The Novel Coupling of Operando Methods: Electrochemical Dilatometry with Mass Spectrometry Using the Example of a Li|Graphite Half Cell
Abstract
1. Introduction
2. Materials and Methods
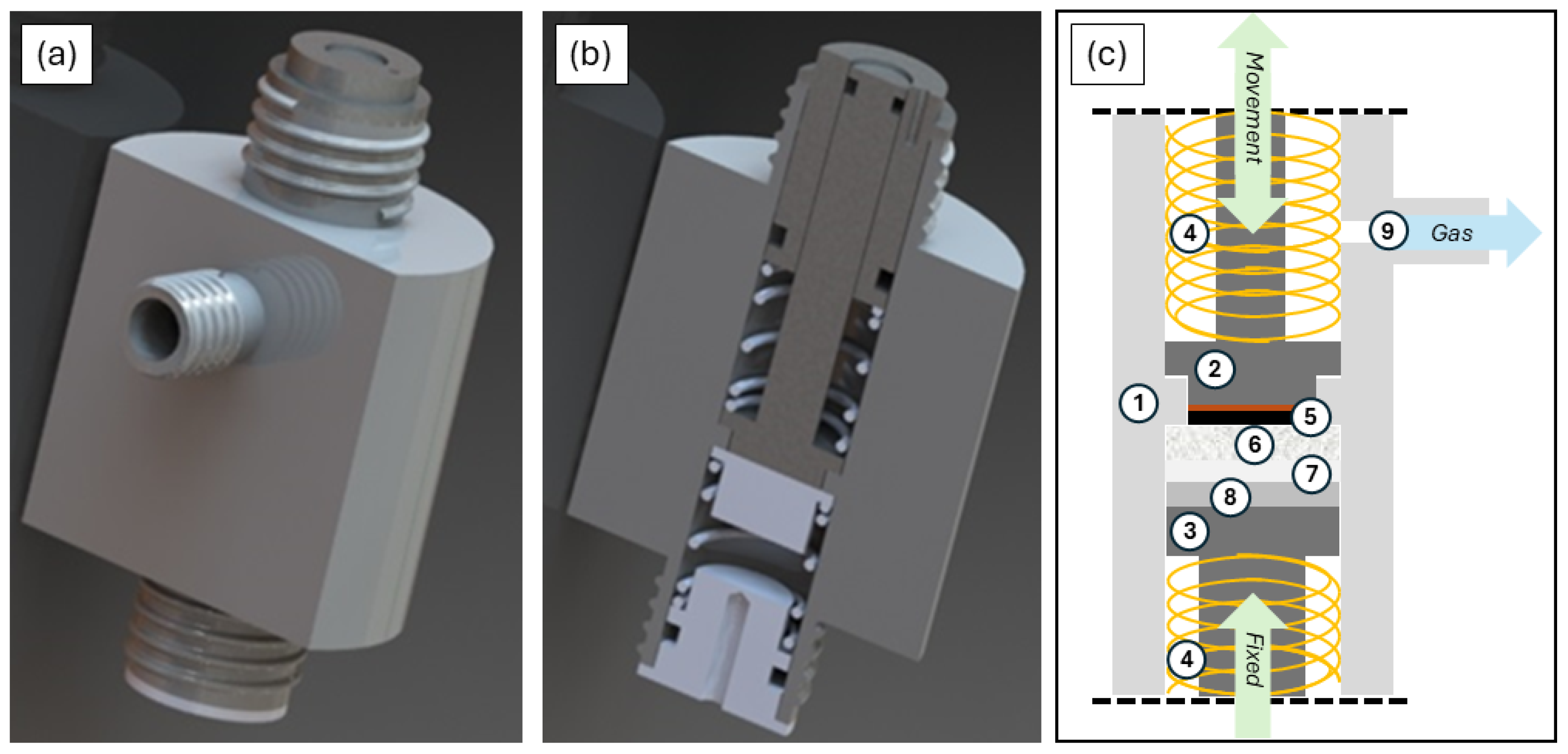
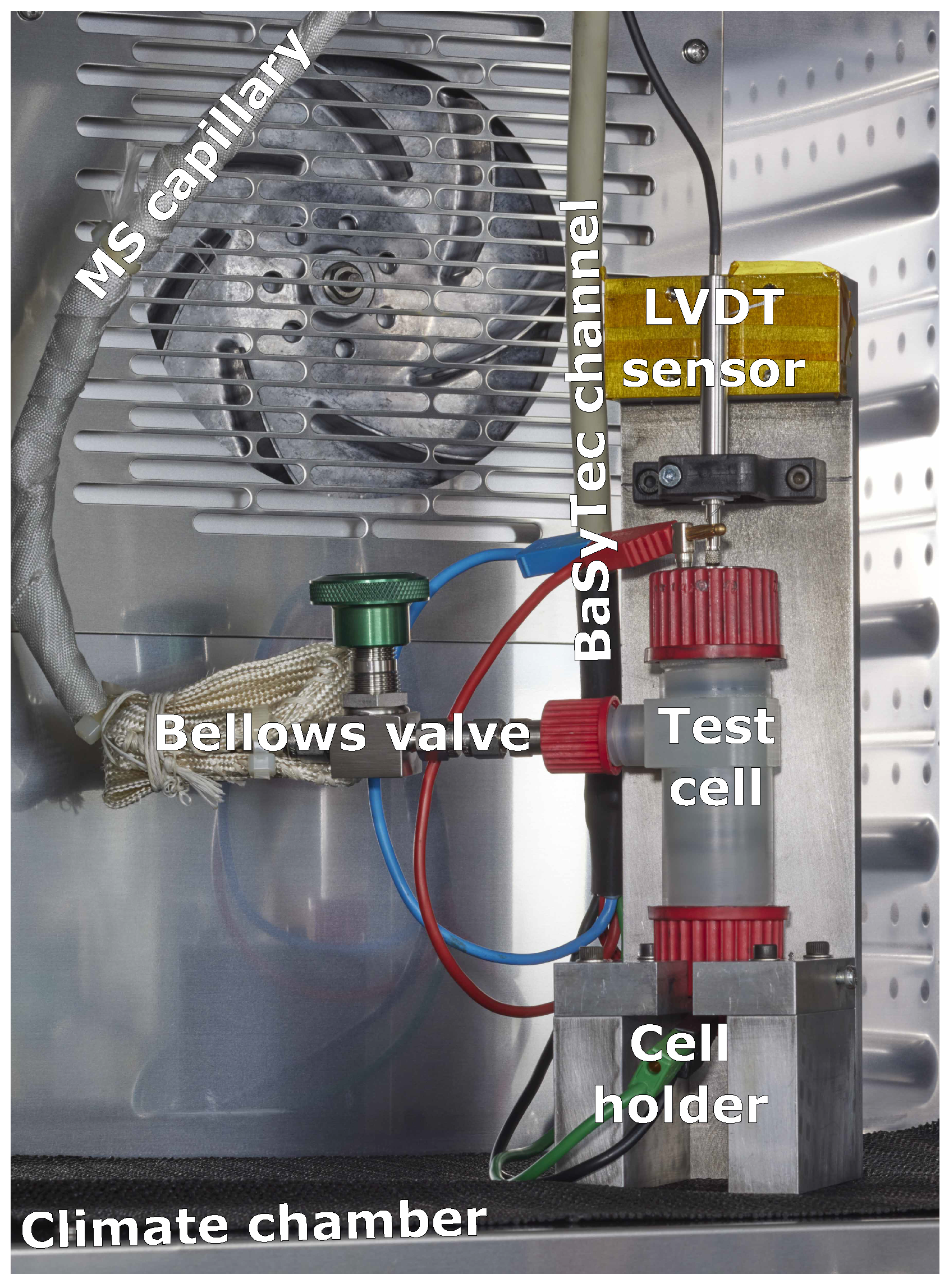
2.1. Combined-Measurement Cell Assembly and Multifunctional Measurement Setup
2.2. Electrochemical Testing
2.3. Operando Electrochemical Dilatometry
2.4. Operando Mass Spectrometry
2.5. Gas Chromatography with Coupled Mass Spectrometry (GC-MS)
3. Results and Discussion
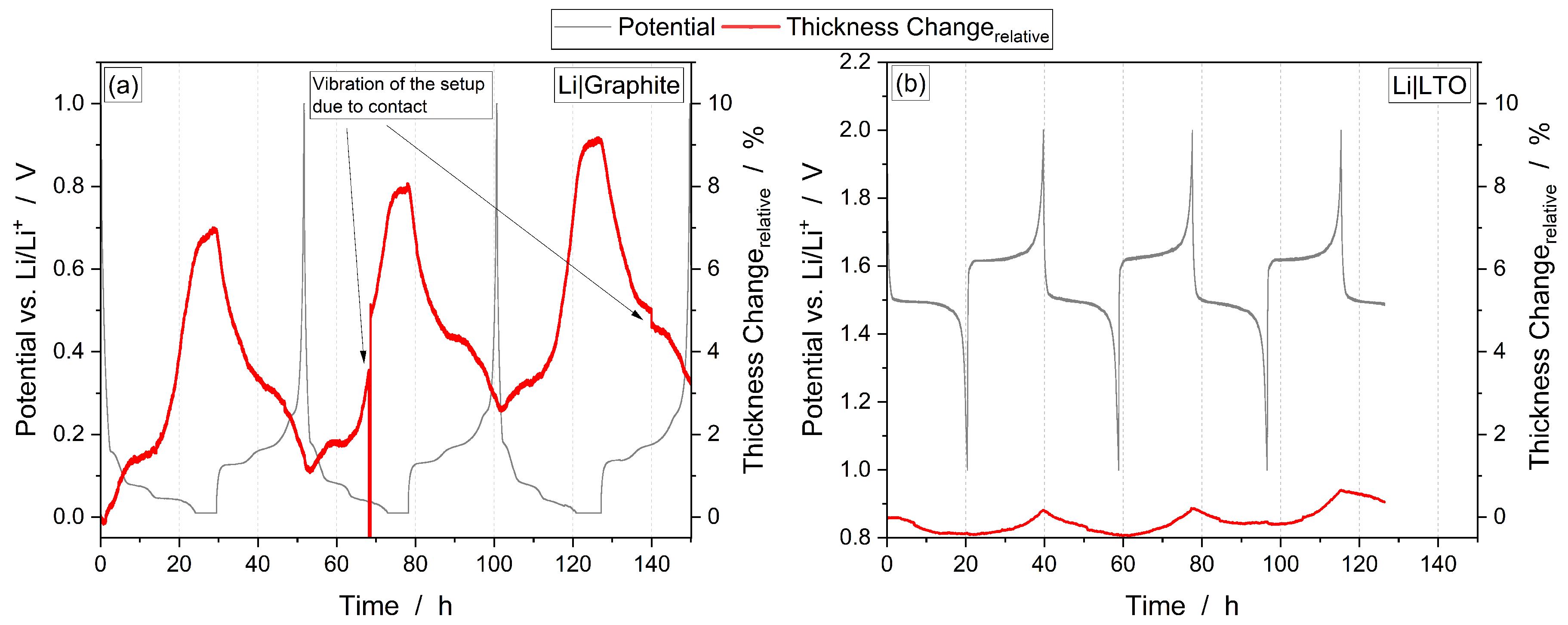
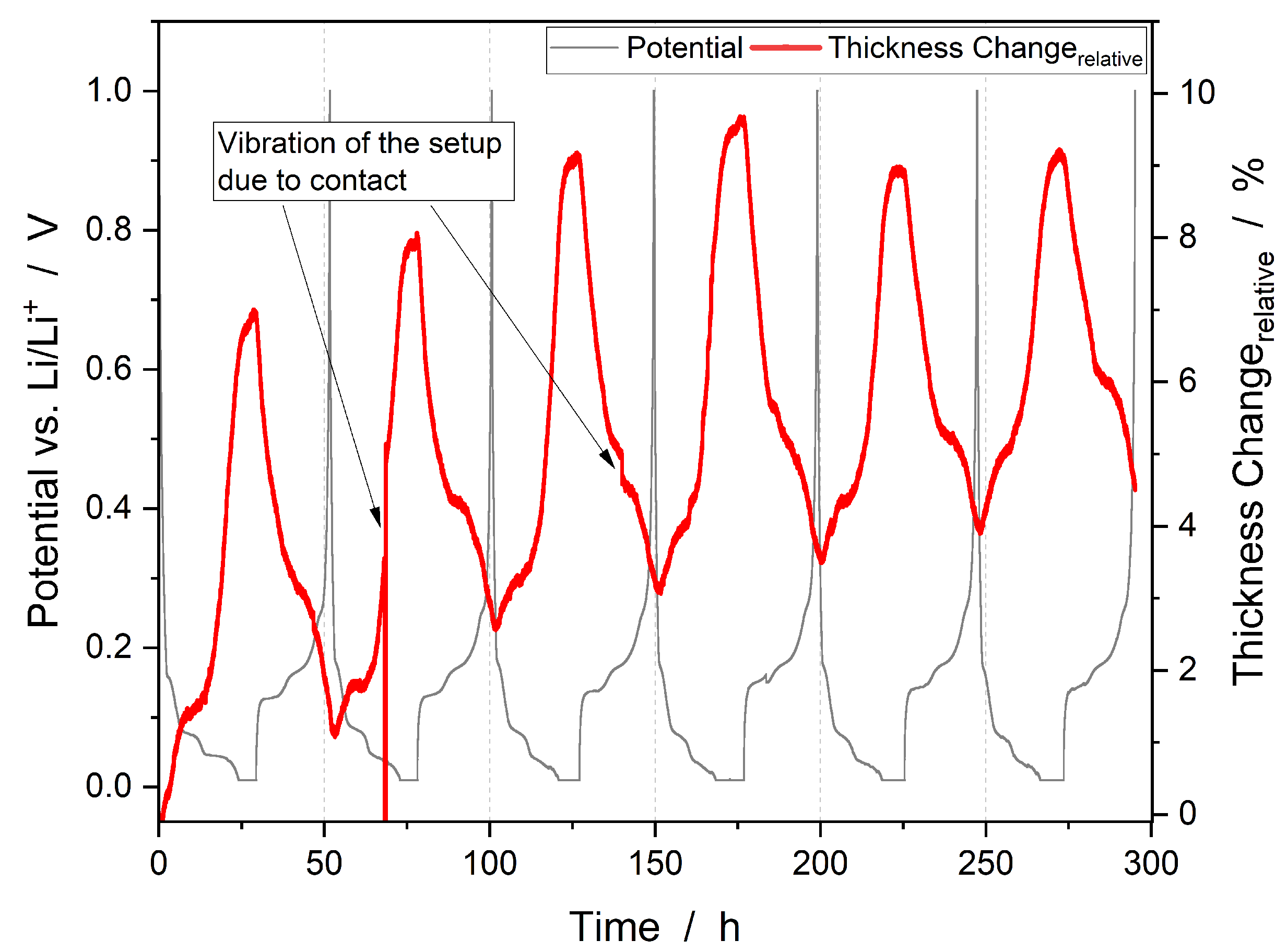
3.1. Combined Operando Dilatometry with Operando Mass Spectrometry
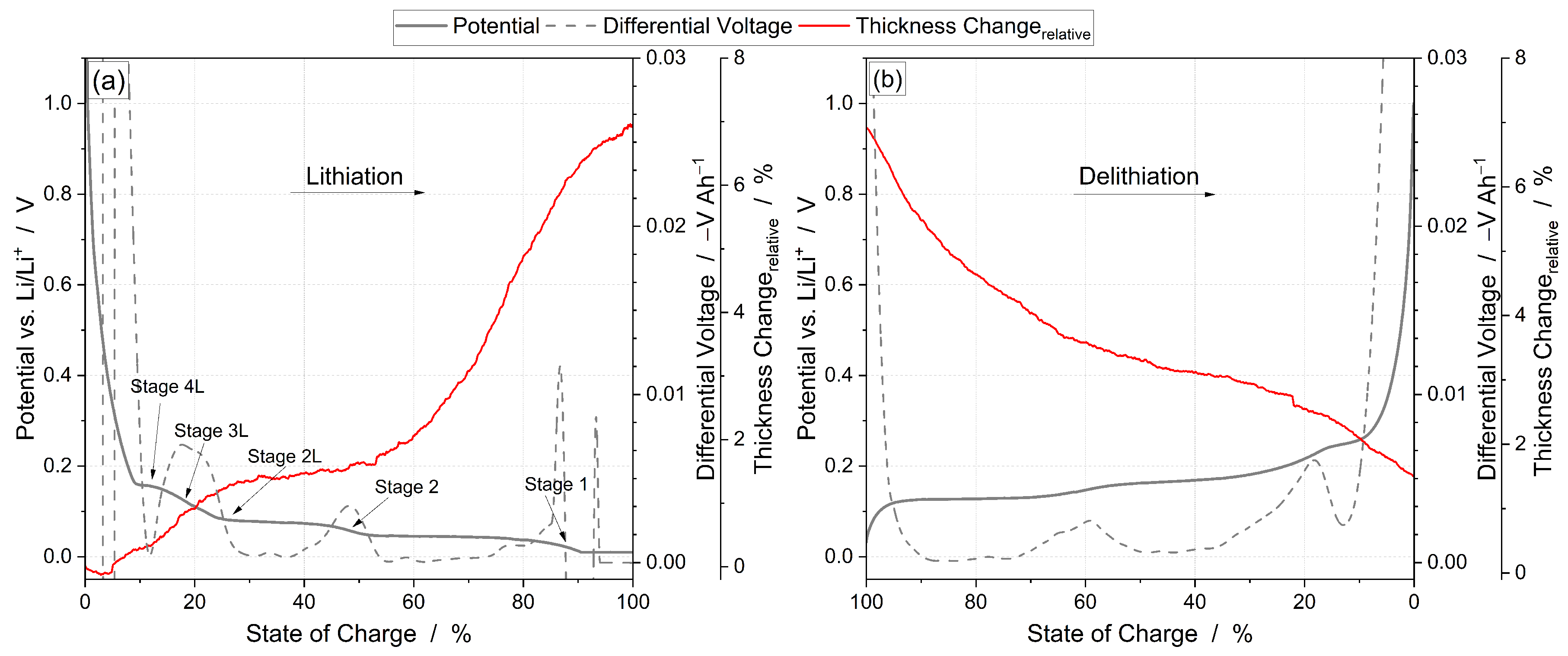
3.2. Thickness Change
3.3. SEI Formation and Electrolyte Degradation
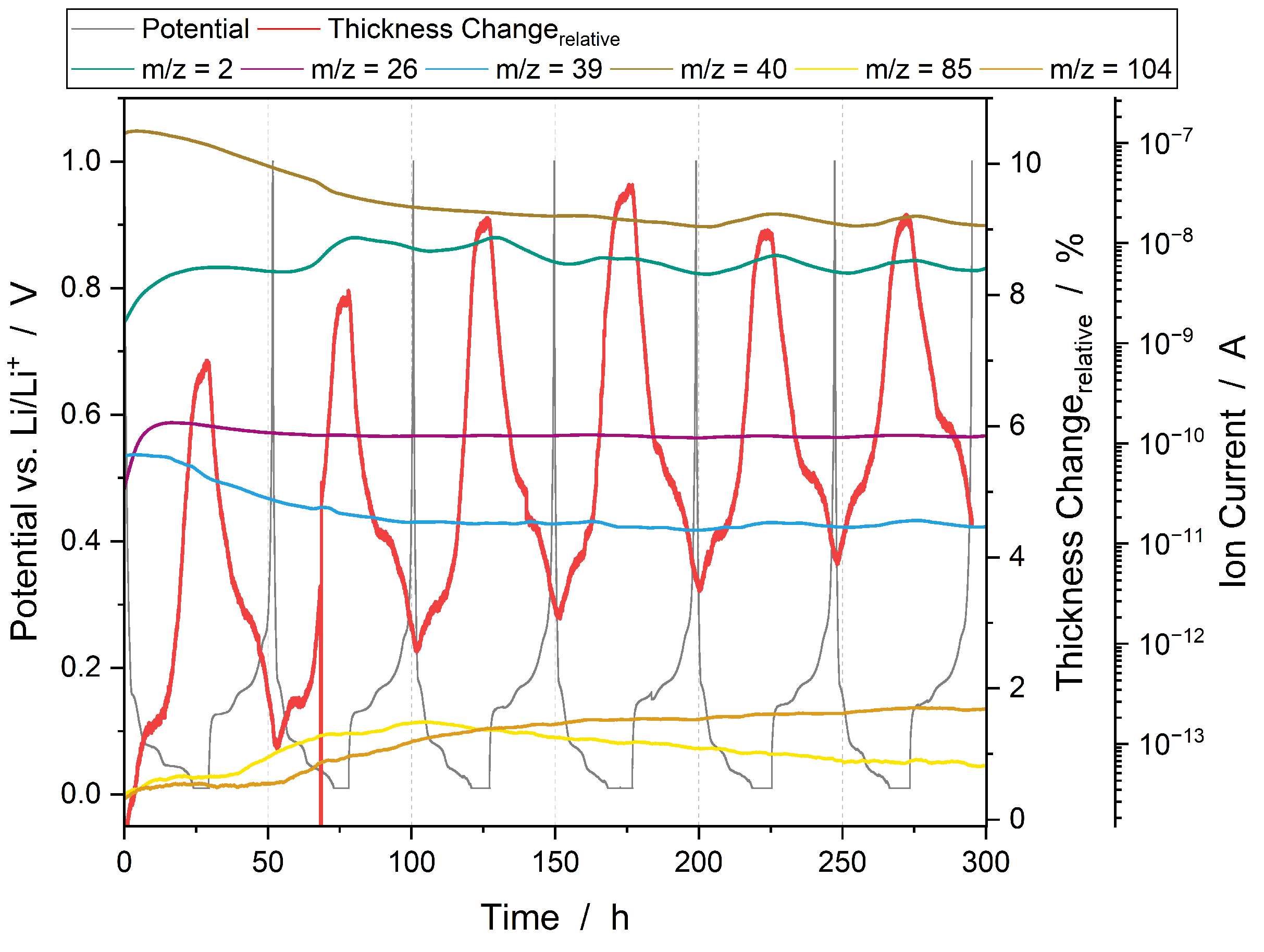
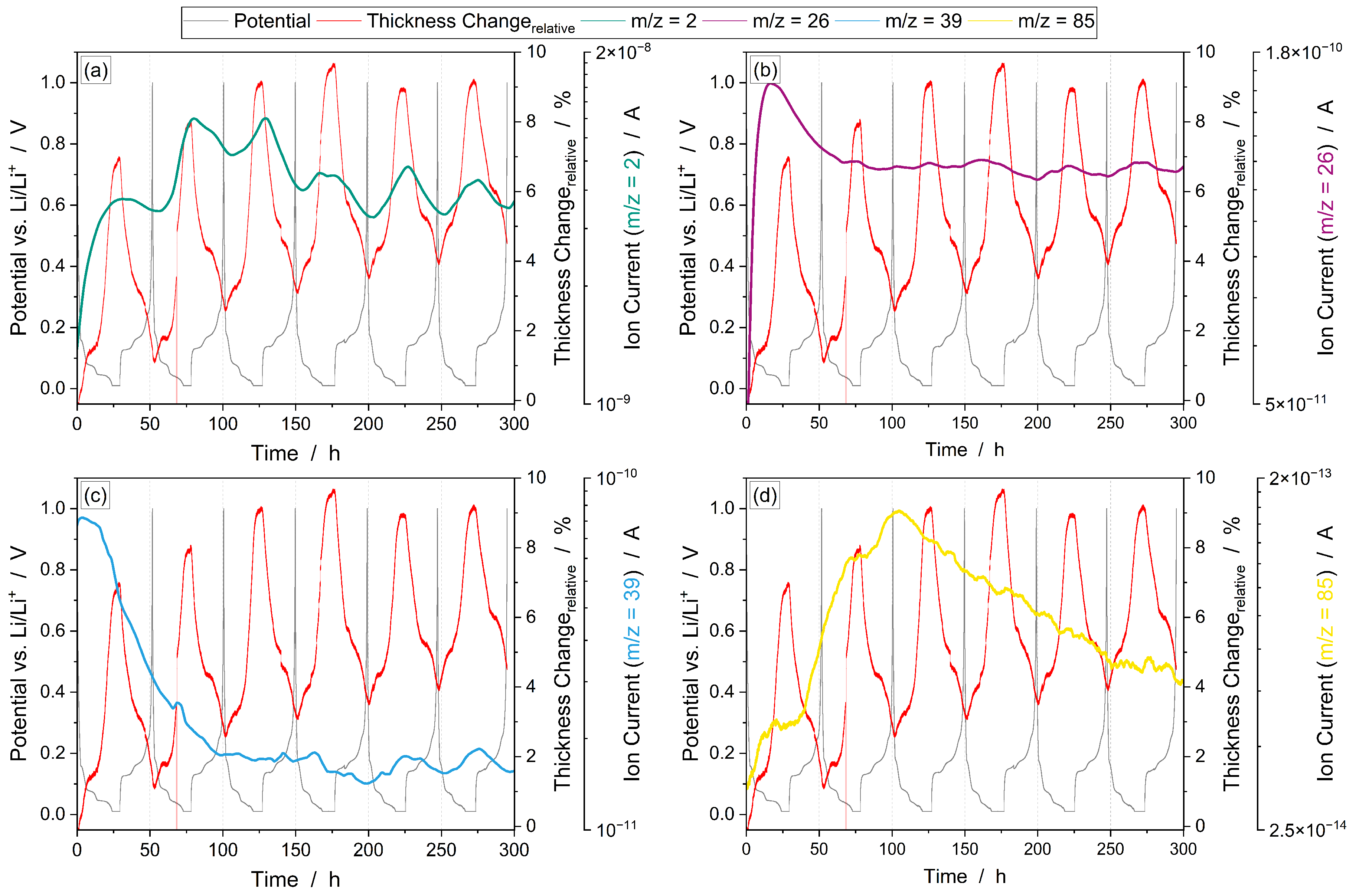
3.3.1. Operando Gas Analysis
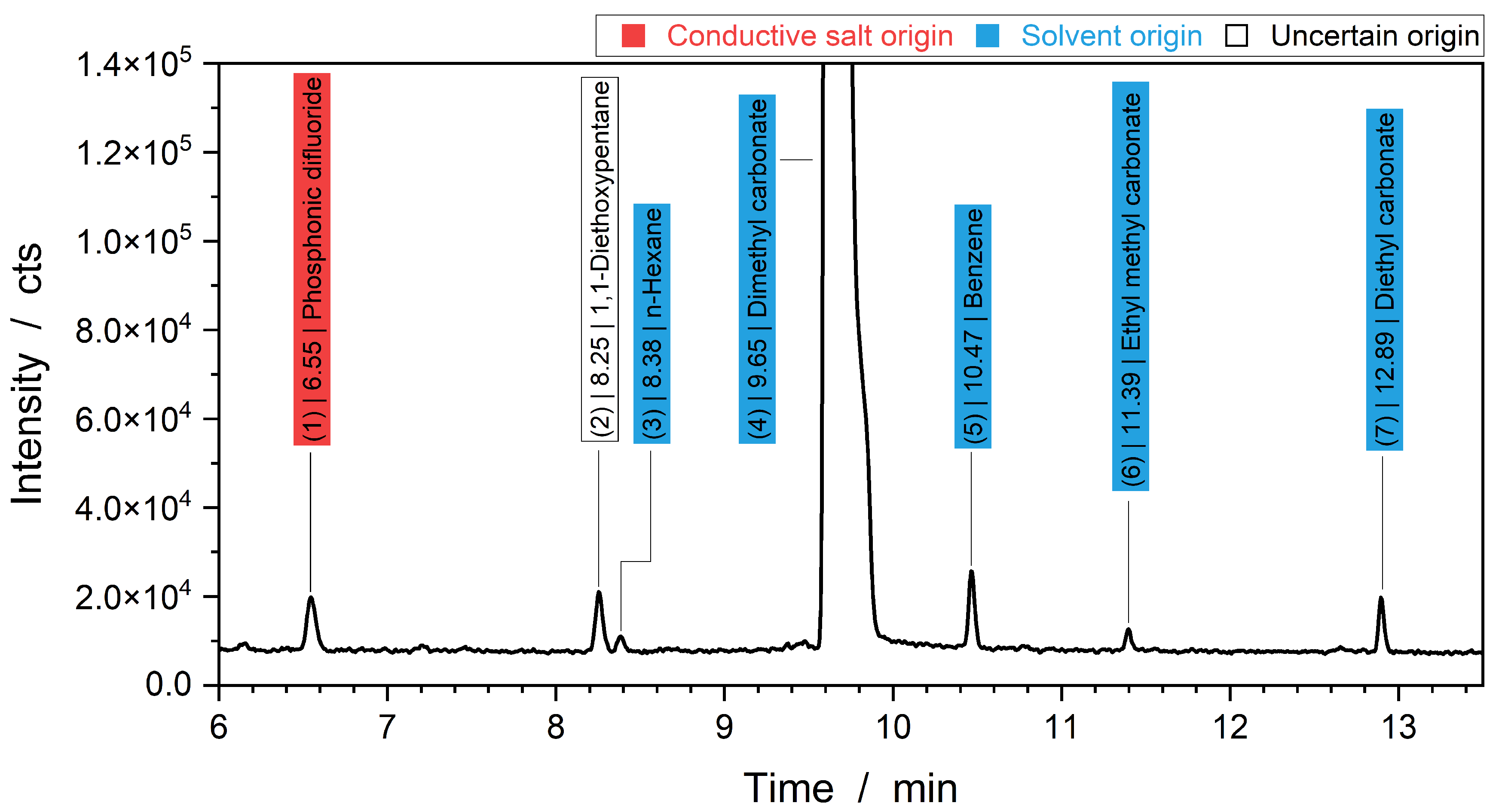
3.3.2. Post-Mortem Gas Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CC | Constant current |
| C-SEM | Continuous secondary electron multiplier |
| CTS | Cell test system |
| CV | Constant voltage |
| DEC | Diethyl carbonate |
| DMC | Dimethyl carbonate |
| DV | Differential voltage |
| EC | Ethylene carbonate |
| EMC | Ethyl methyl carbonate |
| FID | Flame ionization detector |
| GC | Gas chromatography |
| LCV | Lower cut-off voltage |
| LIB | Lithium ion battery |
| LTO | Lithium titanium oxide |
| LVDT | Linear variable differential transformer |
| MID | Multiple ion detection |
| MS | Mass spectrometry |
| NIST | National Institute of Standards and Technology |
| SEI | Solid electrolyte interface |
| SOC | State of charge |
| TCD | Thermal conductivity detector |
| TIC | Total ion current |
| UCV | Upper cut-off voltage |
| VC | Vinylene carbonate |
Appendix A
References
- Edge, J.S.; O’Kane, S.; Prosser, R.; Kirkaldy, N.D.; Patel, A.N.; Hales, A.; Ghosh, A.; Ai, W.; Chen, J.; Yang, J.; et al. Lithium ion battery degradation: What you need to know. Phys. Chem. Chem. Phys. PCCP 2021, 23, 8200–8221. [Google Scholar] [CrossRef] [PubMed]
- Ko, M.; Chae, S.; Cho, J. Challenges in Accommodating Volume Change of Si Anodes for Li-Ion Batteries. ChemElectroChem 2015, 2, 1645–1651. [Google Scholar] [CrossRef] [PubMed]
- Essl, C.; Seifert, L.; Rabe, M.; Fuchs, A. Early Detection of Failing Automotive Batteries Using Gas Sensors. Batteries 2021, 7, 25. [Google Scholar] [CrossRef]
