid electrolyte Li3PO4. AIP Conf. Proc. 2016, 1710, 030047. [Google Scholar] [CrossRef]
- Fan, L.; Wei, S.; Li, S.; Li, Q.; Lu, Y. Recent Progress of the Solid-State Electrolytes for High-Energy Metal-Based Batteries. Adv. Energy Mater. 2018, 8, 1702657. [Google Scholar] [CrossRef]
- Rosen, M.; Hecker, P.; Mann, M.; Ma, Q.; Gross, J.P.; Schwaiger, R.; Guillon, O.; Fattakhova-Rohlfing, D.; Finsterbusch, M. Reducing the environmental footprint of solid-electrolytes—A green synthesis route for LATP. Green Chem. 2024, 26, 2712–2720. [Google Scholar] [CrossRef]
- Saran, S.; Eker, Y.R. Synthesis, structural and conductive properties of Nd doped garnet-type Li7La3Zr2O12 Li-ion conductor. Curr. Appl. Phys. 2022, 41, 1–6. [Google Scholar] [CrossRef]
- Xiao, B.; Li, D.; Dai, X.; Wei, Y.; Liao, Y.; Wang, C.; Ji, F.; Wu, F. Construction of a robust lithium cobalt phosphate layer for enhancement of the electrochemical performance of LiNi0.8Co0.1Mn0.1O2 at high voltage. Electrochim. Acta 2024, 475, 143648. [Google Scholar] [CrossRef]
- Zeng, S.; Ding, X.; He, L.; Li, H.W.; Zhang, Q.; Li, Y. Realizing fast Li-ion conduction of Li3PO4 solid electrolyte at low temperature by mechanochemical formation of lithium-containing dual-shells. Mater. Adv. 2023, 4, 2780–2784. [Google Scholar] [CrossRef]
- Kuwata, N.; Iwagami, N.; Matsuda, Y.; Tanji, Y.; Kawamura, J. Thin Film Batteries with Li3PO4 Solid Electrolyte Fabricated by Pulsed Laser Deposition. ECS Trans. 2009, 16, 53–60. [Google Scholar] [CrossRef]
- Ayu, N.I.P.; Kartini, E.; Prayogi, L.D.; Faisal, M. Supardi, Crystal structure analysis of Li3PO4 powder prepared by wet chemical reaction and solid-state reaction by using X-ray diffraction (XRD). Ionics 2016, 22, 1051–1057. [Google Scholar] [CrossRef]
- Zhao, S.; Jiang, W.; Zhu, X.; Ling, M.; Liang, C. Understanding the synthesis of inorganic solid-state electrolytes for Li ion batteries: Features and progress. Sustain. Mater. Technol. 2022, 33, e00491. [Google Scholar] [CrossRef]
- Method for Recovering Lithium from Lithium Carbonate Precipitation Mother Liquor. CN111533146A. Available online: https://patents.google.com/patent/CN111533146A/zh (accessed on 25 October 2024).
- Liu, W.Y.; Li, C.L.; Fu, Z.W. Stability of Nitrogen-Containing Lithium Phosphate Films in Air. Acta Phys.-Chim. Sin. 2006, 11, 1413–1418. Available online: https://qikan.cqvip.com/Qikan/Article/Detail?id=23426027&from=Qikan_Article_Detail (accessed on 2 October 2024).
- Wu, J. Discussion on the process of preparing lithium dihydrogen phosphate from lithium phosphate tower skin. XinJiang Youse Jinshu 2012, S1, 124–125. Available online: https://qikan.cqvip.com/Qikan/Article/Detail?id=1003444489&from=Qikan_Article_Detail (accessed on 2 October 2024).
