Sodium Polymer Electrolytes: A Review
Abstract
:1. Introduction
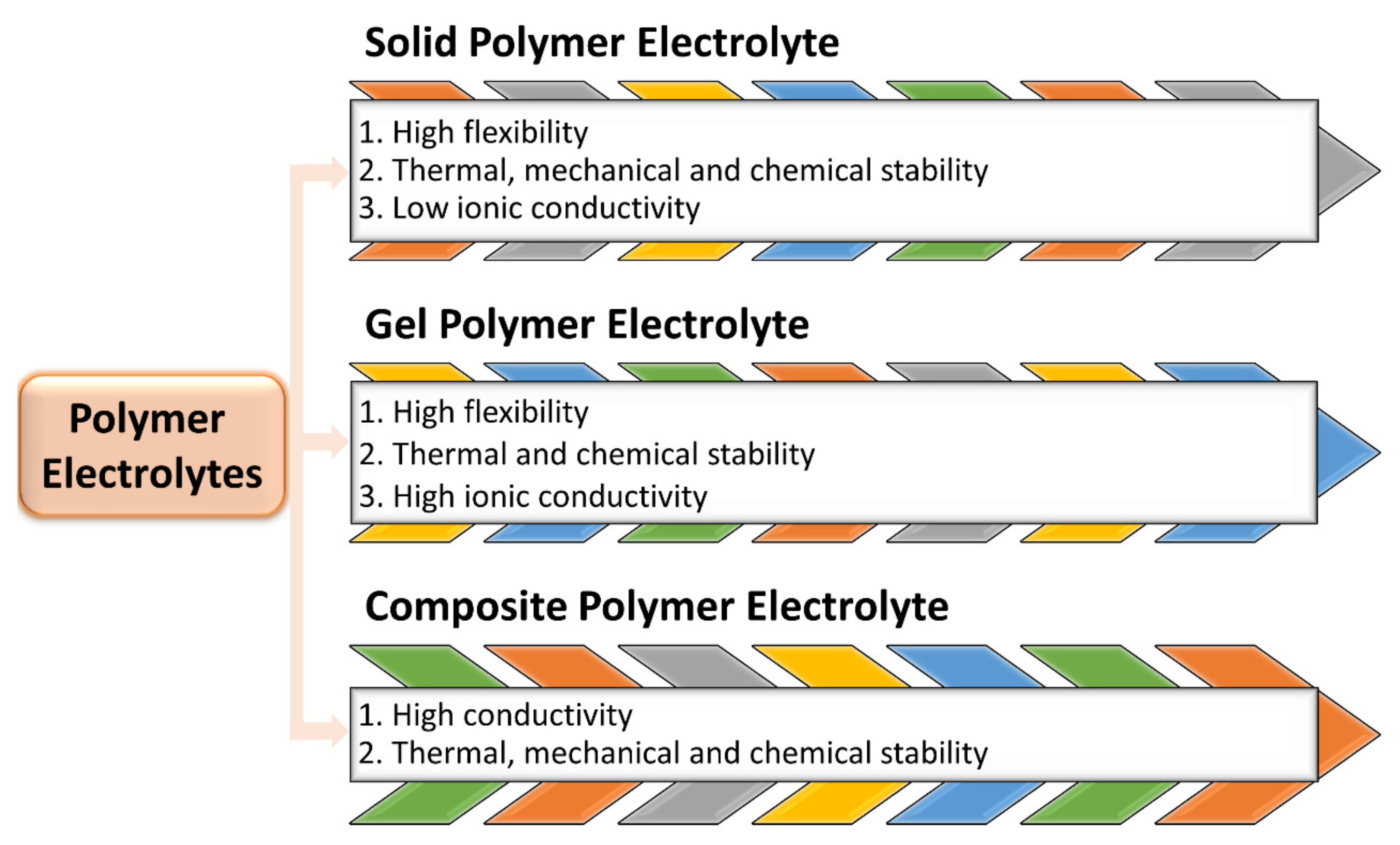
2. Polymer Electrolytes
2.1. Solid Polymers Electrolytes (SPEs)
2.2. Gel Polymers Electrolytes (GPEs)
2.3. Composite Polymer Electrolytes (CPEs)
3. Conclusions and Future Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AC | Activated charcoal |
| AC | Alternating current |
| AFM | Atomic force microscopy |
| AGPE | Amorphous gel polymer electrolyte |
| Al2O3 | Aluminum oxide |
| ASIB | Aqueous sodium ion battery |
| BaTiO3 | Barium titanate |
| BET | Brunauer–Emmett–Teller |
| BMIMBF4 | 1-butyl-3-methylimidazolium tetrafluoroborate |
| BMIM-MS | 1-butyl-3-methylimidazolium methylsulfate |
| CFEM | Composite nanofibrous electrolyte membranes |
| CMC | Carboxy methylcellulose |
| CNT | Carbon nanotubes |
| CPE | Composite polymer electrolyte |
| CPN | Crosslinked polyether network |
| CS | Cornstarch |
| DC | Direct current |
| DEC | Diethyl carbonate |
| DI | Deionized |
| DMA | Dynamic mechanical analysis |
| DMF | Dimethyl formamide |
| DMSO | Dimethylsulfoxide |
| DSC | Differential scanning calorimetry |
| DSSC | Dye-sensitized solar cells |
| EC | Ethylene carbonate |
| EDLC | Electric double-layer capacitor |
| EDS | Energy dispersive spectroscopy |
| EES | Electrochemical energy storage |
| EIS | Electrochemical impedance spectroscopy |
| ESW | Electrochemical stability window |
| Fe(NO3)3 | Ferric nitrate |
| FEC | Fluoroethylenecarbonate |
| FESEM | Field emission scanning electron microscopy |
| FTIR | Fourier transform–infrared spectroscopy |
| FTO | Fluoride/tin oxide |
| GO | Graphene oxide |
| GPC | Gel permeation chromatography |
| GPE | Gel polymer electrolyte |
| HOMO | Highest occupied molecular orbital |
| HPMC | Hydroxypropyl methylcellulose |
| HSCR | Hydrothermal soft chemical reaction |
| H-β-CD | Hyperbranched β-cyclodextrin |
| IL | Ionic liquid |
| IR | Infrared |
| kΩ | Kiloohms |
| La(NO3)3 | Lathanum nitrate |
| LE | Liquid electrolyte |
| LIB | Lithium ion battery |
| LSV | Linear sweep voltammetry |
| LUMO | Lowest unoccupied molecular orbital |
| MC | Methylcellulose |
| MHz | Megahertz |
| MPII | 1-methyl-3-propylimidazolium iodide |
| MW | Molecular weight |
| NaAlO2 | Sodium aluminate |
| NaBFMB | Bis(fluoroallyl)malonato borate salt |
| NaBr | Sodium bromide |
| NaCF3SO3 | Sodium triflate |
| NaCF3SO3 | Sodium trifluoromethanesulfonate |
| NaClO3 | Sodium chlorate |
| NaDFOB | Sodium-difluoro(oxalato)borate |
| NaF | Sodium fluoride |
| NaHSO3 | Sodium hydrogen sulfite |
| NaI | Sodium iodide |
| NaNO3 | Sodium nitrate |
| NaPA | Poly(bis(4-carbonyl benzene sulfonyl)imide-co-2,5-diamino benzesulfonic acid) |
| NaPF2 | Sodium difluorophosphate |
| NaPF6 | Sodium hexafluorophosphate |
| NaPTAB | Sodium-poly(tartaric acid)borate |
| NaSCN | Sodium thiocyanate |
| NaTFSI | Sodium trifluoromethaneslfonimide |
| NGPE | Nanocomposite gel polymer electrolytes |
| NIPS | Non-solvent-induced phase separation |
| NMR | Nuclear magnetic resonance |
| ORB | Organic radical battery |
| P(MVE-alt-MA)) | Poly(methyl vinyl ether-alt-maleic anhydride) |
| PAN | Poly(acrylonitrile) |
| PANI | Polyaniline |
| PC | Propylene carbonate |
| PCPE | Platicizer composite polymer electrolyte |
| PDA | Polydopamine-plastcized polyne |
| PE | Polymer electrolyte |
| PEG | Poly(ethylene glycol) |
| PEGMA | Poly(ethylene glycol) methyl ether methacrylate |
| PEM | Proton exchange membrane |
| PEO | Poly(ethylene) oxide |
| PFSA | Perfluorosulfonic acid |
| PILEs | Polymer ionic liquid electrolytes |
| PMMA | Poly(methyl methacrylate) |
| PPEGMA | Poly(poly(ethylineglycol)methacrylate) |
| PTVE | 2,2,6,6-tetramethylpiperidine-4-yl-1-oxyl vinyl ether |
| PVA | Poly(vinyl alcohol) |
| PVB | Poly(vinyl butyral) |
| PVC | Poly(vinyl chloride) |
| PVDF | Poly(vinylidene fluoride) |
| PVDF-HFP | Poly(vinylidene fluoride hexafluoro propylene) |
| PVP | Poly(vinyl pyrolidone) |
| RT | Room temperature |
| RTIL | Room-temperature ionic liquid |
| SAED | Selected area electron diffraction |
| SIB | Sodium ion battery |
| SEI | Solid electrolyte interphase |
| SEM | Scanning electron microscopy |
| SGPE | Separator-cum gel polymer electrolyte |
| SHE | Standard hydrogen electrode |
| SIC | Single ion-conducting |
| SIL | Solvate ionic liquid |
| SMB | Sodium metal battery |
| SPE | Solid polymer electrolyte |
| TEP | Triethyl phosphate |
| TGA | Thermogravimetric analyzer |
| TG-DTA | Thermogravimetric differential thermal analysis |
| TiO2 | Titanium dioxide |
| TMP | Trimethyl phosphate |
| TMPT | Tri-thiol (trimethylolpropane tris(3-mercapto propionate) |
| VC | Vinylene carbonate |
| VTF | Vogel–Tammann–Fulcher |
| Wh | Watt-hour |
| XPS | X-ray photoelectron spectroscopy |
| XRD | X-ray diffraction |
| ZnFe2O4 | Zinc ferrite |
References
- Yang, Z.; Zhang, J.; Kintner-Meyer, M.C.W.; Lu, X.; Choi, D.; Lemmon, J.P.; Liu, J. Electrochemical Energy Storage for Green Grid. Chem. Rev. 2011, 111, 3577–3613. [Google Scholar] [CrossRef] [PubMed]
- Armand, M.; Tarascon, J.M. Building better batteries. Nature 2008, 451, 652–657. [Google Scholar] [CrossRef] [PubMed]
- Goodenough, J.B.; Park, K.-S. The Li-Ion Rechargeable Battery: A Perspective. J. Am. Chem. Soc. 2013, 135, 1167–1176. [Google Scholar] [CrossRef] [PubMed]
- Tarascon, J.M.; Armand, M. Issues and challenges facing rechargeable lithium batteries. Nature 2001, 414, 359–367. [Google Scholar] [CrossRef] [PubMed]
- Goodenough, J.B.; Kim, Y. Challenges for Rechargeable Li Batteries. Chem. Mater. 2010, 22, 587–603. [Google Scholar] [CrossRef]
- Yang, R.; Zhang, F.; Lei, X.; Zheng, Y.; Zhao, G.; Tang, Y.; Lee, C.-S. Pseudocapacitive Ti-Doped Niobium Pentoxide Nanoflake Structure Design for a Fast Kinetics Anode toward a High-Performance Mg-Ion-Based Dual-Ion Battery. ACS Appl. Mater. Interfaces 2020, 12, 47539–47547. [Google Scholar] [CrossRef]
- Lei, X.; Zheng, Y.; Zhang, F.; Wang, Y.; Tang, Y. Highly stable magnesium-ion-based dual-ion batteries based on insoluble small-molecule organic anode material. Energy Storage Mater. 2020, 30, 34–41. [Google Scholar] [CrossRef]
- Vignarooban, K.; Kushagra, R.; Elango, A.; Badami, P.; Mellander, B.E.; Xu, X.; Tucker, T.G.; Nam, C.; Kannan, A.M. Current trends and future challenges of electrolytes for sodium-ion batteries. Int. J. Hydrogen Energy 2016, 41, 2829–2846. [Google Scholar] [CrossRef]
- Deng, X.; Zou, K.; Cai, P.; Wang, B.; Hou, H.; Zou, G.; Ji, X. Advanced Battery-Type Anode Materials for High-Performance Sodium-Ion Capacitors. Small Methods 2020, 4, 2000401. [Google Scholar] [CrossRef]
- Hwang, J.-Y.; Myung, S.-T.; Sun, Y.-K. Sodium-ion batteries: Present and future. Chem. Soc. Rev. 2017, 46, 3529–3614. [Google Scholar] [CrossRef]
- Peters, J.F.; Peña Cruz, A.; Weil, M. Exploring the Economic Potential of Sodium-Ion Batteries. Batteries 2019, 5, 10. [Google Scholar] [CrossRef]
