Comparative Analysis of Lithium-Ion Batteries for Urban Electric/Hybrid Electric Vehicles
Abstract
1. Introduction
- Advantages:
- -
- Reduced carbon dioxide emissions—EVs and HEVs emit significantly less or no carbon dioxide into the atmosphere compared to conventional cars, making them more environmentally friendly.
- -
- Savings on fuel costs—Using electricity as the main or partial source of energy can significantly reduce fuel costs.
- -
- Low operating costs—Electric motors require less maintenance than conventional internal combustion engines because they have fewer moving parts.
- -
- Quiet operation—EVs are significantly quieter than vehicles with internal combustion engines, reducing noise pollution in urban environments.
- -
- Support from public and public funds—Many governments offer tax breaks, subsidies, and other incentives for the purchase and use of EVs and HEVs.
- Disadvantages:
- -
- Limited range—Many electric vehicles still have a relatively limited range per charge compared to gasoline-powered cars.
- -
- Charging time—Charging an EV battery can take significantly longer than filling up a traditional car with fuel.
- -
- Insufficient charging infrastructure—Many cities still lack a sufficiently developed infrastructure for the fast and convenient charging of electric vehicles.
- -
- A high initial cost—Despite declining prices over time, EVs and HEVs often have higher purchase prices than conventional cars.
- -
- The environmental issues of batteries and other elements of these vehicles—The production and disposal of lithium-ion batteries bring environmental risks and challenges, including the mining of rare metals and waste management.
- -
- Compactness and lightness—For an urban vehicle, it is important that the battery occupies a minimum amount of space and adds as little weight as possible to the vehicle. Lithium-ion batteries are lighter and smaller in size than other types of batteries, which make them suitable for inclusion in the construction of small and maneuverable city cars.
- -
- Modularity—Batteries can be designed into modular systems, allowing them to be easily added or removed from the vehicle for maintenance purposes or capacity upgrades.
- -
- Thermal management—Urban vehicles often operate in stop/start conditions, which can cause the battery temperature to rise. Effective temperature management systems are critical to maintaining optimal performance and extending battery life.
- -
- Fast and flexible charging—Lithium-ion batteries usually support fast charging, which is important for urban vehicles, as they can be quickly recharged during short interruptions in service. Also, batteries are often compatible with different types of charging stations, offering flexibility in urbanized areas with diverse charging infrastructure.
- -
- Durability and reliability—In an urbanized environment where vehicles are used intensively, it is important that batteries have high durability and can withstand multiple charging cycles without a significant reduction in their efficiency.
- -
- Environmental sustainability—Given the increasing regulations to reduce emissions and increase sustainability, lithium-ion batteries offer an environmentally friendly solution, as they do not require the burning of fuels and are less polluting than traditional car batteries.
- Capacity and energy density
- 2.
- Size and weight
- 3.
- Type and chemical composition
- 4.
- Charge and Use Cycles
- 5.
- Terms of use
- Solid-State Batteries
- 2.
- Lithium–sulfur (Li-S) batteries
- 3.
- Improvements in electrode materials
- 4.
- Fast charging
- 5.
- Intelligent Battery Management Systems (BMSs)
- 6.
- Recycling and sustainability
- 7.
- Thermal management systems
2. Goals
3. Materials and Methods
3.1. Types of Li-Ion Cells Used in EVs/HEVs
- Lithium–iron–phosphate (LFP). The active cathode material is LiFePO4. The charging voltage is in the range of 2.1–3.65 V, but it can be up to 3.9 V if doped with yttrium—whose active cathode material is LiFeYPO4. The nominal voltage is in the range of 3.2–3.35 V [24]. These cells have a flash point of 270 °C and, as such, are harder to ignite than NMC or NCA in the case of improper charging or mechanical damage.
