Fine-Tuning Cathode Performance: The Influence of Argon Deposition Pressure on LiMn2O4 Thin Film Electrochemistry for Li-Ion Batteries
Abstract
1. Introduction
2. Materials and Methods
2.1. Substrate Preparation
2.2. Electrode Cathode Deposition
2.3. Physicochemical Characterization
2.4. Battery Assembly and Electrochemical Characterization
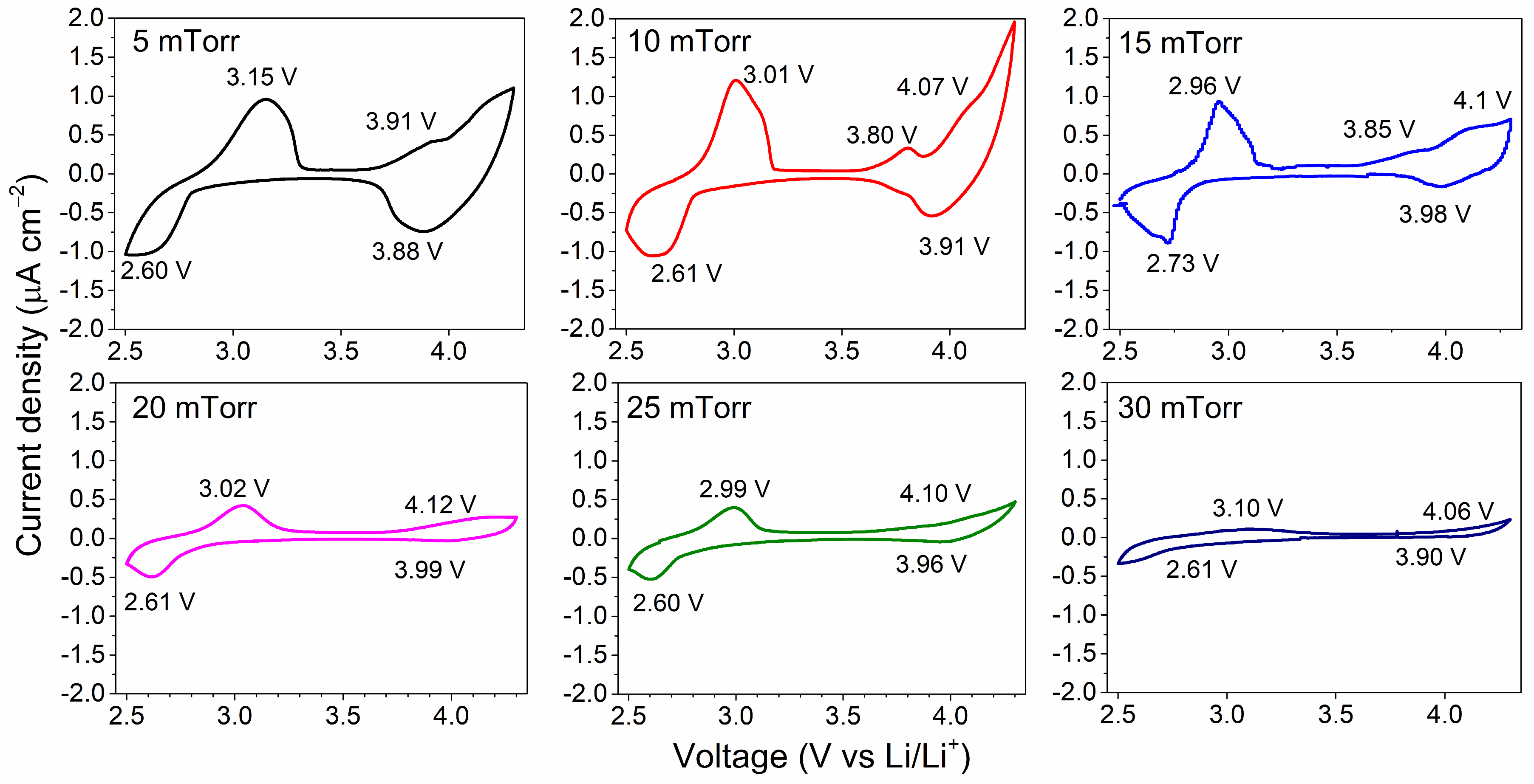
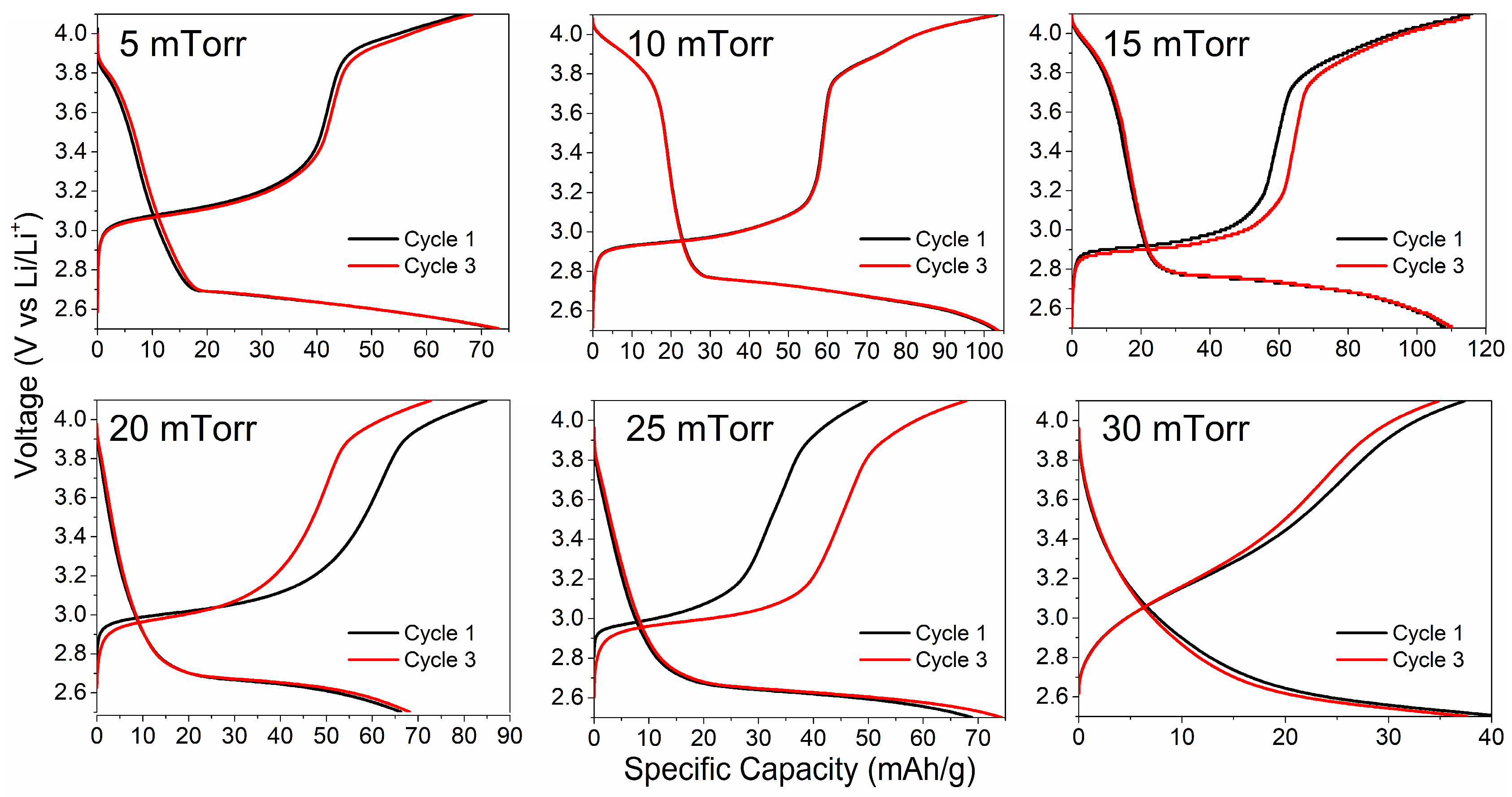
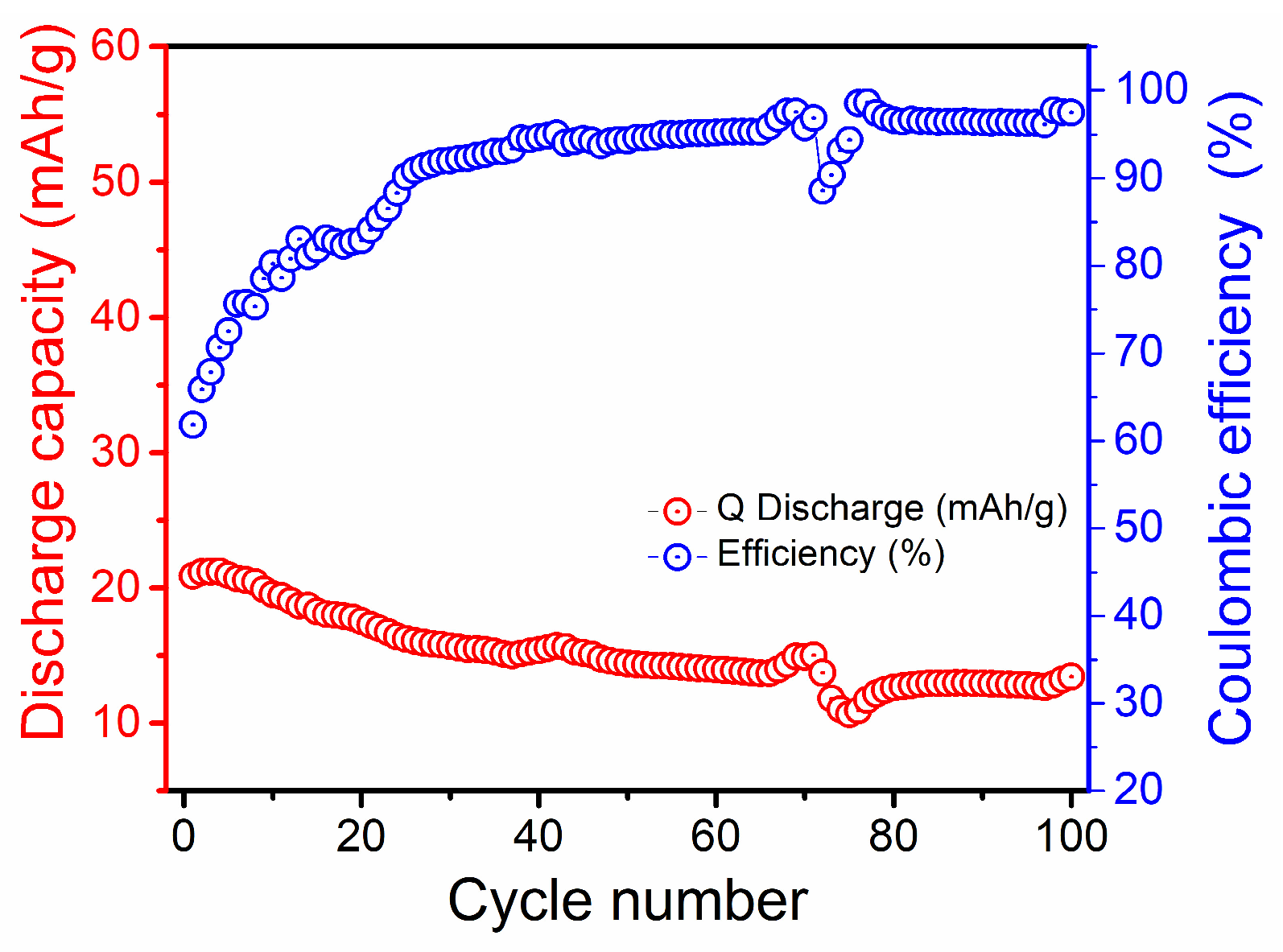
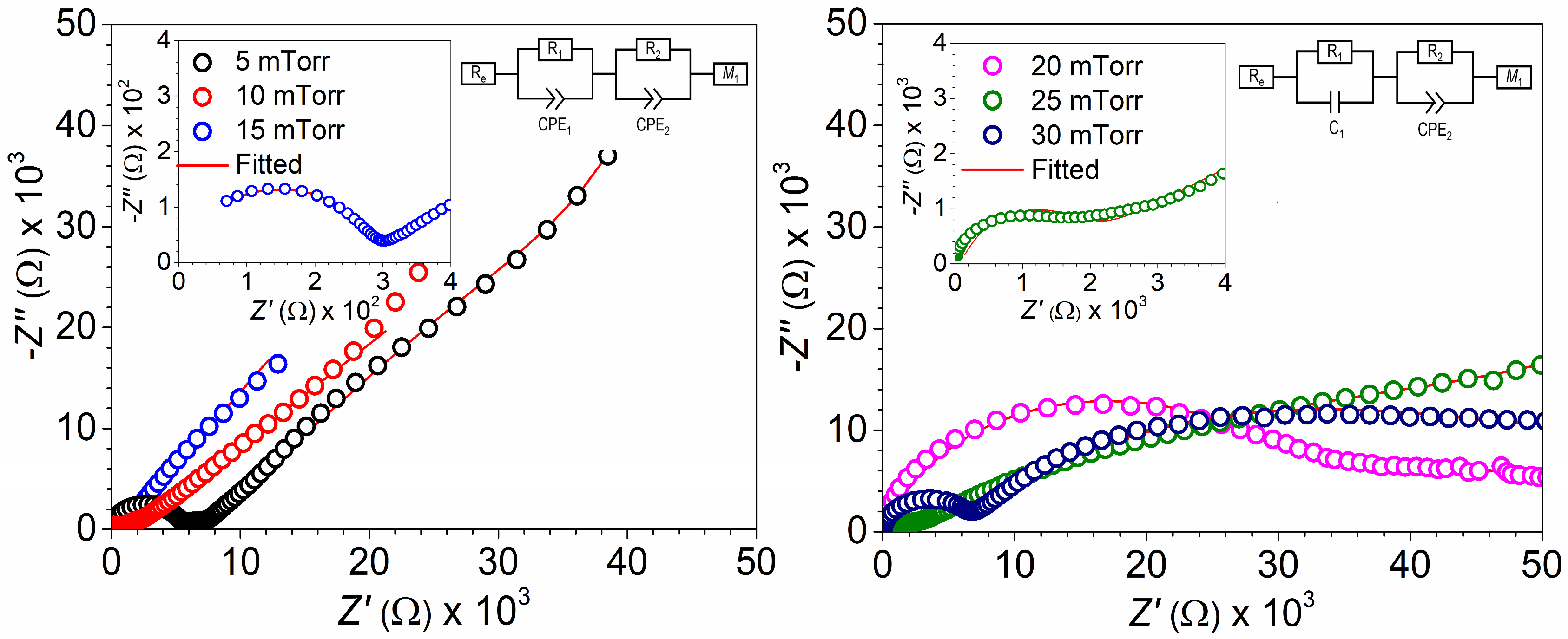
3. Results and Discussion
3.1. Microstructural Properties
3.2. Chemical Properties
3.3. Crystalline Structure Properties
3.4. Electrochemical Performance
4. Conclusions
- Group I (stoichiometric LiMn2O4 cathode materials): This group was obtained in the argon deposition range from 5 to 15 mTorr, where an argon deposition pressure of 15 mTorr leads to the growth of a 30 nm thick LiMn2O4 cathode material which exhibits low charge transfer at both the electrolyte/electrode interface (R1 = 271 Ω) and the surface (R2 = 619 Ω), alongside high lithium-ion diffusion (DLi+ = 2.19 × 10−13 cm2/s), facilitating a high discharge capacity of 116 mAh/g.