- Waldmann, T.; Iturrondobeitia, A.; Kasper, M.; Ghanbari, N.; Aguesse, F.; Bekaert, E.; Daniel, L.; Genies, S.; Gordon, I.J.; Löble, M.W.; et al. Review—Post-Mortem Analysis of Aged Lithium-Ion Batteries: Disassembly Methodology and Physico-Chemical Analysis Techniques. J. Electrochem. Soc. 2016, 163, A2149–A2164. [Google Scholar] [CrossRef]
- Vetter, J.; Novák, P.; Wagner, M.R.; Veit, C.; Möller, K.C.; Besenhard, J.O.; Winter, M.; Wohlfahrt-Mehrens, M.; Vogler, C.; Hammouche, A. Ageing mechanisms in lithium-ion batteries. J. Power Sources 2005, 147, 269–281. [Google Scholar] [CrossRef]
- Pender, J.P.; Jha, G.; Youn, D.H.; Ziegler, J.M.; Andoni, I.; Choi, E.J.; Heller, A.; Dunn, B.S.; Weiss, P.S.; Penner, R.M.; et al. Electrode Degradation in Lithium-Ion Batteries. ACS Nano 2020, 14, 1243–1295. [Google Scholar] [CrossRef]
- Escher, I.; Hahn, M.; Ferrero, G.A.; Adelhelm, P. A Practical Guide for Using Electrochemical Dilatometry as Operando Tool in Battery and Supercapacitor Research. Energy Technol. 2022, 10, 2101120. [Google Scholar] [CrossRef]
- Michael, H.; Jervis, R.; Brett, D.J.L.; Shearing, P.R. Developments in Dilatometry for Characterisation of Electrochemical Devices. Batter. Supercaps 2021, 4, 1378–1396. [Google Scholar] [CrossRef]
- Bauer, M.; Wachtler, M.; Stöwe, H.; Persson, J.V.; Danzer, M.A. Understanding the dilation and dilation relaxation behavior of graphite-based lithium-ion cells. J. Power Sources 2016, 317, 93–102. [Google Scholar] [CrossRef]
- Pegel, H.; von Kessel, O.; Heugel, P.; Deich, T.; Tübke, J.; Birke, K.P.; Sauer, D.U. Volume and thickness change of NMC811|SiOx-graphite large-format lithium-ion cells: From pouch cell to active material level. J. Power Sources 2022, 537, 231443. [Google Scholar] [CrossRef]
- Hahn, M.; Buqa, H.; Ruch, P.W.; Goers, D.; Spahr, M.E.; Ufheil, J.; Novák, P.; Kötz, R. A Dilatometric Study of Lithium Intercalation into Powder-Type Graphite Electrodes. Electrochem. Solid-State Lett. 2008, 11, A151. [Google Scholar] [CrossRef]
- Heugel, P.; Märkle, W.; Deich, T.; von Kessel, O.; Tübke, J. Thickness change and jelly roll deformation and its impact on the aging and lifetime of commercial 18650 cylindrical Li-ion cells with silicon containing anodes and nickel-rich cathodes. J. Energy Storage 2022, 53, 105101. [Google Scholar] [CrossRef]
- Bazlen, S.; Heugel, P.; von Kessel, O.; Commerell, W.; Tübke, J. Influence of charging protocols on the charging capability and aging of lithium-ion cells with silicon-containing anodes. J. Energy Storage 2022, 49, 104044. [Google Scholar] [CrossRef]