- Xu, G.-L.; Amine, R.; Abouimrane, A.; Che, H.; Dahbi, M.; Ma, Z.-F.; Saadoune, I.; Alami, J.; Mattis, W.L.; Pan, F. Challenges in Developing Electrodes, Electrolytes, and Diagnostics Tools to Understand and Advance Sodium-Ion Batteries. Adv. Energy Mater. 2018, 8, 1702403. [Google Scholar] [CrossRef]
- Bella, F.; Colò, F.; Nair, J.R.; Gerbaldi, C. Photopolymer Electrolytes for Sustainable, Upscalable, Safe, and Ambient-Temperature Sodium-Ion Secondary Batteries. ChemSusChem 2015, 8, 3668–3676. [Google Scholar] [CrossRef] [PubMed]
- Nayak, P.K.; Yang, L.; Brehm, W.; Adelhelm, P. From Lithium-Ion to Sodium-Ion Batteries: Advantages, Challenges, and Surprises. Angew. Chem. Int. Ed. 2018, 57, 102–120. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Zhang, H.; Zhou, Q.; Qu, H.; Dong, T.; Zhang, M.; Tang, B.; Zhang, J.; Cui, G. Safety-Enhanced Polymer Electrolytes for Sodium Batteries: Recent Progress and Perspectives. ACS Appl. Mater. Interfaces 2019, 11, 17109–17127. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Sun, X.; Yu, Y.; Ding, C.; Chen, C.; Guan, Y. Research Progress in Sodium-Ion Battery Materials for Energy Storage. Prog. Chem. 2014, 26, 582–591. [Google Scholar]
- Chayambuka, K.; Mulder, G.; Danilov, D.L.; Notten, P.H.L. Sodium-Ion Battery Materials and Electrochemical Properties Reviewed. Adv. Energy Mater. 2018, 8, 1800079. [Google Scholar] [CrossRef]
- Delmas, C. Sodium and Sodium-Ion Batteries: 50 Years of Research. Adv. Energy Mater. 2018, 8, 1703137. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, Q.; Ren, C.; Luo, F.; Ma, Q.; Hu, Y.-S.; Zhou, Z.; Li, H.; Huang, X.; Chen, L. A ceramic/polymer composite solid electrolyte for sodium batteries. J. Mater. Chem. A 2016, 4, 15823–15828. [Google Scholar] [CrossRef]
- Goodenough, J.B.; Hong, H.Y.P.; Kafalas, J.A. Fast Na+-ion transport in skeleton structures. Mater. Res. Bull. 1976, 11, 203–220. [Google Scholar] [CrossRef]
- Qiao, L.; Judez, X.; Rojo, T.; Armand, M.; Zhang, H. Review—Polymer Electrolytes for Sodium Batteries. J. Electrochem. Soc. 2020, 167, 070534. [Google Scholar] [CrossRef]
- Li, Z.-Y.; Li, Z.; Fu, J.-L.; Guo, X. Sodium-ion conducting polymer electrolytes. Rare Met. 2023, 42, 1–16. [Google Scholar] [CrossRef]
- Jacobson, A.J.; Chianelli, R.R.; Rich, S.M.; Whittingham, M.S. Amorphous molybdenum trisulfide: A new lithium battery cathode. Mater. Res. Bull. 1979, 14, 1437–1448. [Google Scholar] [CrossRef]
- Parant, J.-P.; Olazcuaga, R.; Devalette, M.; Fouassier, C.; Hagenmuller, P. Sur quelques nouvelles phases de formule NaxMnO2 (x ≤ 1). J. Solid State Chem. 1971, 3, 1–11. [Google Scholar] [CrossRef]
- Guo, G.; Hong, J.; Cong, C.; Zhou, X.; Zhang, K. Molybdenum disulfide synthesized by hydrothermal method as anode for lithium rechargeable batteries. J. Mater. Sci. 2005, 40, 2557–2559. [Google Scholar] [CrossRef]
- Sarciaux, S.; Le Gal La Salle, A.; Verbaere, A.; Piffard, Y.; Guyomard, D. γ-MnO2 for Li batteries: Part II. Some aspects of the lithium insertion process into γ-MnO2 and electrochemically lithiated γ-LixMnO2 compounds. J. Power Sources 1999, 81–82, 661–665. [Google Scholar] [CrossRef]
- Mauger, A.; Julien, C.M.; Paolella, A.; Armand, M.; Zaghib, K. Building Better Batteries in the Solid State: A Review. Materials 2019, 12, 3892. [Google Scholar] [CrossRef]
- Li, Q.; Chen, J.; Fan, L.; Kong, X.; Lu, Y. Progress in electrolytes for rechargeable Li-based batteries and beyond. Green Energy Environ. 2016, 1, 18–42. [Google Scholar] [CrossRef]
- Eshetu, G.G.; Elia, G.A.; Armand, M.; Forsyth, M.; Komaba, S.; Rojo, T.; Passerini, S. Electrolytes and Interphases in Sodium-Based Rechargeable Batteries: Recent Advances and Perspectives. Adv. Energy Mater. 2020, 10, 2000093. [Google Scholar] [CrossRef]
- Zhu, Z.; Chen, J. Review—Advanced Carbon-Supported Organic Electrode Materials for Lithium (Sodium)-Ion Batteries. J. Electrochem. Soc. 2015, 162, A2393. [Google Scholar] [CrossRef]
- Fenton, D.E.; Parker, J.M.; Wright, P.V. Complexes of alkali metal ions with poly(ethylene oxide). Polymer 1973, 14, 589. [Google Scholar] [CrossRef]
- Zhou, D.; Shanmukaraj, D.; Tkacheva, A.; Armand, M.; Wang, G. Polymer Electrolytes for Lithium-Based Batteries: Advances and Prospects. Chem 2019, 5, 2326–2352. [Google Scholar] [CrossRef]
- Skaarup, S.; West, K.; Zachau-Christiansen, B. Mixed phase solid electrolytes. Solid State Ionics 1988, 28–30, 975–978. [Google Scholar] [CrossRef]
- Hoang Huy, V.P.; So, S.; Hur, J. Inorganic Fillers in Composite Gel Polymer Electrolytes for High-Performance Lithium and Non-Lithium Polymer Batteries. Nanomaterials 2021, 11, 614. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Zhu, Z.; Wang, L.; Wang, S.; Li, H.; Tao, Z.; Shi, J.; Guan, L.; Chen, J. Quasi-Solid-State Rechargeable Lithium-Ion Batteries with a Calix[4]quinone Cathode and Gel Polymer Electrolyte. Angew. Chem. Int. Ed. 2013, 52, 9162–9166. [Google Scholar] [CrossRef] [PubMed]
- Vasudevan, S.; Dwivedi, S.; Balaya, P. Overview and perspectives of solid electrolytes for sodium batteries. Int. J. Appl. Ceram. Technol. 2023, 20, 563–584. [Google Scholar] [CrossRef]
- Ahmad, H.; Kubra, K.T.; Butt, A.; Nisar, U.; Iftikhar, F.J.; Ali, G. Recent progress, challenges, and perspectives in the development of solid-state electrolytes for sodium batteries. J. Power Sources 2023, 581, 233518. [Google Scholar] [CrossRef]
- Zheng, J.; Li, W.; Liu, X.; Zhang, J.; Feng, X.; Chen, W. Progress in Gel Polymer Electrolytes for Sodium-Ion Batteries. Energy Environ. Mater. 2023, 6, e12422. [Google Scholar] [CrossRef]
- Aruchamy, K.; Ramasundaram, S.; Divya, S.; Chandran, M.; Yun, K.; Oh, T.H. Gel Polymer Electrolytes: Advancing Solid-State Batteries for High-Performance Applications. Gels 2023, 9, 585. [Google Scholar] [CrossRef]
- Maurya, D.K.; Dhanusuraman, R.; Guo, Z.; Angaiah, S. Composite polymer electrolytes: Progress, challenges, and future outlook for sodium-ion batteries. Adv. Compos. Hybrid Mater. 2022, 5, 2651–2674. [Google Scholar] [CrossRef]
- Zhang, J.; Yue, L.; Hu, P.; Liu, Z.; Qin, B.; Zhang, B.; Wang, Q.; Ding, G.; Zhang, C.; Zhou, X. Taichi-inspired rigid-flexible coupling cellulose-supported solid polymer electrolyte for high-performance lithium batteries. Sci. Rep. 2014, 4, 6272. [Google Scholar] [CrossRef]
- Tsuchida, E.; Ohno, H.; Tsunemi, K. Conduction of lithium ions in polyvinylidene fluoride and its derivatives—I. Electrochim. Acta 1983, 28, 591–595. [Google Scholar] [CrossRef]
- Das, S.; Ghosh, A. Effect of plasticizers on ionic conductivity and dielectric relaxation of PEO-LiClO4 polymer electrolyte. Electrochim. Acta 2015, 171, 59–65. [Google Scholar] [CrossRef]
- Tiwari, T.; Srivastava, N.; Srivastava, P.C. Ion Dynamics Study of Potato Starch + Sodium Salts Electrolyte System. Int. J. Electrochem. 2013, 2013, 670914. [Google Scholar] [CrossRef]
- Aragón, M.J.; Gutiérrez, J.; Klee, R.; Lavela, P.; Alcántara, R.; Tirado, J.L. On the effect of carbon content for achieving a high performing Na3V2(PO4)3/C nanocomposite as cathode for sodium-ion batteries. J. Electroanal. Chem. 2017, 784, 47–54. [Google Scholar] [CrossRef]