- Group II (non-stoichiometric LiMn2O4 cathode materials with lithium deficiency): This group was obtained in the argon deposition range from 20 to 30 mTorr. The deposition of LiMn2O4 cathode materials with lithium deficiency at the lower end of the range (30 mTorr) resulted in a 30 nm thick cathode material with an unbalanced Mn4+/Mn3+ ratio of 0.2. This led to a decrease in the oxidation/reduction peaks in cyclic voltammograms at of high-voltage regime (3.7 to 4.1 V vs. Li/Li+) and a reduction in the plateau region in the charge/discharge curve, ultimately resulting in a moderate discharge capacity of 40 mAh/g.
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rambabu, A.; Krupanidhi, S.B.; Barpanda, P. An overview of nanostructured Li-based thin film micro-batteries. Proc. Indian Natl. Sci. Acad. 2019, 85, 121–142. [Google Scholar] [CrossRef]
- Yu, Y.; Gong, M.; Dong, C.; Xu, X. Thin-film deposition techniques in surface and interface engineering of solid-state lithium batteries. Next Nanotechnol. 2023, 3–4, 100028. [Google Scholar] [CrossRef]
- Zhou, Y.N.; Xue, M.Z.; Fu, Z.W. Nanostructured thin film electrodes for lithium storage and all-solid-state thin-film lithium batteries. J. Power Sources 2013, 23, 310–332. [Google Scholar] [CrossRef]
- Moitzheim, S.; Put, B.; Vereecken, P.M. Advances in 3D thin-film Li-ion batteries. Adv. Mater. Interfaces 2019, 6, 1900805. [Google Scholar] [CrossRef]
- Sun, K.; Wei, T.S.; Ahn, B.Y.; Seo, J.Y.; Dillon, S.J.; Lewis, J.A. 3D printing of interdigitated Li-ion microbattery architectures. Adv. Mater. 2013, 25, 4539–4543. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, B.; Li, Q.; Cartmell, S.; Ferrara, S.; Deng, Z.D.; Xiao, J. Lithium and lithium-ion batteries for applications in microelectronic devices: A review. J. Power Sources 2015, 286, 330–345. [Google Scholar] [CrossRef]
- Liu, L.; Weng, Q.; Lu, X.; Sun, X.; Zhang, L.; Schmidt, O.G. Advances on Microsized On-Chip Lithium-Ion Batteries. Small 2017, 13, 1701847. [Google Scholar] [CrossRef]
- Wu, T.; Dai, W.; Ke, M.; Huang, Q.; Lu, L. All-Solid-State Thin Film μ-Batteries for Microelectronics. Adv. Sci. 2021, 8, 2100774. [Google Scholar] [CrossRef] [PubMed]
- Meunier, G.; Dormoy, R.; Levasseur, A. New Positive Electrode Materials for Lithium Thin Film Secondary Batteries. Mater. Sci. Eng. B 1989, 83, 19–23. [Google Scholar] [CrossRef]
- Jones, S.D.; Akridge, J.R. A thin-film solid-state microbattery. J. Power Sources 1993, 43-44, 505–551. [Google Scholar] [CrossRef]
- Fu, W.; Wang, Y.; Kong, K.; Kim, D.; Wang, F.; Yushin, G. Materials and Processing of Lithium-Ion Battery Cathodes. Nanoenergy Adv. 2023, 3, 138–154. [Google Scholar] [CrossRef]
- Chakraborty, A.; Kunnikuruvan, S.; Kumar, S.; Markovsky, B.; Aurbach, D.; Dixit, M.; Major, D.T. Layered Cathode Materials for Lithium-Ion Batteries: Review of Computational Studies on LiNi1−x−yCoxMnyO2 and LiNi1−x−yCoxAlyO2. Chem. Mater. 2020, 32, 915–952. [Google Scholar] [CrossRef]
- Ammundsen, B.; Paulsen, J. Novel Lithium-Ion Cathode Materials Based on Layered Manganese Oxides. Adv. Mater. 2001, 13, 943–956. [Google Scholar] [CrossRef]
