Intrinsic Safety Risk Control and Early Warning Methods for Lithium-Ion Power Batteries
Abstract
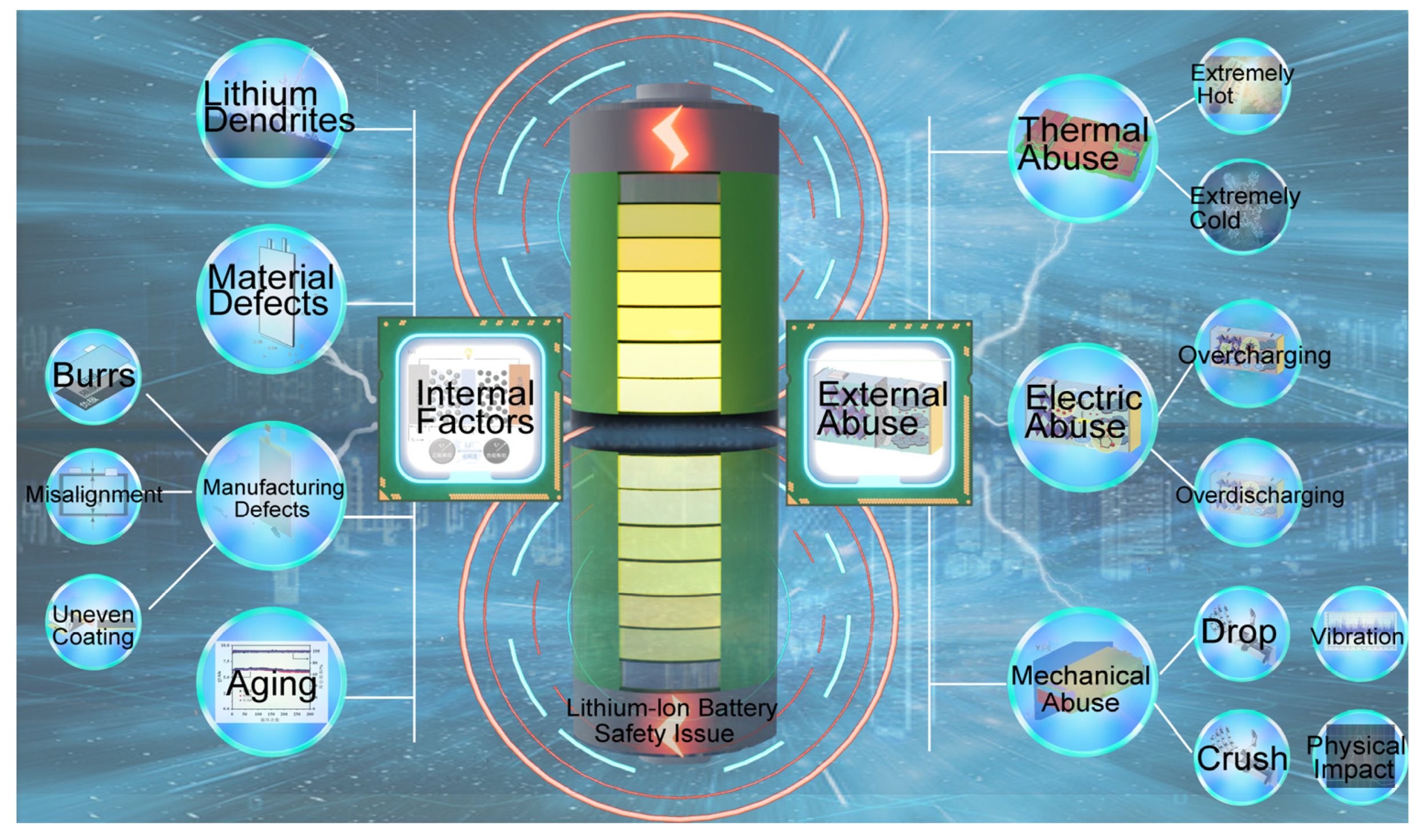
1. Introduction
2. Intrinsic LIB Safety Risk Control from a Materials Perspective
2.1. Improvement of Cathode Materials in Lithium-Ion Power Batteries
2.1.1. Surface Coating
2.1.2. Material Structural Optimization
2.2. Improvement of Anode Materials
2.2.1. Material Structural Optimization
2.2.2. New Anode Materials
2.3. Improvement of Electrolytes
2.3.1. Use of Additives
2.3.2. Development of New Electrolytes
2.4. Improvement of Separators
2.4.1. Coating Modification
2.4.2. Development of New Materials
3. Early Warning Systems for Safety Risk
3.1. Analysis of Battery Failure Scenarios
- (1)
- Nonstandard operating environments during manufacturing that may lead to foreign objects entering the battery;
- (2)
- External mechanical damage;
- (3)
- Anomalies caused by overcharging and overdischarging;
- (4)
- The impact of environmental conditions, such as extreme temperatures, on battery performance.