- Aziz, S.B.; Brevik, I.; Hamsan, M.H.; Brza, M.A.; Nofal, M.M.; Abdullah, A.M.; Rostam, S.; Al-Zangana, S.; Muzakir, S.K.; Kadir, M.F.Z. Compatible Solid Polymer Electrolyte Based on Methyl Cellulose for Energy Storage Application: Structural, Electrical, and Electrochemical Properties. Polymers 2020, 12, 2257. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Zhou, W.; Park, K.; Goodenough, J.B. A Sodium-Ion Battery with a Low-Cost Cross-Linked Gel-Polymer Electrolyte. Adv. Energy Mater. 2016, 6, 1600467. [Google Scholar] [CrossRef]
- Wang, Y.; Song, S.; Xu, C.; Hu, N.; Molenda, J.; Lu, L. Development of solid-state electrolytes for sodium-ion battery–A short review. Nano Mater. Sci. 2019, 1, 91–100. [Google Scholar] [CrossRef]
- Moon, S.-H.; Kim, Y.H.; Cho, D.-C.; Shin, E.-C.; Lee, D.; Im, W.B.; Lee, J.-S. Sodium ion transport in polymorphic scandium NASICON analog Na3Sc2(PO4)3 with new dielectric spectroscopy approach for current-constriction effects. Solid State Ion. 2016, 289, 55–71. [Google Scholar] [CrossRef]
- Faiz, H.; Zainuddin, S.K.; Kamarudin, K.; Kok Sheng, C.; Abdullah, M.A. Ion-conducting polymer electrolyte films based on poly (Sodium 4-styrenesulfonate) complexed with ammonium nitrate: Studies based on morphology, structural and electrical spectroscopy. Malays. J. Anal. Sci. 2018, 22, 238–248. [Google Scholar]
- Jinisha, B.; Anilkumar, K.M.; Manoj, M.; Abhilash, A.; Pradeep, V.S.; Jayalekshmi, S. Poly (ethylene oxide) (PEO)-based, sodium ion-conducting‚ solid polymer electrolyte films, dispersed with Al2O3 filler, for applications in sodium ion cells. Ionics 2018, 24, 1675–1683. [Google Scholar] [CrossRef]
- Anantha, P.S.; Hariharan, K. Physical and ionic transport studies on poly(ethylene oxide)–NaNO3 polymer electrolyte system. Solid State Ion. 2005, 176, 155–162. [Google Scholar] [CrossRef]
- Hashmi, S.A.; Chandra, S. Experimental investigations on a sodium-ion-conducting polymer electrolyte based on poly(ethylene oxide) complexed with NaPF6. Mater. Sci. Eng. B 1995, 34, 18–26. [Google Scholar] [CrossRef]
- Sreekanth, T.; Jaipal Reddy, M.; Ramalingaiah, S.; Subba Rao, U.V. Ion-conducting polymer electrolyte based on poly (ethylene oxide) complexed with NaNO3 salt-application as an electrochemical cell. J. Power Sources 1999, 79, 105–110. [Google Scholar] [CrossRef]
- Chandrasekaran, R.; Selladurai, S. Preparation and characterization of a new polymer electrolyte (PEO:NaClO3) for battery application. J. Solid State Electrochem. 2001, 5, 355–361. [Google Scholar] [CrossRef]
- Siva Kumar, J.; Subrahmanyam, A.R.; Jaipal Reddy, M.; Subba Rao, U.V. Preparation and study of properties of polymer electrolyte system (PEO+NaClO3). Mater. Lett. 2006, 60, 3346–3349. [Google Scholar] [CrossRef]
- Mohan, V.M.; Raja, V.; Sharma, A.K.; Narasimha Rao, V.V.R. Ion transport and battery discharge characteristics of polymer electrolyte based on PEO complexed with NaFeF4 salt. Ionics 2006, 12, 219–226. [Google Scholar] [CrossRef]
- Mohan, V.M.; Bhargav, P.B.; Raja, V.; Sharma, A.K.; Narasimha Rao, V.V.R. Optical and Electrical Properties of Pure and Doped PEO Polymer Electrolyte Films. Soft Mater. 2007, 5, 33–46. [Google Scholar] [CrossRef]
- Bhide, A.; Hariharan, K. Ionic transport studies on (PEO)6:NaPO3 polymer electrolyte plasticized with PEG400. Eur. Polym. J. 2007, 43, 4253–4270. [Google Scholar] [CrossRef]
- Bhide, A.; Hariharan, K. A new polymer electrolyte system (PEO)n:NaPO3. J. Power Sources 2006, 159, 1450–1457. [Google Scholar] [CrossRef]
- Sasikala, U.T.; Kumar, P.N.; Rao, V.V.R.N.; Sharma, A.K. Structural, Electrical and Parametric studies of a PEO based Polymer Electrolyte for battery Applications. Int. J. Eng. Sci. Adv. Technol. 2012, 2, 722–730. [Google Scholar]
- Ma, Q.; Liu, J.; Qi, X.; Rong, X.; Shao, Y.; Feng, W.; Nie, J.; Hu, Y.-S.; Li, H.; Huang, X. A new Na[(FSO2)(n-C4F9SO2)N]-based polymer electrolyte for solid-state sodium batteries. J. Mater. Chem. A 2017, 5, 7738–7743. [Google Scholar] [CrossRef]
- Herath, H.M.A.; Seneviratne, V.A. Electrical and Thermal studies on Sodium based polymer electrolyte. Procedia Eng. 2017, 215, 124–129. [Google Scholar] [CrossRef]
- Arya, A.; Sharma, A.L. Tailoring of the structural, morphological, electrochemical, and dielectric properties of solid polymer electrolyte. Ionics 2019, 25, 1617–1632. [Google Scholar] [CrossRef]
- Pritam; Arya, A.; Sharma, A.L. Dielectric relaxations and transport properties parameter analysis of novel blended solid polymer electrolyte for sodium-ion rechargeable batteries. J. Mater. Sci. 2019, 54, 7131–7155. [Google Scholar] [CrossRef]
- Pritam; Arya, A.; Sharma, A.L. Selection of best composition of Na+ ion conducting PEO-PEI blend solid polymer electrolyte based on structural, electrical, and dielectric spectroscopic analysis. Ionics 2020, 26, 745–766. [Google Scholar] [CrossRef]
- Zhang, Q.; Lu, Y.; Yu, H.; Yang, G.; Liu, Q.; Wang, Z.; Chen, L.; Hu, Y.-S. PEO-NaPF6 Blended Polymer Electrolyte for Solid State Sodium Battery. J. Electrochem. Soc. 2020, 167, 070523. [Google Scholar] [CrossRef]
- Youcef, H.B.; Orayech, B.; Del Amo, J.M.L.; Bonilla, F.; Shanmukaraj, D.; Armand, M. Functionalized cellulose as quasi single-ion conductors in polymer electrolyte for all-solid–state Li/Na and LiS batteries. Solid State Ion. 2020, 345, 115168. [Google Scholar] [CrossRef]
- Kim, Y.; Künzel, M.; Steinle, D.; Dong, X.; Kim, G.-T.; Varzi, A.; Passerini, S. Anode-less seawater batteries with a Na-ion conducting solid-polymer electrolyte for power to metal and metal to power energy storage. Energy Environ. Sci. 2022, 15, 2610–2618. [Google Scholar] [CrossRef]
- Chandra, A.; Chandra, A.; Dhundhel, R.S.; Bhatt, A. Sodium-ion-conducting solid polymer electrolyte: Temperature-dependent ionic parameters and solid-state polymer battery fabrication. Indian J. Phys. 2022, 96, 1069–1074. [Google Scholar] [CrossRef]
- Subba Reddy, C.V.; Han, X.; Zhu, Q.-Y.; Mai, L.-Q.; Chen, W. Conductivity and discharge characteristics of (PVC+NaClO4) polymer electrolyte systems. Eur. Polym. J. 2006, 42, 3114–3120. [Google Scholar] [CrossRef]
- Muhammad, F.H.; Subban, R.H.Y.; Winie, T. Charge carrier density and mobility of poly(vinyl chloride)-based polymer electrolyte using impedance spectroscopy. Mater. Today Proc. 2017, 4 Pt C, 5130–5137. [Google Scholar] [CrossRef]
- Bhargav, P.B.; Mohan, V.M.; Sharma, A.K.; Rao, V.V.R.N. Structural and electrical studies of sodium iodide doped poly(vinyl alcohol) polymer electrolyte films for their application in electrochemical cells. Ionics 2007, 13, 173–178. [Google Scholar] [CrossRef]
- Bhargav, P.B.; Mohan, V.M.; Sharma, A.K.; Rao, V.V.R.N. Structural and electrical properties of pure and NaBr doped poly (vinyl alcohol) (PVA) polymer electrolyte films for solid-state battery applications. Ionics 2007, 13, 441–446. [Google Scholar] [CrossRef]
- Duraikkan, V.; Sultan, A.B.; Nallaperumal, N.; Shunmuganarayanan, A. Structural, thermal and electrical properties of polyvinyl alcohol/poly(vinyl pyrrolidone)–sodium nitrate solid polymer blend electrolyte. Ionics 2018, 24, 139–151. [Google Scholar] [CrossRef]