- Huang, Y.; Dong, Y.; Li, S.; Lee, J.; Wang, C.; Zhu, Z.; Xue, W.; Li, Y.; Li, J. Lithium Manganese Spinel Cathodes for Lithium-Ion Batteries. Adv. Energy Mater. 2020, 11, 2000997. [Google Scholar] [CrossRef]
- Thackeray, M.M.; David, W.I.; Bruce, P.G.; Goodenough, J.B. Lithium insertion into manganese spinels. Mater. Res. Bull. 1983, 18, 461. [Google Scholar] [CrossRef]
- Thackeray, M.M.; Johnson, P.J.; De Picciotto, L.A.; Bruce, P.G.; Goodenough, J.B. Electrochemical extraction of lithium from LiMn2O4. Mater. Res. Bull. 1984, 19, 179. [Google Scholar] [CrossRef]
- Ma, Y.; Li, L.; Qian, J.; Qu, W.; Luo, R.; Wu, F.; Chen, R. Materials and structure engineering by magnetron sputtering for advanced lithium batteries. Energy Storage Mater. 2021, 39, 203–224. [Google Scholar] [CrossRef]
- Ugalde-Vázquez, R.M.; Ambriz-Vargas, F.; Morales-Morales, F.; Hernández-Sebastián, N.; Benítez-Lara, A.; Cabrera-Sierra, R.; Gomez-Yañez, C. Effect of argon sputtering pressure on the electrochemical performance of LiFePO4 cathode. J. Eur. Ceram. Soc. 2023, 43, 407–418. [Google Scholar] [CrossRef]
- Ye, R.; Ohta, K.; Baba, M. Fabrication and Characterization of LiMn2O4 Thin Films for Flexible Thin Film Lithium ion Batteries: Effect of thermal Annealing. Int. J. Eng. Sci. Technol. 2021, 8, 2394–3661. [Google Scholar] [CrossRef]
- Chen, H.C.; Jan, D.J.; Lin, B.C.; Hsueh, T.H. Nanostructure distortion improvement of Al doped spinel LiMn2O4 films deposited by RF magnetron sputtering for flexible high-voltage lithium ion batteries. Mater. Res. Bull. 2021, 140, 111313. [Google Scholar] [CrossRef]
- Hsueh, T.H.; Yu, Y.Q.; Jan, D.J.; Su, C.H.; Chang, S.M. Checkerboard deposition of lithium manganese oxide spinel (LiMn2O4) by RF magnetron sputtering on a stainless steel in all-solid-state thin film battery. IOP Conf. Ser. Mater. Sci. Eng. 2018, 324, 012004. [Google Scholar] [CrossRef]
- Prasad, K.H.; Vinoth, S.; Ratnakar, A.; Venkateswarlu, M.; Satyanarayana, N. Satyanarayana. Structural and Electrical Conductivity studies of Spinel LiMn2O4 Cathode films grown by RF Sputtering. Mater. Today Proc. 2016, 3, 4046–4051. [Google Scholar] [CrossRef]
- Kong, W.Y.; Yim, H.; Yoon, S.J.; Nahm, S.; Choi, J.W. Electrochemical Properties of Sn-Substituted LiMn2O4 Thin Films Prepared by Radio-Frequency Magnetron Sputtering. J. Nanosci. Nanotechnol. 2013, 13, 3288–3292. [Google Scholar] [CrossRef] [PubMed]
- Jayanth Babu, K.; Jeevan Kumar, P.; Hussain, O.M. Microstructural and electrochemical properties of rf-sputtered LiMn2O4 thin film cathodes. Appl. Nanosci. 2012, 2, 401–407. [Google Scholar] [CrossRef][Green Version]
- Zhu, J.; Zeng, K.; Lu, L. Cycling effects on surface morphology, nanomechanical and interfacial reliability of LiMn2O4 cathode in thin film lithium ion batteries. Electrochim. Acta. 2012, 68, 52–59. [Google Scholar] [CrossRef]
- Isai, M.; Nakamura, K.; Hosokawa, T.; Sakai, S.; Hosoe, S. Preparation of LiMn2O4 Films by RF Magnetron Sputtering Method. Trans. Mat. Res. Soc. Jpn. 2009, 34, 355–358. [Google Scholar] [CrossRef][Green Version]
- Hwang, B.J.; Wang, C.Y.; Cheng, M.Y.; Santhanam, R. Structure, Morphology, and Electrochemical Investigation of LiMn2O4 Thin Film Cathodes Deposited by Radio Frequency Sputtering for Lithium Microbatteries. J. Phys. Chem. C 2009, 113, 11373–11380. [Google Scholar] [CrossRef]