- Holzapfel, M.; Buqa, H.; Hardwick, L.J.; Hahn, M.; Würsig, A.; Scheifele, W.; Novák, P.; Kötz, R.; Veit, C.; Petrat, F.M. Nano silicon for lithium-ion batteries. Electrochim. Acta 2006, 52, 973–978. [Google Scholar] [CrossRef]
- Jahn, L.; Katzer, F.; Danzer, M.A. Combined dilatometry and voltage analysis for a reliable detection of lithium deposition on graphitic anodes. J. Power Sources 2022, 520, 230870. [Google Scholar] [CrossRef]
- Zhang, L.; Tsolakidou, C.; Mariyappan, S.; Tarascon, J.M.; Trabesinger, S. Unraveling gas evolution in sodium batteries by online electrochemical mass spectrometry. Energy Storage Mater. 2021, 42, 12–21. [Google Scholar] [CrossRef]
- Holzapfel, M.; Würsig, A.; Scheifele, W.; Vetter, J.; Novák, P. Oxygen, hydrogen, ethylene and CO2 development in lithium-ion batteries. J. Power Sources 2007, 174, 1156–1160. [Google Scholar] [CrossRef]
- Seitzinger, C.L.; Sacci, R.L.; Coyle, J.E.; Apblett, C.A.; Hays, K.A.; Armstrong, R.R.; Rogers, A.M.; Armstrong, B.L.; Bennet, T.H.; Neale, N.R.; et al. Intrinsic Chemical Reactivity of Silicon Electrode Materials: Gas Evolution. Chem. Mater. 2020, 32, 3199–3210. [Google Scholar] [CrossRef]
- Metzger, M.; Strehle, B.; Solchenbach, S.; Gasteiger, H.A. Origin of H2 Evolution in LIBs: H2O Reduction vs. Electrolyte Oxidation. J. Electrochem. Soc. 2016, 163, A798–A809. [Google Scholar] [CrossRef]
- Bernhard, R.; Metzger, M.; Gasteiger, H.A. Gas Evolution at Graphite Anodes Depending on Electrolyte Water Content and SEI Quality Studied by On-Line Electrochemical Mass Spectrometry. J. Electrochem. Soc. 2015, 162, A1984–A1989. [Google Scholar] [CrossRef]
- Groher, C.; Cupid, D.M.; Mautner, A.; Rosenberg, E.; Kahr, J. Operando GC/MS for the investigation of different decomposition pathways during solid electrolyte interphase (SEI) formation with SEI forming additives. J. Power Sources 2024, 605, 234481. [Google Scholar] [CrossRef]
- Gachot, G.; Ribière, P.; Mathiron, D.; Grugeon, S.; Armand, M.; Leriche, J.B.; Pilard, S.; Laruelle, S. Gas chromatography/mass spectrometry as a suitable tool for the Li-ion battery electrolyte degradation mechanisms study. Anal. Chem. 2011, 83, 478–485. [Google Scholar] [CrossRef] [PubMed]
- Geisler, J.; Pfeiffer, L.; Ferrero, G.A.; Axmann, P.; Adelhelm, P. Setup Design and Data Evaluation for DEMS in Sodium Ion Batteries, Demonstrated on a Mn–Rich Cathode Material. Batter. Supercaps 2024, 7, e202400006. [Google Scholar] [CrossRef]
