Critical Review on High-Safety Lithium-Ion Batteries Modified by Self-Terminated Oligomers with Hyperbranched Architectures
Abstract
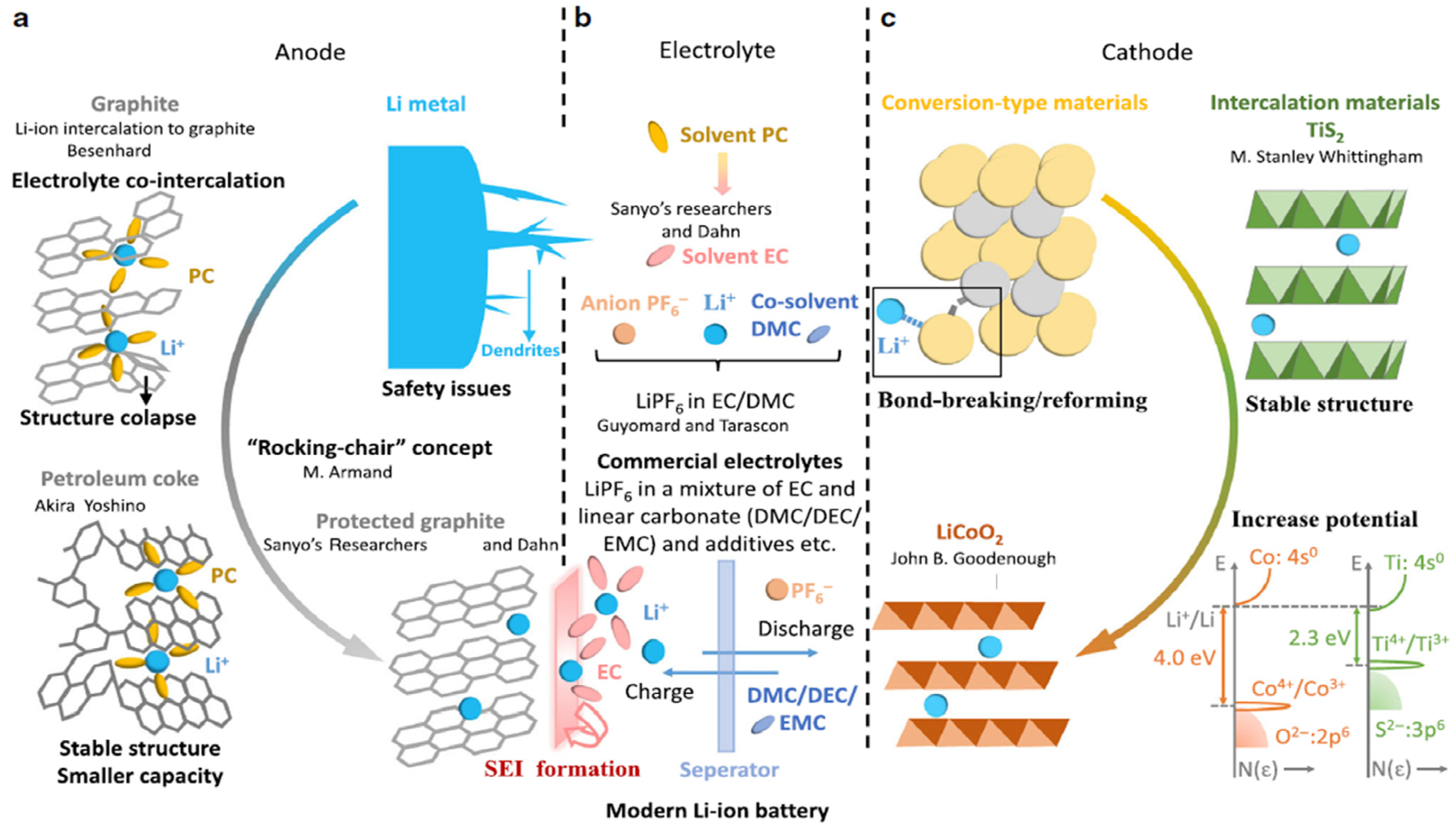
:1. Introduction
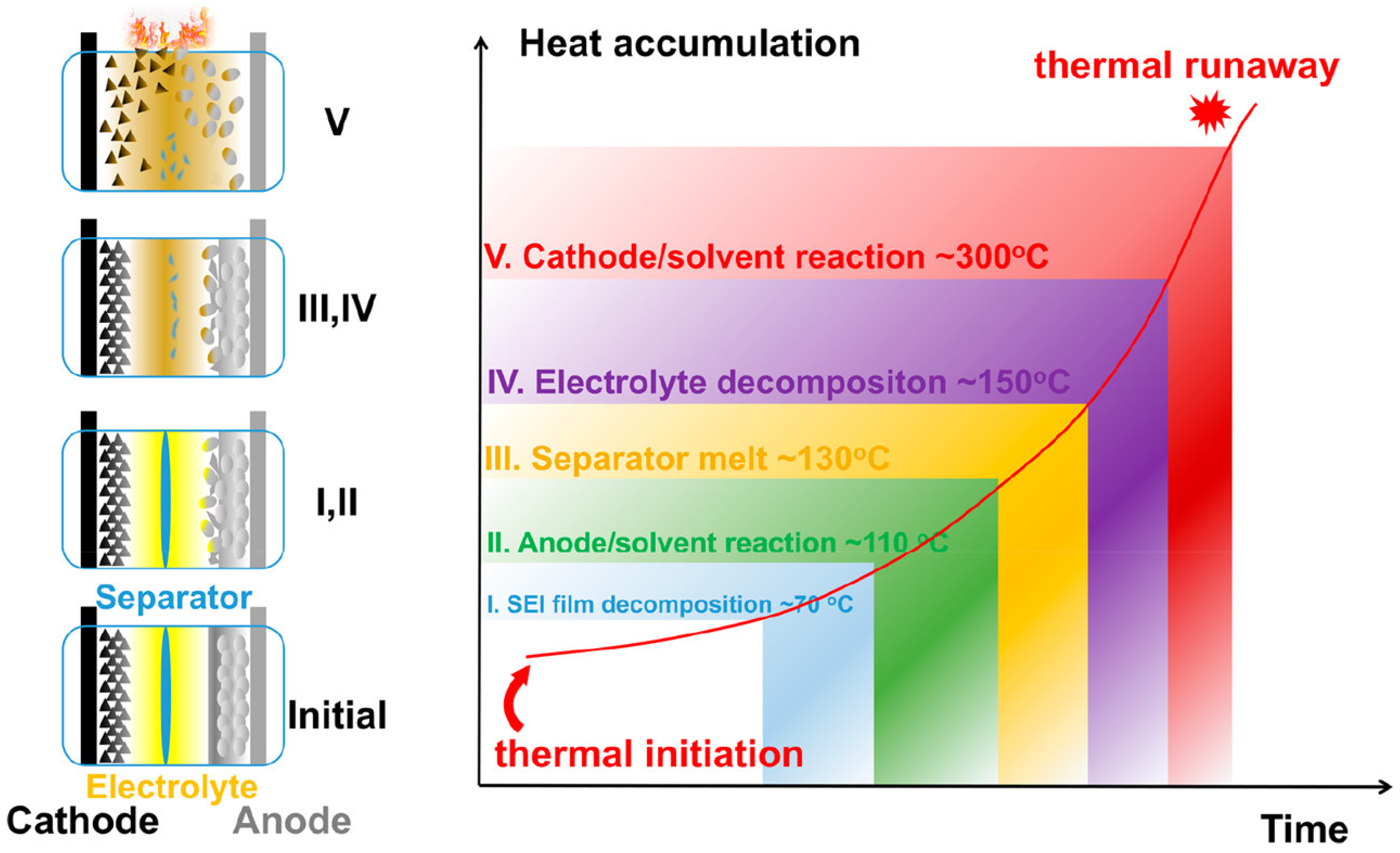
1.1. Overview of the Current State of STOBA for Lithium-Ion Battery Technology
1.2. Background and Motivation for the Use of STOBA Materials in LIBs
2. Synthesis and Characterization of STOBA Materials
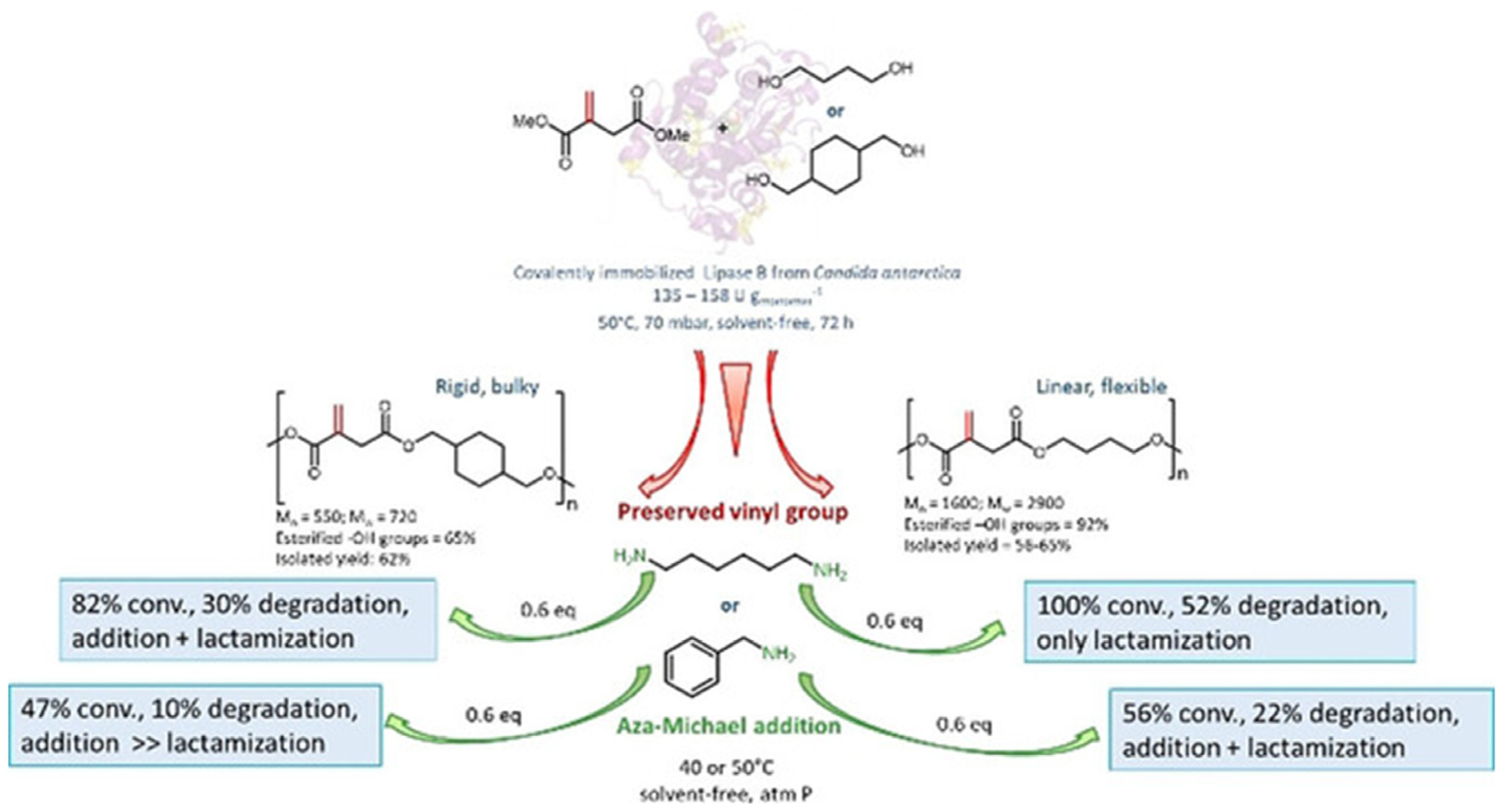
2.1. Synthesis of STOBA Materials: Effect of Solvents, Monomers, and Initiators
2.2. Characterization Techniques for STOBA Materials
3. Electrochemical Properties of STOBA Materials
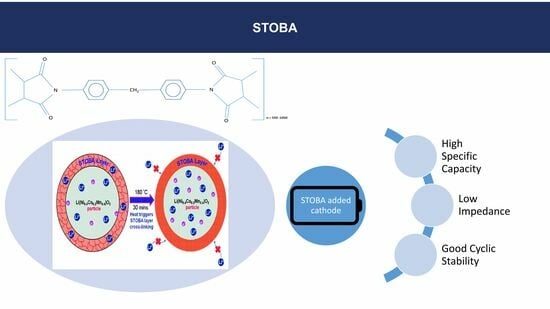
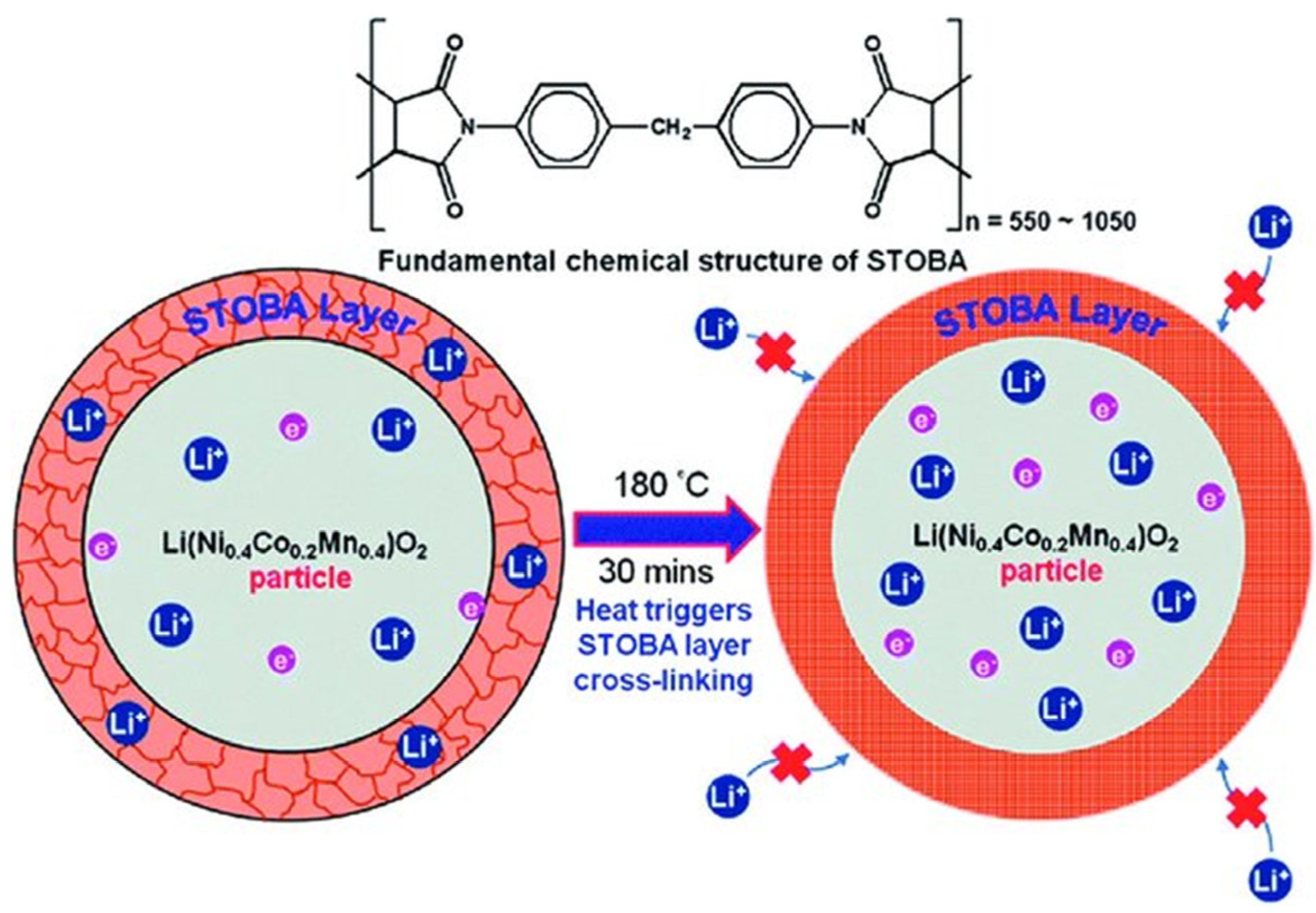
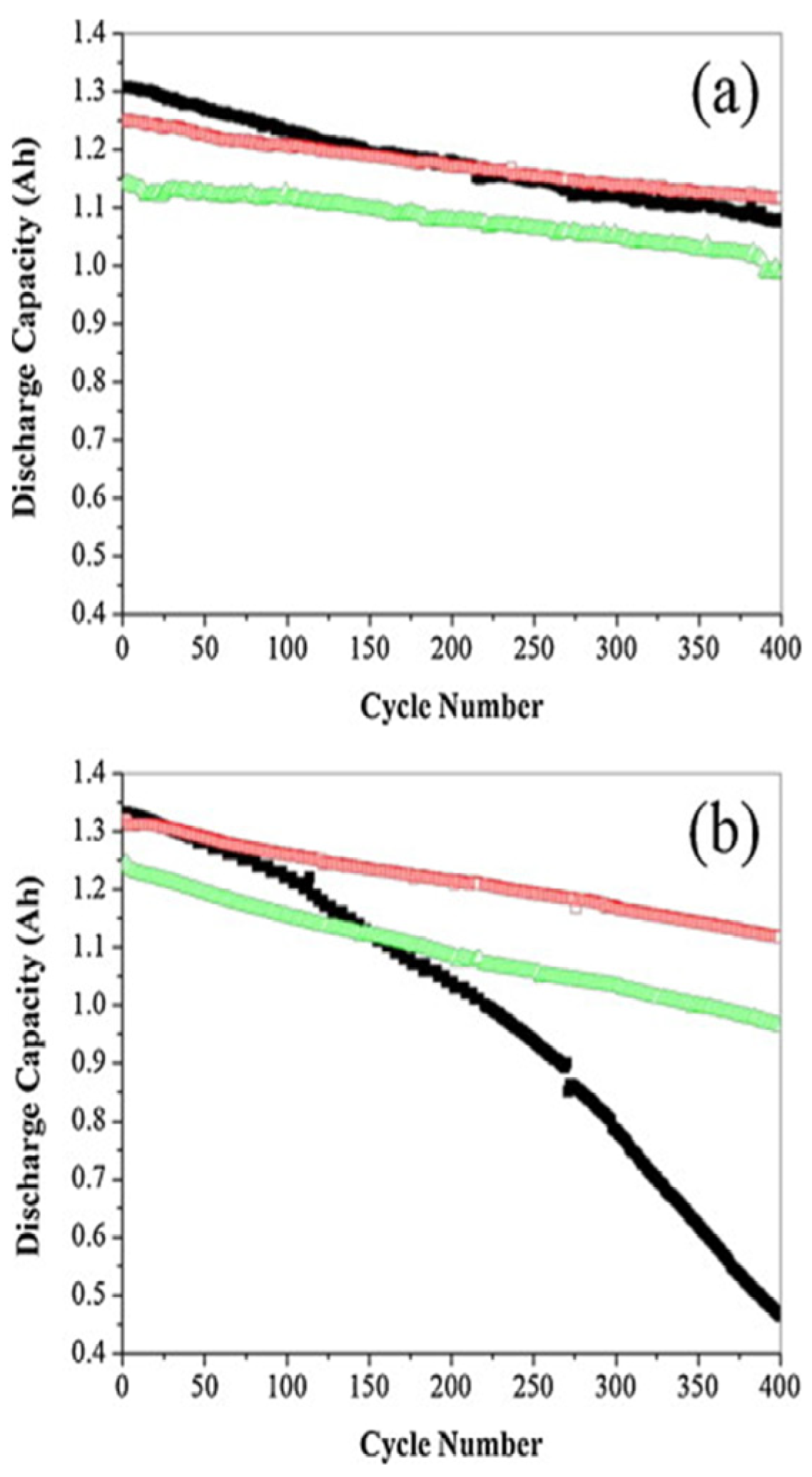
3.1. Effect of STOBA Additive on Specific Capacity, Impedance, and Cycling Stability
3.2. Explanation of How STOBA Materials Contribute to Electrochemical Properties
4. Applications of STOBA Materials in LIBs
4.1. Portable Electronics, EVs, and Renewable Energy Storage Systems
4.2. Optimization of STOBA Materials for LIBs
5. Conclusions and Future Work
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Parlikar, A.; Schott, M.; Godse, K.; Kucevic, D.; Jossen, A.; Hesse, H. High-power electric vehicle charging: Low-carbon grid integration pathways with stationary lithium-ion battery systems and renewable generation. Appl. Energy 2023, 333, 120541–120557. [Google Scholar] [CrossRef]
- Pražanová, A.; Knap, V.; Stroe, D.I. Literature Review, Recycling of Lithium-Ion Batteries from Electric Vehicles, Part II: Environmental and Economic Perspective. Energies 2022, 15, 7356. [Google Scholar] [CrossRef]
- San Román, T.G.; Momber, I.; Abbad, M.R.; Sánchez Miralles, A. Regulatory framework and business models for charging plug-in electric vehicles: Infrastructure, agents, and commercial relationships. Energy Pol. 2011, 39, 6360–6375. [Google Scholar] [CrossRef]
- Rangarajan, S.S.; Sundararaj, S.P.; Sudhakar, A.V.V.; Siva, C.K.; Subramaniam, U.; Collins, E.R.; Senjyu, T. Lithium-Ion Batteries—The Crux of Electric Vehicles with Opportunities and Challenges. Clean Technol. 2022, 4, 908–930. [Google Scholar] [CrossRef]
- Fang, R.; Chen, K.; Yin, L.; Sun, Z.; Li, F.; Cheng, H.M. The Regulating Role of Carbon Nanotubes and Graphene in Lithium-Ion and Lithium–Sulfur Batteries. Adv. Mater. 2019, 31, 1800863–1800884. [Google Scholar] [CrossRef]
- Wu, F.; Maier, J.; Yu, Y. Guidelines and trends for next-generation rechargeable lithium and lithium-ion batteries. Chem. Soc. Rev. 2020, 49, 1569–1614. [Google Scholar] [CrossRef]
- Wagner, R.; Preschitschek, N.; Passerini, S.; Leker, J.; Winter, M. Current research trends and prospects among the various materials and designs used in lithium-based batteries. J. Appl. Electrochem. 2013, 43, 481–496. [Google Scholar] [CrossRef]
- Cavers, H.; Molaiyan, P.; Abdollahifar, M.; Lassi, U.; Kwade, A. Perspectives on Improving the Safety and Sustainability of High Voltage Lithium-Ion Batteries Through the Electrolyte and Separator Region. Adv. Energy Mater. 2022, 12, 2200147–2200179. [Google Scholar] [CrossRef]