- Xie, J.; Tanaka, T.; Imanishi, N.; Matsumura, T.; Hirano, A.; Takeda, Y.; Yamamoto, O. Yamamoto. Li-ion transport kinetics in LiMn2O4 thin films prepared by radio frequency magnetron sputtering. J. Power Sources 2008, 180, 576–581. [Google Scholar] [CrossRef]
- Thomann, A.L.; Caillard, A.; Raza, M.; El Mokh, M.; Cormier, P.A.; Konstantinidis, S. Energy flux measurements during magnetron sputter deposition processes. Surf. Coat. Technol. 2019, 377, 124887. [Google Scholar] [CrossRef]
- Jameel, D. Thin Film Deposition Processes. J. Mod. Phys. A 2015, 1, 193–199. [Google Scholar]
- Julien, C.M.; Mauger, A.; Hussain, O.M. Sputtered LiCoO2 Cathode Materials for All-Solid-State Thin-Film Lithium Microbatteries. Materials 2019, 12, 2687. [Google Scholar] [CrossRef]
- Albrecht, D.; Wulfmeier, H.; Fritze, H. Preparation and Characterization of c-LiMn2O4 Thin Filmsprepared by Pulsed Laser Deposition for Lithium-Ion Batteries. Energy Technol. 2016, 4, 1558–1564. [Google Scholar] [CrossRef]
- Chen, Z.; Zhang, W.; Yang, Z. A review on cathode materials for advanced lithium ion batteries: Microstructure designs and performance regulations. Nanotechnology 2020, 31, 012001. [Google Scholar] [CrossRef] [PubMed]
- Shirazi Moghadam, Y.; El Kharbachi, A.; Diemant, T.; Melinte, G.; Hu, Y.; Fichtner, M. Toward Better Stability and Reversibility of the Mn4+/Mn2+ Double Redox Activity in Disordered Rocksalt Oxyfluoride Cathode Materials. Chem. Mater. 2021, 33, 8235–8247. [Google Scholar] [CrossRef]
- Aghilizadeh, N.; Sari, A.H.; Dorranian, D. Role of Ar/O2 mixture on structural, compositional and optical properties of thin copper oxide films deposited by DC magnetron sputtering. Theor. Appl. Phys. 2017, 11, 285–290. [Google Scholar] [CrossRef]
- Tarascon, J.M.; Guyomard, D. Li Metal-Free Rechargeable Batteries Based on Lil+xMn2O4 Cathodes ( 0 ≤ x ≤ 1 ) and Carbon Anodes. J. Electrochem. Soc. 1991, 138, 2864–2868. [Google Scholar] [CrossRef]
- Lee, J.; Kitchaev, D.A.; Kwon, D.H.; Lee, C.W.; Papp, J.K.; Liu, Y.S.; Lun, Z.; Clément, R.J.; Shi, T.; McCloskey, B.D.; et al. Reversible Mn2+/Mn4+ double redox in lithium-excess cathode materials. Nature 2018, 556, 185–190. [Google Scholar] [CrossRef] [PubMed]
- Poolcharuansin, P.; Chingsungnoen, A.; Pasaja, N.; Horprathum, M.; Bradley, J.W. Measurement of negative ion fluxes during DC reactive magnetron sputtering of Ti in Ar/O2 atmosphere using a magnetic-filtering probe. Vacuum 2021, 194, 110549. [Google Scholar] [CrossRef]
- Vargas, F.A.; Nouar, R.; Bacar, Z.S.; Higuera, B.; Porter, R.; Sarkissian, A.; Thomas, R.; Ruediger, A. On-axis radio frequency magnetron sputtering of stoichiometric BaTiO3 target: Localized re-sputtering and substrate etching during thin film growth. Thin Solid Film. 2015, 596, 77–82. [Google Scholar] [CrossRef]
- Ramana, C.V.; Massot, M.; Julien, C.M. XPS and Raman spectroscopic characterization of LiMn2O4 spinels. Surf. Interface Anal. 2005, 37, 412–416. [Google Scholar] [CrossRef]
- Julien, C.M.; Massot, M. Lattice Vibrations of Materials for Lithium Rechargeable Batteries I. Lithium Manganese Oxide Spinel. Mater. Sci. Eng. B 2003, 97, 217–230. [Google Scholar] [CrossRef]
