Effect of Mixing Intensity on Electrochemical Performance of Oxide/Sulfide Composite Electrolytes
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
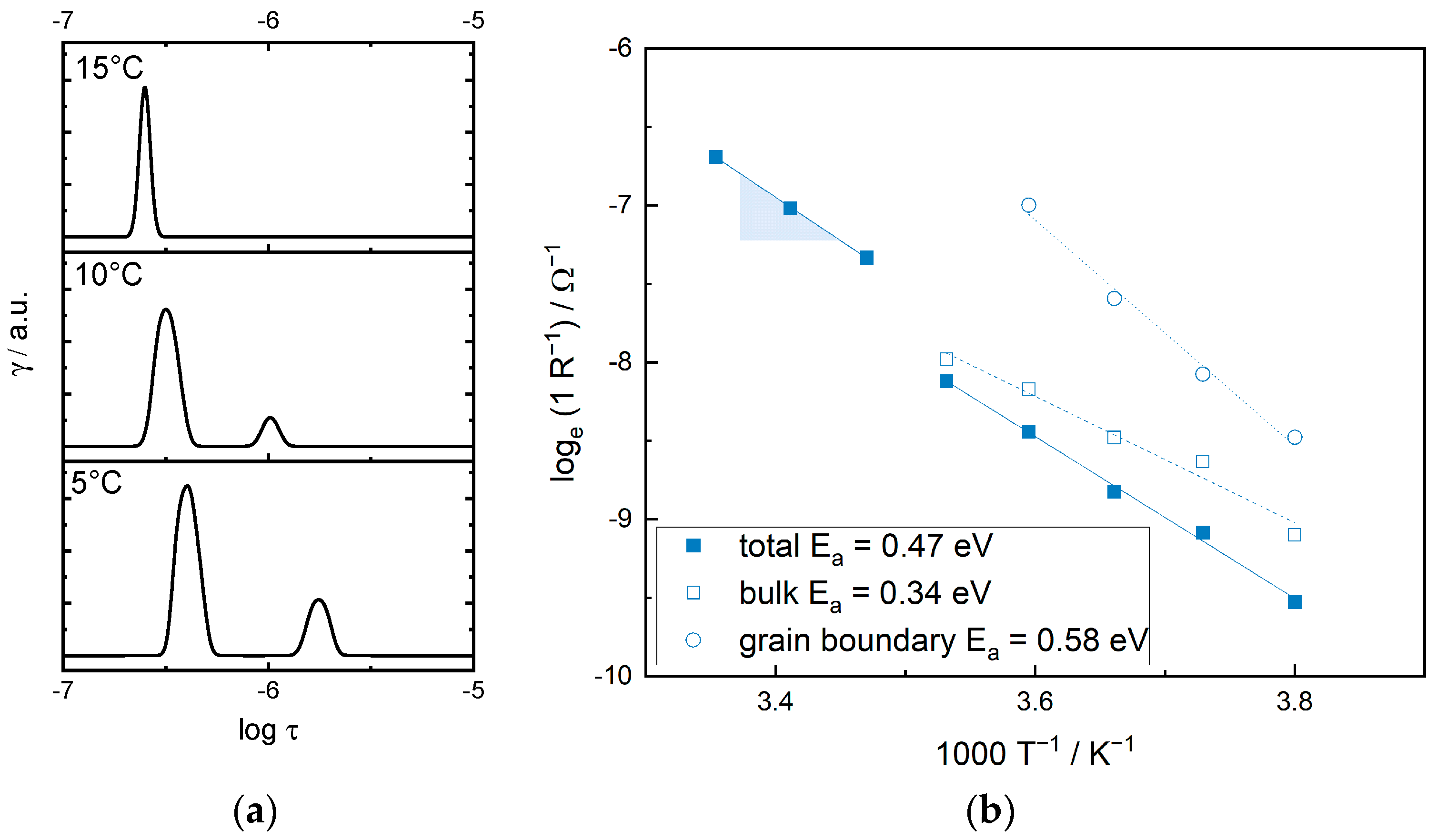
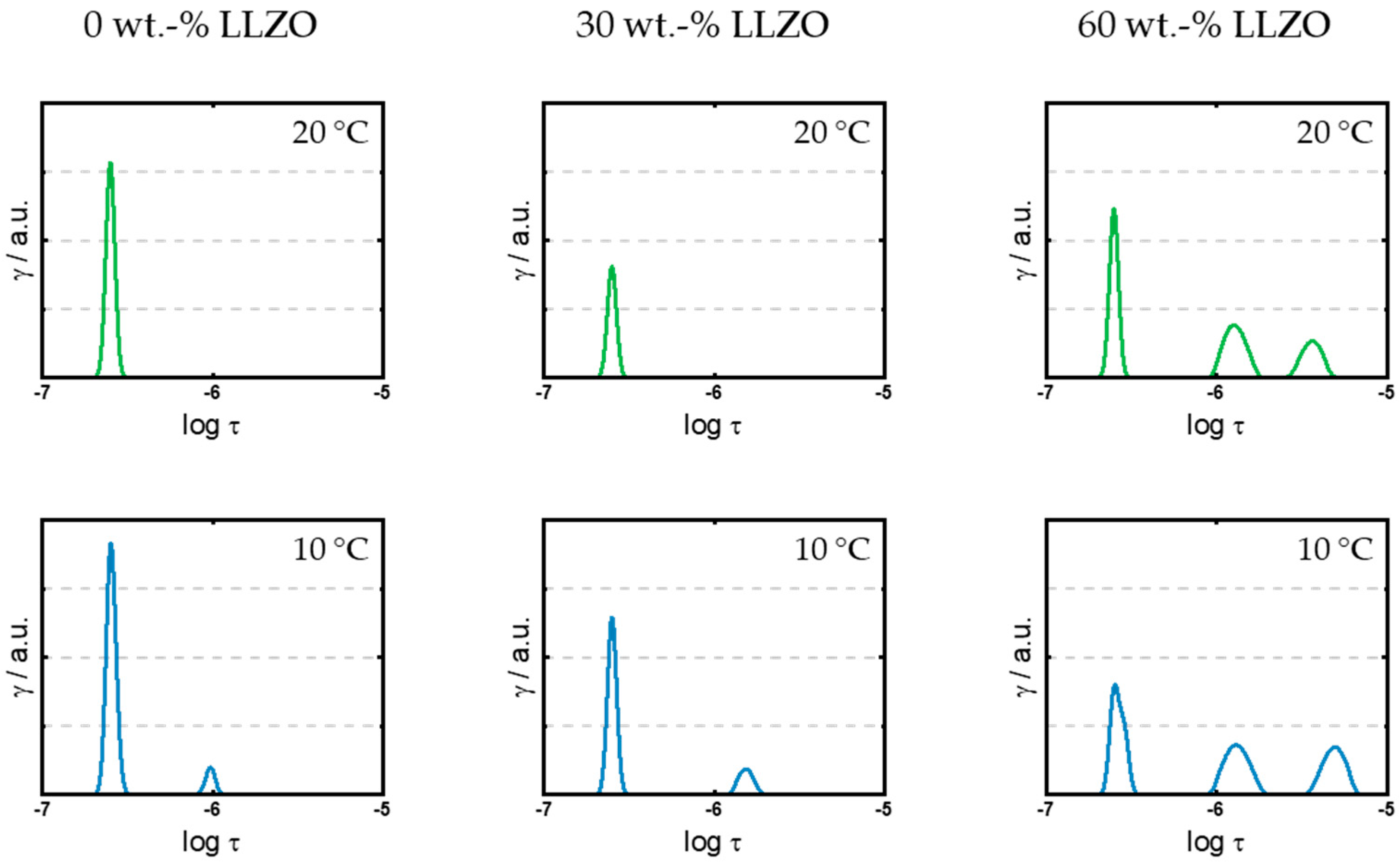
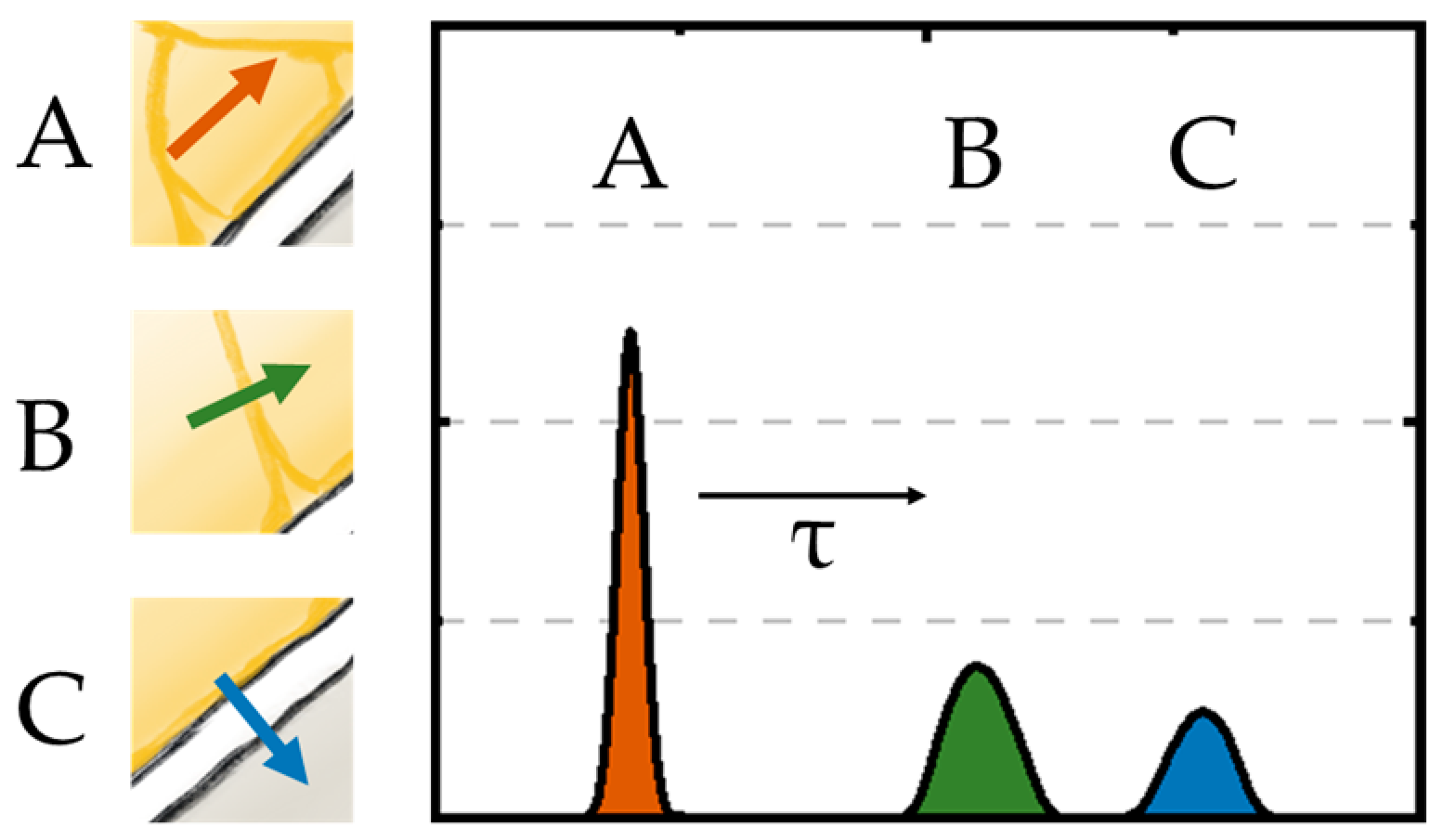
3.1. Quantification of the Polarization Processes within the Hybrid Separator
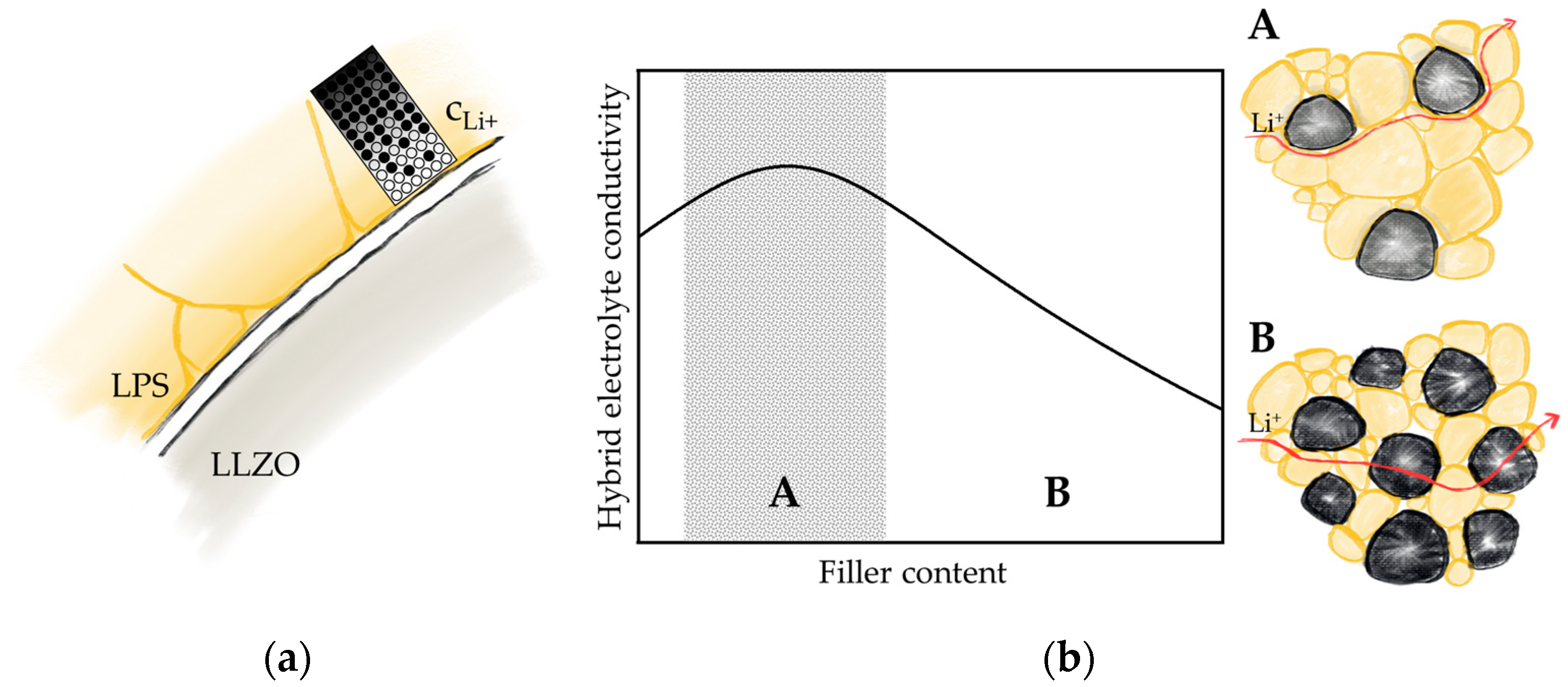
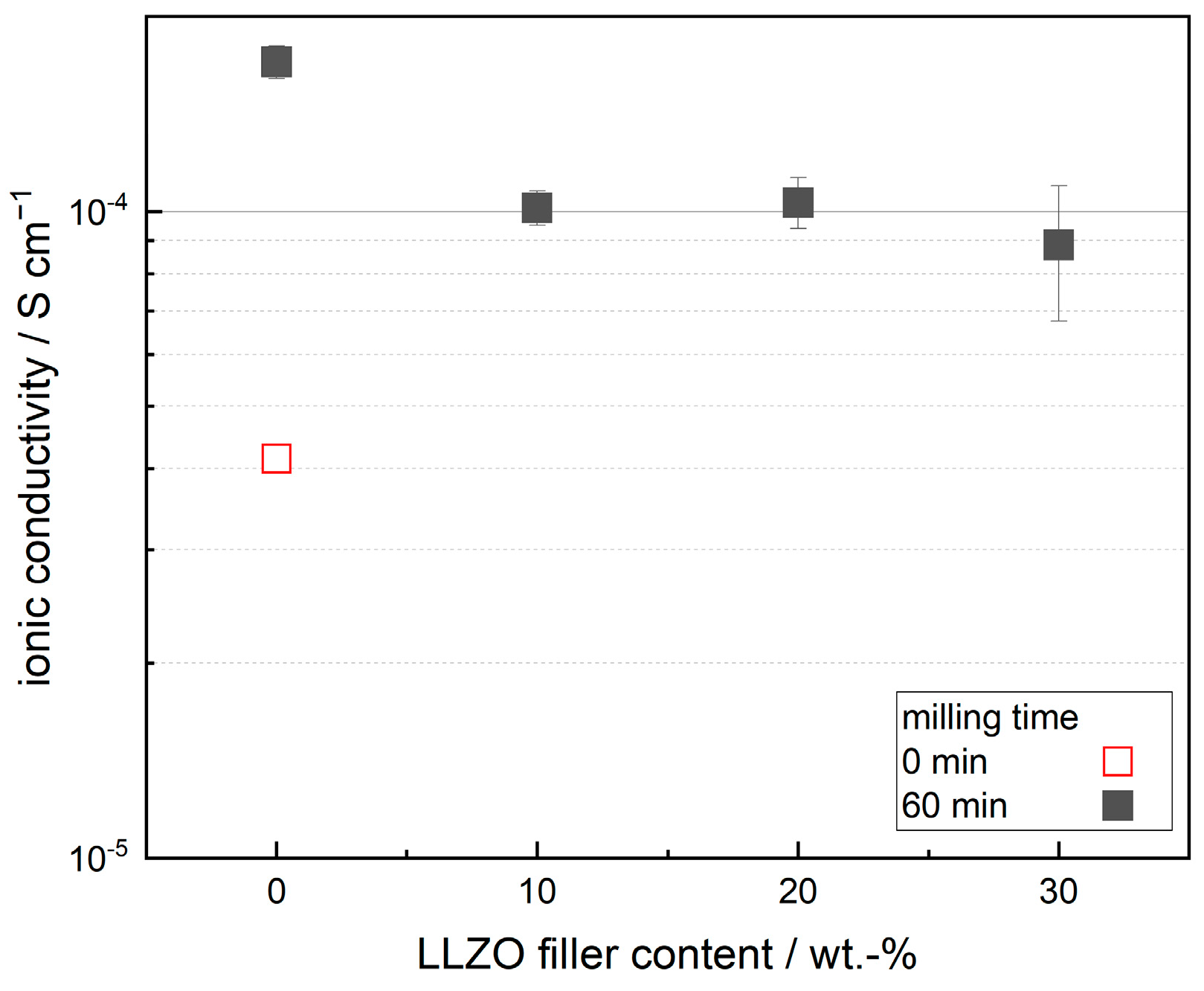
3.2. Impact of Filler Particles on Ionic Conductivity
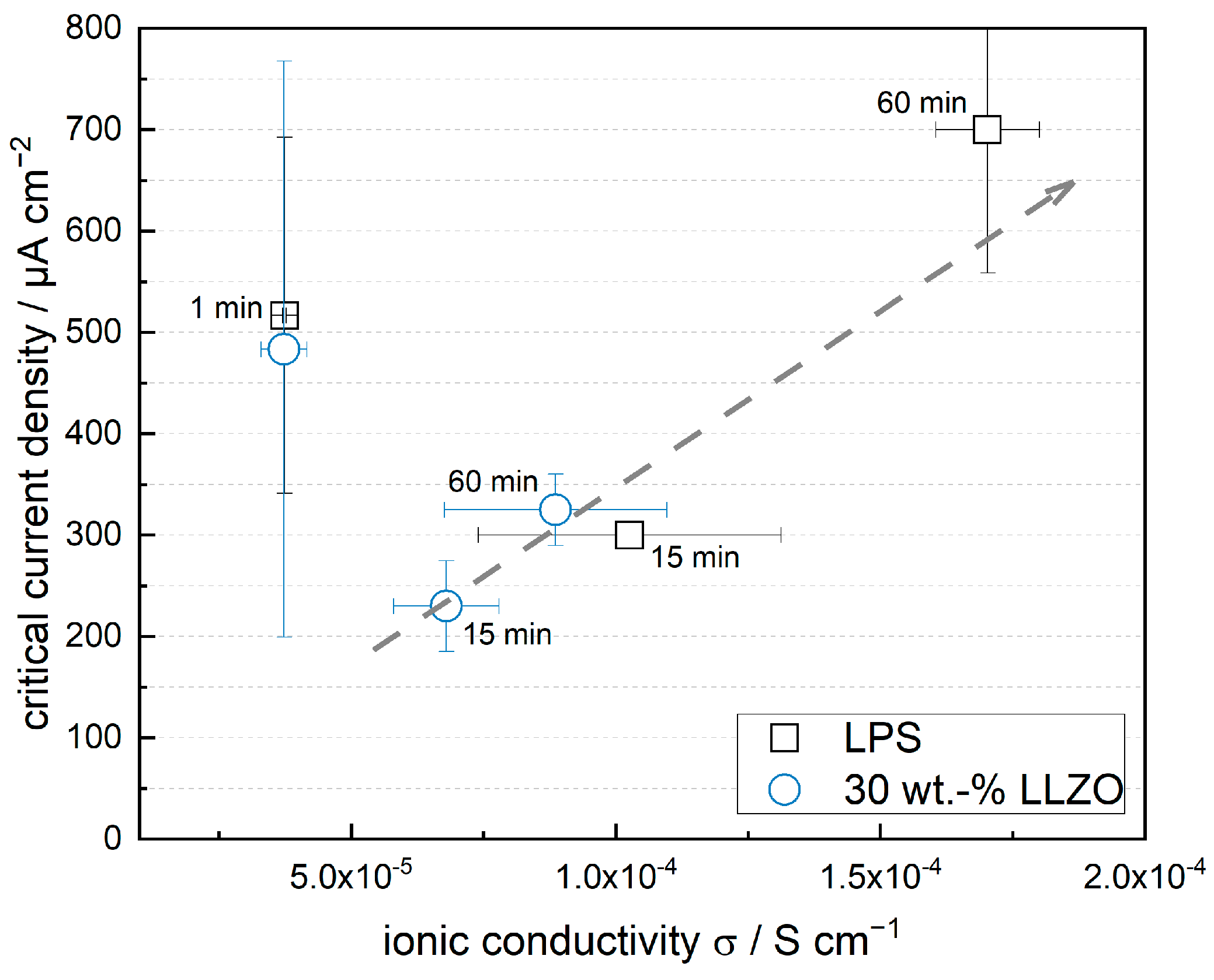
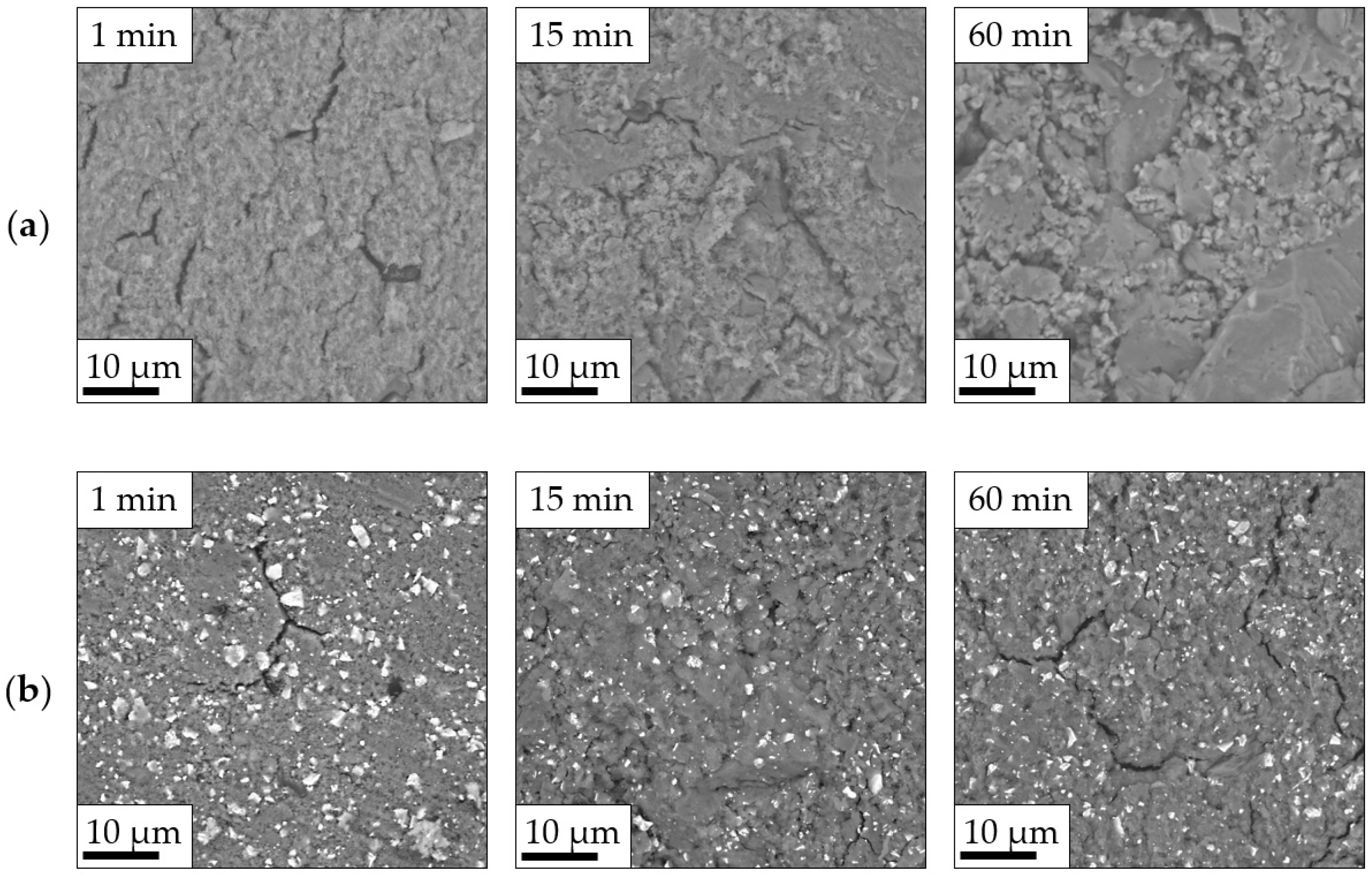
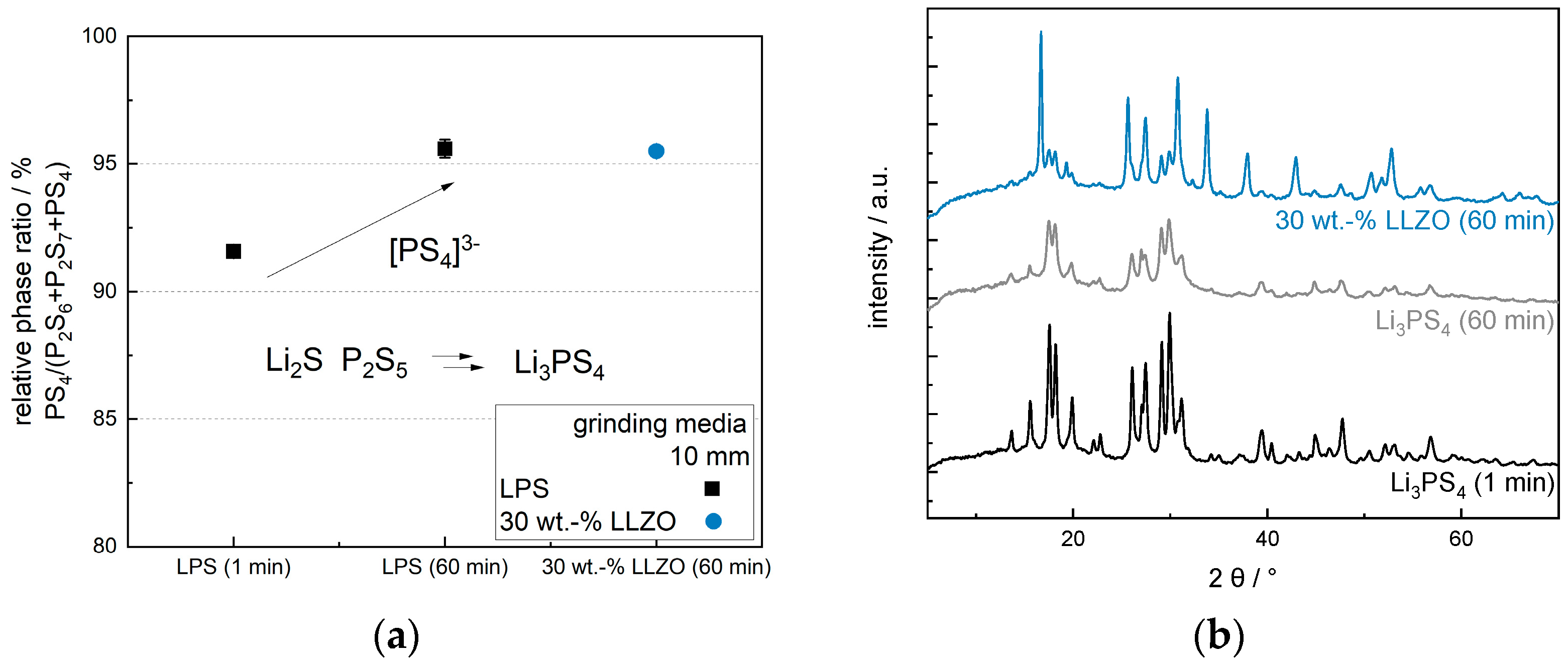
3.3. Impact of Optimized Mixing Parameters on Ionic Conductivity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zheng, F.; Kotobuki, M.; Song, S.; Lai, M.O.; Lu, L. Review on solid electrolytes for all-solid-state lithium-ion batteries. J. Power Sources 2018, 389, 198–213. [Google Scholar] [CrossRef]
- Janek, J.; Zeier, W.G. A solid future for battery development. Nat. Energy 2016, 1, 1167. [Google Scholar] [CrossRef]
- Placke, T.; Kloepsch, R.; Dühnen, S.; Winter, M. Lithium ion, lithium metal, and alternative rechargeable battery technologies: The odyssey for high energy density. J. Solid. State Electrochem. 2017, 21, 1939–1964. [Google Scholar] [CrossRef]
- Niu, C.; Liu, D.; Lochala, J.A.; Anderson, C.S.; Cao, X.; Gross, M.E.; Xu, W.; Zhang, J.-G.; Whittingham, M.S.; Xiao, J.; et al. Balancing interfacial reactions to achieve long cycle life in high-energy lithium metal batteries. Nat. Energy 2021, 6, 723–732. [Google Scholar] [CrossRef]
- Irfan, M.; Atif, M.; Yang, Z.; Zhang, W. Recent advances in high performance conducting solid polymer electrolytes for lithium-ion batteries. J. Power Sources 2020, 486, 229378. [Google Scholar] [CrossRef]
- Asano, T.; Yubuchi, S.; Sakuda, A.; Hayashi, A.; Tatsumisago, M. Electronic and Ionic Conductivities of LiNi1/3Mn1/3Co1/3O2-Li3PS4 Positive Composite Electrodes for All-Solid-State Lithium Batteries. J. Electrochem. Soc. 2017, 164, A3960–A3963. [Google Scholar] [CrossRef]