- Chombo, P.V.; Laoonual, Y. A review of safety strategies of a Li-ion battery. J. Power Sources 2020, 478, 228649–228666. [Google Scholar] [CrossRef]
- Chen, Y.; Kang, Y.; Zhao, Y.; Wang, L.; Liu, J.; Li, Y.; Liang, Z.; He, X.; Li, X.; Tavajohi, N.; et al. A review of lithium-ion battery safety concerns: The issues, strategies, and testing standards. J. Energy Chem. 2021, 59, 83–99. [Google Scholar] [CrossRef]
- Qiu, Y.; Jiang, F. A review on passive and active strategies of enhancing the safety of lithium-ion batteries. Int. J. Heat. Mass Trans. 2022, 184, 122288–122301. [Google Scholar] [CrossRef]
- Liu, Y.; Duan, Q.; Xu, J.; Li, H.; Sun, J.; Wang, Q. Experimental study on a novel safety strategy of lithium-ion battery integrating fire suppression and rapid cooling. J. Energy Storage 2020, 28, 101185–101193. [Google Scholar] [CrossRef]
- Md Said, M.S.; Mohd Tohir, M.Z. Visual and thermal imaging of lithium-ion battery thermal runaway induced by mechanical impact. J. Loss Prev. Proc. Indust. 2022, 79, 104854–104861. [Google Scholar] [CrossRef]
- Goodman, J.K.S.; Miller, J.T.; Kreuzer, S.; Forman, J.; Wi, S.; Choi, J.M.; Oh, B.; White, K. Lithium-ion cell response to mechanical abuse: Three-point bend. J. Energy Storage 2020, 28, 101244–101254. [Google Scholar] [CrossRef]
- Feng, X.; Ouyang, M.; Liu, X.; Lu, L.; Xia, Y.; He, X. Thermal runaway mechanism of lithium ion battery for electric vehicles: A review. Energy Storage Mater. 2018, 10, 246–267. [Google Scholar] [CrossRef]
- Feng, X.; Ren, D.; He, X.; Ouyang, M. Mitigating Thermal Runaway of Lithium-Ion Batteries. Joule 2020, 4, 743–770. [Google Scholar] [CrossRef]
- Xu, B.; Lee, J.; Kwon, D.; Kong, L.; Pecht, M. Mitigation strategies for Li-ion battery thermal runaway: A review. Renew. Sustain. Energy Rev. 2021, 150, 111437–111459. [Google Scholar] [CrossRef]
- Larsson, F.; Andersson, P.; Mellander, B.E. Lithium-Ion Battery Aspects on Fires in Electrified Vehicles on the Basis of Experimental Abuse Tests. Batteries 2016, 2, 9. [Google Scholar] [CrossRef]
- Raijmakers, L.H.J.; Danilov, D.L.; Eichel, R.A.; Notten, P.H.L. A review on various temperature-indication methods for Li-ion batteries. Appl. Energy 2019, 240, 918–945. [Google Scholar] [CrossRef]
- Gabryelczyk, A.; Ivanov, S.; Bund, A.; Lota, G. Corrosion of aluminium current collector in lithium-ion batteries: A review. J. Energy Storage 2021, 43, 103226–103247. [Google Scholar] [CrossRef]
- Yuan, M.; Liu, K. Rational design on separators and liquid electrolytes for safer lithium-ion batteries. J. Energy Chem. 2020, 43, 58–70. [Google Scholar] [CrossRef]
- Mohanty, D.; Chen, S.Y.; Hung, I.M. Effect of Lithium Salt Concentration on Materials Characteristics and Electrochemical Performance of Hybrid Inorganic/Polymer Solid Electrolyte for Solid-State Lithium-Ion Batteries. Batteries 2022, 8, 173. [Google Scholar] [CrossRef]