3.2. Early Warning Systems for Safety
3.2.1. Early Warning Models
3.2.2. External Sensors Application
3.2.3. Application of Embedded Sensors
Pressure Sensors
Gas Sensors
Temperature Sensors
Integrated Sensor Systems
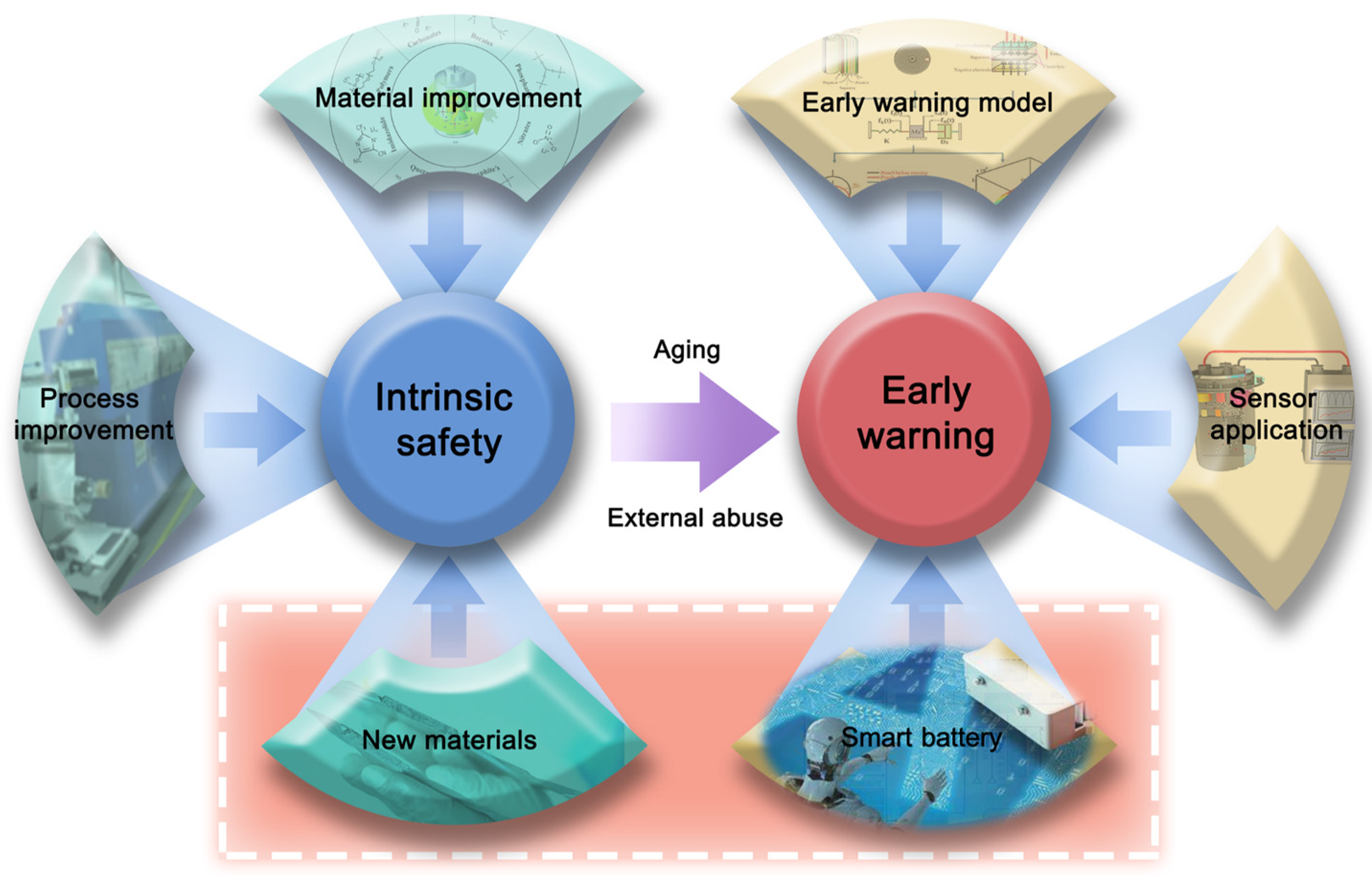
4. Conclusions
- (1)
- Development of thermal runaway inhibition materials: Investigate the internal mechanisms of thermal runaway in battery systems and develop materials capable of suppressing such events. This includes the integration of safety mechanisms to prevent overcharging and short-circuiting, culminating in the creation of novel battery systems that address inherent issues of thermal instability and combustible components in existing battery materials.