- Heugel, P.; Petit, J.; Klein, F.; Tübke, J. Investigation of the Influence of Silicon Oxide Content on Electrolyte Degradation, Gas Evolution, and Thickness Change in Silicon Oxide/Graphite Composite Anodes for Li-Ion Cells Using Operando Techniques. Batteries 2023, 9, 449. [Google Scholar] [CrossRef]
- Kreissl, J.J.A.; Petit, J.; Oppermann, R.; Cop, P.; Gerber, T.; Joos, M.; Abert, M.; Tübke, J.; Miyazaki, K.; Abe, T.; et al. Electrochemical Lithiation/Delithiation of ZnO in 3D-Structured Electrodes: Elucidating the Mechanism and the Solid Electrolyte Interphase Formation. ACS Appl. Mater. Interfaces 2021, 13, 35625–35638. [Google Scholar] [CrossRef]
- Winter, M. The Solid Electrolyte Interphase—The Most Important and the Least Understood Solid Electrolyte in Rechargeable Li Batteries. Z. Phys. Chem. 2009, 223, 1395–1406. [Google Scholar] [CrossRef]
- Rumberg, B.; Epding, B.; Stradtmann, I.; Kwade, A. Identification of Li ion battery cell aging mechanisms by half-cell and full-cell open-circuit-voltage characteristic analysis. J. Energy Storage 2019, 25, 100890. [Google Scholar] [CrossRef]
- Heiskanen, S.K.; Kim, J.; Lucht, B.L. Generation and Evolution of the Solid Electrolyte Interphase of Lithium-Ion Batteries. Joule 2019, 3, 2322–2333. [Google Scholar] [CrossRef]
- Gerasimov, M.; Soto, F.A.; Wagner, J.; Baakes, F.; Guo, N.; Ospina-Acevedo, F.; Röder, F.; Balbuena, P.B.; Krewer, U. Species Distribution During Solid Electrolyte Interphase Formation on Lithium Using MD/DFT-Parameterized Kinetic Monte Carlo Simulations. J. Phys. Chem. C 2023, 127, 4872–4886. [Google Scholar] [CrossRef]
- Zhang, B.; Metzger, M.; Solchenbach, S.; Payne, M.; Meini, S.; Gasteiger, H.A.; Garsuch, A.; Lucht, B.L. Role of 1,3-Propane Sultone and Vinylene Carbonate in Solid Electrolyte Interface Formation and Gas Generation. J. Phys. Chem. C 2015, 119, 11337–11348. [Google Scholar] [CrossRef]
- Nie, M.; Chalasani, D.; Abraham, D.P.; Chen, Y.; Bose, A.; Lucht, B.L. Lithium Ion Battery Graphite Solid Electrolyte Interphase Revealed by Microscopy and Spectroscopy. J. Phys. Chem. C 2013, 117, 1257–1267. [Google Scholar] [CrossRef]
- Ravdel, B.; Abraham, K.; Gitzendanner, R.; DiCarlo, J.; Lucht, B.; Campion, C. Thermal stability of lithium-ion battery electrolytes. J. Power Sources 2003, 119–121, 805–810. [Google Scholar] [CrossRef]
- Terborg, L.; Nowak, S.; Passerini, S.; Winter, M.; Karst, U.; Haddad, P.R.; Nesterenko, P.N. Ion chromatographic determination of hydrolysis products of hexafluorophosphate salts in aqueous solution. Anal. Chim. Acta 2012, 714, 121–126. [Google Scholar] [CrossRef] [PubMed]