- Wu, C.; Lou, J.; Zhang, J.; Chen, Z.; Kakar, A.; Emley, B.; Ai, Q.; Guo, H.; Liang, Y.; Lou, J.; et al. Current status and future directions of all-solid-state batteries with lithium metal anodes, sulfide electrolytes, and layered transition metal oxide cathodes. Nano Energy 2021, 87, 106081. [Google Scholar] [CrossRef]
- Lian, P.-J.; Zhao, B.-S.; Zhang, L.-Q.; Xu, N.; Wu, M.-T.; Gao, X.-P. Inorganic sulfide solid electrolytes for all-solid-state lithium secondary batteries. J. Mater. Chem. A 2019, 7, 20540–20557. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, W.; Chen, X.; Das, D.; Ruess, R.; Gautam, A.; Walther, F.; Ohno, S.; Koerver, R.; Zhang, Q.; et al. Influence of Crystallinity of Lithium Thiophosphate Solid Electrolytes on the Performance of Solid-State Batteries. Adv. Energy Mater. 2021, 11, 2100654. [Google Scholar] [CrossRef]
- Zhang, Q.; Cao, D.; Ma, Y.; Natan, A.; Aurora, P.; Zhu, H. Sulfide-Based Solid-State Electrolytes: Synthesis, Stability, and Potential for All-Solid-State Batteries. Adv. Mater. 2019, 31, e1901131. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Kim, D.; Bliem, R.; Vardar, G.; Waluyo, I.; Hunt, A.; Wright, J.T.; Katsoudas, J.P.; Yildiz, B. Thermally Driven Interfacial Degradation between Li7La3Zr2O12 Electrolyte and LiNi0.6Mn0.2Co0.2O2 Cathode. Chem. Mater. 2020, 32, 9531–9541. [Google Scholar] [CrossRef]
- Miara, L.; Windmüller, A.; Tsai, C.-L.; Richards, W.D.; Ma, Q.; Uhlenbruck, S.; Guillon, O.; Ceder, G. About the Compatibility between High Voltage Spinel Cathode Materials and Solid Oxide Electrolytes as a Function of Temperature. ACS Appl. Mater. Interfaces 2016, 8, 26842–26850. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Connell, J.G.; Tepavcevic, S.; Zapol, P.; Garcia-Mendez, R.; Taylor, N.J.; Sakamoto, J.; Ingram, B.J.; Curtiss, L.A.; Freeland, J.W.; et al. Dopant-Dependent Stability of Garnet Solid Electrolyte Interfaces with Lithium Metal. Adv. Energy Mater. 2019, 9, 1803440. [Google Scholar] [CrossRef]