- (2)
- Electrical–thermal–pressure–gas coupled safety warning model: Construct a comprehensive warning model that integrates electrical, thermal, pressure, and gas dynamics. Develop an array-type multicore chip warning module to enhance the precision, timeliness, and reliability of safety alerts, addressing the limitations of delay, false alarms, and sensitivity in single-signal warning systems.
- (3)
- Big data and AI integration in battery management: Utilize big data and artificial intelligence to refine commercial battery management systems and supplement these systems with sensor arrays. Focus on building a comprehensive database delineating normal and thermal runaway battery states. Develop neural-network-based analytical models for more accurate recognition of battery states and predictive analysis of potential future battery conditions.
- (4)
- Smart batteries with embedded sensors: Fabricate intelligent battery systems equipped with embedded sensors for real-time monitoring of internal resistance, temperature, and gas emission. Incorporate these insights into the warning model to facilitate direct and early detection of potential safety hazards.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ren, D.; Feng, X.; Lu, L.; Li, J.; Ouyang, M. Comparison of the Overcharge Behaviors of Lithium-ion Batteries Under Different Test Conditions. Energy Procedia 2019, 158, 4921–4926. [Google Scholar] [CrossRef]
- Sun, P.; Bisschop, R.; Niu, H.; Huang, X. A Review of Battery Fires in Electric Vehicles. Fire Technol. 2020, 56, 1361–1410. [Google Scholar] [CrossRef]
- Zhu, X.; Wang, Z.; Wang, Y.; Wang, H.; Wang, C.; Tong, L.; Yi, M. Overcharge investigation of large format lithium-ion pouch cells with Li(Ni0.6Co0.2Mn0.2)O2 cathode for electric vehicles: Thermal runaway features and safety management method. Energy 2019, 169, 868–880. [Google Scholar] [CrossRef]
- Larsson, F.; Andersson, P.; Blomqvist, P.; Mellander, B.-E. Toxic fluoride gas emissions from lithium-ion battery fires. Sci. Rep. 2017, 7, 10018. [Google Scholar] [CrossRef]
- Feng, X.; Fang, M.; He, X.; Ouyang, M.; Lu, L.; Wang, H.; Zhang, M. Thermal runaway features of large format prismatic lithium ion battery using extended volume accelerating rate calorimetry. J. Power Sources 2014, 255, 294–301. [Google Scholar] [CrossRef]
- Hu, G.; Huang, P.; Bai, Z.; Wang, Q.; Qi, K. Comprehensively analysis the failure evolution and safety evaluation of automotive lithium ion battery. eTransportation 2021, 10, 100140. [Google Scholar] [CrossRef]
- Mc Carthy, K.; Gullapalli, H.; Ryan, K.M.; Kennedy, T. Review—Use of Impedance Spectroscopy for the Estimation of Li-ion Battery State of Charge, State of Health and Internal Temperature. J. Electrochem. Soc. 2021, 168, 080517. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, L.; Sun, F.; Wang, Z. An Overview on Thermal Safety Issues of Lithium-ion Batteries for Electric Vehicle Application. IEEE Access 2018, 6, 23848–23863. [Google Scholar] [CrossRef]
- Wang, G.; Kong, D.; Ping, P.; Wen, J.; He, X.; Zhao, H.; He, X.; Peng, R.; Zhang, Y.; Dai, X. Revealing particle venting of lithium-ion batteries during thermal runaway: A multi-scale model toward multiphase process. eTransportation 2023, 16, 100237. [Google Scholar] [CrossRef]