- Stich, M.; Göttlinger, M.; Kurniawan, M.; Schmidt, U.; Bund, A. Hydrolysis of LiPF6 in Carbonate-Based Electrolytes for Lithium-Ion Batteries and in Aqueous Media. J. Phys. Chem. C 2018, 122, 8836–8842. [Google Scholar] [CrossRef]
- Han, J.G.; Kim, K.; Lee, Y.; Choi, N.S. Scavenging Materials to Stabilize LiPF6 -Containing Carbonate-Based Electrolytes for Li-Ion Batteries. Adv. Mater. 2019, 31, e1804822. [Google Scholar] [CrossRef]
- Kanayama, K.; Takahashi, S.; Nakamura, H.; Tezuka, T.; Maruta, K. Experimental and modeling study on pyrolysis of ethylene carbonate/dimethyl carbonate mixture. Combust. Flame 2022, 245, 112359. [Google Scholar] [CrossRef]
- Han, K.S.; Lee, M.S.; Kim, N.; Choi, D.; Chae, S.; Ryu, J.; Piccini, G.; Rousseau, R.; Thomsen, E.C. Lithium-ion hopping weakens thermal stability of LiPF6 carbonate electrolytes. Cell Rep. Phys. Sci. 2024, 5, 101768. [Google Scholar] [CrossRef]
- Ein-Eli, Y. Dimethyl carbonate (DMC) electrolytes—The effect of solvent purity on Li–ion intercalation into graphite anodes. Electrochem. Commun. 2002, 4, 644–648. [Google Scholar] [CrossRef]
- National Institute of Standards and Technology. The NIST Mass Spectral Search Program 2.3; National Institute of Standards and Technology: Gaithersburg, MD, USA, 2017. [Google Scholar]
- Sauerteig, D.; Ivanov, S.; Reinshagen, H.; Bund, A. Reversible and irreversible dilation of lithium-ion battery electrodes investigated by in-situ dilatometry. J. Power Sources 2017, 342, 939–946. [Google Scholar] [CrossRef]
- Daubinger, P.; Ebert, F.; Hartmann, S.; Giffin, G.A. Impact of electrochemical and mechanical interactions on lithium-ion battery performance investigated by operando dilatometry. J. Power Sources 2021, 488, 229457. [Google Scholar] [CrossRef]
- Ivanov, S.; Sauerteig, D.; Dimitrova, A.; Krischok, S.; Bund, A. Irreversible dilation of graphite composite anodes influenced by vinylene carbonate. J. Power Sources 2020, 457, 228020. [Google Scholar] [CrossRef]
- An, S.J.; Li, J.; Daniel, C.; Mohanty, D.; Nagpure, S.; Wood, D.L. The state of understanding of the lithium-ion-battery graphite solid electrolyte interphase (SEI) and its relationship to formation cycling. Carbon 2016, 105, 52–76. [Google Scholar] [CrossRef]
- Wang, A.; Kadam, S.; Li, H.; Shi, S.; Qi, Y. Review on modeling of the anode solid electrolyte interphase (SEI) for lithium-ion batteries. Npj Comput. Mater. 2018, 4, 15. [Google Scholar] [CrossRef]
- Ohzuku, T.; Iwakoshi, Y.; Sawai, K. Formation of Lithium–Graphite Intercalation Compounds in Nonaqueous Electrolytes and Their Application as a Negative Electrode for a Lithium Ion (Shuttlecock) Cell. J. Electrochem. Soc. 1993, 140, 2490. [Google Scholar] [CrossRef]