- Aziz, S.B.; Nofal, M.M.; Abdulwahid, R.T.; Ghareeb, H.O.; Dannoun, E.M.A.; Abdullah, R.M.; Hamsan, M.H.; Kadir, M.F.Z. Plasticized Sodium-Ion Conducting PVA Based Polymer Electrolyte for Electrochemical Energy Storage—EEC Modeling, Transport Properties, and Charge-Discharge Characteristics. Polymers 2021, 13, 803. [Google Scholar] [CrossRef] [PubMed]
- Khanmirzaei, M.H.; Ramesh, S.; Ramesh, K. Polymer electrolyte based dye-sensitized solar cell with rice starch and 1-methyl-3-propylimidazolium iodide ionic liquid. Mater. Des. 2015, 85, 833–837. [Google Scholar] [CrossRef]
- Binti Shahrudin, S.; Ahmad, A.H. Electrical Analysis of Cornstarch-Based Polymer Electrolyte Doped with NaCl. Solid State Phenom. 2017, 268, 347–351. [Google Scholar] [CrossRef]
- Awang, F.; Hassan, M.; Kamarudin, K. Effect of Sodiumbisulfite on corn starch solid polymer electrolyte. Malays. J. Anal. Sci. 2021, 25, 224–233. [Google Scholar]
- Asnawi, A.S.F.M.; Aziz, S.B.; Brevik, I.; Brza, M.A.; Yusof, Y.M.; Alshehri, S.M.; Ahamad, T.; Kadir, M.F.Z. The Study of Plasticized Sodium-Ion Conducting Polymer Blend Electrolyte Membranes Based on Chitosan/Dextran Biopolymers: Ion Transport, Structural, Morphological and Potential Stability. Polymers 2021, 13, 383. [Google Scholar] [CrossRef]
- Rani, N.S.; Sannappa, J.; Demappa, T.; Mahadevaiah, A. Structural, thermal, and electrical studies of sodium iodide (NaI)-doped hydroxypropyl methylcellulose (HPMC) polymer electrolyte films. Ionics 2014, 20, 201–207. [Google Scholar] [CrossRef]
- Abiddin, J.F.B.; Ahmad, A. Fourier transform infrared spectroscopy and electrical characterization of methylcellulose based solid polymer electrolyte doped with sodium iodide. J. Teknol. 2015, 76, 41–45. [Google Scholar]
- El Sayed, A.M.; Khabiri, G. Spectroscopic, Optical and Dielectric Investigation of (Mg, Cu, Ni, or Cd) Acetates’ Influence on Carboxymethyl Cellulose Sodium Salt/Polyvinylpyrrolidone Polymer Electrolyte Films. J. Electron. Mater. 2020, 49, 2381–2392. [Google Scholar] [CrossRef]
- Shetty, S.K.; Ismayil, N.; Shetty, G. Enhancement of Electrical and Optical Properties of Sodium Bromide Doped Carboxymethyl Cellulose Biopolymer Electrolyte Films. J. Macromol. Sci. Part B 2020, 59, 235–247. [Google Scholar] [CrossRef]
- Singh, V.K.; Shalu; Chaurasia, S.K.; Singh, R.K. Development of ionic liquid mediated novel polymer electrolyte membranes for application in Na-ion batteries. RSC Adv. 2016, 6, 40199–40210. [Google Scholar] [CrossRef]
- Zhang, J.; Wen, H.; Yue, L.; Chai, J.; Ma, J.; Hu, P.; Ding, G.; Wang, Q.; Liu, Z.; Cui, G. In Situ Formation of Polysulfonamide Supported Poly(ethylene glycol) Divinyl Ether Based Polymer Electrolyte toward Monolithic Sodium Ion Batteries. Small 2017, 13, 1601530. [Google Scholar] [CrossRef] [PubMed]
- Pan, Q.; Li, Z.; Zhang, W.; Zeng, D.; Sun, Y.; Cheng, H. Single ion conducting sodium ion batteries enabled by a sodium ion exchanged poly(bis(4-carbonyl benzene sulfonyl)imide-co-2,5-diamino benzesulfonic acid) polymer electrolyte. Solid State Ion. 2017, 300, 60–66. [Google Scholar] [CrossRef]
- Chen, S.; Feng, F.; Yin, Y.; Che, H.; Liao, X.-Z.; Ma, Z.-F. A solid polymer electrolyte based on star-like hyperbranched β-cyclodextrin for all-solid-state sodium batteries. J. Power Sources 2018, 399, 363–371. [Google Scholar] [CrossRef]
- Janakiraman, S.; Surendran, A.; Biswal, R.; Ghosh, S.; Anandhan, S.; Venimadhav, A. Electrospun electroactive polyvinylidene fluoride-based fibrous polymer electrolyte for sodium ion batteries. Mater. Res. Express 2019, 6, 086318. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, M.; Chen, Z.; Du, X.; Huang, S.; Tang, B.; Dong, T.; Wu, H.; Yu, Z.; Zhang, J. Flame-retardant quasi-solid polymer electrolyte enabling sodium metal batteries with highly safe characteristic and superior cycling stability. Nano Res. 2019, 12, 2230–2237. [Google Scholar] [CrossRef]
- Liu, K.; Xie, Y.; Yang, Z.; Kim, H.-K.; Dzwiniel, T.L.; Yang, J.; Xiong, H.; Liao, C. Design of a Single-Ion Conducting Polymer Electrolyte for Sodium-Ion Batteries. J. Electrochem. Soc. 2021, 168, 120543. [Google Scholar] [CrossRef]
- Law, H.M.; Yu, J.; Kwok, S.C.T.; Zhou, G.; Robson, M.J.; Wu, J.; Ciucci, F. A hybrid dual-salt polymer electrolyte for sodium metal batteries with stable room temperature cycling performance. Energy Storage Mater. 2022, 46, 182–191. [Google Scholar] [CrossRef]
- Martinez-Cisneros, C.S.; Pandit, B.; Levenfeld, B.; Varez, A.; Sanchez, J.-Y. Flexible solvent-free polymer electrolytes for solid-state Na batteries. J. Power Sources 2023, 559, 232644. [Google Scholar] [CrossRef]
- Olmedo-Martínez, J.L.; Fdz De Anastro, A.; Martínez-Ibañez, M.; Müller, A.J.; Mecerreyes, D. Polyethylene Oxide/Sodium Sulfonamide Polymethacrylate Blends as Highly Conducting Single-Ion Solid Polymer Electrolytes. Energy Fuels 2023, 37, 5519–5529. [Google Scholar] [CrossRef]
- Cheng, X.; Pan, J.; Zhao, Y.; Liao, M.; Peng, H. Gel Polymer Electrolytes for Electrochemical Energy Storage. Adv. Energy Mater. 2018, 8, 1702184. [Google Scholar] [CrossRef]
- Feuillade, G.; Perche, P. Ion-conductive macromolecular gels and membranes for solid lithium cells. J. Appl. Electrochem. 1975, 5, 63–69. [Google Scholar] [CrossRef]
- Baskoro, F.; Wong, H.Q.; Yen, H.-J. Strategic Structural Design of a Gel Polymer Electrolyte toward a High Efficiency Lithium-Ion Battery. ACS Appl. Energy Mater. 2019, 2, 3937–3971. [Google Scholar] [CrossRef]
- Liang, S.; Yan, W.; Wu, X.; Zhang, Y.; Zhu, Y.; Wang, H.; Wu, Y. Gel polymer electrolytes for lithium ion batteries: Fabrication, characterization and performance. Solid State Ion. 2018, 318, 2–18. [Google Scholar] [CrossRef]
- Hsueh, M.-F.; Huang, C.-W.; Wu, C.-A.; Kuo, P.-L.; Teng, H. The Synergistic Effect of Nitrile and Ether Functionalities for Gel Electrolytes Used in Supercapacitors. J. Phys. Chem. C 2013, 117, 16751–16758. [Google Scholar] [CrossRef]
- Ostrovskii, D.; Brodin, A.; Torell, L.M.; Appetecchi, G.B.; Scrosati, B. Molecular and ionic interactions in poly(acrylonitrile)- and poly(methylmetacrylate)-based gel electrolytes. J. Chem. Phys. 1998, 109, 7618–7624. [Google Scholar] [CrossRef]
- Zhu, M.; Wu, J.; Wang, Y.; Song, M.; Long, L.; Siyal, S.H.; Yang, X.; Sui, G. Recent advances in gel polymer electrolyte for high-performance lithium batteries. J. Energy Chem. 2019, 37, 126–142. [Google Scholar] [CrossRef]
- Vincent, C.A. Applications of electroactive polymers. Edited by B. Scrosati. Chapman and Hall, London, 1993. pp. 354 price £40.00. ISBN 0-412-41430-9. Polym. Int. 1994, 33, 343. [Google Scholar] [CrossRef]
- Sannier, L.; Bouchet, R.; Rosso, M.; Tarascon, J.M. Evaluation of GPE performances in lithium metal battery technology by means of simple polarization tests. J. Power Sources 2006, 158, 564–570. [Google Scholar] [CrossRef]
- Groce, F.; Gerace, F.; Dautzemberg, G.; Passerini, S.; Appetecchi, G.B.; Scrosati, B. Synthesis and characterization of highly conducting gel electrolytes. Electrochim. Acta 1994, 39, 2187–2194. [Google Scholar] [CrossRef]