- Feng, T.; Guo, W.; Li, Q.; Meng, Z.; Liang, W. Life cycle assessment of lithium nickel cobalt manganese oxide batteries and lithium iron phosphate batteries for electric vehicles in China. J. Energy Storage 2022, 52, 104767–104778. [Google Scholar] [CrossRef]
- Nitta, N.; Wu, F.; Lee, J.T.; Yushin, G. Li-ion battery materials: Present and future. Mater. Today 2015, 18, 252–264. [Google Scholar] [CrossRef]
- Hagen, M.; Hanselmann, D.; Ahlbrecht, K.; Maça, R.; Gerber, D.; Tübke, J. Lithium–Sulfur Cells: The Gap between the State-of-the-Art and the Requirements for High Energy Battery Cells. Adv. Energy Mater. 2015, 5, 1401986–1401996. [Google Scholar] [CrossRef]
- Koerver, R.; Aygün, I.; Leichtwei, T.; Dietrich, C.; Zhang, W.; Binder, J.O.; Hartmann, P.; Zeier, W.G.; Janek, J. Capacity Fade in Solid-State Batteries: Interphase Formation and Chemomechanical Processes in Nickel-Rich Layered Oxide Cathodes and Lithium Thiophosphate Solid Electrolytes. Chem. Mater. 2017, 29, 5574–5582. [Google Scholar] [CrossRef]
- Costa, C.M.; Barbosa, J.C.; Gonçalves, R.; Castro, H.; Campo, F.J.G.; Lanceros-Méndez, S. Recycling and environmental issues of lithium-ion batteries: Advances, challenges and opportunities. Energy Storage Mater. 2021, 37, 433–465. [Google Scholar] [CrossRef]
- Mukherjee, A.B.; Zevenhoven, R.; Brodersen, J.; Hylander, L.D.; Bhattacharya, P. Mercury in waste in the European Union: Sources, disposal methods and risks. Res. Conserv. Recycl. 2004, 42, 155–182. [Google Scholar] [CrossRef]
- Huang, L.; Liu, L.; Lu, L.; Feng, X.; Han, X.; Li, W.; Zhang, M.; Li, D.; Liu, X.; Sauer, D.U.; et al. A review of the internal short circuit mechanism in lithium-ion batteries: Inducement, detection and prevention. Int. J. Energy Res. 2021, 45, 15797–15831. [Google Scholar] [CrossRef]
- Pham, Q.T.; Hsu, J.M.; Pan, J.P.; Wang, T.H.; Chern, C.S. Synthesis and characterization of phenylsiloxane-modified bismaleimide/barbituric acid-based polymers with 3-aminopropyltriethoxysilane as the coupling agent. Polymer Int. 2013, 62, 1045–1052. [Google Scholar] [CrossRef]
- Lai, X.; Jin, C.; Yi, W.; Han, X.; Feng, X.; Zheng, Y.; Ouyang, M. Mechanism, modeling, detection, and prevention of the internal short circuit in lithium-ion batteries: Recent advances and perspectives. Energy Storage Mater. 2021, 35, 470–499. [Google Scholar] [CrossRef]
- Wen, L.; Liang, J.; Chen, J.; Chu, Z.Y.; Cheng, H.M.; Li, F. Smart Materials and Design toward Safe and Durable Lithium Ion Batteries. Small Methods 2019, 3, 1900323–1900354. [Google Scholar] [CrossRef]
- Finegan, D.P.; Darcy, E.; Keyser, M.; Tjaden, B.; Heenan, T.M.M.; Jervis, R.; Baily, J.J.; Malik, R.; Vo, N.T.; Magdysyuk, O.V.; et al. Characterising thermal runaway within lithium-ion cells by inducing and monitoring internal short circuits. Energy Environ. Sci. 2017, 10, 1377–1388. [Google Scholar] [CrossRef]
- Zhu, L.; Ding, G.; Han, Q.; Yang, X.; Xie, L.; Cao, X. Review—Recent Developments in Safety-Enhancing Separators for Lithium-Ion Batteries. J. Electrochem. Soc. 2021, 168, 100524–100542. [Google Scholar] [CrossRef]