- Baddour-Hadjean, R.; Pereira-Ramos, J.P. Microspectrometry Applied to the Study of Electrode Materials for Lithium Batteries. Chem. Rev. 2010, 110, 1278–1319. [Google Scholar] [CrossRef] [PubMed]
- Slautin, B.; Alikin, D.; Rosato, D.; Pelegov, D.; Shur, V.; Kholkin, A. Local Study of Lithiation and Degradation Paths in LiMn2O4 Battery Cathodes: Confocal Raman Microscopy Approach. Batteries 2018, 4, 21. [Google Scholar] [CrossRef]
- Paolone, A.; Sacchetti, A.; Corridoni, T.; Postorino, P.; Cantelli, R.; Rousse, G.; Masquelier, C. MicroRaman spectroscopy on LiMn2O4: Warnings on laser-induced thermal decomposition. Solid State Ion. 2004, 170, 135–138. [Google Scholar] [CrossRef]
- Haruna, A.B.; Barrett, D.H.; Rodella, C.B.; Erasmus, R.M.; Venter, A.M.; Sentsho, Z.N.; Ozoemena, K.I. Microwave irradiation suppresses the Jahn-Teller distortion in Spinel LiMn2O4 cathode material for lithium-ion batteries. Electrochim. Acta 2022, 426, 140786. [Google Scholar] [CrossRef]
- Ma, S.; Noguchi, H.; Yoshio, M. Cyclic voltammetric study on stoichiometric spinel LiMn2O4 electrode at elevated temperature. J. Power Sources 2001, 97–98, 385–388. [Google Scholar] [CrossRef]
- Knyazev, A.V.; Mączka, M.; Smirnova, N.N.; Knyazeva, S.S.; Chernorukov, N.G.; Ptak, M.; Shushunov, A.N. Study of the phase transition and thermodynamic functions of LiMn2O4. Thermochim. Acta 2014, 593, 58–64. [Google Scholar] [CrossRef]
- Radzi, Z.I.; Arifin, K.H.; Kufian, M.Z.; Balakrishnan, V.; Raihan, S.R.S.; Abd Rahim, N.; Subramaniam, R. Review of spinel LiMn2O4 cathode materials under high cut-off voltage in lithium-ion batteries: Challenges and strategies. J. Electroanal. Chem. 2022, 920, 116623. [Google Scholar] [CrossRef]
- Kim, S.; Kumar, V.; Seo, D.; Park, Y.; Kim, J.; Kim, H.; Kim, J.; Hong, J.; Kang, K. Invited Paper: Preparation and Electrochemical Characterization of Doped Spinel LiMn1.88Ge0.1Li0.02O4 Cathode Material. Electron. Mater. Lett. 2011, 7, 105–108. [Google Scholar] [CrossRef]
- Yu, X.; Qian, K.; Du, L.; Zhang, J.; Lu, N.; Miao, Z.; Li, Y.; Kobayashi, H.; Yan, X.; Li, R. A strong Jahn–Teller distortion in Mn3O4–MnO heterointerfaces for enhanced silver catalyzed formaldehyde reforming into hydrogen. Sustain. Energy Fuels 2022, 6, 3068–3077. [Google Scholar] [CrossRef]
- Labyedh, N.; Mattelaer, F.; Detavernier, C.; Vereecken, P.M. 3D LiMn2O4 thin-film electrodes for high rate all solid-state lithium and Li-ion microbatteries. J. Mater. Chem. A 2019, 7, 18996–19007. [Google Scholar] [CrossRef]
- Schweikert, N.; Hahn, H.; Indris, S. Cycling behaviour of Li/Li4Ti5O12 cells studied by electrochemical impedance spectroscopy. Phys. Chem. Chem. Phys. 2011, 13, 6234–6240. [Google Scholar] [CrossRef] [PubMed]
- Huger, E.; Uxa, D.; Schmidt, H. Electrochemical (PITT, EIS) and Analytical (SIMS) Determination of Li Diffusivities at the Onset of Charging LiNi0.33Mn0.33Co0.33O2. Electrodes. J. Phys. Chem. C 2024, 128, 7408–7423. [Google Scholar] [CrossRef]
- Berg, H.; Thomas, J.O. Neutron diffraction study of electrochemically delithiated LiMn2O4 spinel. Solid State Ion. 1999, 123, 227–234. [Google Scholar] [CrossRef]