3.2. Stack Assembly
- Li-ion battery consisting of 24 series connected prismatic LFP cells with chemistry
- Li-ion battery stack consisting of 676 cylindrical NCA cells with chemistry
3.3. Experimental Vehicle
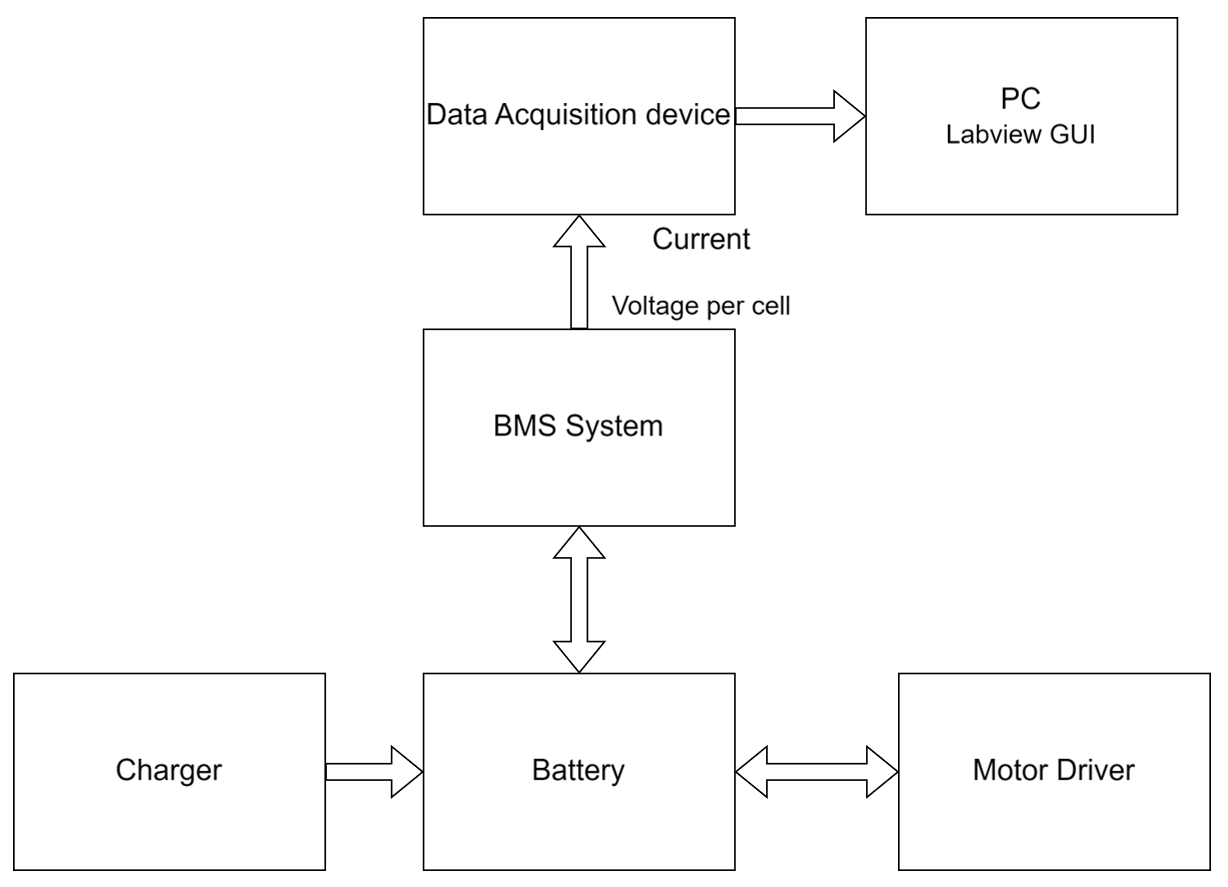
3.4. Intelligent Module System for Battery Control—BMS
- To protect the individual cells from damage due to over discharge or overcharge.
- To prolong the life of the battery by optimally equilibrizing the charge in each cell of the battery.
- To keep the battery in a state which allows for its optimal use.
- Voltage measurement. Each ECU in the local BMS modules is shown in Figure 3a. Circuits are used for precise voltage measurement, and they are sent through an interface to the central microprocessor in the control panel.