- Ramakumar, S.; Deviannapoorani, C.; Dhivya, L.; Shankar, L.S.; Murugan, R. Lithium garnets: Synthesis, structure, Li + conductivity, Li + dynamics and applications. Prog. Mater. Sci. 2017, 88, 325–411. [Google Scholar] [CrossRef]
- Feng, J.; Wang, L.; Chen, Y.; Wang, P.; Zhang, H.; He, X. PEO based polymer-ceramic hybrid solid electrolytes: A review. Nano Converg. 2021, 8, 1–12. [Google Scholar] [CrossRef]
- Ji, X.; Zhang, Y.; Cao, M.; Gu, Q.; Wang, H.; Yu, J.; Guo, Z.-H.; Zhou, X. Advanced inorganic/polymer hybrid electrolytes for all-solid-state lithium batteries. J. Adv. Ceram. 2022, 11, 835–861. [Google Scholar] [CrossRef]
- Gao, J.; Zhao, Y.-S.; Shi, S.-Q.; Li, H. Lithium-ion transport in inorganic solid state electrolyte. Chin. Phys. B 2016, 25, 018211. [Google Scholar] [CrossRef]
- Minami, T.; Hayashi, A.; Tatsumisago, M. Preparation and characterization of lithium ion-conducting oxysulfide glasses. Solid. State Ionics 2000, 136–137, 1015–1023. [Google Scholar] [CrossRef]
- Hayashi, A.; Hama, S.; Morimoto, H.; Tatsumisago, M.; Minami, T. Preparation of Li2S-P2S5 Amorphous Solid Electrolytes by Mechanical Milling. J. Am. Ceram. Soc. 2001, 84, 477–479. [Google Scholar] [CrossRef]
- Hofer, M.; Grube, M.; Burmeister, C.F.; Michalowski, P.; Zellmer, S.; Kwade, A. Effective mechanochemical synthesis of sulfide solid electrolyte Li3PS4 in a high energy ball mill by process investigation. Adv. Powder Technol. 2023, 34, 104004. [Google Scholar] [CrossRef]
- Mizuno, F.; Hayashi, A.; Tadanaga, K.; Tatsumisago, M. New, Highly Ion-Conductive Crystals Precipitated from Li2S-P2S5 Glasses. Adv. Mater. 2005, 17, 918–921. [Google Scholar] [CrossRef]
- Seino, Y.; Ota, T.; Takada, K.; Hayashi, A.; Tatsumisago, M. A sulphide lithium super ion conductor is superior to liquid ion conductors for use in rechargeable batteries. Energy Environ. Sci. 2014, 7, 627–631. [Google Scholar] [CrossRef]