- Wu, S.; Zhang, M.; Zan, C.; Zhou, H. Process of Deep Recovery of Lithium from the Mother Liquor of Lithium Carbonate by Phosphate Precipitation. J. Tianjin Univ. Sci. Technol. 2023, 38, 35–41. [Google Scholar] [CrossRef]
- Yao, X.; Wang, Y.; Xu, M.; Shuying, S.U.N. Preparation of lithium phosphate from waste liquid of retired lithium batteries. Chem. Ind. Eng. 2024, 41, 179–190. [Google Scholar] [CrossRef]
- Ishigaki, N.; Akimoto, J. Room temperature synthesis and phase transformation of lithium phosphate Li3PO4 as solid electrolyte. J. Asian Ceram. Soc. 2021, 9, 452–458. [Google Scholar] [CrossRef]
- Puente, P.M.G.; Song, S.; Cao, S.; Rannalter, L.Z.; Pan, Z.; Xiang, X.; Shen, Q.; Chen, F. Garnet-type solid electrolyte: Advances of ionic transport performance and its application in all-solid-state batteries. J. Adv. Ceram. 2021, 10, 933–972. [Google Scholar] [CrossRef]
- Farrukh, M.A.; Butt, K.M.; Chong, K.K.; Chang, W.S. Photoluminescence emission behavior on the reduced band gap of Fe doping in CeO2-SiO2 nanocomposite and photophysical properties. J. Saudi Chem. Soc. 2019, 23, 561–575. [Google Scholar] [CrossRef]
- Del Mar Graciani, M.; Rodríguez, A.; Muñoz, M.; Moyá, M.L. Micellar solutions of sulfobetaine surfactants in water-ethylene glycol mixtures: Surface tension, fluorescence, spectroscopic, conductometric, kinetic studies. Langmuir 2005, 21, 7161–7169. [Google Scholar] [CrossRef]
- Silva, J.M.R.; de Morais Araújo, A.M.; da Costa Evangelista, J.P.; da Silva, D.R.; Gondim, A.D.; de Araujo, A.S. Evaluation of the kinetic and thermodynamic parameters in catalytic pyrolysis process of sunflower oil using Al-MCM-41 and zeolite H-ZSM-5. Fuel 2023, 333, 126225. [Google Scholar] [CrossRef]
- Bhardwaj, G.; Kumar, M.; Mishra, P.K.; Upadhyay, S.N. Kinetic analysis of the slow pyrolysis of paper wastes. Biomass Convers. Biorefinery 2023, 13, 3087–3100. [Google Scholar] [CrossRef]
- Zakariyaou, S.Y.; Ye, H.; Oumarou, A.D.M.; Aziz, M.S.A.; Ke, S. Characterization of Equilibrium Catalysts from the Fluid Catalytic Cracking Process of Atmospheric Residue. Catalysts 2023, 13, 1483. [Google Scholar] [CrossRef]
- Keffer, C.; Mighell, A.; Mauer, F.; Swanson, H.; Block, S. The Crystal Structure of Twinned Low-Temperature Lithium Phosphate. Inorg. Chem. 1967, 6, 119–125. [Google Scholar] [CrossRef]
- Lander, L.; Reynaud, M.; Carrasco, J.; Katcho, N.A.; Bellin, C.; Polian, A.; Baptiste, B.; Rousse, G.; Tarascon, J.M. Unveiling the electrochemical mechanisms of Li2Fe(SO4)2 polymorphs by neutron diffraction and density functional theory calculations. Phys. Chem. Chem. Phys. 2016, 18, 14509–14519. [Google Scholar] [CrossRef]
- Huang, Y.X.; Liu, J.Y.; Mi, J.X.; Zhao, J.T. (Ga0.71B0.29)PO4 with a high-cristobalite-type structure refined from powder data. Acta Crystallogr. Sect. E Struct. Rep. Online 2010, 66, i4. [Google Scholar] [CrossRef]
- Carbon|XPS Periodic Table|Thermo Fisher Scientific|Thermo Fisher Scientific—CN. Available online: https://www.thermofisher.cn/cn/zh/home/materials-science/learning-center/periodic-table/non-metal/carbon.html (accessed on 18 November 2024).
- Carbon Spectra—Li2CO3—Lithium Carbonate. Available online: https://xpsdatabase.net/carbon-spectra-li2co3-lithium-carbonate/ (accessed on 18 November 2024).