- Current and state of charge (SOC) measurement. A resistive shunt measures the current though the cells. A counter counts the charge accumulated/extracted for a certain time interval, which is known as CC (Coulomb counting) [27,32,33]. The SOC measured in this way is obtained by very accurately integrating the measured current in the circuit to avoid many of the errors. The algorithm used for determining the current to actively balance the battery packs uses Formula (1):
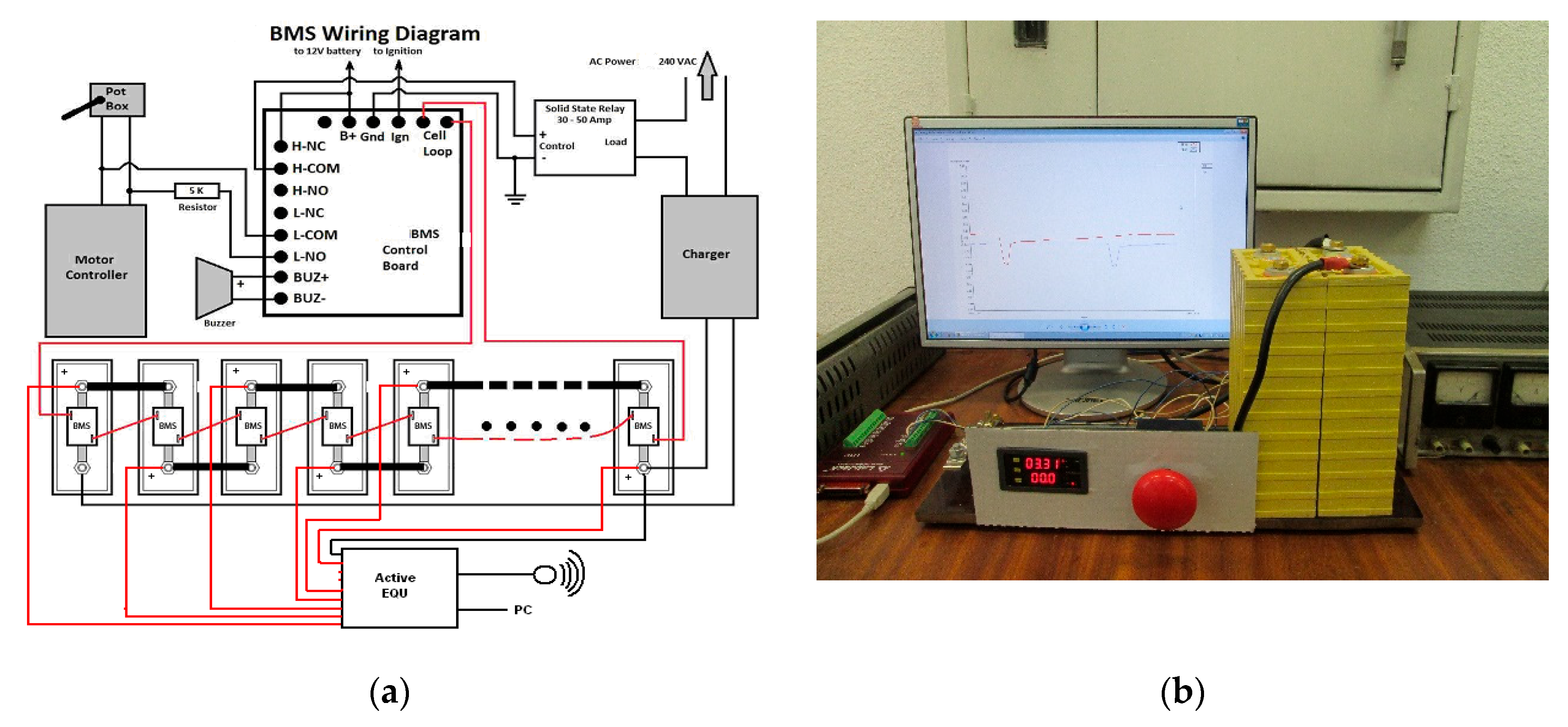
- Cell balancing. The purpose of cell balancing is to increase the battery’s performance without overcharging or over-discharging it. Its essence is to make the SOC levels of the cells closer to each other. In the developed smart BMS, a combined passive and active cell balancing method is selected with a decentralized arrangement of the modular BMS, as shown in Figure 3a. The purpose of using passive equalizers is to achieve fast SOC equalization during charging by applying charge shunting to the fastest charging cells until their voltages are within ±0.01 V of the normal cells’ OCV voltage, V. Balancing with active EQUs works during battery charging and discharging as well as in standby mode, actively transferring a current of up to 1 A from the higher voltage cells to the lower voltage cells until the voltages equalize. Through the GUI, modules with local passive and active EQUs signal the problems to the central BMS module, where a decision will be made about when to start balancing with the EQU and accurately report the state of charge (SOC) [14,16,17,35].