- Lu, S.; Kosaka, F.; Shiotani, S.; Tsukasaki, H.; Mori, S.; Otomo, J. Optimization of lithium ion conductivity of Li2S-P2S5 glass ceramics by microstructural control of crystallization kinetics. Solid. State Ionics 2021, 362, 115583. [Google Scholar] [CrossRef]
- Garcia-Mendez, R.; Smith, J.G.; Neuefeind, J.C.; Siegel, D.J.; Sakamoto, J. Correlating Macro and Atomic Structure with Elastic Properties and Ionic Transport of Glassy Li2S-P2S5 (LPS) Solid Electrolyte for Solid-State Li Metal Batteries. Adv. Energy Mater. 2020, 10, 7. [Google Scholar] [CrossRef]
- Shahi, K.; Wagner, J. Anomalous ionic conduction in AgBr–AgI mixed crystals and multiphase systems. J. Phys. Chem. Solids 1982, 43, 713–722. [Google Scholar] [CrossRef]
- Maier, J. Control parameters for electrochemically relevant materials: The significance of size and complexity. Faraday Discuss. 2014, 176, 17–29. [Google Scholar] [CrossRef] [PubMed]
- Rangasamy, E.; Sahu, G.; Keum, J.K.; Rondinone, A.J.; Dudney, N.J.; Liang, C. A high conductivity oxide–sulfide composite lithium superionic conductor. J. Mater. Chem. A 2014, 2, 4111–4116. [Google Scholar] [CrossRef]
- Hood, Z.D.; Wang, H.; Li, Y.; Pandian, A.S.; Parans Paranthaman, M.; Liang, C. The “filler effect”: A study of solid oxide fillers with β-Li3PS4 for lithium conducting electrolytes. Solid State Ion. 2015, 283, 75–80. [Google Scholar] [CrossRef]
- Batzer, M.; Voges, K.; Wang, W.; Michalowski, P.; Kwade, A. Systematic evaluation of materials and recipe for scalable processing of sulfide-based solid-state batteries. Mater. Today Commun. 2022, 30, 103189. [Google Scholar] [CrossRef]
- Heins, T.P.; Schlüter, N.; Schröder, U. Electrode-Resolved Monitoring of the Ageing of Large-Scale Lithium-Ion Cells by using Electrochemical Impedance Spectroscopy. ChemElectroChem 2017, 4, 2921–2927. [Google Scholar] [CrossRef]
- Wan, T.H.; Saccoccio, M.; Chen, C.; Ciucci, F. Influence of the Discretization Methods on the Distribution of Relaxation Times Deconvolution: Implementing Radial Basis Functions with DRTtools. Electrochimica Acta 2015, 184, 483–499. [Google Scholar] [CrossRef]