- Wang, F.; Hu, E.; Sun, W.; Gao, T.; Ji, X.; Fan, X.; Han, F.; Yang, X.Q.; Xu, K.; Wang, C. A rechargeable aqueous Zn2+-battery with high power density and a long cycle-life. Energy Environ. Sci. 2018, 11, 3168–3175. [Google Scholar] [CrossRef]
- Wu, H.B.; Chen, J.S.; Hng, H.H.; Lou, X.W. Nanostructured metal oxide-based materials as advanced anodes for lithium-ion batteries. Nanoscale 2012, 4, 2526–2542. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Wang, D.; Zhao, Z.; Han, J.; Zhang, K.; Cui, X.; Xu, Z. Pore-forming Technology Development of Polymer Separators for Power Lithium-ion Battery. In Proceedings of the SAE-China Congress 2014: Selected Papers; Lecture Notes in Electrical Engineering; Springer: Berlin/Heidelberg, Germany, 2015; pp. 71–80. [Google Scholar]
- Cui, X.; Mi, X.; Cao, T.; Jiang, T.; Chen, H.; Zhao, Z.; Zhang, K. Studies on the Working Mode of Hyperbranched New Materials STOBA in Lithium-ion Battery Cathode Materials. In Proceedings of the SAE-China Congress 2014: Selected Papers; Lecture Notes in Electrical Engineering; Springer: Berlin/Heidelberg, Germany, 2015; pp. 81–88. [Google Scholar]
- Wang, Y.W.; Shu, C.M. Hazard Characterizations of Li-Ion Batteries: Thermal Runaway Evaluation by Calorimetry Methodology. In Rechargeable Batteries: Materials, Technologies and New Trends, 1st ed.; Zhang, Z., Zhang, S., Eds.; Springer International Publishing: Cham, Switzerland, 2015; pp. 419–454. [Google Scholar]
- Shi, X.; Simanova, E.; Schönherr, J.; Schreiber, L. Effects of Accelerators on Mobility of 14C-2,4-Dichlorophenoxy Butyric Acid in Plant Cuticles Depends on Type and Concentration of Accelerator. J. Agric. Food Chem. 2005, 53, 2207–2212. [Google Scholar] [CrossRef] [PubMed]
- Ma, P.; Dai, C.; Wang, H.; Li, Z.; Liu, H.; Li, W.; Yang, C. A review on high temperature resistant polyimide films: Heterocyclic structures and nanocomposites. Comp. Commun. 2019, 16, 84–93. [Google Scholar] [CrossRef]
- Guarneri, A.; Cutifani, V.; Cespugli, M.; Pellis, A.; Vasallo, R.; Asaro, F.; Ebert, C.; Gardossi, L. Functionalization of Enzymatically Synthesized Rigid Poly(itaconate)s via Post-Polymerization Aza-Michael Addition of Primary Amines. Adv. Synth. Catal. 2019, 361, 2559–2573. [Google Scholar] [CrossRef]
- Writer, B. Ionic Conductivity, Polymer Electrolyte, Membranes, Electrochemical Stability, Separators. In Lithium-Ion Batteries: A Machine-Generated Summary of Current Research, 1st ed.; Writer, B., Ed.; Springer International Publishing: Cham, Switzerland, 2019; pp. 163–193. [Google Scholar]
- Pintauer, T.; Braunecker, W.; Collange, E.; Poli, R.; Matyjaszewski, K. Determination of Rate Constants for the Activation Step in Atom Transfer Radical Polymerization Using the Stopped-Flow Technique. Macromolecules 2004, 37, 2679–2682. [Google Scholar] [CrossRef]
- Sun, G.; Cheng, C.; Wooley, K.L. Reversible Addition Fragmentation Chain Transfer Polymerization of 4-Vinylbenzaldehyde. Macromolecules 2007, 40, 793–795. [Google Scholar] [CrossRef] [PubMed]
- Martinez, H.; Ren, N.; Matta, M.E.; Hillmyer, M.A. Ring-opening metathesis polymerization of 8-membered cyclic olefins. Polym. Chem. 2014, 5, 3507–3532. [Google Scholar] [CrossRef]