4. Methods for Testing Battery Systems (LESMSs)
4.1. Preliminary Tests
- Discharge characteristics of NCA and LFP cells. One fully charged LFP and NCA.
- Full charge/discharge cycle with 0.25 C. Every charge cycle could be separated into 4 different phases: discharge, pauses, charging, and pauses. By applying the LabVIEW graphical program, we can control the phase change boundary condition.
4.2. Qualification Tests
- Initial charging of the system. Only after the cells are balanced on the same voltage level and after checking the internal resistance and technical characteristics is it possible to assemble them together with the BMS into a functional LESMS.
- Constant current discharge. Fully charged LESMSs undergo a constant current discharge with current I~0.25 C depending on the SOC, where C is the capacity of the LESMS—in the case for both systems, the current is, on average, about 20 A. They are discharged to establish the characteristics of the discharge curves and the operating zones of the systems (Figure 7a).
- Weak cell identification in LFP or weak module in NCA. With the BMS, the general EQU operations of LFP or NCA cells can be counted, and this information can be used to identify the weak cell (module). A weak cell always requires more EQU operations regardless of whether it is in the process of charging or discharging. The LESMS is only loaded to the levels of the weak cell LFP (or the weak module NCA, respectively) to prevent overcharging and degradation.
- Testing under a constant load. A test is carried out to verify that the LESMS can deliver the specified power under a load when required. The load is usually representative of the expected conditions under which the system may be used (Figure 7b).
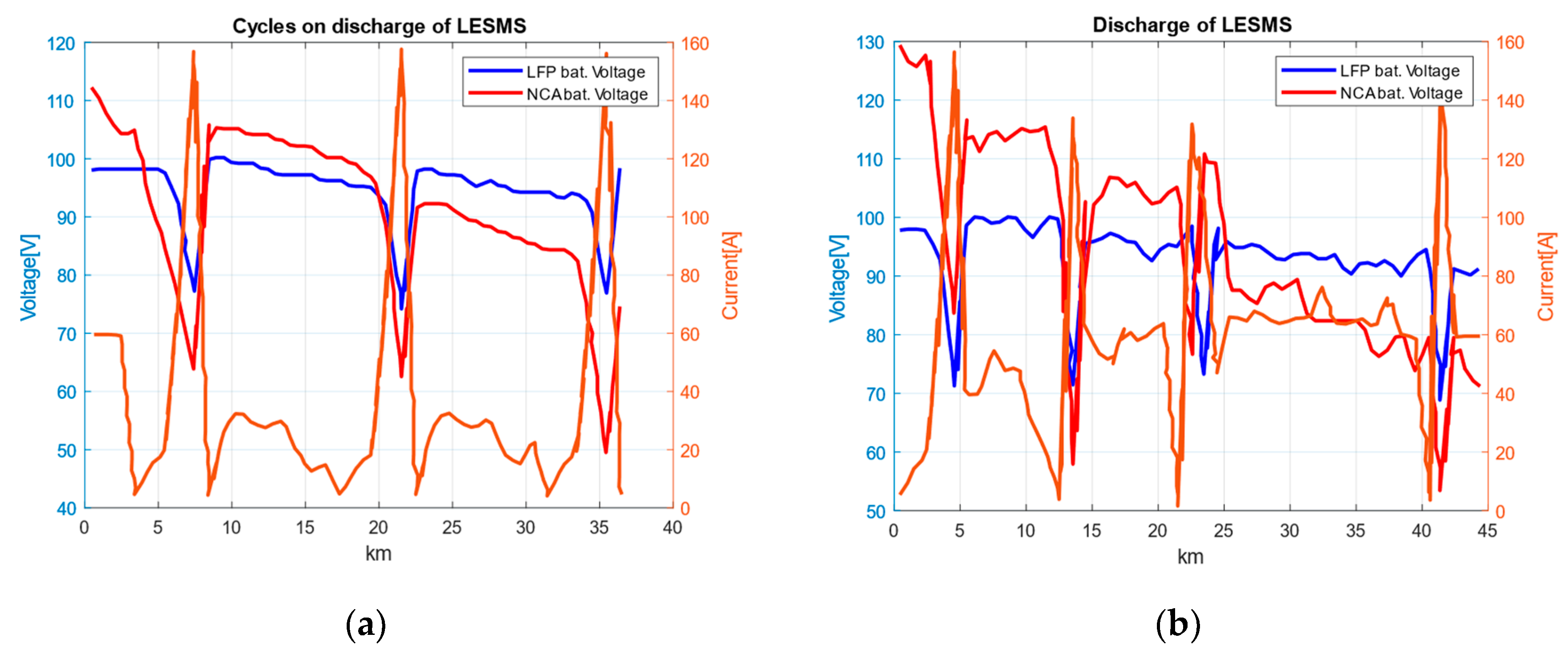