- Wang, L.; Xie, L.-Q.; Tian, G.-Y.; He, X.-M. Lithium ion battery safety accidents: Safety issues or reliability issues. Energy Storage Sci. Technol. 2021, 10, 1–6. [Google Scholar]
- Yuan, S.; Lai, Q.; Duan, X.; Wang, Q. Carbon-based materials as anode materials for lithium-ion batteries and lithium- ion capacitors: A review. J. Energy Storage 2023, 61, 106716. [Google Scholar] [CrossRef]
- Nitou, M.V.M.; Pang, Y.; Wan, Z.; Li, W.; Zhong, Z.; Muhammad, W.; Muhammad, S.; Muhammad, S.; Niu, Y.; Lv, W. LiFePO4 as a dual-functional coating for separators in lithium-ion batteries: A new strategy for improving capacity and safety. J. Energy Chem. 2023, 86, 490–498. [Google Scholar] [CrossRef]
- Cai, L.; Li, Z.; Zhang, S.; Prenger, K.; Naguib, M.; Pol, V.G. Safer lithium-ion battery anode based on Ti3C2Tz MXene with thermal safety mechanistic elucidation. Chem. Eng. J. 2021, 419, 129387. [Google Scholar] [CrossRef]
- Chiba, K.; Yoshizawa, A.; Isogai, Y. Thermal safety diagram for lithium-ion battery using single-crystal and polycrystalline particles LiNi0.8Co0.1Mn0.1O2. J. Energy Storage 2020, 32, 101775. [Google Scholar] [CrossRef]
- Hou, J.; Qu, S.; Yang, M.; Zhang, J. Materials and electrode engineering of high capacity anodes in lithium ion batteries. J. Power Sources 2020, 450, 0378–7753. [Google Scholar] [CrossRef]
- Fan, Y.; Zhang, H.; Liu, M.; Zhang, J.; Xiao, X.; Ouyang, L. Controllable hydrogen generation at low temperatures and safety evaluation for tin anode materials of spent lithium-ion batteries. J. Alloys Compd. 2023, 947, 169548. [Google Scholar] [CrossRef]
- Pushparaj, R.I.; Kumar, A.R.; Xu, G. Enhancing safety in lithium-ion batteries with additive-based liquid electrolytes: A critical review. J. Energy Storage 2023, 72, 108493. [Google Scholar] [CrossRef]
- Ji, W.; Li, H.; Li, W.; He, Z.; Zhao, J. Novelty method based on thermal trigger mechanism for high energy density lithium-ion battery safety. J. Energy Storage 2023, 64, 107231. [Google Scholar] [CrossRef]
- Chen, Z.; Shen, H.; Zhu, Y.; Hua, M.; Pan, X.; Liu, Y.; Ji, H.; Bolliev, M.; Jiang, J. Advanced low-flammable pyrrole ionic liquid electrolytes for high safety lithium-ion batteries. J. Energy Storage 2023, 72, 108289. [Google Scholar] [CrossRef]
- Liu, M.-C.; Liu, Q.-S.; Quan, Y.-Z.; Yu, J.-L.; Wu, G.; Wang, X.-L.; Wang, Y.-Z. Phosphorus-silicon-integrated electrolyte additive boosts cycling performance and safety of high-voltage lithium-ion batteries. Chin. Chem. Lett. 2023, 109123. [Google Scholar] [CrossRef]
- Lei, S.; Zeng, Z.; Wu, Y.; Liu, M.; Cheng, S.; Xie, J. Non-coordinating flame retardants with varied vapor pressures enabling biphasic fire-extinguishing electrolyte for high safety lithium-ion batteries. Chem. Eng. J. 2023, 463, 142181. [Google Scholar] [CrossRef]
- Long, M.-C.; Wu, G.; Wang, X.-L.; Wang, Y.-Z. Self-adaptable gel polymer electrolytes enable high-performance and all-round safety lithium ion batteries. Energy Storage Mater. 2022, 53, 62–71. [Google Scholar] [CrossRef]
- Costa, C.M.; Lee, Y.-H.; Kim, J.-H.; Lee, S.-Y.; Lanceros-Méndez, S. Recent advances on separator membranes for lithium-ion battery applications: From porous membranes to solid electrolytes. Energy Storage Mater. 2019, 22, 346–375. [Google Scholar] [CrossRef]