- Al-Kadhi, N.S.; Hefnawy, M.A.; Alamro, F.S.; Pashameah, R.A.; Ahmed, H.A.; Medany, S.S. Polyaniline-Supported Nickel Oxide Flower for Efficient Nitrite Electrochemical Detection in Water. Polymers 2023, 15, 1804. [Google Scholar] [CrossRef]
- Alamro, F.S.; Medany, S.S.; Al-Kadhi, N.S.; Ahmed, H.A.; Hefnawy, M.A. Modified NiFe2O4-Supported Graphene Oxide for Effective Urea Electrochemical Oxidation and Water Splitting Applications. Molecules 2024, 29, 1215. [Google Scholar] [CrossRef]
- Shulga, Y.M.; Baskakov, S.A.; Knerelman, E.I.; Davidova, G.I.; Badamshina, E.R.; Shulga, N.Y.; Skryleva, E.A.; Agapov, A.L.; Voylov, D.N.; Sokolov, A.P.; et al. Carbon nanomaterial produced by microwave exfoliation of graphite oxide: New insights. RSC Adv. 2014, 4, 587–592. [Google Scholar] [CrossRef]
- Lu, Y.C.; Crumlin, E.J.; Veith, G.M.; Harding, J.R.; Mutoro, E.; Baggetto, L.; Dudney, N.J.; Liu, Z.; Shao-Horn, Y. In Situ Ambient Pressure X-ray Photoelectron Spectroscopy Studies of Lithium-Oxygen Redox Reactions. Sci. Rep. 2012, 2, 715. [Google Scholar] [CrossRef]
- Yin, X.; Sun, X.; Li, D.; Xie, W.; Mao, Y.; Liu, Z.; Liu, Z. 2D/2D Phosphorus-Doped g-C3N4/Bi2WO6 Direct Z-Scheme Heterojunction Photocatalytic System for Tetracycline Hydrochloride (TC-HCl) Degradation. Int. J. Environ. Res. Public. Health 2022, 19, 14935. [Google Scholar] [CrossRef]
- Liu, X.; Yu, M.; Wu, S.; Gong, J. Composite nanoarchitectonics for efficient lithium storage by encapsulating black phosphorus quantum dots in cobalt/iron based Prussian blue analogues. J. Alloys Compd. 2023, 969, 172291. [Google Scholar] [CrossRef]
- Chatterjee, T.; Raul, C.K.; Mandal, S.; Pradhan, S.K.; Meikap, A.K. Effect of surfactant-assisted hierarchical growth of cupric oxide-hydroxyapatite nanocomposite on the dielectric and electrical transport behavior. Phys. B Condens. Matter 2023, 650, 414560. [Google Scholar] [CrossRef]
- Jung, W.D.; Jeon, M.; Shin, S.S.; Kim, J.S.; Jung, H.G.; Kim, B.K.; Lee, J.H.; Chung, Y.C.; Kim, H. Functionalized Sulfide Solid Electrolyte with Air-Stable and Chemical-Resistant Oxysulfide Nanolayer for All-Solid-State Batteries. ACS Omega 2020, 5, 26015–26022. [Google Scholar] [CrossRef]
- Chen, K.; Tang, Y.; Zhang, S.; Hao, X.; Zhao, X.; Cheng, L.Q.; Xiao, Y.; Wen, Z. Promoted Stability and Reaction Kinetics in Ni-Rich Cathodes via Mechanical Fusing Multifunctional LiZr2(PO4)3 Nanocrystals for High Mass Loading All-Solid-State Lithium Batteries. ACS Appl. Mater. Interfaces 2024, 16, 45459–45472. [Google Scholar] [CrossRef]
- Fontecha, D.; Nuwayhid, R.B.; Kozen, A.C.; Stewart, D.M.; Rubloff, G.W.; Gregorczyk, K.E. Low temperature plasma-enhanced atomic layer deposition of sodium phosphorus oxynitride with tunable nitrogen content. J. Vac. Sci. Technol. A 2022, 40, 032403. [Google Scholar] [CrossRef]
- López, E.O.; Bernardo, P.L.; Checca, N.R.; Rossi, A.L.; Mello, A.; Ellis, D.E.; Rossi, A.M.; Terra, J. Hydroxyapatite and lead-substituted hydroxyapatite near-surface structures: Novel modelling of photoemission lines from X-ray photoelectron spectra. Appl. Surf. Sci. 2022, 571, 151310. [Google Scholar] [CrossRef]
- Wang, Y.; Zheng, X.; Qu, Q.; Liu, G.; Battglia, V.S.; Zheng, H. A novel maleic acid/graphite composite anode for lithium ion batteries with high energy and power density. Carbon. 2018, 132, 420–429. [Google Scholar] [CrossRef]
- Oxygen|Thermo Fisher Scientific XPS Periodic Table|Thermo Fisher Scientific|Thermo Fisher Scientific—CN. Available online: https://www.thermofisher.cn/cn/zh/home/materials-science/learning-center/periodic-table/non-metal/oxygen.html (accessed on 18 November 2024).