- Testing under a dynamic load. The tests for cyclic and dynamically changing loads are performed with the test vehicle. For simplicity in testing, no recovery is applied. The operation of a “pure” electric car under different road conditions is simulated. For cyclic loading, the test car’s movement on a test site with simulated stopping and starting actions at a traffic light at a certain distance is carried out in order to establish the suitability of the components for application in joint operation (Figure 6a). Figure 6b presents a graph of the current and voltage under a dynamic load of LESMS at a 50 km mileage with a maximum speed of 60 km/h in real urban conditions typical of an urban EV/HEV.
5. Experimental Results and Discussion
5.1. The Advantages and Disadvantages of the System
5.2. Results and Discussion of Preliminary Testing
- Weak cell identification. Figure 5a shows the voltage ΔVcell of two fully charged LFP cells, and Figure 5b shows the same ΔVcell of two fully charged NCA cells during the internal impedance measurement according to Formulas (7) and (8). In Figure 5a, the slower OCV relaxation is seen in one of the LFP-type cells; therefore, it has a higher internal resistance. This cell was identified as being weaker than the normal LFP cell. In the two tested cells of the NCA type, the ΔVcell pulses and the voltage, V, at OCV are the same; therefore, both cells have a normal capacity (Figure 5b).
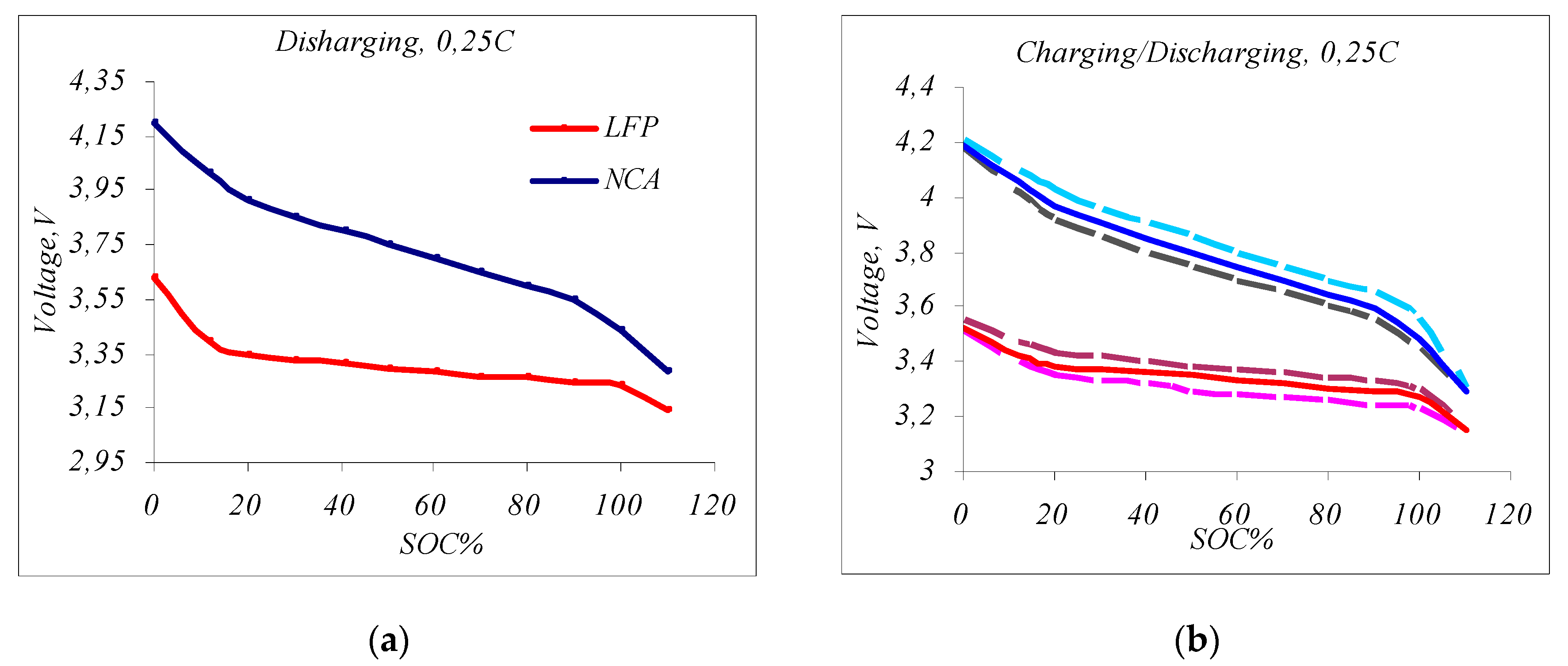
- Discharge and full charge/discharge cycle of LFP and NCA cells. Figure 5a shows the graphs of the discharge voltage versus the SOC. The discharge graph of the small cylindrical NCA cell type is marked with a blue line, and the discharge graph of a large prismatic LFP cell type is constructed with a red line. The voltage difference of the NCA cell at the end and the beginning of the working area is 0.65 V, and the voltage difference of the LFP cell is 0.25 V. Therefore, the electrochemical characteristics of the two cells differ. For example, estimating the state of charge in NCA-type cells becomes much easier by measuring only the voltage at the OCV, while in LFP-type cells, due to the small slope of the discharge curve, estimating the SOC by only measuring the voltage is not a precise enough method. Therefore, in the SOC determination algorithms, for LFP-type cells, it is also necessary to measure the amount of