- Hashmi, S.A.; Kumar, A.; Tripathi, S.K. Experimental studies on poly methyl methacrylate-based gel polymer electrolytes for application in electrical double layer capacitors. J. Phys. D Appl. Phys. 2007, 40, 6527. [Google Scholar] [CrossRef]
- Michot, T.; Nishimoto, A.; Watanabe, M. Electrochemical properties of polymer gel electrolytes based on poly(vinylidene fluoride) copolymer and homopolymer. Electrochim. Acta 2000, 45, 1347–1360. [Google Scholar] [CrossRef]
- Stallworth, P.E.; Greenbaum, S.G.; Croce, F.; Slane, S.; Salomon, M. Lithium-7 NMR and ionic conductivity studies of gel electrolytes based on poly(methylmethacrylate). Electrochim. Acta 1995, 40, 2137–2141. [Google Scholar] [CrossRef]
- Karuppasamy, K.; Theerthagiri, J.; Vikraman, D.; Yim, C.-J.; Hussain, S.; Sharma, R.; Maiyalagan, T.; Qin, J.; Kim, H.-S. Ionic Liquid-Based Electrolytes for Energy Storage Devices: A Brief Review on Their Limits and Applications. Polymers 2020, 12, 918. [Google Scholar] [CrossRef]
- Wang, X.; Liu, Z.; Wang, Y.; Chen, J.; Mao, Z.; Wang, D. Conductive Na2Zn2TeO6 Filler Modified Gel Polymer Electrolyte Membranes for Application in Sodium-Ions Batteries. ChemElectroChem 2020, 7, 5021–5028. [Google Scholar] [CrossRef]
- Wang, P.; Zhang, H.; Chai, J.; Liu, T.; Hu, R.; Zhang, Z.; Li, G.; Cui, G. A novel single-ion conducting gel polymer electrolyte based on polymeric sodium tartaric acid borate for elevated-temperature sodium metal batteries. Solid State Ion. 2019, 337, 140–146. [Google Scholar] [CrossRef]
- Wang, X.; Wang, X.; Chen, J.; Zhao, Y.; Mao, Z.; Wang, D. Durable sodium battery composed of conductive Ti3C2Tx MXene modified gel polymer electrolyte. Solid State Ion. 2021, 365, 115655. [Google Scholar] [CrossRef]
- Vo, D.T.; Do, H.N.; Nguyen, T.T.; Nguyen, T.T.H.; Tran, V.M.; Okada, S.; Le, M.L.P. Sodium ion conducting gel polymer electrolyte using poly(vinylidene fluoride hexafluoropropylene). Mater. Sci. Eng. B 2019, 241, 27–35. [Google Scholar] [CrossRef]
- Harshlata; Mishra, K.; Rai, D.K. Studies on ionic liquid-based nanocomposite gel polymer electrolyte and its application in sodium battery. Mater. Sci. Eng. B 2021, 267, 115098. [Google Scholar] [CrossRef]
- Chen, G.; Zhang, K.; Liu, Y.; Ye, L.; Gao, Y.; Lin, W.; Xu, H.; Wang, X.; Bai, Y.; Wu, C. Flame-retardant gel polymer electrolyte and interface for quasi-solid-state sodium ion batteries. Chem. Eng. J. 2020, 401, 126065. [Google Scholar] [CrossRef]
- Komaba, S.; Ishikawa, T.; Yabuuchi, N.; Murata, W.; Ito, A.; Ohsawa, Y. Fluorinated Ethylene Carbonate as Electrolyte Additive for Rechargeable Na Batteries. ACS Appl. Mater. Interfaces 2011, 3, 4165–4168. [Google Scholar] [CrossRef]
- Shen, W.; Li, H.; Guo, Z.; Wang, C.; Li, Z.; Xu, Q.; Liu, H.; Wang, Y.; Xia, Y. Double-Nanocarbon Synergistically Modified Na3V2(PO4)3: An Advanced Cathode for High-Rate and Long-Life Sodium-Ion Batteries. ACS Appl. Mater. Interfaces 2016, 8, 15341–15351. [Google Scholar] [CrossRef] [PubMed]
- Lonchakova, O.V.; Semenikhin, O.A.; Zakharkin, M.V.; Karpushkin, E.A.; Sergeyev, V.G.; Antipov, E.V. Efficient gel-polymer electrolyte for sodium-ion batteries based on poly(acrylonitrile-co-methyl acrylate). Electrochim. Acta 2020, 334, 135512. [Google Scholar] [CrossRef]
- Tian, L.-Y.; Huang, X.-B.; Tang, X.-Z. Single-ionic gel polymer electrolyte based on polyvinylidene fluoride and fluorine-containing ionomer. Eur. Polym. J. 2004, 40, 735–742. [Google Scholar] [CrossRef]
- Parveen, S.; Sehrawat, P.; Hashmi, S.A. Triglyme-based solvate ionic liquid gelled in a polymer: A novel electrolyte composition for sodium-ion battery. Mater. Today Commun. 2022, 31, 103392. [Google Scholar] [CrossRef]
- Van Nghia, N.; Long, P.D.; Tan, T.A.; Jafian, S.; Hung, I.M. Electrochemical Performance of a V2O5 Cathode for a Sodium Ion Battery. J. Electron. Mater. 2017, 46, 3689–3694. [Google Scholar] [CrossRef]
- Kumar, D.; Hashmi, S.A. Ionic liquid based sodium ion conducting gel polymer electrolytes. Solid State Ion. 2010, 181, 416–423. [Google Scholar] [CrossRef]
- Kumar, D.; Yadav, N.; Mishra, K.; Shahid, R.; Arif, T.; Kanchan, D.K. Sodium ion conducting flame-retardant gel polymer electrolyte for sodium batteries and electric double layer capacitors (EDLCs). J. Energy Storage 2022, 46, 103899. [Google Scholar] [CrossRef]
- Mishra, R.; Singh, S.K.; Gupta, H.; Tiwari, R.K.; Meghnani, D.; Patel, A.; Tiwari, A.; Tiwari, V.K.; Singh, R.K. Polar β-Phase PVdF-HFP-Based Freestanding and Flexible Gel Polymer Electrolyte for Better Cycling Stability in a Na Battery. Energy Fuels 2021, 35, 15153–15165. [Google Scholar] [CrossRef]
- Mishra, K.; Garg, A.; Sharma, R.; Gautam, R.; Pundir, S.S. Effect of blending of PMMA on PVdF-HFP + NaCF3SO3-EC-PC gel polymer electrolyte. Mater. Today Proc. 2019, 12, 621–627. [Google Scholar] [CrossRef]
- Chauhan, A.K.; Kumar, D.; Mishra, K.; Singh, A. Performance enhancement of Na+ ion conducting porous gel polymer electrolyte using NaAlO2 active filler. Mater. Today Commun. 2021, 26, 101713. [Google Scholar] [CrossRef]
- Janakiraman, S.; Agrawal, A.; Biswal, R.; Venimadhav, A. An amorphous polyvinylidene fluoride-co-hexafluoropropylene based gel polymer electrolyte for sodium-ion cells. Appl. Surf. Sci. Adv. 2021, 6, 100139. [Google Scholar] [CrossRef]
- Janakiraman, S.; Padmaraj, O.; Ghosh, S.; Venimadhav, A. A porous poly (vinylidene fluoride-co-hexafluoropropylene) based separator-cum-gel polymer electrolyte for sodium-ion battery. J. Electroanal. Chem. 2018, 826, 142–149. [Google Scholar] [CrossRef]
- Yang, L.; Jiang, Y.; Liang, X.; Lei, Y.; Yuan, T.; Lu, H.; Liu, Z.; Cao, Y.; Feng, J. Novel Sodium–Poly(tartaric acid)Borate-Based Single-Ion Conducting Polymer Electrolyte for Sodium–Metal Batteries. ACS Appl. Energy Mater. 2020, 3, 10053–10060. [Google Scholar] [CrossRef]
- Feng, J.; An, Y.; Ci, L.; Xiong, S. Nonflammable electrolyte for safer non-aqueous sodium batteries. J. Mater. Chem. A 2015, 3, 14539–14544. [Google Scholar] [CrossRef]
- Liu, X.; Jiang, X.; Zhong, F.; Feng, X.; Chen, W.; Ai, X.; Yang, H.; Cao, Y. High-Safety Symmetric Sodium-Ion Batteries Based on Nonflammable Phosphate Electrolyte and Double Na3V2(PO4)3 Electrodes. ACS Appl. Mater. Interfaces 2019, 11, 27833–27838. [Google Scholar] [CrossRef]
- Park, T.-H.; Park, M.-S.; Ban, A.H.; Lee, Y.-S.; Kim, D.-W. Nonflammable Gel Polymer Electrolyte with Ion-Conductive Polyester Networks for Sodium Metal Cells with Excellent Cycling Stability and Enhanced Safety. ACS Appl. Energy Mater. 2021, 4, 10153–10162. [Google Scholar] [CrossRef]
- Niu, Y.-B.; Yin, Y.-X.; Wang, W.-P.; Wang, P.-F.; Ling, W.; Xiao, Y.; Guo, Y.-G. In Situ Copolymerizated Gel Polymer Electrolyte with Cross-Linked Network for Sodium-Ion Batteries. CCS Chem. 2020, 2, 589–597. [Google Scholar] [CrossRef]
- Farhana, N.K.; Khanmirzaei, M.H.; Ramesh, S.; Ramesh, K. Exploration on polypropylene carbonate polymer for gel polymer electrolyte preparation and dye-sensitized solar cell application. J. Appl. Polym. Sci. 2017, 134, 45091. [Google Scholar] [CrossRef]