- Luiso, S.; Fedkiw, P. Lithium-ion battery separators: Recent developments and state of art. Curr. Opin. Electrochem. 2020, 20, 99–107. [Google Scholar] [CrossRef]
- Li, S.; Wu, Y.; Ma, X.; Hu, J.; Song, Q.; Shen, X.; Zhang, W. Organic phase change composite separators to enhance the safety performance of lithium-ion batteries. J. Power Sources 2023, 584, 233620. [Google Scholar] [CrossRef]
- Roh, Y.; Kim, D.; Jin, D.; Kim, D.; Han, C.; Choi, J.; Lee, H.; Lee, Y.-G.; Lee, Y.M. Enhanced safety of lithium ion batteries through a novel functional separator with encapsulated flame retardant and hydroxide ceramics. Chem. Eng. J. 2023, 474, 145937. [Google Scholar] [CrossRef]
- Liu, Z.; Peng, Y.; Meng, T.; Yu, L.; Wang, S.; Hu, X. Thermal-triggered fire-extinguishing separators by phase change materials for high-safety lithium-ion batteries. Energy Storage Mater. 2022, 47, 445–452. [Google Scholar] [CrossRef]
- Xiao, Y.; Fu, A.; Zou, Y.; Huang, L.; Wang, H.; Su, Y.; Zheng, J. High safety lithium-ion battery enabled by a thermal-induced shutdown separator. Chem. Eng. J. 2022, 438, 135550. [Google Scholar] [CrossRef]
- Chen, X.; Chen, S.; Lin, Y.; Wu, K.; Lu, S. Multi-functional ceramic-coated separator for lithium-ion batteries safety tolerance improvement. Ceram. Int. 2020, 46, 24689–24697. [Google Scholar] [CrossRef]
- Gou, H.; Li, W.; Yang, Y.; Li, X.; Cui, H.; Liu, Y.; Wang, J.; Kakimov, A.; Wang, J.; Shi, W.; et al. Porous skeleton-stabilized Co/N–C coated separator for boosting lithium-ion batteries stability and safety. J. Power Sources 2021, 499, 229933. [Google Scholar] [CrossRef]
- Lv, P.; Zhang, D.; Lin, Y.; Shi, H.; Xie, S.; Sun, Q.; Chen, X.; He, Y.; Tang, C. Polyethylene ceramic separators with semi-interpenetrating polymer network boosting fast-charging cycle capacity retention and safety for lithium-ion batteries. J. Power Sources 2023, 570, 233022. [Google Scholar] [CrossRef]
- Liao, C.; Mu, X.; Han, L.; Li, Z.; Zhu, Y.; Lu, J.; Wang, H.; Song, L.; Kan, Y.; Hu, Y. A flame-retardant, high ionic-conductivity and eco-friendly separator prepared by papermaking method for high-performance and superior safety lithium-ion batteries. Energy Storage Mater. 2022, 48, 123–132. [Google Scholar] [CrossRef]
- Yu, Y.; Jia, G.; Zhao, L.; Xiang, H.; Hu, Z.; Xu, G.; Zhu, M. Flexible and heat-resistant polyphenylene sulfide ultrafine fiber hybrid separators for high-safety lithium-ion batteries. Chem. Eng. J. 2023, 452, 139112. [Google Scholar] [CrossRef]
- Tang, W.; Liu, Q.; Luo, N.; Chen, F.; Fu, Q. High safety and electrochemical performance electrospun para-aramid nanofiber composite separator for lithium-ion battery. Compos. Sci. Technol. 2022, 225, 109479. [Google Scholar] [CrossRef]
- Lin, G.; Bai, Z.; Liu, C.; Liu, S.; Han, M.; Huang, Y.; Liu, X. Mechanically robust, nonflammable and surface cross-linking composite membranes with high wettability for dendrite-proof and high-safety lithium-ion batteries. J. Membr. Sci. 2022, 647, 120262. [Google Scholar] [CrossRef]