- Ohno, S.; Banik, A.; Dewald, G.F.; Kraft, M.A.; Krauskopf, T.; Minafra, N.; Till, P.; Weiss, M.; Zeier, W.G. Materials design of ionic conductors for solid state batteries. Prog. Energy 2020, 2, 022001. [Google Scholar] [CrossRef]
- Bron, P.; Johansson, S.; Zick, K.; der Guenne, J.S.A.; Dehnen, S.; Roling, B. Li10SnP2S12: An Affordable Lithium Superionic Conductor. J. Am. Chem. Soc. 2013, 135, 15694–15697. [Google Scholar] [CrossRef] [PubMed]
- Bron, P.; Dehnen, S.; Roling, B. Li10Si0.3Sn0.7P2S12—A low-cost and low-grain-boundary-resistance lithium superionic conductor. J. Power Sources 2016, 329, 530–535. [Google Scholar] [CrossRef]
- Hüttl, J.; Seidl, C.; Auer, H.; Nikolowski, K.; Görne, A.L.; Arnold, M.; Heubner, C.; Wolter, M.; Michaelis, A. Ultra-low LPS/LLZO interfacial resistance—Towards stable hybrid solid-state batteries with Li-metal anodes. Energy Storage Mater. 2021, 40, 259–267. [Google Scholar] [CrossRef]
- de Klerk, N.J.J.; van der Maas, E.; Wagemaker, M. Analysis of Diffusion in Solid-State Electrolytes through MD Simulations, Improvement of the Li-Ion Conductivity in β-Li3PS4 as an Example. ACS Appl. Energy Mater. 2018, 1, 3230–3242. [Google Scholar] [CrossRef]
- Ghidiu, M.; Schlem, R.; Zeier, W.G. Pyridine Complexes as Tailored Precursors for Rapid Synthesis of Thiophosphate Superionic Conductors. Batter. Supercaps 2021, 4, 607–611. [Google Scholar] [CrossRef]
- Samsinger, R.F.; Schopf, S.O.; Schuhmacher, J.; Treis, P.; Schneider, M.; Roters, A.; Kwade, A. Influence of the Processing on the Ionic Conductivity of Solid-State Hybrid Electrolytes Based on Glass-Ceramic Particles Dispersed in PEO with LiTFSI. J. Electrochem. Soc. 2020, 167, 120538. [Google Scholar] [CrossRef]
- Duan, H.; Zheng, H.; Zhou, Y.; Xu, B.; Liu, H. Stability of garnet-type Li ion conductors: An overview. Solid. State Ionics 2018, 318, 45–53. [Google Scholar] [CrossRef]
- Gu, Z.; Ma, J.; Zhu, F.; Liu, T.; Wang, K.; Nan, C.-W.; Li, Z.; Ma, C. Atomic-scale study clarifying the role of space-charge layers in a Li-ion-conducting solid electrolyte. Nat. Commun. 2023, 14, 1632. [Google Scholar] [CrossRef]