- Khan, Z.; Park, S.O.; Yang, J.; Park, S.; Shanker, R.; Song, H.K.; Kim, Y.; Kwak, S.K.; Ko, H. Binary N,S-doped carbon nanospheres from bio-inspired artificial melanosomes: A route to efficient air electrodes for seawater batteries. J. Mater. Chem. A Mater. 2018, 6, 24459–24467. [Google Scholar] [CrossRef]
- García-Bordejé, E.; Dongil, A.B.; Conesa, J.M.; Guerrero-Ruiz, A.; Rodríguez-Ramos, I. Dual functional materials based on Ni and different alkaline metals on alumina for the cyclic stepwise CO2 capture and methanation. Chem. Eng. J. 2023, 472, 144953. [Google Scholar] [CrossRef]
- Fang, R.; Li, Y.; Wu, N.; Xu, B.; Liu, Y.; Manthiram, A.; Goodenough, J.B. Ultra-Thin Single-Particle-Layer Sodium Beta-Alumina-Based Composite Polymer Electrolyte Membrane for Sodium-Metal Batteries. Adv. Funct. Mater. 2022, 33, 2211229. [Google Scholar] [CrossRef]
- Foucaud, Y.; Badawi, M.; Filippov, L.O.; Barres, O.; Filippova, I.V.; Lebègue, S. Synergistic adsorptions of Na2CO3 and Na2SiO3 on calcium minerals revealed by spectroscopic and ab initio molecular dynamics studies. Chem. Sci. 2019, 10, 9928–9940. [Google Scholar] [CrossRef] [PubMed]
- Savinova, E.R.; Zemlyanov, D.Y.; Scheybal, A.; Schlögl, R.; Doblhofer, K. Ex Situ X-ray Photoelectron Spectroscopy Study of the Interface between a Ag(111) Electrode and an Alkaline Electrolyte. 2. Structure of the Double Layer. Langmuir 1999, 15, 6552–6556. [Google Scholar] [CrossRef]
- He, X.; Zhu, Y.; Mo, Y. Origin of fast ion diffusion in super-ionic conductors. Nat. Commun. 2017, 8, 15893. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.A.; Holzwarth, N.A.W. Li Ion Diffusion Mechanisms in the Crystalline Electrolyte γ-Li3PO4. J. Electrochem. Soc. 2007, 154, A999–A1004. [Google Scholar] [CrossRef]