- Lee, H.W.; Muralidharan, P.; Ruffo, R.; Mari, C.M.; Cui, Y.; Kim do, K. Ultrathin Spinel LiMn2O4 Nanowires as High Power Cathode Materials for Li-Ion Batteries. Nano Lett. 2010, 10, 3852–3856. [Google Scholar] [CrossRef] [PubMed]
- Kiani, M.A.; Mousavi, M.F.; Rahmanifar, M.S. Synthesis of Nano- and Micro-Particles of LiMn2O4: Electrochemical Investigation and Assessment as a Cathode in Li Battery. Int. J. Electrochem. Sci. 2011, 6, 2581–2595. [Google Scholar] [CrossRef]
- Tang, W.; Liu, L.L.; Tian, S.; Li, L.; Li, L.L.; Yue, Y.B.; Bai, Y.; Wu, Y.P.; Zhu, K.; Holze, R. LiMn2O4 nanorods as a super-fast cathode material for aqueous rechargeable lithium batteries. Electrochem. Commun. 2011, 13, 1159–1162. [Google Scholar] [CrossRef]
- Sadeghi, B.; Sarraf-Mamoory, R.; Shahverdi, H.R. Surface Modification of LiMn2O4 for Lithium Batteries by Nanostructured LiFePO4 Phosphate. J. Nanomater. 2012, 2012, 743236. [Google Scholar] [CrossRef]
- Karaal, Ş.; Köse, H.; Aydin, A.O.; Akbulut, H. The effect of LiBF4 concentration on the discharge and stability of LiMn2O4 half cell Li ion. Mater. Sci. Semicond. Process. 2015, 38, 397–403. [Google Scholar] [CrossRef]
- Jian-Kun, T.; Fu-Cheng, W.; Battaglia, V.S.; Hai-Lang, Z. Synthesis and Electrochemical Performance of Nanosized Multiple-doped LiMn2O4 Prepared at Low Temperature for Li- ion Battery. Int. J. Electrochem. Sci. 2014, 9, 931–942. [Google Scholar] [CrossRef]
- Han, C.-G.; Zhu, C.; Saito, G.; Akiyama, T. Improved electrochemical properties of LiMn2O4 with the Bi and La co-doping for lithium-ion batteries. RSC Adv. 2015, 5, 73315–73322. [Google Scholar] [CrossRef]
- Nkosi, F.P.; Jafta, C.J.; Kebede, M.; le Roux, L.; Mathe, M.K.; Ozoemena, K.I. Microwave-assisted optimization of the manganese redox states for enhanced capacity and capacity retention of LiAlxMn2−xO4 (x = 0 and 0.3) spinel materials. RSC Adv. 2015, 5, 32256–32262. [Google Scholar] [CrossRef]
- Taniguchi, I.; Lim, C.K.; Song, D.; Wakihara, M. Particle morphology and electrochemical performances of spinel LiMn2O4 powders synthesized using ultrasonic spray pyrolysis method. Solid State Ion. 2002, 146, 239–247. [Google Scholar] [CrossRef]
- Subramania, A.; Karthick, S.N.; Angayarkanni, N. Preparation and electrochemical behaviour of LiMn2O4 thin film by spray pyrolysis method. Thin Solid Films 2008, 516, 8295–8298. [Google Scholar] [CrossRef]

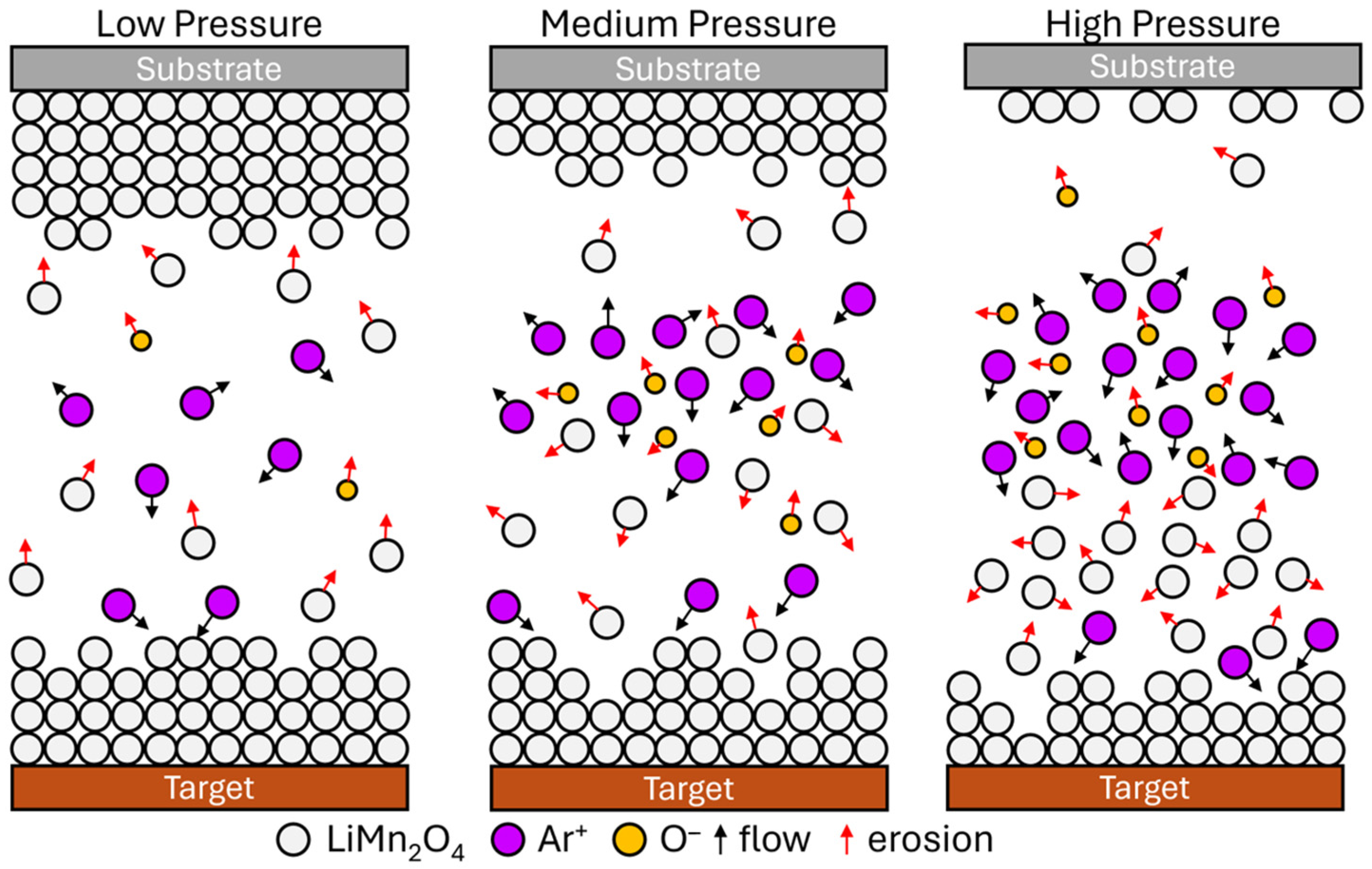
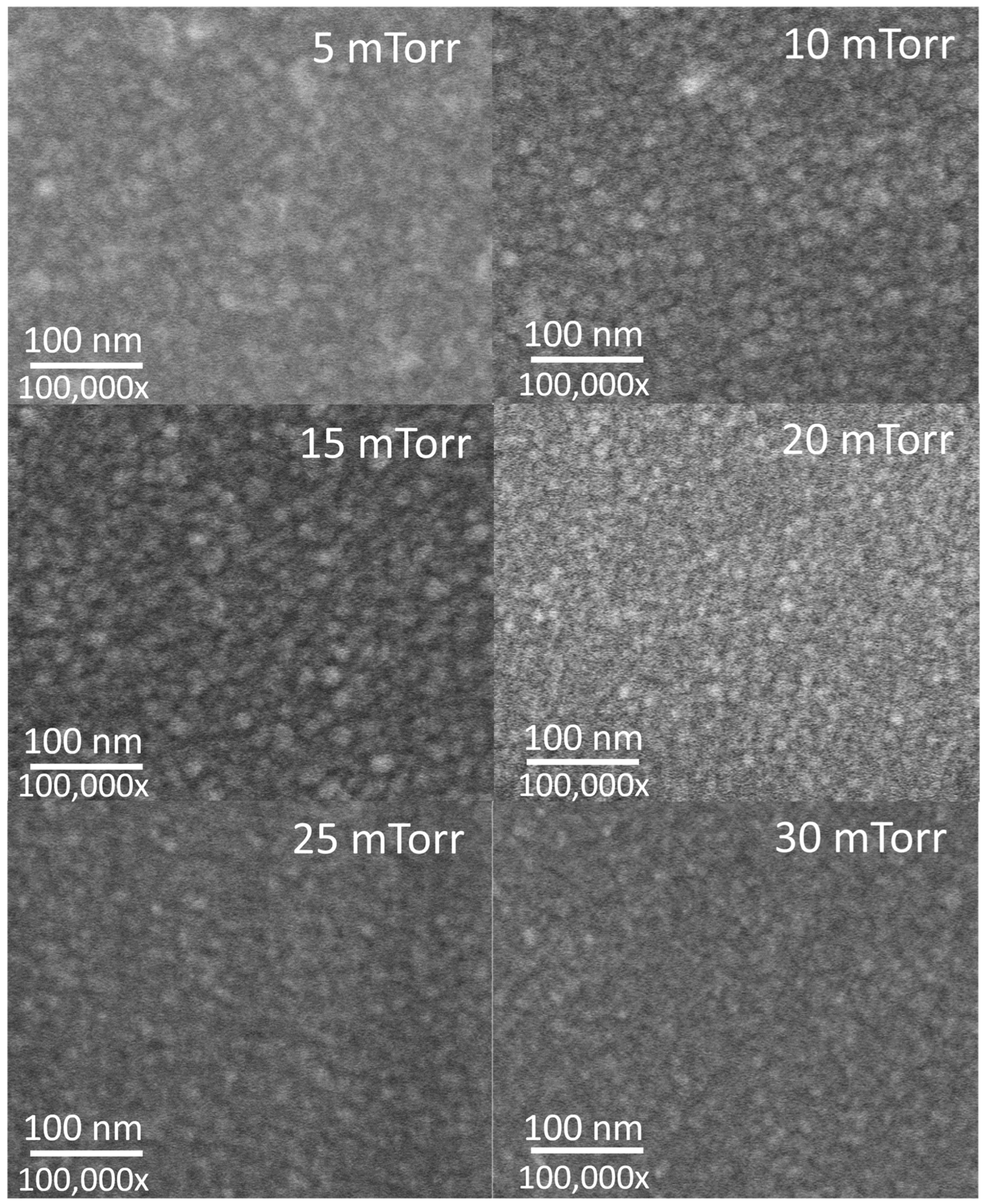
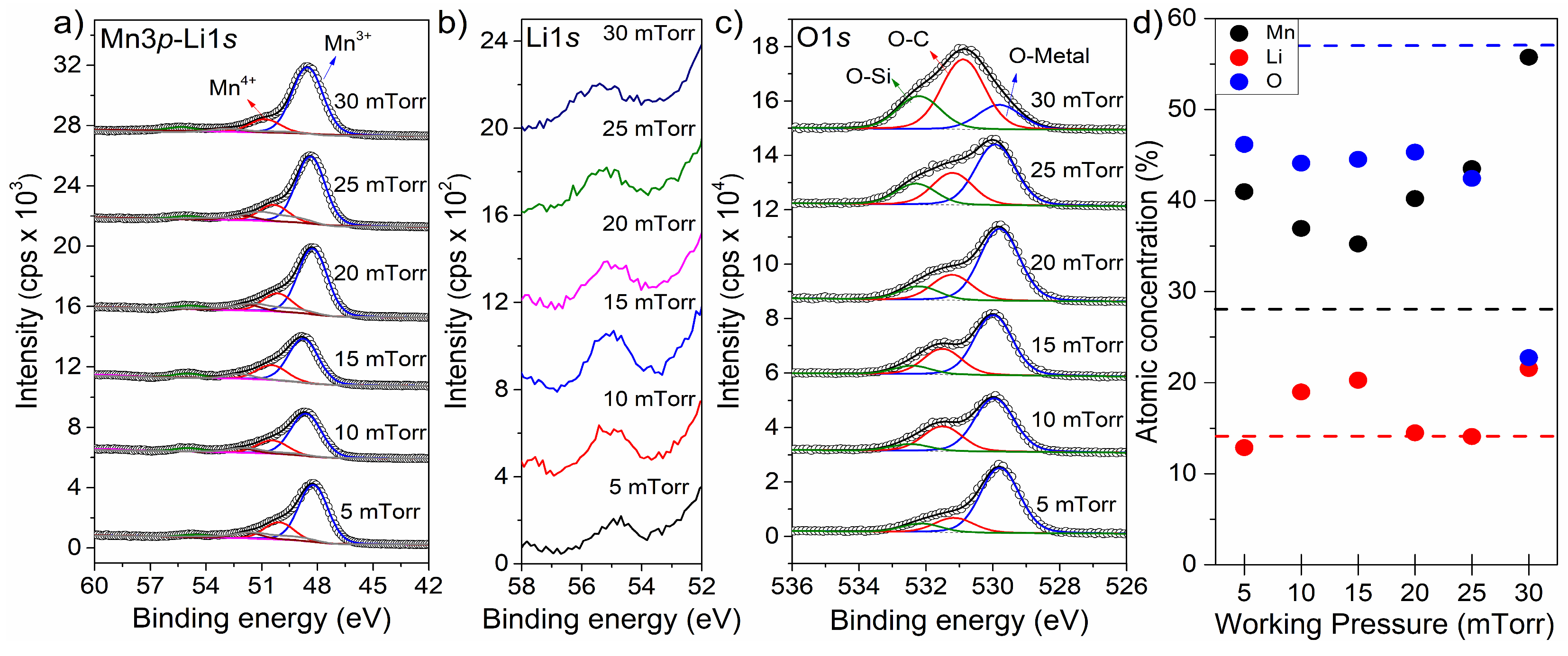
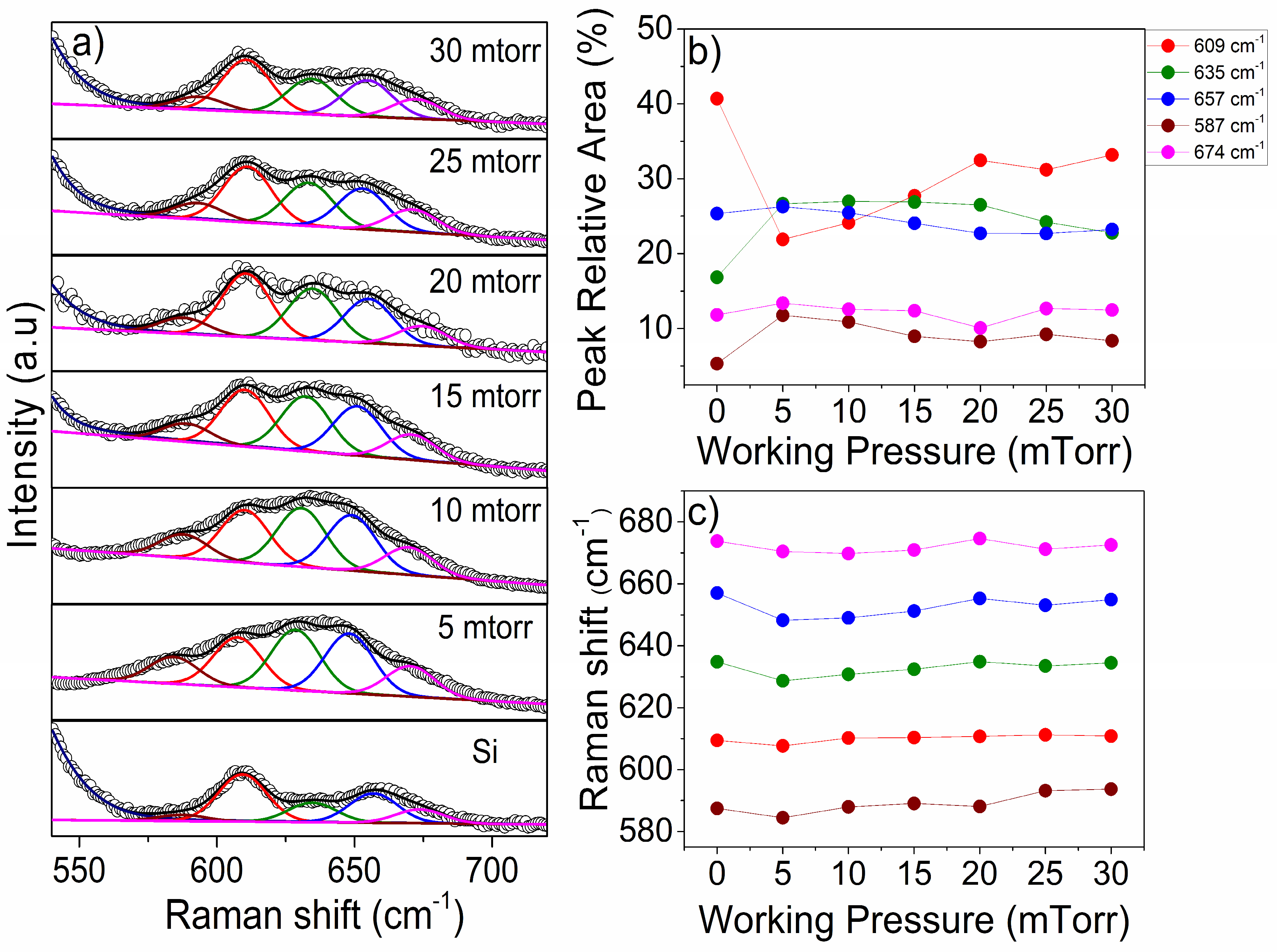
| Gas | Power (W) | Substrate | Pressure (mTorr) | Discharge Capacity | Reference |
|---|---|---|---|---|---|
| Ar | 100 | Stainless steel | 7 | 2.75 μAh/cm2 (5 μA/cm2) | [19] 2021 |
| Ar | 150 | Stainless steel | 10 | 45 μAh/cm2-μm (0.5 C) | [20] 2021 |
| Ar | 91 | Stainless steel | 5 | 107.8 μAh (11 μA/cm2) | [21] 2018 |
| Ar | 100 | Ti/Si (100) | 1 | - | [22] 2016 |
| Ar/O2 | - | Pt/Ti/SiO2/Si (100) | 5–20 | 27 μAh/cm2 -μm (13 μA/cm2) | [23] 2013 |
| Ar/O2 | 100 | Si/SiO2/Ti/Au | 2 | 44 μAh/cm2-μm | [24] 2012 |
| Ar/O2 | 100 | Ti | 10 | 57 μAh/cm2-μm (10 μA/cm2) | [25] 2012 |
| Ar/O2 | 100 | Al | 37 | - | [26] 2009 |
| Ar/O2 | 100 | Si wafer and Al | 12 | 60.9 mAh/g (0.1C) | [27] 2009 |