- Wang, L.; Xie, R.; Chen, B.; Yu, X.; Ma, J.; Li, C.; Hu, Z.; Sun, X.; Xu, C.; Dong, S.; et al. In-situ visualization of the space-charge-layer effect on interfacial lithium-ion transport in all-solid-state batteries. Nat. Commun. 2020, 11, 5889. [Google Scholar] [CrossRef]
- Takada, K.; Ohno, T.; Ohta, N.; Ohnishi, T.; Tanaka, Y. Positive and Negative Aspects of Interfaces in Solid-State Batteries. ACS Energy Lett. 2018, 3, 98–103. [Google Scholar] [CrossRef]
- Takada, K.; Ohta, N.; Zhang, L.; Xu, X.; Hang, B.T.; Ohnishi, T.; Osada, M.; Sasaki, T. Interfacial phenomena in solid-state lithium battery with sulfide solid electrolyte. Solid. State Ionics 2012, 225, 594–597. [Google Scholar] [CrossRef]
- Haruyama, J.; Sodeyama, K.; Han, L.; Takada, K.; Tateyama, Y. Space–Charge Layer Effect at Interface between Oxide Cathode and Sulfide Electrolyte in All-Solid-State Lithium-Ion Battery. Chem. Mater. 2014, 26, 4248–4255. [Google Scholar] [CrossRef]
- Cheng, Z.; Liu, M.; Ganapathy, S.; Li, C.; Li, Z.; Zhang, X.; He, P.; Zhou, H.; Wagemaker, M. Revealing the Impact of Space-Charge Layers on the Li-Ion Transport in All-Solid-State Batteries. Joule 2020, 4, 1311–1323. [Google Scholar] [CrossRef]
- Maier, J. Ionic conduction in space charge regions. Prog. Solid State Chem. 1995, 23, 171–263. [Google Scholar] [CrossRef]
- Huo, H.; Li, X.; Sun, Y.; Lin, X.; Doyle-Davis, K.; Liang, J.; Gao, X.; Li, R.; Huang, H.; Guo, X.; et al. Li2CO3 effects: New insights into polymer/garnet electrolytes for dendrite-free solid lithium batteries. Nano Energy 2020, 73, 104836. [Google Scholar] [CrossRef]
- Biao, J.; Han, B.; Cao, Y.; Li, Q.; Zhong, G.; Ma, J.; Chen, L.; Yang, K.; Mi, J.; Deng, Y.; et al. Inhibiting Formation and Reduction of Li2 CO3 to LiCx at Grain Boundaries in Garnet Electrolytes to Prevent Li Penetration. Adv. Mater. 2023, 35, e2208951. [Google Scholar] [CrossRef]