- Long, M.-C.; Duan, P.-H.; Gao, Y.; Wang, X.-L.; Wu, G.; Wang, Y.-Z. Boosting safety and performance of lithium-ion battery enabled by cooperation of thermotolerant fire-retardant composite membrane and nonflammable electrolyte. Chem. Eng. J. 2022, 432, 134394. [Google Scholar] [CrossRef]
- Zhang, S.; Luo, J.; Du, M.; Hui, H.; Sun, Z. Safety and cycling stability enhancement of cellulose paper-based lithium-ion battery separator by aramid nanofibers. Eur. Polym. J. 2022, 171, 111222. [Google Scholar] [CrossRef]
- Chen, J.; Kang, T.; Cui, Y.; Xue, J.; Xu, H.; Nan, J. Nonflammable and thermally stable glass fiber/polyacrylate (GFP) separator for lithium-ion batteries with enhanced safety and lifespan. J. Power Sources 2021, 496, 229862. [Google Scholar] [CrossRef]
- Liu, X.-T.; Zhang, B.; Wu, Y.-N.; Chen, J.-L.; Fang, M.-L.; Wang, L.; Wang, L. The effects of polybenzimidazole nanofiber separator on the safety and performance of lithium-ion batteries: Characterization and analysis from the perspective of mechanism. J. Power Sources 2020, 475, 228624. [Google Scholar] [CrossRef]
- Wu, D.; Dong, N.; Wang, R.; Qi, S.; Liu, B.; Wu, D. In situ construction of High-safety and Non-flammable polyimide “Ceramic” Lithium-ion battery separator via SiO2 Nano-Encapsulation. Chem. Eng. J. 2021, 420, 129992. [Google Scholar] [CrossRef]
- Li, L.; Zhou, M.; Xiong, R.; Wang, X.; Shen, G.; Sun, S.; Chen, Y.; Huang, T.; Zhou, H.; Zhang, Y. High-safety poly(ethylene-co-vinyl acetate)/poly(ether ether ketone)/poly(ethylene-co-vinyl acetate) composite separator with the thermal shutdown feature for lithium-ion battery. J. Membr. Sci. 2023, 687, 122059. [Google Scholar] [CrossRef]
- Su, W.; Zhong, G.-B.; Shen, J.-N.; Wang, C.; Xu, J.-L.; He, Y.-J.; Ma, Z.-F. The progress in fault diagnosis technology for lithium-ion batteries. Energy Storage Sci. Technol. 2019, 8, 225–236. [Google Scholar]
- Zou, B.; Zhang, L.; Xue, X.; Tan, R.; Jiang, P.; Ma, B.; Song, Z.; Hua, W. A Review on the Fault and Defect Diagnosis of Lithium-Ion Battery for Electric Vehicles. Energies 2023, 16, 5507. [Google Scholar] [CrossRef]
- Lai, X.; Jin, C.; Yi, W.; Han, X.; Feng, X.; Zheng, Y.; Ouyang, M. Mechanism, Modeling, Detection and Prevention of the Internal Short Circuit in Lithium-Ion Batteries: Recent Advances and Perspectives. Energy Storage Mater. 2021, 35, 470–499. [Google Scholar] [CrossRef]
- Samanta, A.; Chowdhuri, S.; Williamson, S.S. Machine Learning-Based Data-Driven Fault Detection/Diagnosis of Lithium-Ion Battery: A Critical Review. Electronics 2021, 10, 1309. [Google Scholar] [CrossRef]
- Jia, Y.; Li, J.; Yao, W.; Li, Y.; Xu, J. Precise and fast safety risk classification of lithium-ion batteries based on machine learning methodology. J. Power Sources 2022, 548, 232064. [Google Scholar] [CrossRef]
- González, I.; Calderón, A.J.; Folgado, F.J. IoT real time system for monitoring lithium-ion battery long-term operation in microgrids. J. Energy Storage 2022, 51, 104596. [Google Scholar] [CrossRef]
- Wang, W.; Li, Y.; Cheng, L.; Zuo, F.; Yang, S. Safety performance and failure prediction model of cylindrical lithium-ion battery. J. Power Sources 2020, 451, 227755. [Google Scholar] [CrossRef]