- Chen, J.; Spear, S.K.; Huddleston, J.G.; Rogers, R.D. Polyethylene glycol and solutions of polyethylene glycol as green reaction media. Green Chem. 2005, 7, 64–82. [Google Scholar]
- Aoshima, H.; Uchiyama, M.; Satoh, K.; Kamigaito, M. Interconvertible Living Radical and Cationic Polymerization through Reversible Activation of Dormant Species with Dual Activity. Angew. Chem. Int. Ed. 2014, 53, 10932–10936. [Google Scholar] [CrossRef] [PubMed]
- Zhao, K.; Song, H.; Duan, X.; Wang, Z.; Liu, J.; Ba, X. Novel Chemical Cross-Linked Ionogel Based on Acrylate Terminated Hyperbranched Polymer with Superior Ionic Conductivity for High Performance Lithium-Ion Batteries. Polymers 2019, 11, 444. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Qiu, W.; Qin, F.; Fang, H.; Hadjiev, V.G.; Litvinov, D.; Bao, J. Identification of Cobalt Oxides with Raman Scattering and Fourier Transform Infrared Spectroscopy. J. Phys. Chem. C 2016, 120, 4511–4516. [Google Scholar] [CrossRef]
- Hari, B.S.; Kumar, K.V.M.; Krishnamurthy, K.; Kumar, P.S.; Gobinath, V.K.; Sachinbala, R.; Rajasekar, R. Influence of graphene oxide on the morphological and mechanical behaviour of compatibilized low density polyethylene nanocomposites. Mater. Today Proceed. 2021, 39, 1487–1493. [Google Scholar]
- Martinez-Cisneros, C.S.; Fernandez, A.; Antonelli, C.; Levenfeld, B.; Varez, A.; Vezzu, K.; Di Noto, V.; Sanchez, J.Y. Opening the door to liquid-free polymer electrolytes for calcium batteries. Electrochim. Acta 2020, 353, 136525–136535. [Google Scholar] [CrossRef]
- Tian, X.; Yi, Y.; Fang, B.; Yang, P.; Wang, T.; Liu, P.; Qu, L.; Li, M.; Zhang, S. Design Strategies of Safe Electrolytes for Preventing Thermal Runaway in Lithium Ion Batteries. Chem. Mater. 2020, 32, 9821–9848. [Google Scholar] [CrossRef]
- Xie, J.; Lu, Y.C. A retrospective on lithium-ion batteries. Nat. Commun. 2020, 11, 2499–2502. [Google Scholar] [CrossRef]
- Thalji, M.R.; Ibrahim, A.A.; Ali, G.A.M. Cutting-edge development in dendritic polymeric materials for biomedical and energy applications. Eur. Polym. J. 2021, 160, 110770–110794. [Google Scholar] [CrossRef]
- Yue, L.; Ma, J.; Zhang, J.; Zhao, J.; Dong, S.; Liu, Z.; Cui, G.; Chen, L. All solid-state polymer electrolytes for high-performance lithium ion batteries. Energy Storage Mater. 2016, 5, 139–164. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, Y.; Ma, S.; Duan, R.; Zhao, Y.; Zhang, Y.; Liu, Z.; Li, C. Low temperature performance enhancement of high-safety Lithium–Sulfur battery enabled by synergetic adsorption and catalysis. Electrochim. Acta 2020, 353, 136470–136480. [Google Scholar] [CrossRef]
- Haregewoin, A.M.; Wotango, A.S.; Hwang, B.J. Electrolyte additives for lithium ion battery electrodes: Progress and perspectives. Energy Environ. Sci. 2016, 9, 1955–1988. [Google Scholar] [CrossRef]
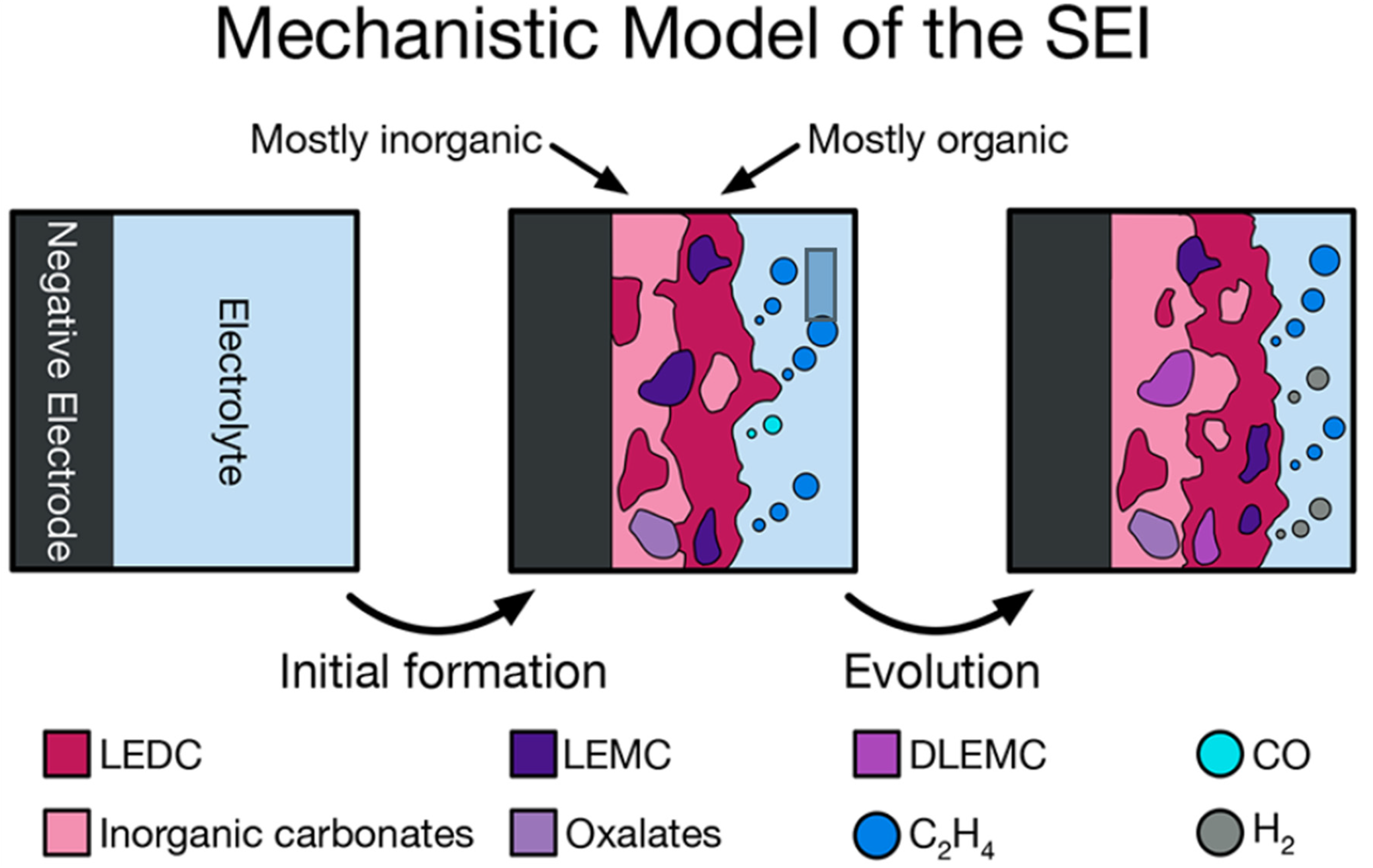