electricity Q (CC) or apply the Kalman filter [15,27,33,35]. In Figure 4b, LFP-type and NCA-type cell voltages are shown as a function of the SOC during the charge/discharge process with 0.25 C current pauses. This allows for a direct comparison of the voltage levels between charge and discharge at the same states of charge. The charging and discharging times are stopped for a short time after every 10% change in capacity to measure and analyze the stress relaxation at the OCV. The upper dashed curves of the cycles represent charging (light blue for the NCA cell and brown for the LFP cell), and the lower dashed curves (black for NCA and light red for LFP, respectively) represent their discharge with 1 min cut-off pauses when the OCV is read. Cell voltage measurements during the test cycle show that the cells do not reach equilibrium at the end of the pauses if the response step has a pause of 1 min, which is very small. Therefore, the equilibrium voltage at the OCV cannot be directly measured at this pause. For full relaxation to the OCV level (equilibrium voltage), longer pauses are required, especially at higher charge/discharge currents and at large LFP cell formats (of the order of 10–15 min). This makes the test difficult and pointless because it extends the time taken to conduct the test cycle to several days. It is necessary to speed up the execution time of the cycle. For this purpose, the continuous averages represent an interpolation between the voltage levels at the end of the 1 min breaks. The blue and red solid curves in the center of the cycles are the averages of this region. Initially, the true equilibrium is assumed to be the OCV. We assume that this approach of estimating the OCV by calculating the average value of the voltage is accurate enough for the charge/discharge cycle with a current in the 0.25–1 C range, where the current most often varies in small city EV/HEV applications; therefore, the continuous curves represent the actual discharge/charge characteristics of the respective Li-ion battery types, and no breaks are required during the test.