- Fill, A.; Zhan, M.; Stapf, N.; Hemmerling, J.; Avdyli, A.; Petit, J.; Heugel, P.; Tübke, J.; Birke, K.P. Electro-mechanical Li-Ion Cell Model considering the composite Graphite-Silicon Structure of the negative Electrode. In Proceedings of the 2024 7th International Conference on Electrical Engineering and Green Energy (CEEGE), Los Angeles, CA, USA, 28 June–1 July 2024; IEEE: Piscataway, NJ, USA, 2024; pp. 186–192. [Google Scholar] [CrossRef]
- Lory, P.F.; Mathieu, B.; Genies, S.; Reynier, Y.; Boulineau, A.; Hong, W.; Chandesris, M. Probing Silicon Lithiation in Silicon-Carbon Blended Anodes with a Multi-Scale Porous Electrode Model. J. Electrochem. Soc. 2020, 167, 120506. [Google Scholar] [CrossRef]
- Assefa, T.A.; Suzana, A.F.; Wu, L.; Koch, R.J.; Li, L.; Cha, W.; Harder, R.J.; Bozin, E.S.; Wang, F.; Robinson, I.K. Imaging the Phase Transformation in Single Particles of the Lithium Titanate Anode for Lithium-Ion Batteries. ACS Appl. Energy Mater. 2021, 4, 111–118. [Google Scholar] [CrossRef]
- Mukai, K.; Kato, Y.; Nakano, H. Understanding the Zero-Strain Lithium Insertion Scheme of Li[Li1/3Ti5/3]O4: Structural Changes at Atomic Scale Clarified by Raman Spectroscopy. J. Phys. Chem. C 2014, 118, 2992–2999. [Google Scholar] [CrossRef]
- Dahn. Phase diagram of LixC6. Phys. Rev. B Condens. Matter 1991, 44, 9170–9177. [Google Scholar] [CrossRef]
- Asenbauer, J.; Eisenmann, T.; Kuenzel, M.; Kazzazi, A.; Chen, Z.; Bresser, D. The success story of graphite as a lithium-ion anode material—Fundamentals, remaining challenges, and recent developments including silicon (oxide) composites. Sustain. Energy Fuels 2020, 4, 5387–5416. [Google Scholar] [CrossRef]
- Schmitt, C.; Kube, A.; Wagner, N.; Friedrich, K.A. Understanding the Influence of Temperature on Phase Evolution during Lithium–Graphite (De-)Intercalation Processes: An Operando X-ray Diffraction Study. ChemElectroChem 2022, 9, e202101342. [Google Scholar] [CrossRef]
- Schweidler, S.; de Biasi, L.; Schiele, A.; Hartmann, P.; Brezesinski, T.; Janek, J. Volume Changes of Graphite Anodes Revisited: A Combined Operando X-ray Diffraction and In Situ Pressure Analysis Study. J. Phys. Chem. C 2018, 122, 8829–8835. [Google Scholar] [CrossRef]
- Belharouak, I.; Koenig, G.M.; Amine, K. Electrochemistry and safety of Li4Ti5O12 and graphite anodes paired with LiMn2O4 for hybrid electric vehicle Li-ion battery applications. J. Power Sources 2011, 196, 10344–10350. [Google Scholar] [CrossRef]