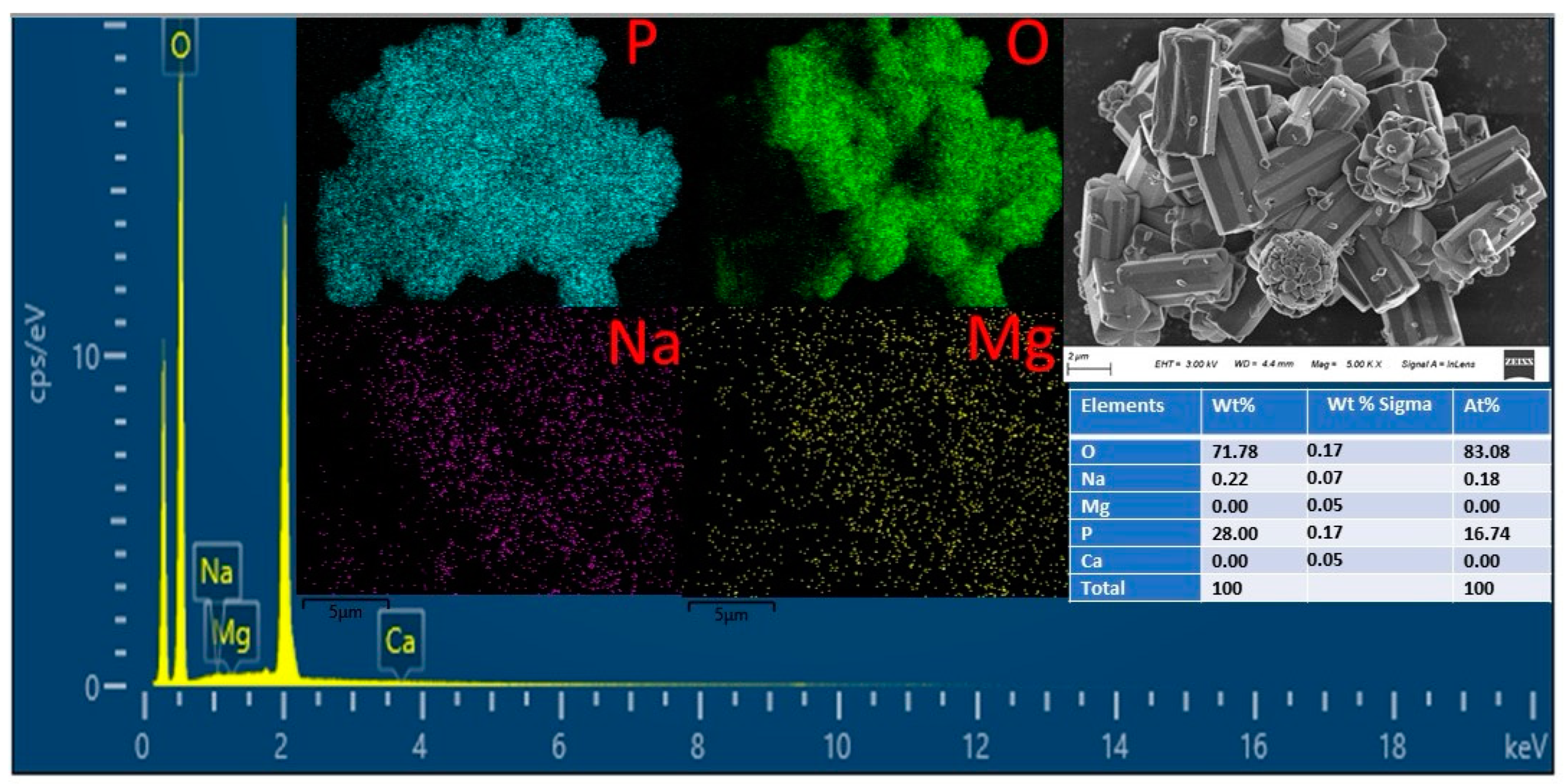
| Lithium Source | Phosphate Source | pH Control Reagent | Reaction Temperature °C | Reaction Time/min | pH | Ref. |
|---|---|---|---|---|---|---|
| Li2CO3 | Na3PO4•10H2O | HCl; NaOH | 70–78 | 20–25 | 12.5–13 | [12] |
| LiOH•H2O | NH4H2PO4 | - | 200 | 60 | - | [13] |
| LiOH | H3PO4 | - | >80 | - | 7.5 | [14] |
| Li2CO3 | Na3PO4•10H2O | HCl | >90 | 60 | >12 | [15] |
| Spent battery | H3PO4 | - | 70–90 | 60–120 | 7–8 | [16] |
| Samples | Li2CO3 | Na3PO4.12H2O | pH Control Reagent | Reaction Temperature/°C | Reaction Time/min | pH | Li3PO4 (g) | Yield (%) |
|---|---|---|---|---|---|---|---|---|
| LPO-1 | 3 g | 9.942 g | HCl-NaOH | 78 | 40 min | 12 | 2.40 | 79.19 |
| LPO-2 | 9.942 g | 78 | 50 min | 12.5 | 2.37 | 78.20 | ||
| LPO-3 | 9.942 g | 78–92 | 1 h 30 min | 13 | 2.50 | 82.49 | ||
| LPO-4 | 10 g | 78–90 | 1 h 30 min | 12.7 | 2.85 | 93.49 |
| Li2CO3 | ||||
|---|---|---|---|---|
| Element | Intensity | Concentration (C) (mg/L) | with Equation (1) (%) | with Equation (2) (g/L) |
| Al | 396.163 | 0.242 | 0.239 | 2.390 |
| As | 188.984 | −0.320 | −0.316 | −3.163 |
| B | 249.778 | −0.082 | −0.081 | −0.816 |
| Ba | 233.533 | 0.002 | 0.002 | 0.020 |
| Bi | 223.065 | −0.009 | −0.009 | −0.096 |
| Ca | 317.941 | 0.358 | 0.353 | 3.531 |
| Cd | 214.443 | −0.003 | −0.003 | −0.039 |
| Cr | 283.568 | 0.001 | 0.0015 | 0.015 |
| Co | 228.618 | 0.001 | 0.001 | 0.018 |
| Cu | 324.762 | 0.001 | 0.001 | 0.016 |
| Fe | 238.205 | 0.125 | 0.123 | 1.236 |
| K | 766.51 | 0.124 | 0.123 | 1.230 |
| Li | 670.803 | 18.460 | 18.205 | 182.059 |
| Mg | 285.217 | 0.134 | 0.132 | 1.323 |
| Mn | 257.615 | 0.011 | 0.011 | 0.110 |
| Na | 589.606 | 1.150 | 1.134 | 11.344 |
| Ni | 231.605 | 0.003 | 0.003 | 0.036 |