- Katzenmeier, L.; Carstensen, L.; Bandarenka, A.S. Li+ Conductivity of Space Charge Layers Formed at Electrified Interfaces Between a Model Solid-State Electrolyte and Blocking Au-Electrodes. ACS Appl. Mater. Interfaces 2022, 14, 15811–15817. [Google Scholar] [CrossRef]
- Schlem, R.; Burmeister, C.F.; Michalowski, P.; Ohno, S.; Dewald, G.F.; Kwade, A.; Zeier, W.G. Energy Storage Materials for Solid-State Batteries: Design by Mechanochemistry. Adv. Energy Mater. 2021, 11, 2101022. [Google Scholar] [CrossRef]
- Burmeister, C.F.; Hofer, M.; Molaiyan, P.; Michalowski, P.; Kwade, A. Characterization of Stressing Conditions in a High Energy Ball Mill by Discrete Element Simulations. Processes 2022, 10, 692. [Google Scholar] [CrossRef]
- Kwade, A. A Stressing Model for the Description and Optimization of Grinding Processes. Chem. Eng. Technol. 2003, 26, 199–205. [Google Scholar] [CrossRef]
- Zou, C.; Yang, L.; Luo, K.; Tao, X.; Yi, L.; Liu, X.; Luo, Z.; Wang, X. Ionic conductivity and interfacial stability of Li6PS5Cl–Li6.5La3Zr1.5Ta0.5O12 composite electrolyte. J. Solid State Electrochem. 2021, 25, 2513–2525. [Google Scholar] [CrossRef]
- Diallo, M.S.; Shi, T.; Zhang, Y.; Peng, X.; Shozib, I.; Wang, Y.; Miara, L.J.; Scott, M.C.; Tu, Q.H.; Ceder, G. Effect of solid-electrolyte pellet density on failure of solid-state batteries. Nat. Commun. 2024, 15, 858. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Zhao, C.-Z.; Yuan, H.; Cheng, X.-B.; Huang, J.-Q.; Zhang, Q. Critical Current Density in Solid-State Lithium Metal Batteries: Mechanism, Influences, and Strategies. Adv. Funct. Mater. 2021, 31, 479. [Google Scholar] [CrossRef]
- Garcia-Mendez, R.; Mizuno, F.; Zhang, R.; Arthur, T.S.; Sakamoto, J. Effect of Processing Conditions of 75Li2S-25P2S5 Solid Electrolyte on its DC Electrochemical Behavior. Electrochim. Acta 2017, 237, 144–151. [Google Scholar] [CrossRef]
- Poudel, T.P.; Deck, M.J.; Wang, P.; Hu, Y.-Y. Transforming Li3PS4 Via Halide Incorporation: A Path to Improved Ionic Conductivity and Stability in All-Solid-State Batteries. Adv. Funct. Mater. 2024, 34, 299. [Google Scholar] [CrossRef]