- Spotte-Smith, E.W.S.; Kam, R.L.; Barter, D.; Xie, X.; Hou, T.; Dwaraknath, S.; Blau, S.M.; Persson, K.A. Toward a Mechanistic Model of Solid–Electrolyte Interphase Formation and Evolution in Lithium-Ion Batteries. ACS Energy Lett. 2022, 7, 1446–1453. [Google Scholar] [CrossRef]
- Kowalczyk, S.; Dębowski, M.; Iuliano, A.; Brzeski, S.; Plichta, A. Synthesis of (Hyper)Branched Monohydroxyl Alkoxysilane Oligomers toward Silanized Urethane Prepolymers. Molecules 2012, 27, 2790–2806. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Zheng, S.; Ren, D.; He, X.; Wang, L.; Cui, H.; Liu, X.; Jin, C.; Zhang, F.; Xu, C.; et al. Investigating the thermal runaway mechanisms of lithium-ion batteries based on thermal analysis database. Appl. Energy 2019, 246, 53–64. [Google Scholar] [CrossRef]
- Liu, K.; Liu, Y.; Lin, D.; Pei, A.; Cui, Y. Materials for lithium-ion battery safety. Sci. Adv. 2018, 4, 9820–9830. [Google Scholar] [CrossRef]
- Liu, H.M.; Saikia, D.; Wu, H.C.; Su, C.Y.; Wang, T.H.; Li, Y.H.; Pan, J.P.; Kao, H.M. Towards an understanding of the role of hyper-branched oligomers coated on cathodes, in the safety mechanism of lithium-ion batteries. RSC Adv. 2014, 4, 56147–56155. [Google Scholar] [CrossRef]
- Duh, Y.S.; Sun, Y.; Lin, X.; Zheng, J.; Wang, W.; Wang, Y.; Lin, X.; Jiang, X.; Zheng, Z.; Zheng, S.; et al. Characterization on thermal runaway of commercial 18650 lithium-ion batteries used in electric vehicles: A review. J. Energy Storage 2021, 41, 102888–102902. [Google Scholar] [CrossRef]
- Pham, Q.T.; Hsu, J.M.; Shao, W.J.; Wang, F.M.; Chern, C.S. Mechanisms and kinetics of isothermal polymerization of N,N′-bismaleimide-4,4′-diphenylmethane with 5,5-dimethylbarbituric acid in the presence of triphenylphosphine. Thermochim. Acta 2017, 655, 234–241. [Google Scholar] [CrossRef]
- Pham, Q.T.; Hsu, J.M.; Shao, W.J.; Zhan, Y.X.; Wang, F.M.; Chern, C.S. Mechanisms and kinetics of non-isothermal polymerization of N,N′-bismaleimide-4,4′-diphenylmethane with 1,3-dimethylbarbituric acid. Thermochim. Acta 2017, 658, 31–37. [Google Scholar] [CrossRef]
- Li, Y.H.; Lee, M.L.; Wang, F.M.; Yang, C.R.; Chu, P.P.J.; Pan, J.P. Electrochemical characterization of a branched oligomer as a high-temperature and long-cycle-life additive for lithium-ion batteries. Electrochim. Acta 2012, 85, 72–77. [Google Scholar] [CrossRef]
- Li, Y.H.; Lee, M.L.; Wang, F.M.; Yang, C.R.; Chu, P.P.J.; Yau, S.L.; Pan, J.P. Electrochemical performance and safety features of high-safety lithium ion battery using novel branched additive for internal short protection. Appl. Surf. Sci. 2012, 261, 306–311. [Google Scholar] [CrossRef]
- Lin, C.C.; Wu, H.C.; Pan, J.P.; Su, C.Y.; Wang, T.H.; Sheu, H.S.; Wu, N.L. Investigation on suppressed thermal runaway of Li-ion battery by hyper-branched polymer coated on cathode. Electrochim. Acta 2013, 101, 11–17. [Google Scholar] [CrossRef]
- Wen, Y.C.; Sue, Y.C.; Yang, S.Y.; Pan, J.P.; Wang, T.H.; Jia, H.W. NMR spectroscopy study on N,N′-bismaleimide-4,4′-diphenylmethane and barbituric acid synthesis reaction mechanism in N,N′-dimethylformamide solvent. RSC Adv. 2017, 7, 651–661. [Google Scholar] [CrossRef]