- Krishna Jyothi, N.; Vijaya Kumar, K.; Sunita Sundari, G.; Narayana Murthy, P. Ionic conductivity and battery characteristic studies of a new PAN-based Na+ ion conducting gel polymer electrolyte system. Indian J. Phys. 2016, 90, 289–296. [Google Scholar] [CrossRef]
- Zhang, Y.; Bakenov, Z.; Tan, T.; Huang, J. Polyacrylonitrile-Nanofiber-Based Gel Polymer Electrolyte for Novel Aqueous Sodium-Ion Battery Based on a Na4Mn9O18 Cathode and Zn Metal Anode. Polymers 2018, 10, 853. [Google Scholar] [CrossRef]
- Mei, W.; Wang, X.; Wang, Y.; Chen, J.; Mao, Z.; Wang, D. Conductive Na3Sc2P3O12 filler with different crystal phases modified gel polymer electrolyte membranes for sodium ions batteries. J. Solid State Chem. 2021, 302, 122459. [Google Scholar] [CrossRef]
- Boehm, L.; Delbecq, C.J.; Hutchinson, E.; Susman, S. Fast ion conduction and phase transitions in various preparations of Na3Sc2P3O12. Solid State Ion. 1981, 5, 311–314. [Google Scholar] [CrossRef]
- Kim, J.I.; Chung, K.Y.; Park, J.H. Design of a porous gel polymer electrolyte for sodium-ion batteries. J. Membr. Sci. 2018, 566, 122–128. [Google Scholar] [CrossRef]
- Kim, H.W.; Kim, H.-J.; Byeon, H.; Kim, J.; Yang, J.W.; Kim, Y.; Kim, J.-K. Binder-free organic cathode based on nitroxide radical polymer-functionalized carbon nanotubes and gel polymer electrolyte for high-performance sodium organic polymer batteries. J. Mater. Chem. A 2020, 8, 17980–17986. [Google Scholar] [CrossRef]
- Shin, W.-K.; Cho, J.; Kannan, A.G.; Lee, Y.-S.; Kim, D.-W. Cross-linked Composite Gel Polymer Electrolyte using Mesoporous Methacrylate-Functionalized SiO2 Nanoparticles for Lithium-Ion Polymer Batteries. Sci. Rep. 2016, 6, 26332. [Google Scholar] [CrossRef]
- Aravindan, V.; Vickraman, P.; Sivashanmugam, A.; Thirunakaran, R.; Gopukumar, S. Comparison among the performance of LiBOB, LiDFOB and LiFAP impregnated polyvinylidenefluoride-hexafluoropropylene nanocomposite membranes by phase inversion for lithium batteries. Curr. Appl. Phys. 2013, 13, 293–297. [Google Scholar] [CrossRef]
- Shim, J.; Kim, H.J.; Kim, B.G.; Kim, Y.S.; Kim, D.-G.; Lee, J.-C. 2D boron nitride nanoflakes as a multifunctional additive in gel polymer electrolytes for safe, long cycle life and high rate lithium metal batteries. Energy Environ. Sci. 2017, 10, 1911–1916. [Google Scholar] [CrossRef]
- Zhao, Y.; Liu, H.; Meng, X.; Liu, A.; Chen, Y.; Ma, T. A cross-linked tin oxide/polymer composite gel electrolyte with adjustable porosity for enhanced sodium-ion batteries. Chem. Eng. J. 2022, 431, 133922. [Google Scholar] [CrossRef]
- Shi, J.; Xiong, H.; Yang, Y.; Shao, H. Nano-sized oxide filled composite PEO/PMMA/P(VDF-HFP) gel polymer electrolyte for rechargeable lithium and sodium batteries. Solid State Ion. 2018, 326, 136–144. [Google Scholar] [CrossRef]
- Dong, T.; Zhang, J.; Xu, G.; Chai, J.; Du, H.; Wang, L.; Wen, H.; Zang, X.; Du, A.; Jia, Q. A multifunctional polymer electrolyte enables ultra-long cycle-life in a high-voltage lithium metal battery. Energy Environ. Sci. 2018, 11, 1197–1203. [Google Scholar] [CrossRef]
- Zhu, Y.; Yang, Y.; Fu, L.; Wu, Y. A porous gel-type composite membrane reinforced by nonwoven: Promising polymer electrolyte with high performance for sodium-ion batteries. Electrochim. Acta 2017, 224, 405–411. [Google Scholar] [CrossRef]
- Pu, W.; He, X.; Wang, L.; Jiang, C.; Wan, C. Preparation of PVDF–HFP microporous membrane for Li-ion batteries by phase inversion. J. Membr. Sci. 2006, 272, 11–14. [Google Scholar] [CrossRef]
- Evans, J.; Vincent, C.A.; Bruce, P.G. Electrochemical measurement of transference numbers in polymer electrolytes. Polymer 1987, 28, 2324–2328. [Google Scholar] [CrossRef]
- Lei, D.; He, Y.-B.; Huang, H.; Yuan, Y.; Zhong, G.; Zhao, Q.; Hao, X.; Zhang, D.; Lai, C.; Zhang, S. Cross-linked beta alumina nanowires with compact gel polymer electrolyte coating for ultra-stable sodium metal battery. Nat. Commun. 2019, 10, 4244. [Google Scholar] [CrossRef] [PubMed]
- Kumar, D.; Hashmi, S.A. Ion transport and ion–filler-polymer interaction in poly(methyl methacrylate)-based, sodium ion conducting, gel polymer electrolytes dispersed with silica nanoparticles. J. Power Sources 2010, 195, 5101–5108. [Google Scholar] [CrossRef]
- Kim, J.I.; Choi, Y.; Chung, K.Y.; Park, J.H. A Structurable Gel-Polymer Electrolyte for Sodium Ion Batteries. Adv. Funct. Mater. 2017, 27, 1701768. [Google Scholar] [CrossRef]
- Gao, H.; Guo, B.; Song, J.; Park, K.; Goodenough, J.B. A Composite Gel–Polymer/Glassfiber Electrolyte for Sodium-Ion Batteries. Adv. Energy Mater. 2015, 5, 1402235. [Google Scholar] [CrossRef]
- Wang, L.; Lu, Y.; Liu, J.; Xu, M.; Cheng, J.; Zhang, D.; Goodenough, J.B. A Superior Low-Cost Cathode for a Na-Ion Battery. Angew. Chem. Int. Ed. 2013, 52, 1964–1967. [Google Scholar] [CrossRef]
- Park, K.; Cho, J.H.; Shanmuganathan, K.; Song, J.; Peng, J.; Gobet, M.; Greenbaum, S.; Ellison, C.J.; Goodenough, J.B. New battery strategies with a polymer/Al2O3 separator. J. Power Sources 2014, 263, 52–58. [Google Scholar] [CrossRef]
- Jiang, Y.; Zhou, X.; Li, D.; Cheng, X.; Liu, F.; Yu, Y. Highly Reversible Na Storage in Na3V2(PO4)3 by Optimizing Nanostructure and Rational Surface Engineering. Adv. Energy Mater. 2018, 8, 1800068. [Google Scholar] [CrossRef]
- Luo, C.; Shen, T.; Ji, H.; Huang, D.; Liu, J.; Ke, B.; Wu, Y.; Chen, Y.; Yan, C. Mechanically Robust Gel Polymer Electrolyte for an Ultrastable Sodium Metal Battery. Small 2020, 16, 1906208. [Google Scholar] [CrossRef]
- Luo, C.; Shen, T.; Ke, B.; Wu, Y.; Chen, Y. Ultra-small Na3V2(PO4)3 nanoparticles decorated MOFs-derived carbon enabling fast charge transfer for high-rate sodium storage. Solid State Ion. 2019, 342, 115061. [Google Scholar] [CrossRef]
- Mishra, K.; Arif, T.; Kumar, R.; Kumar, D. Effect of Al2O3 nanoparticles on ionic conductivity of PVdF-HFP/PMMA blend-based Na-ion conducting nanocomposite gel polymer electrolyte. J. Solid State Electrochem. 2019, 23, 2401–2409. [Google Scholar] [CrossRef]
- Yao, P.; Yu, H.; Ding, Z.; Liu, Y.; Lu, J.; Lavorgna, M.; Wu, J.; Liu, X. Review on Polymer-Based Composite Electrolytes for Lithium Batteries. Front. Chem. 2019, 7, 522. [Google Scholar] [CrossRef]
- Yabuuchi, N.; Kubota, K.; Dahbi, M.; Komaba, S. Research Development on Sodium-Ion Batteries. Chem. Rev. 2014, 114, 11636–11682. [Google Scholar] [CrossRef]
- Das, S.K.; Mandal, S.S.; Bhattacharyya, A.J. Ionic conductivity, mechanical strength and Li-ion battery performance of mono-functional and bi-functional (“Janus”) “soggy sand” electrolytes. Energy Environ. Sci. 2011, 4, 1391–1399. [Google Scholar] [CrossRef]
- Cao, J.; Wang, L.; He, X.; Fang, M.; Gao, J.; Li, J.; Deng, L.; Chen, H.; Tian, G.; Wang, J. In situ prepared nano-crystalline TiO2–poly(methyl methacrylate) hybrid enhanced composite polymer electrolyte for Li-ion batteries. J. Mater. Chem. A 2013, 1, 5955–5961. [Google Scholar] [CrossRef]