- Stöffler, H.; Zinkevich, T.; Yavuz, M.; Hansen, A.-L.; Knapp, M.; Bednarčík, J.; Randau, S.; Richter, F.H.; Janek, J.; Ehrenberg, H.; et al. Amorphous versus Crystalline Li3PS4: Local Structural Changes during Synthesis and Li Ion Mobility. J. Phys. Chem. C 2019, 123, 10280–10290. [Google Scholar] [CrossRef]
- Yamamoto, K.; Yang, S.; Takahashi, M.; Ohara, K.; Uchiyama, T.; Watanabe, T.; Sakuda, A.; Hayashi, A.; Tatsumisago, M.; Muto, M.; et al. High Ionic Conductivity of Liquid-Phase-Synthesized Li3PS4 Solid Electrolyte, Comparable to That Obtained via Ball Milling. ACS Appl. Energy Mater. 2021, 4, 2275–2281. [Google Scholar] [CrossRef]
- Self, E.C.; Hood, Z.D.; Brahmbhatt, T.; Delnick, F.M.; Meyer, H.M.; Yang, G.; Rupp, J.L.M.; Nanda, J. Solvent-Mediated Synthesis of Amorphous Li3PS4/Polyethylene Oxide Composite Solid Electrolytes with High Li + Conductivity. Chem. Mater. 2020, 32, 8789–8797. [Google Scholar] [CrossRef]
- Kaiser, N.; Spannenberger, S.; Schmitt, M.; Cronau, M.; Kato, Y.; Roling, B. Ion transport limitations in all-solid-state lithium battery electrodes containing a sulfide-based electrolyte. J. Power Sources 2018, 396, 175–181. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gerstenberg, J.; Steckermeier, D.; Kwade, A.; Michalowski, P. Effect of Mixing Intensity on Electrochemical Performance of Oxide/Sulfide Composite Electrolytes. Batteries 2024, 10, 95. https://doi.org/10.3390/batteries10030095
Gerstenberg J, Steckermeier D, Kwade A, Michalowski P. Effect of Mixing Intensity on Electrochemical Performance of Oxide/Sulfide Composite Electrolytes. Batteries. 2024; 10(3):95. https://doi.org/10.3390/batteries10030095
Chicago/Turabian StyleGerstenberg, Jessica, Dominik Steckermeier, Arno Kwade, and Peter Michalowski. 2024. "Effect of Mixing Intensity on Electrochemical Performance of Oxide/Sulfide Composite Electrolytes" Batteries 10, no. 3: 95. https://doi.org/10.3390/batteries10030095