- Su, H.L.; Hsu, J.M.; Pan, J.P.; Chern, C.S. Silica nanoparticles modified with vinyltriethoxysilane and their copolymerization with N,N′-bismaleimide-4,4′-diphenylmethane. J. Appl. Polymer Sci. 2007, 103, 3600–3608. [Google Scholar] [CrossRef]
- Wang, F.M.; Cheng, H.M.; Wu, H.C.; Chu, H.Y.; Cheng, C.S.; Yang, C.R. Novel SEI formation of maleimide-based additives and its improvement of capability and cyclicability in lithium ion batteries. Electrochim. Acta 2009, 54, 3344–3351. [Google Scholar] [CrossRef]
- Su, H.L.; Hsu, J.M.; Pan, J.P.; Wang, T.H.; Chern, C.S. Effects of solvent basicity on free radical polymerizations of N,N′-bismaleimide-4,4′-diphenylmethane initiated by barbituric acid. J. Appl. Polymer Sci. 2010, 117, 596–603. [Google Scholar] [CrossRef]
- Su, H.L.; Hsu, J.M.; Pan, J.P.; Wang, T.H.; Yu, F.E.; Chern, C.S. Kinetic and structural studies of the polymerization of N,N′-bismaleimide-4,4′-diphenylmethane with barbituric acid. Polymer Eng. Sci. 2011, 51, 1188–1197. [Google Scholar] [CrossRef]
- Yu, F.E.; Hsu, J.M.; Pan, J.P.; Wang, T.H.; Chern, C.S. Kinetics of Michael addition polymerizations of n,n′-bismaleimide-4,4′-diphenylmethane with barbituric acid. Polymer Eng. Sci. 2013, 53, 204–211. [Google Scholar] [CrossRef]
- Pham, Q.T.; Hsu, J.M.; Pan, J.P.; Wang, T.H.; Chern, C.S. Non-isothermal degradation kinetics of N,N′-bismaleimide-4,4′-diphenylmethane/barbituric acid based polymers in the presence of hydroquinone. J. Appl. Polymer Sci. 2013, 130, 1923–1930. [Google Scholar] [CrossRef]
- Pham, Q.T.; Hsu, J.M.; Pan, J.P.; Wang, T.H.; Chern, C.S. Non-isothermal degradation of bisphenol A diglycidyl ether diacrylate-based polymers. Thermochim. Acta 2013, 573, 10–17. [Google Scholar] [CrossRef]
- Pham, Q.T.; Hsu, J.M.; Pan, J.P.; Wang, T.H.; Chern, C.S. Kinetics of free radical polymerization of bisphenol A diglycidyl ether diacrylate initiated by barbituric acid. Thermochim. Acta 2013, 573, 121–129. [Google Scholar] [CrossRef]
- Yang, J.; Zhao, P.; Shang, Y.; Wang, L.; He, X.; Fang, M.; Wang, J. Improvement in High-voltage Performance of Lithium-ion Batteries Using Bismaleimide as an Electrolyte Additive. Electrochim. Acta 2014, 121, 264–269. [Google Scholar] [CrossRef]
- Yu, F.E.; Hsu, J.M.; Pan, J.P.; Wang, T.H.; Chiang, Y.C.; Lin, W.; Jiang, J.C.; Chern, C.S. Effect of solvent proton affinity on the kinetics of michael addition polymerization of n,n′-bismaleimide-4,4′-diphenylmethane with barbituric acid. Polymer Eng. Sci. 2014, 54, 559–568. [Google Scholar] [CrossRef]
- Pham, Q.T.; Yu, F.E.; Hsu, J.M.; Chiang, C.H.; Chern, C.S. Kinetics of polymerization of N,N′-bismaleimide-4,4′-diphenylmethane, barbituric acid and aminopropyl phenylsiloxane oligomer. J. Taiwan Inst. Chem. Eng. 2016, 67, 88–97. [Google Scholar] [CrossRef]
- Yu, F.E.; Lee, H.Y.; Hsu, J.M.; Pan, J.P.; Wang, T.H.; Chen, Z.Y.; Chern, C.S. Effects of initial composition and temperature on the kinetics of polymerizations of N,N′-bismaleimide-4,4′-diphenylmethane with barbituric acid. J. Taiwan Inst. Chem. Eng. 2015, 52, 181–190. [Google Scholar] [CrossRef]