- Belharouak, I.; Koenig, G.M.; Tan, T.; Yumoto, H.; Ota, N.; Amine, K. Performance Degradation and Gassing of Li4Ti5O12/LiMn2O4 Lithium-Ion Cells. J. Electrochem. Soc. 2012, 159, A1165–A1170. [Google Scholar] [CrossRef]
- Bernhard, R.; Meini, S.; Gasteiger, H.A. On-Line Electrochemical Mass Spectrometry Investigations on the Gassing Behavior of Li4Ti5O12 Electrodes and Its Origins. J. Electrochem. Soc. 2014, 161, A497–A505. [Google Scholar] [CrossRef]
- Lanz, M.; Novák, P. DEMS study of gas evolution at thick graphite electrodes for lithium-ion batteries: The effect of gamma-butyrolactone. J. Power Sources 2001, 102, 277–282. [Google Scholar] [CrossRef]
- Pritzl, D.; Solchenbach, S.; Wetjen, M.; Gasteiger, H.A. Analysis of Vinylene Carbonate (VC) as Additive in Graphite/LiNi0.5Mn1.5O4 Cells. J. Electrochem. Soc. 2017, 164, A2625–A2635. [Google Scholar] [CrossRef]
- Kriston, A.; Adanouj, I.; Ruiz, V.; Pfrang, A. Quantification and simulation of thermal decomposition reactions of Li-ion battery materials by simultaneous thermal analysis coupled with gas analysis. J. Power Sources 2019, 435, 226774. [Google Scholar] [CrossRef]
- Plakhotnyk, A.V.; Ernst, L.; Schmutzler, R. Hydrolysis in the system LiPF6—Propylene carbonate—Dimethyl carbonate—H2O. J. Fluor. Chem. 2005, 126, 27–31. [Google Scholar] [CrossRef]
- Sloop, S.E.; Pugh, J.K.; Wang, S.; Kerr, J.B.; Kinoshita, K. Chemical Reactivity of PF5 and LiPF6 in Ethylene Carbonate/Dimethyl Carbonate Solutions. Electrochem. Solid-State Lett. 2001, 4, A42. [Google Scholar] [CrossRef]
- Terborg, L.; Weber, S.; Blaske, F.; Passerini, S.; Winter, M.; Karst, U.; Nowak, S. Investigation of thermal aging and hydrolysis mechanisms in commercial lithium ion battery electrolyte. J. Power Sources 2013, 242, 832–837. [Google Scholar] [CrossRef]
- Tasaki, K.; Kanda, K.; Nakamura, S.; Ue, M. Decomposition of LiPF6 and Stability of PF5 in Li-Ion Battery Electrolytes. J. Electrochem. Soc. 2003, 150, A1628. [Google Scholar] [CrossRef]
- Stenzel, Y.; Horsthemke, F.; Winter, M.; Nowak, S. Chromatographic Techniques in the Research Area of Lithium Ion Batteries: Current State-of-the-Art. Separations 2019, 6, 26. [Google Scholar] [CrossRef]
- Kahr, J.; Groher, C.; Schierer, V.; Rosenberg, E.; Jahn, M. Operando gas chromatography mass spectrometry for the continuous study of overcharge-induced electrolyte decomposition in lithium-ion batteries. J. Power Sources 2024, 615, 235038. [Google Scholar] [CrossRef]