| Ar/O2 | 30 | Au/silica glass | 3 | - | [28] 2008 |
| Deposition Condition | Temperature (°C) | Time (h) | Power (W) | Target/Substrate Distance (cm) | Working Pressure (mTorr) |
|---|---|---|---|---|---|
| A | 25 | 1.5 | 150 | 10 | 5 |
| B | 10 | ||||
| C | 15 | ||||
| D | 20 | ||||
| E | 25 | ||||
| F | 30 |
| Working Pressure (mTorr) | Thickness (nm) | Grain Size (nm) | Characteristic Mass (mg) | Sheet Resistance (Ω) | Resistivity (S/cm) |
|---|---|---|---|---|---|
| 5 | 69.00 ± 1.00 | 14.99 ± 1.75 | 0.057 ± 0.0004 | 1.81 × 108 ± 7.39 × 106 | 1250.9 ± 51.0 |
| 10 | 47.17 ± 0.38 | 13.28 ± 1.30 | 0.040 ± 0.0008 | 1.08 × 108 ± 1.59 × 107 | 506.3 ± 74.8 |
| 15 | 30.56 ± 0.87 | 12.12 ± 1.84 | 0.026 ± 0.0003 | 4.19 × 108 ± 9.55 × 107 | 1299.3 ± 296.1 |
| 20 | 22.13 ± 0.49 | 9.95 ± 2.15 | 0.019 ± 0.0018 | 4.27 × 108 ± 4.23 × 107 | 939.8 ± 93.1 |
| 25 | 19.45 ± 0.93 | 9.61 ± 1.98 | 0.016 ± 0.0006 | 2.97 × 108 ± 1.25 × 108 | 564.6 ± 237.2 |
| 30 | 16.55 ± 0.45 | 8.84 ± 1.11 | 0.014 ± 0.0006 | 5.53 × 108 ± 5.99 × 107 | 940.3 ± 101.8 |
| Working Pressure (mTorr) | Mn (3p) at. % | Li (1s) at. % | O (1s) at. % | Mn/Li |
|---|---|---|---|---|
| 5 | 41.0 | 12.9 | 46.2 | 3.18 |
| 10 | 36.9 | 19.0 | 44.1 | 1.94 |
| 15 | 35.2 | 20.3 | 44.5 | 1.74 |
| 20 | 40.2 | 14.5 | 45.3 | 2.78 |
| 25 | 43.5 | 14.1 | 42.4 | 3.09 |
| 30 | 55.7 | 21.5 | 22.7 | 2.59 |
| Working Pressure (mTorr) | Re (Ω) | R1 (Ω) | R2 (Ω) | DLi+ (cm2/s) |
|---|---|---|---|---|
| 5 | 3.33 | 5076.19 | 1598.63 | 5.19 × 10−12 |
| 10 | 3.51 | 761.02 | 952.24 | 3.22 × 10−12 |
| 15 | 6.62 | 271.5 | 619.5 | 2.19 × 10−13 |
| 20 | 4.70 | 25,720.90 | 27,629.58 | 3.65 × 10−14 |
| 25 | 3.99 | 1380 | 70,908.00 | 3.70 × 10−13 |
| 30 | 5.25 | 5236.94 | 48,529.51 | 1.34 × 10−14 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ambriz-Vargas, F.; Garza-Hernández, R.; Martínez-Flores, J.S.; Aguirre-Tostado, F.S.; Martínez-Guerra, E.; Quevedo-López, M. Fine-Tuning Cathode Performance: The Influence of Argon Deposition Pressure on LiMn2O4 Thin Film Electrochemistry for Li-Ion Batteries. Batteries 2024, 10, 449. https://doi.org/10.3390/batteries10120449
Ambriz-Vargas F, Garza-Hernández R, Martínez-Flores JS, Aguirre-Tostado FS, Martínez-Guerra E, Quevedo-López M. Fine-Tuning Cathode Performance: The Influence of Argon Deposition Pressure on LiMn2O4 Thin Film Electrochemistry for Li-Ion Batteries. Batteries. 2024; 10(12):449. https://doi.org/10.3390/batteries10120449
Chicago/Turabian StyleAmbriz-Vargas, Fabián, Raquel Garza-Hernández, José Salvador Martínez-Flores, Francisco Servando Aguirre-Tostado, Eduardo Martínez-Guerra, and Manuel Quevedo-López. 2024. "Fine-Tuning Cathode Performance: The Influence of Argon Deposition Pressure on LiMn2O4 Thin Film Electrochemistry for Li-Ion Batteries" Batteries 10, no. 12: 449. https://doi.org/10.3390/batteries10120449
APA StyleAmbriz-Vargas, F., Garza-Hernández, R., Martínez-Flores, J. S., Aguirre-Tostado, F. S., Martínez-Guerra, E., & Quevedo-López, M. (2024). Fine-Tuning Cathode Performance: The Influence of Argon Deposition Pressure on LiMn2O4 Thin Film Electrochemistry for Li-Ion Batteries. Batteries, 10(12), 449. https://doi.org/10.3390/batteries10120449