- Pham, Q.T.; Hsu, J.M.; Wang, F.M.; Huang, X.C.; Tesemma, M.; Chern, C.S. Kinetics of nucleation-controlled polymerization of N,N′-bismaleimide-4,4′- diphenylmethane/barbituric acid. Thermochim. Acta 2016, 641, 1–7. [Google Scholar] [CrossRef]
- MacNeil, D.D.; Dahn, J.R. Test of Reaction Kinetics Using Both Differential Scanning and Accelerating Rate Calorimetries As Applied to the Reaction of LixCoO2 in Non-aqueous Electrolyte. J. Phys. Chem. A 2001, 105, 4430–4439. [Google Scholar] [CrossRef]
- Pham, Q.T.; Hsu, J.M.; Wang, F.M.; Chen, M.P.; Chern, C.S. Isothermal polymerization kinetics of N,N′-bismaleimide-4,4′- diphenylmethane with cyanuric acid. Thermochim. Acta 2017, 647, 30–35. [Google Scholar] [CrossRef]
- Pham, Q.T.; Zhan, Y.X.; Wang, F.M.; Chern, C.S. Mechanisms and kinetics of non-isothermal polymerization of N,N′-bismaleimide-4,4′-diphenylmethane with barbituric acid in dimethyl sulfoxide. Thermochim. Acta 2019, 676, 139–144. [Google Scholar] [CrossRef]
- Wang, F.M.; Alemu, T.; Yeh, N.H.; Wang, X.C.; Lin, Y.W.; Hsu, C.C.; Chang, Y.J.; Liu, C.H.; Chuang, C.I.; Hsiao, L.H.; et al. Interface Interaction Behavior of Self-Terminated Oligomer Electrode Additives for a Ni-Rich Layer Cathode in Lithium-Ion Batteries: Voltage and Temperature Effects. ACS Appl. Mater. Interfaces 2019, 11, 39827–39840. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.S.; Pham, Q.T.; Yang, C.C.; Chern, C.S.; Babulal, L.M.; Seenivasan, M.; Brunklaus, G.; Placke, T.; Hwang, B.J.; Winter, M. Study of electrochemical performance and thermal property of LiNi0.5Co0.2Mn0.3O2 cathode materials coated with a novel oligomer additive for high-safety lithium-ion batteries. Chem. Eng. J. 2021, 405, 126727–126742. [Google Scholar] [CrossRef]
- Pham, Q.T.; Chern, C.S. Applications of polymers in lithium-ion batteries with enhanced safety and cycle life. J. Polymer Res. 2022, 29, 124–151. [Google Scholar] [CrossRef]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohanty, D.; Hung, I.-M.; Hsieh, C.-T.; Pan, J.-P.; Liu, W.-R. Critical Review on High-Safety Lithium-Ion Batteries Modified by Self-Terminated Oligomers with Hyperbranched Architectures. Batteries 2024, 10, 65. https://doi.org/10.3390/batteries10020065
Mohanty D, Hung I-M, Hsieh C-T, Pan J-P, Liu W-R. Critical Review on High-Safety Lithium-Ion Batteries Modified by Self-Terminated Oligomers with Hyperbranched Architectures. Batteries. 2024; 10(2):65. https://doi.org/10.3390/batteries10020065
Chicago/Turabian StyleMohanty, Debabrata, I-Ming Hung, Chien-Te Hsieh, Jing-Pin Pan, and Wei-Ren Liu. 2024. "Critical Review on High-Safety Lithium-Ion Batteries Modified by Self-Terminated Oligomers with Hyperbranched Architectures" Batteries 10, no. 2: 65. https://doi.org/10.3390/batteries10020065
APA StyleMohanty, D., Hung, I.-M., Hsieh, C.-T., Pan, J.-P., & Liu, W.-R. (2024). Critical Review on High-Safety Lithium-Ion Batteries Modified by Self-Terminated Oligomers with Hyperbranched Architectures. Batteries, 10(2), 65. https://doi.org/10.3390/batteries10020065