- Wei, G.; Huang, R.; Zhang, G.; Jiang, B.; Zhu, J.; Guo, Y.; Han, G.; Wei, X.; Dai, H. A comprehensive insight into the thermal runaway issues in the view of lithium-ion battery intrinsic safety performance and venting gas explosion hazards. Appl. Energy 2023, 349, 121651. [Google Scholar] [CrossRef]
- Jin, Y.; Zheng, Z.; Wei, D.; Jiang, X.; Lu, H.; Sun, L.; Tao, F.; Guo, D.; Liu, Y.; Gao, J.; et al. Detection of micro-scale Li dendrite via H2 gas capture for early safety warning. Joule 2020, 4, 1714–1729. [Google Scholar] [CrossRef]
- Song, Y.; Lyu, N.; Shi, S.; Jiang, X.; Jin, Y. Safety warning for lithium-ion batteries by module-space air-pressure variation under thermal runaway conditions. J. Energy Storage 2022, 56, 105911. [Google Scholar] [CrossRef]
- Su, T.; Lyu, N.; Zhao, Z.; Wang, H.; Jin, Y. Safety warning of lithium-ion battery energy storage station via venting acoustic signal detection for grid application. J. Energy Storage 2021, 38, 102498. [Google Scholar] [CrossRef]
- Lyu, S.; Li, N.; Sun, L.; Jiao, S.; Chen, H.; Song, W.-L. Rapid operando gas monitor for commercial lithium-ion batteries: Gas evolution and relation with electrode materials. J. Energy Chem. 2022, 72, 14–25. [Google Scholar] [CrossRef]
- Samanta, A.; Williamson, S.S. A Comprehensive Review of Lithium-Ion Cell Temperature Estimation Techniques Applicable to Health-Conscious Fast Charging and Smart Battery Management Systems. Energies 2021, 14, 5960. [Google Scholar] [CrossRef]
- Zhang, G.; Cao, L.; Ge, S.; Wang, C.-Y.; Shaffer, C.E.; Rahn, C.D. In situ measurement of radial temperature distributions in cylindrical Li-ion cells. J. Electrochem. Soc. 2014, 161, A1499–A1507. [Google Scholar] [CrossRef]
- Huang, J.; Blanquer, L.A.; Bonefacino, J.; Logan, E.R.; Corte, D.A.D.; Delacourt, C.; Gallant, B.M.; Boles, S.T.; Dahn, J.R.; Tam, H.-Y.; et al. Operando decoding of chemical and thermal events in commercial Na(Li)-ion cells via optical sensors. Nat. Energy 2020, 5, 674–683. [Google Scholar] [CrossRef]
- Cui, Y.; Shi, D.; Wang, Z.; Mou, L.; Ou, M.; Fan, T.; Bi, S.; Zhang, X.; Yu, Z.; Fang, Y. Thermal runaway early warning and risk estimation based on gas production characteristics of different types of lithium-ion batteries. Batteries 2023, 9, 438. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cui, Y.; Shen, X.; Zhang, H.; Yin, Y.; Yu, Z.; Shi, D.; Fang, Y.; Xu, R. Intrinsic Safety Risk Control and Early Warning Methods for Lithium-Ion Power Batteries. Batteries 2024, 10, 62. https://doi.org/10.3390/batteries10020062
Cui Y, Shen X, Zhang H, Yin Y, Yu Z, Shi D, Fang Y, Xu R. Intrinsic Safety Risk Control and Early Warning Methods for Lithium-Ion Power Batteries. Batteries. 2024; 10(2):62. https://doi.org/10.3390/batteries10020062
Chicago/Turabian StyleCui, Yi, Xueling Shen, Hang Zhang, Yanping Yin, Zhanglong Yu, Dong Shi, Yanyan Fang, and Ran Xu. 2024. "Intrinsic Safety Risk Control and Early Warning Methods for Lithium-Ion Power Batteries" Batteries 10, no. 2: 62. https://doi.org/10.3390/batteries10020062
APA StyleCui, Y., Shen, X., Zhang, H., Yin, Y., Yu, Z., Shi, D., Fang, Y., & Xu, R. (2024). Intrinsic Safety Risk Control and Early Warning Methods for Lithium-Ion Power Batteries. Batteries, 10(2), 62. https://doi.org/10.3390/batteries10020062