| P | 213.621 | 0.101 | 0.099 | 0.999 |
| Pb | 220.36 | −0.009 | −0.009 | −0.097 |
| Se | 196.032 | 0.059 | 0.058 | 0.582 |
| Sr | 407.78 | 0.003 | 0.003 | 0.034 |
| V | 290.886 | 0.0006 | 0.0006 | 0.006 |
| Zn | 213.861 | 0.047 | 0.047 | 0.473 |
| Sample | Weigh of Empty Glass (A) | Weigh of Sample (B) | (A + B) | Weigh After Drying (C) | Water Content (%) |
|---|---|---|---|---|---|
| Li2CO3 | 42.10 | 16 | 58.10 | 57.10 | 6.25 |
| Samples | SBET (m2g−1) | St-Plot-Ext (m2g−1) | SLangmuir (m2g−1) | Vtotal (cm3g−1) | Vmicro (cm3g−1) | Size |
|---|---|---|---|---|---|---|
| LPO-4 | 9.029 | 19.69 | 28.189 | 0.022 | - | 10.08 nm |
| Samples | Phase | a (Å) | b (Å) | C (Å) | Cell Volume (Å3) | Density (g/cm3) |
|---|---|---|---|---|---|---|
| LPO-1 | β-Li3PO4 | 6.115 | 5.2394 | 4.855 | 155.56 | 2.472 |
| LPO-2 | β-Li3PO4 | 6.115 | 5.2394 | 4.854 | 155.56 | 2.471 |
| LPO-3 | β-Li3PO4 | 6.115 | 5.2394 | 4.854 | 155.56 | 2.471 |
| LPO-4 | β-Li3PO4 | 6.115 | 5.2394 | 4.854 | 155.56 | 2.471 |
| LPO-1 | LPO-2 | LPO-3 | LPO-4 | ||
|---|---|---|---|---|---|
| Binding Energy (eV) | Li 1s | 54.8 | 54.8 | 54.7 | 54.8 |
| P 2p | 133.0 | 133.05 | 133.0 | 133.1 | |
| O 1s | 530.9 | 530.87 | 530.9 | 531.0 | |
| C 1s | 284.8 | 284.8 | 284.8 | 284.7 | |
| Na 1s | 1071.4 | 1071.4 | 1071.6 | 1071.8 | |
| Atomic ratio | Li/P | 3.1 | 3.2 | 3.0 | 3.0 |
| O/P | 4.3 | 4.3 | 4.2 | 4.1 | |
| Na/P | 0.84 | 0.66 | 0.52 | 0.06 |
| Spectra | Binding Energy (eV) | Assignment | Refs. |
|---|---|---|---|
| C 1s | 284.65/284.50/284.72 | C-C | [27,28] |
| 285.41/285.32 | C-O | [29,30] | |
| 288.75 | OC=O | [27] | |
| 289.00/289.85 | C=O | [27,31] | |
| 285.12/286.26 | C-O-C | [27] | |
| Li 1s | 54.84 | Li2CO3 | [32] |
| P 2p | 133.27 | P–N | [33] |