- Croce, F.; Appetecchi, G.B.; Persi, L.; Scrosati, B. Nanocomposite polymer electrolytes for lithium batteries. Nature 1998, 394, 456–458. [Google Scholar] [CrossRef]
- Ni’mah, Y.L.; Cheng, M.-Y.; Cheng, J.H.; Rick, J.; Hwang, B.-J. Solid-state polymer nanocomposite electrolyte of TiO2/PEO/NaClO4 for sodium ion batteries. J. Power Sources 2015, 278, 375–381. [Google Scholar] [CrossRef]
- Verma, H.; Mishra, K.; Rai, D.K. Sodium ion conducting nanocomposite polymer electrolyte membrane for sodium-ion batteries. J. Solid State Electrochem. 2020, 24, 521–532. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, X.; Liu, S.; Tao, Z.; Chen, J. A novel PMA/PEG-based composite polymer electrolyte for all-solid-state sodium-ion batteries. Nano Res. 2018, 11, 6244–6251. [Google Scholar] [CrossRef]
- Zhang, Z.; Xu, K.; Rong, X.; Hu, Y.-S.; Li, H.; Huang, X.; Chen, L. Na3.4Zr1.8Mg0.2Si2PO12 filled poly(ethylene oxide)/Na(CF3SO2)2N as flexible composite polymer electrolyte for solid-state sodium batteries. J. Power Sources 2017, 372, 270–275. [Google Scholar] [CrossRef]
- Jian, Z.; Zhao, L.; Pan, H.; Hu, Y.-S.; Li, H.; Chen, W.; Chen, L. Carbon coated Na3V2(PO4)3 as novel electrode material for sodium ion batteries. Electrochem. Commun. 2012, 14, 86–89. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, Q.; Shi, J.; Chu, Y.S.; Yu, X.; Xu, K.; Ge, M.; Yan, H.; Li, W.; Gu, L. A Self-Forming Composite Electrolyte for Solid-State Sodium Battery with Ultralong Cycle Life. Adv. Energy Mater. 2017, 7, 1601196. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, H.; Gao, G.; Qi, L. Nanofiber membrane based on ionic liquids as high-performance polymer electrolyte for sodium electrochemical device. Ionics 2013, 19, 1595–1602. [Google Scholar] [CrossRef]
- Farmer, V.; Welton, T. The oxidation of alcohols in substituted imidazolium ionic liquids using ruthenium catalysts. Green Chem. 2002, 4, 97–102. [Google Scholar] [CrossRef]
- Raghavan, P.; Zhao, X.; Manuel, J.; Shin, C.; Heo, M.-Y.; Ahn, J.-H.; Ryu, H.-S.; Ahn, H.-J.; Noh, J.-P.; Cho, G.-B. Electrochemical studies on polymer electrolytes based on poly(vinylidene fluoride-co-hexafluoropropylene) membranes prepared by electrospinning and phase inversion—A comparative study. Mater. Res. Bull. 2010, 45, 362–366. [Google Scholar] [CrossRef]
- Song, S.; Dong, Z.; Fernandez, C.; Wen, Z.; Hu, N.; Lu, L. Nanoporous ceramic-poly(ethylene oxide) composite electrolyte for sodium metal battery. Mater. Lett. 2019, 236, 13–15. [Google Scholar] [CrossRef]
- Lee, S.; Park, S.-J.; Kim, S. Thermal and Electrical Conducting Property of Sodium Polymer Electrolyte Containing Barium Titanate Filler. J. Nanosci. Nanotechnol. 2017, 17, 5768–5770. [Google Scholar] [CrossRef]
- Serra Moreno, J.; Armand, M.; Berman, M.B.; Greenbaum, S.G.; Scrosati, B.; Panero, S. Composite PEOn:NaTFSI polymer electrolyte: Preparation, thermal and electrochemical characterization. J. Power Sources 2014, 248, 695–702. [Google Scholar] [CrossRef]
- Perrier, M.; Besner, S.; Paquette, C.; Vallée, A.; Lascaud, S.; Prud’homme, J. Mixed-alkali effect and short-range interactions in amorphous poly(ethylene oxide) electrolytes. Electrochim. Acta 1995, 40, 2123–2129. [Google Scholar] [CrossRef]
- Appetecchi, G.B.; Croce, F.; Dautzenberg, G.; Mastragostino, M.; Ronci, F.; Scrosati, B.; Soavi, F.; Zanelli, A.; Alessandrini, F.; Prosini, P.P. Composite Polymer Electrolytes with Improved Lithium Metal Electrode Interfacial Properties: I. Elechtrochemical Properties of Dry PEO-LiX Systems. J. Electrochem. Soc. 1998, 145, 4126. [Google Scholar] [CrossRef]
- Kunteppa, H.; Roy, A.S.; Koppalkar, A.R.; Ambika Prasad, M.V.N. Synthesis and morphological change in poly(ethylene oxide)–sodium chlorate based polymer electrolyte complex with polyaniline. Phys. B Condens. Matter 2011, 406, 3997–4000. [Google Scholar] [CrossRef]
- Chen, S.; Feng, F.; Che, H.; Yin, Y.; Ma, Z.-F. High-performance solid-state sodium batteries enabled by boron-contained 3D composite polymer electrolyte. Chem. Eng. J. 2021, 406, 126736. [Google Scholar] [CrossRef]
- Sun, L.; Xie, Y.; Liao, X.-Z.; Wang, H.; Tan, G.; Chen, Z.; Ren, Y.; Gim, J.; Tang, W.; He, Y.-S. Insight into Ca-Substitution Effects on O3-Type NaNi1/3Fe1/3Mn1/3O2 Cathode Materials for Sodium-Ion Batteries Application. Small 2018, 14, 1704523. [Google Scholar] [CrossRef]
- Chen, S.; Che, H.; Feng, F.; Liao, J.; Wang, H.; Yin, Y.; Ma, Z.-F. Poly(vinylene carbonate)-Based Composite Polymer Electrolyte with Enhanced Interfacial Stability To Realize High-Performance Room-Temperature Solid-State Sodium Batteries. ACS Appl. Mater. Interfaces 2019, 11, 43056–43065. [Google Scholar] [CrossRef]
- Ahad, N.; Saion, E.; Gharibshahi, E. Structural, Thermal, and Electrical Properties of PVA-Sodium Salicylate Solid Composite Polymer Electrolyte. J. Nanomater. 2012, 2012, 857569. [Google Scholar] [CrossRef]
- Yu, X.; Xue, L.; Goodenough, J.B.; Manthiram, A. A High-Performance All-Solid-State Sodium Battery with a Poly(ethylene oxide)–Na3Zr2Si2PO12 Composite Electrolyte. ACS Mater. Lett. 2019, 1, 132–138. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Z.; Zheng, F.; Sun, J.; Oh, J.A.S.; Wu, T.; Chen, G.; Huang, Q.; Kotobuki, M.; Zeng, K. Ferroelectric Engineered Electrode-Composite Polymer Electrolyte Interfaces for All-Solid-State Sodium Metal Battery. Adv. Sci. 2022, 9, 2105849. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Z.; Sun, J.; Zheng, F.; Kotobuki, M.; Wu, T.; Zeng, K.; Lu, L. Flexible, stable, fast-ion-conducting composite electrolyte composed of nanostructured Na-super-ion-conductor framework and continuous Poly(ethylene oxide) for all-solid-state Na battery. J. Power Sources 2020, 454, 227949. [Google Scholar] [CrossRef]
- Wang, Y.; Yao, K.; Sharifzadeh Mirshekarloo, M.; Tay, F.E.H. Effects and Mechanism of Combinational Chemical Agents on Solution-Derived K0.5Na0.5NbO3 Piezoelectric Thin Films. J. Am. Ceram. Soc. 2016, 99, 1631–1636. [Google Scholar] [CrossRef]
- Dimri, M.C.; Kumar, D.; Aziz, S.B.; Mishra, K. ZnFe2O4 nanoparticles assisted ion transport behavior in a sodium ion-conducting polymer electrolyte. Ionics 2021, 27, 1143–1157. [Google Scholar] [CrossRef]
- Chauhan, A.K.; Mishra, K.; Kumar, D.; Singh, A. Enhancing Sodium Ion Transport in a PEO-Based Solid Polymer Electrolyte System with NaAlO2 Active Fillers. J. Electron. Mater. 2021, 50, 5122–5133. [Google Scholar] [CrossRef]
- Yao, Y.; Liu, Z.; Wang, X.; Chen, J.; Wang, X.; Wang, D.; Mao, Z. Promoted ion conductivity of sodium salt–poly(ethylene oxide) polymer electrolyte induced by adding conductive beta-alumina and application in all-solid-state sodium batteries. J. Mater. Sci. 2021, 56, 9951–9960. [Google Scholar] [CrossRef]
- Mallaiah, Y.; Jeedi, V.R.; Swarnalatha, R.; Raju, A.; Narender Reddy, S.; Sadananda Chary, A. Impact of polymer blending on ionic conduction mechanism and dielectric properties of sodium based PEO-PVdF solid polymer electrolyte systems. J. Phys. Chem. Solids 2021, 155, 110096. [Google Scholar] [CrossRef]