- Nedjalkov, A.; Meyer, J.; Köhring, M.; Doering, A.; Angelmahr, M.; Dahle, S.; Sander, A.; Fischer, A.; Schade, W. Toxic Gas Emissions from Damaged Lithium Ion Batteries—Analysis and Safety Enhancement Solution. Batteries 2016, 2, 5. [Google Scholar] [CrossRef]
- Daubinger, P.; Göttlinger, M.; Hartmann, S.; Giffin, G.A. Consequences of Different Pressures and Electrolytes on the Irreversible Expansion of Lithium Metal Half Cells. Batter. Supercaps 2023, 6, e202200452. [Google Scholar] [CrossRef]
- Mukai, K.; Yamada, I. High-pressure study of Li[Li1/3Ti5/3]O4 spinel. Inorg. Chem. Front. 2018, 5, 1941–1949. [Google Scholar] [CrossRef]
| Command | Parameter | Termination | Comment |
|---|---|---|---|
| Pause | t > 27 h | Settle time | |
| Cycle-Start | |||
| Discharge | I = −0.05 C | U = 10 mV | CC Discharge (Lithiation) |
| Discharge | U = 10 mV I = −0.05 C | I > −0.01 C t > 10 h | CV Discharge (Lithiation) |
| Charge | I = 0.05 C | U = 1.0 V | CC Charge (Delithiation) |
| Charge | U = 1.0 V I = 0.05 C | I < 0.01 C t > 10 h | CV Charge (Delithiation) |
| Cycle-End | Count = 6 | Repeat for 6 cycles | |
| Pause | t > 5 min |
| Peak Number | Retention Time [min] | Compound Name |
|---|---|---|
| 1 | 6.55 | Phosphonic difluoride |
| 2 | 8.25 | 1,1-Diethoxyopentane |
| 3 | 8.38 | n-Hexane |
| 4 | 9.65 | Dimethyl carbonate (DMC) |
| 5 | 10.47 | Benzene |
| 6 | 11.39 | Ethyl methyl carbonate (EMC) |
| 7 | 12.89 | Diethyl carbonate (DEC) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Petit, J.; Heugel, P.; Geiger, S.; Klein, F.; Tübke, J. The Novel Coupling of Operando Methods: Electrochemical Dilatometry with Mass Spectrometry Using the Example of a Li|Graphite Half Cell. Batteries 2024, 10, 445. https://doi.org/10.3390/batteries10120445
Petit J, Heugel P, Geiger S, Klein F, Tübke J. The Novel Coupling of Operando Methods: Electrochemical Dilatometry with Mass Spectrometry Using the Example of a Li|Graphite Half Cell. Batteries. 2024; 10(12):445. https://doi.org/10.3390/batteries10120445
Chicago/Turabian StylePetit, Jan, Philipp Heugel, Sebastian Geiger, Franziska Klein, and Jens Tübke. 2024. "The Novel Coupling of Operando Methods: Electrochemical Dilatometry with Mass Spectrometry Using the Example of a Li|Graphite Half Cell" Batteries 10, no. 12: 445. https://doi.org/10.3390/batteries10120445
APA StylePetit, J., Heugel, P., Geiger, S., Klein, F., & Tübke, J. (2024). The Novel Coupling of Operando Methods: Electrochemical Dilatometry with Mass Spectrometry Using the Example of a Li|Graphite Half Cell. Batteries, 10(12), 445. https://doi.org/10.3390/batteries10120445