| 132.77/132.95 | P 2p1/2 | [34,35] | |
| 133.27/133.52/133.20/132.85 | PO43− | [36,37,38] | |
| 132.32 | P 2p3/2 | [39] | |
| 133.00 | C–P–O | [40] | |
| O 1s | 531.56 | metal carbonate | [41] |
| 530.86 | metal oxide | [41] | |
| Na 1s | 1071.7/1070.86 | Na+ | [42] |
| 1071.16/1073.13 | Sodium oxides (NaAlO) | [43] | |
| 1071.89 | SBA | [44] | |
| 1071.94 | Na-O | [45] | |
| 1071.50 | Na | [46] |
| Phases | T°C Range | Loss (Wt.%) | Ea (eV) | ∆S | ∆H | ∆G | Fitting Equations | A (M.s−1) |
|---|---|---|---|---|---|---|---|---|
| 1 | RT–208.7 °C | 1.22 | 0.38 | −206.502 | 1.49 × 10+8 | 1.49 × 10+8 | Y = −4.4647x + 7.498 R2 = 80.56 | 163.89 |
| 2 | 208.7–414.8 °C | 4.02 | 0.19 | −261.54 | 1.1 × 10+8 | 1.1 × 10+8 | Y = −2.3067x + 2.608 R2 = 98.92 | 0.31 |
| 3 | 414.8–820.89 °C | 8.07 | 0.29 | −255.374 | 2.58 × 10+8 | 2.58 × 10+8 | Y = −3.4057x + 4.352 R2 = 95.09 | 1.04 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zakariyaou, S.Y.; Ye, H.; Jiang, C. Synthesis and Characterization of Lithium Phosphate (Li3PO4) as a Solid Electrolyte. Batteries 2024, 10, 429. https://doi.org/10.3390/batteries10120429
Zakariyaou SY, Ye H, Jiang C. Synthesis and Characterization of Lithium Phosphate (Li3PO4) as a Solid Electrolyte. Batteries. 2024; 10(12):429. https://doi.org/10.3390/batteries10120429
Chicago/Turabian StyleZakariyaou, Seybou Yacouba, Hua Ye, and Chongwen Jiang. 2024. "Synthesis and Characterization of Lithium Phosphate (Li3PO4) as a Solid Electrolyte" Batteries 10, no. 12: 429. https://doi.org/10.3390/batteries10120429
APA StyleZakariyaou, S. Y., Ye, H., & Jiang, C. (2024). Synthesis and Characterization of Lithium Phosphate (Li3PO4) as a Solid Electrolyte. Batteries, 10(12), 429. https://doi.org/10.3390/batteries10120429