5.3. Results and Discussion on Qualification Testing
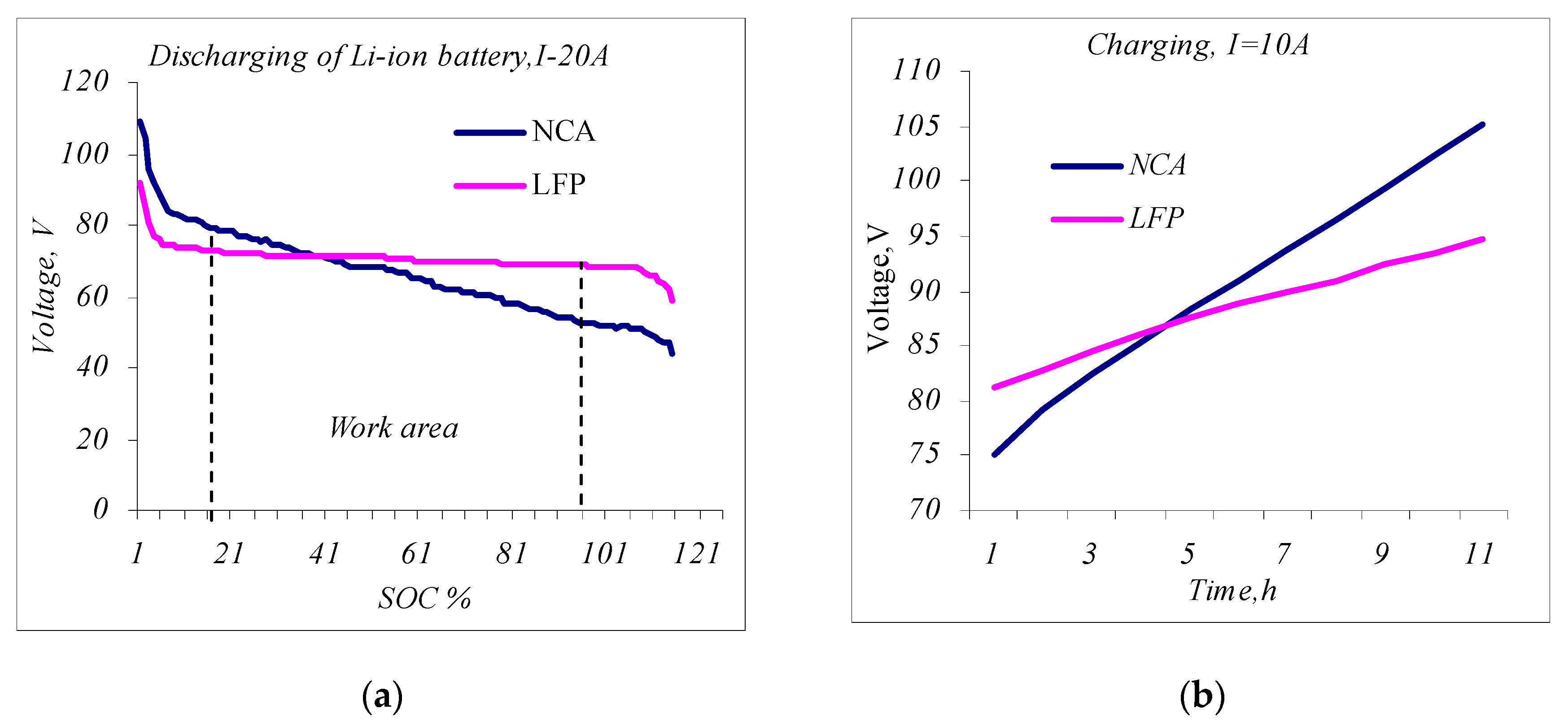
- The constant current discharge of the LESMS. Fully charged LESMS stacks are discharged with a constant current of I~0.25 C—this equals 20 A, on average, for both cells. In Figure 7a, the discharge curves of the two LESMSs are shown as a function of the SOC. The blue line is the discharge curve of the NCA-type LESMS, and the red line is the discharge curve of the LFP-type system. The visualization of Figure 7a makes it possible to determine the OCV voltage operating zones of the two systems required for the following functional tests. The operating range of the SOC should be between 15% and 90% for NCA and between 10 and 100% for LFP.
- Constant load tests. For this test, the LESMS is loaded with a constant load to verify its use for the application. The LESMSs are charged and then discharged with a constant current of 10 A ranging from 15% to 90% SOC. In Figure 7b, the charging diagram of the two LESMSs is given in the operating range of 15% to 90% SOC for a time of 10 h, as determined by the control system. The visualization shows the higher energy efficiency and steeper curve of the LESMS based on NCA cells (the blue line). Therefore, the NCA system was found to have a higher specific energy of up to 260 Wh/kg, and the LFP-type system was found to have a specific energy of 90 Wh/kg [23,36].