- Pradhan, D.K.; Samantaray, B.K.; Choudhary, R.N.P.; Thakur, A.K. Effect of plasticizer on microstructure and electrical properties of a sodium ion conducting composite polymer electrolyte. Ionics 2005, 11, 95–102. [Google Scholar] [CrossRef]









| Year | SPE | Temp | Conductivity | Ref |
|---|---|---|---|---|
| 1995 | PEO + NaPF4 | 25 °C | 5 × 10−6 S·cm−1 | [53] |
| 1999 | PEO + NaNO3 | 29 °C | 2.83 × 10−6 S·cm−1 | [54] |
| 2001 | PEO + NaClO3 + PEG | 105 °C | 9.47 × 10−4 S·cm−1 | [55] |
| 2005 | PEO + NaNO3 | 25 °C | 3.5 × 10−6 S·cm−1 | [52] |
| 2006 | PEO + NaClO3 | ~30 °C | ~10−7 S·cm−1 | [56] |
| 2006 | PEO + NaFeF4 | 25 °C | 1.37 × 10−7 S·cm−1 | [57] |
| 2006 | PVC + NaClO4 | - | - | [71] |
| 2006 | PVA + NaBr | - | 1.12 × 10−6 S·cm−1 | [74] |
| 2007 | PEO + NaLaF4 | 25 °C | 3.9 × 10−7 S cm−1 | [58] |
| 2007 | (PEO)6:NaPO3 + PEG400 | 36 °C | 8.9 × 10−7 S cm−1 | |
| 2007 | PVA + NaI | - | 1.02 × 10−5 S·cm−1 | [73] |
| 2012 | PEO + NaF | - | 4.73 × 10−7 S cm−1 | [61] |
| 2013 | Potato starch + NaSCN | - | 1.12 × 10−4 S·cm−1 | [44] |
| 2014 | HPMC + NaI | 40 °C | 1.126 × 10−6 S·cm−1 | [81] |
| 2015 | MC + NaI | 60 °C | 2.7 × 10−5 S·cm−1 | [82] |
| 2015 | BEMA/PEGMA + NaClO4 | 20 °C | 5.1 × 10−3 S·cm−1 | [13] |
| 2015 | MPII + RS + NaI | - | 1.20 × 10−3 S·cm−1 | [77] |
| 2016 | PEO + sodium methylsulfate salt + BMIM-MS | 75 °C | 4 × 10−4 S·cm−1 | [85] |
| 2017 | PEO + NaFNFSI | 80 °C | 3.36 × 10−4 S·cm−1 | [62] |
| 2017 | PEO + NaI | 25 °C | 7.1 × 10−5 S·cm−1 | [63] |
| 2017 | PVC + NaI | 25 °C | 2.9 × 10−8 S·cm−1 | [72] |
| 2017 | Tri(ethylene glycol) divinyl ether + LiBF4 | Ambient temperature | 1.2 × 10−3 S·cm−1 | [86] |
| 2017 | Poly(bis(4-carbonyl benzene sulfonyl)imide-co-2,5-diamino benzesulfonic acid) + NaPA | 80 °C | 4.1 × 10−4 S·cm−1 | [87] |
| 2017 | CS + NaCl | - | 1.72 × 10−5 S·cm−1 | [78] |
| 2018 | PVA + NaNO3 + PVP | 25 °C | 1.25 × 10−5 S·cm−1 | [75] |
| 2018 | Oligo(methyl methacrylate)-block-oligo(ethylene glycol) methyl ether methacrylate + H-β-CD + NaTFSI | 60 °C | 1.3 × 10−4 S·cm−1 | [88] |
| 2019 | PEO + NaPF6 | 25 °C | 2 × 10−5 S·cm−1 | [64] |
| 2019 | PEO + NaNO3 + PVP | 100 °C | 2.90 × 10−4 S·cm−1 | [65] |
| 2019 | PVDF + NaPF6 | Ambient temperature | 1.08 × 10−3 S·cm−1 | [89] |
| 2019 | (P(MVE-alt-MA) + TEP/VC + NaClO4 | 25 °C | 2.2 × 10−4 S·cm−1 | [90] |
| 2020 | PEO + PEI + NaPF6 | 100 °C | 5.2 × 10−4 S·cm−1 | [66] |
| 2020 | PEO + NaPF6 + succinonitrile | 80 °C | 6.3 × 10−4 S·cm−1 | [67] |
| 2020 | PEO + Na(FSI-ethyl cellulose) | 80 °C | ~10−4 S·cm−1 | [68] |
| 2020 | CMC-Na + PVP | - | 1.81 × 10−4 S·cm−1 | [83] |
| 2020 | CMC + NaBr | 25 °C | 5.15 × 10−4 S·cm−1 | [84] |
| 2020 | MC + NaI | - | 3.01 × 10−3 S·cm−1 | [46] |
| 2021 | PVA + NaI + Glycerol | 25 °C | 1.17 × 10−3 S·cm−1 | [76] |
| 2021 | CS-NaHSO3 | 25 °C | 2.22 × 10−4 S·cm−1 | [79] |
| 2021 | Chitosan/Dextran + NaCF3SO3 | 25 °C | 6.10 × 10−5 S·cm−1 | [80] |
| 2021 | TMPT crosslinked with NaBFMB | 30 °C | 2 × 10−3 S cm−1 | [91] |
| 2022 | PEO + NaFSI + Pyr14FSI | 25 °C | 1 × 10−3 S·cm−1 | [69] |
| 2022 | PEO + NaCl | 25 °C | 4.5 × 10−6 S·cm−1 | [70] |
| 2022 | PVDF + NaTFSI + NaDFOB | 23 °C | 3.1 × 10−4 S·cm−1 | [92] |
| 2023 | PEO + NaClO4 + NaCF3SO3 | 90 °C | 10−3 S·cm−1 | [93] |
| 2023 | PEO + PNaMTFSI | 85 °C | 7.74 × 10−5 S·cm−1 | [94] |
| Year | GPE | Temp | Conductivity | Ref |
|---|---|---|---|---|
| 1981, 2021 | PVDF-HFP/PMMA- | [136,137] | ||
| γ-Na4Sc2P2SiO12 | - | 2.783 × 10−3 S·cm−1 | ||
| β-Na3.5Sc2P2.5Si0.5O12 | - | 2.072 × 10−3 S·cm−1 | ||
| α-Na3Sc2P3O12 | - | 1.92 × 10−3 S·cm−1 | ||
| 2004 | PVDF + PSVE-Na + PC + VA | Ambient temperature | Above 10−4 S.cm−1 | [118] |
| 2010 | PVDF-HFP + NaTf + EMITf | ∼27 °C | 5.74 × 10−3 S·cm−1 | [121] |
| 2016 | PAN + NaI + EC + DMF | 29 °C | 2.35 × 10−4 S·cm−1 | [134] |
| 2017 | PPC + NaI + EC:PC | 25 °C | 2.01 × 10−3 S·cm−1 | [133] |
| 2017 | P(VDF-HFP) + NaClO4 + PP | Ambient temperature | 8.2 × 10−4 S·cm−1 | [146] |
| 2018 | P(VDF-co-HFP) + NaClO4 + EC + DEC | - | 1.13 × 10−3 S·cm−1 | [127] |
| 2019 | PVDF-HFP + NaCF3SO3 + PMMA | 25 °C | ~6.1 × 10−4 S·cm−1 | [124] |
| 2019 | PSP + NaClO4 + PSTB + PVCA | 25 °C | 1 × 10−4 S·cm−1 | [110] |
| 2019 | PVDF-HFP + NaClO4 + PC + FEC | 25 °C | 1.91 × 10−3 S·cm−1 | [112] |
| 2019 | PVDF-HFP/PMMA + NaCF3SO3 + EC + PC | 7.5×10−4 S·cm−1 | [157] | |
| 2020 | PVDF-HFP/PMMA + Na2Zn2TeO6 + ED + DMC | 25 °C | 2.52 × 10−3 S·cm−1 | [109] |
| 2020 | PPEGMA + NaTFSI + TEP | 27 °C | 9.1 × 10−4 S·cm−1 | [114] |
| 2020 | poly(acrylonitrile-co-methyl acrylate) + NaClO4 + PC | 25 °C | 1.8 × 10−3 S·cm−1 | [117] |
| 2020 | PVDF-HFP + NaPTAB + EC + PC | 25 °C | 9.4 × 10−5 S·cm−1 | [128] |
| 2020 | CPN + NaPF6 + PC | 25 °C | 8.2 × 10−4 S·cm−1 | [132] |
| 2020 | 2-GPH + NaClO4 + EC + PC | - | 2.3 × 10−3 S·cm−1 | [156] |
| 2021 | PVDF-HFP/PMMA + Ti3C2Tx MXene + NaClO4 + EC + DMC | - | 3.28 × 10−3 S·cm−1 | [111] |
| 2021 | PVDF-HFP + NaCF3SO3 + BMImCF3SO3 | 25 °C | 4.0 × 10−4 S·cm−1 | [113] |
| 2021 | PVDF-HFP + NaTFSI + EMIMTFSI | - | 1.9 × 10−3 S·cm−1 | [123] |
| 2021 | PVDF-HFP + NaAlO2 + EC + PC | - | 6.8 × 10−4 mS·cm−1 | [125] |
| 2021 | PVDF-co-HFP + NaPF6 + EC + PC | 25 °C | 1.28 × 10−3 S·cm−1 | [126] |
| 2021 | PCL-TA + NaFSI + TMP | - | 6.3 × 10−3 S·cm−1 | [131] |
| 2022 | PVDF-HFP + NaClO4 + G3 | - | 2.54 × 10−3 S·cm−1 | [119] |
| 2022 | TMP + NaPF6 + EC + PC | - | ~1.40 × 10−3 S·cm−1 | [123] |
| 2022 | PVDF-HFP-PSGGSE- SnO2 + NaClO4 + EC + DMC | 70 °C | 2.32 × 10−4 S·cm−1 | [143] |
| Year | CPE | Temp | Conductivity | Ref |
|---|---|---|---|---|
| 2013 | P(VDF-HFP) + NaSCN + BMIMBF4 Not mentioned | 25 °C | 5.6 × 10−5 S·cm−1 | [170] |
| 2014 | PEO + NaTFSI + SiO2 | >75 °C | ~10−3 S·cm−1 | [175] |
| 2015 | PEO + NaClO4 + TiO2 | 25 °C | 1.35 × 10−4 S·cm−1 | [164] |
| 2017 | PEO + NaClO4 + BaTiO3 | 25 °C | 1.0 × 10−5 S·cm−1 | [174] |
| 2017 | PEO + NaTFSI + NASICON | 80 °C | 2.8 mS·cm−1 | [167] |
| 2018 | PMMA/PEG + NaClO4 + Al2O3 | 70 °C | 1.46 × 10−4 S·cm−1 | [166] |
| 2018 | PEO + NaNO3 + Al2O3 | 25 °C | 1.86 × 10−4 S·cm−1 | [51] |
| 2019 | PEO + NaClO4 + Na3Zr2Si2PO12 | 60 °C | 5.6 × 10−4 S·cm−1 | [183] |
| 2020 | PVDF-HFP + NaPF6 + TiO2 + EC | 25 °C | 1.3 × 10−3 S·cm−1 | [165] |
| 2021 | PEGMA + NaTF + B-HEMA + TEGDME | 40 °C | 2.57 × 10−4 S·cm−1 | [179] |
| 2021 | PEO + NaCF3SO3 + ZnFe2O4 | 25 °C | 6 × 10−5 S·cm−1 | [187] |
| 2021 | PEO + NaClO4 + NaAlO2 | 25 °C | 1.6 × 10−5 S·cm−1 | [188] |
| 2021 | PEO + NaClO4 + β-Al2O3 | 60 °C | 3.95 × 10−4 S·cm−1 | [189] |
| 2022 | PEO + NaTFSI + KNN-Na3Zr2Si2PO12 | Ambient temperature | 7.9 × 10−5 S·cm−1 | [184] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kumar, S.; Raghupathy, R.; Vittadello, M. Sodium Polymer Electrolytes: A Review. Batteries 2024, 10, 73. https://doi.org/10.3390/batteries10030073
Kumar S, Raghupathy R, Vittadello M. Sodium Polymer Electrolytes: A Review. Batteries. 2024; 10(3):73. https://doi.org/10.3390/batteries10030073
Chicago/Turabian StyleKumar, Sumit, Rajesh Raghupathy, and Michele Vittadello. 2024. "Sodium Polymer Electrolytes: A Review" Batteries 10, no. 3: 73. https://doi.org/10.3390/batteries10030073