- Cyclic load testing. Figure 6a shows the results of a few cycles with the dynamic discharge of the system composed of LFP and NCA cells. A diagram of the changes in the current and voltage of the two systems under cyclic loads for a certain distance traveled (on a test site) is presented. In Figure 6b, the variations in the current and voltage of the LFP- and NCA-type LESMSs under a load in real urban conditions at a distance of 50 km with a maximum speed of 60 km/h are presented. The currents and voltages are recorded directly from the motor controller via a graphical user interface (GUI) and the LabVIEW graphical program.
5.4. Comparative Analysis
- A comparison of the characteristics of the two types of LESMSs (volume, geometric dimensions, costs, and prices of components and systems). At approximately the same power (~8 kWh), the geometric dimensions of the LFP-type LESMS are approximately twice the size and weight of those of the NCA-type LESMS; therefore, it has twice the energy density and energy efficiency. The assembly costs and the cost of the components (except for the cost of the Li-ion cells) and BMS are about the same for both LESMSs. Recently, in view of the high current price increase for cobalt, nickel, and manganese LFP-type cells, regardless of their lower energy efficiency and complex SOC determination algorithm, they are also starting to be implemented in EV/NEV applications [18,37]. Due to reasons of a modern geopolitical nature, the metals used to manufacture NMC and NCA cells—nickel, cobalt, manganese, and aluminum—have risen sharply in price, and from there, the prices of high-voltage battery systems based on nickel and cobalt have risen sharply [23]. Therefore, for example, “Tesla has announced that it is switching to LFP batteries for its standard range models such as Model 3 and Model Y” [18,37]. The main arguments for the implementation of LFP batteries are the availability of cheap cathode material and the competitive price [23]. Phosphate is much easier to obtain and, for now, reliably cheaper. This means that iron phosphate LESMSs can be much cheaper than cobalt-type NMS and NCA systems and, in addition to solar PV systems, they can successfully displace them in low-cost and urban EV/HEV applications [18,20].
- A comparison based on the preliminary test. It was found that there are significant differences in the electrochemical characteristics of the tested cells, for example, the operating area of the discharge/charge characteristic curve of the NCA-type cells has a greater slope than the almost flat characteristic curve in the operating area of the LFP-type cell (Figure 4a). This means that the SOC estimation of the NCA-type cell is more accurate than that of the LFP-type cell. Correctly calculating the remaining battery capacity is significantly more difficult with LFP cells due to their flatter discharge/charge voltage characteristic. For this reason, it is recommended that LFP-type cells are periodically charged to a 100% SOC so that the BMS can accurately calculate the battery capacity by measuring the amount of electricity, Q, through the remaining capacity, CC, rather than relying solely on the voltage measurement. For NCA-type cells, the voltage measurement is sufficient to estimate the SOC and the remaining capacity. From tests to determine the internal resistance of the cells, it was found that at a 100% SOC, the normal internal resistance of the tested LFP cells was 0.39Ω, and that of the small NCA cells was 0.035Ω. NCA cells have been found to last for 500 to 1000 full charge/discharge cycles, while LFP cells last for 2000 to 6000 full cycles without degrading [24,29]. A disadvantage of LFP cells is their low energy efficiency compared to NCA cells and the difficulty in determining their state of charge (SOC). These problems can be solved during the development and implementation of intelligent control systems with a more complex algorithm, including, in addition to the voltage measurement, the integration of the amount of electricity (Q) through the CC or through an algorithm based on the Kalman filter (KF) [15]. Cells with a higher OCV voltage that reached full charge faster were also found to have higher internal resistance or not be well balanced.
- A comparison based on real exploration tests. The voltage of a no-load current (OCV) of an LESMS is not a reliable measure of its ability to deliver a current. As the battery ages or degrades due to improper use, its internal resistance increases. This reduces the ability to accept and hold a charge, but the OCV idle voltage will still show as normal despite the reduced battery capacity. Comparing the actual internal resistance to the internal resistance of a new battery will show any deterioration in battery functionality; therefore, the internal resistance should be measured and compared periodically.
- Suggestions for integration. Recently, low-voltage LESMSs with LFP-type cells, in addition to a solar PV, can also be successfully implemented in low-budget urban vehicles—such as rickshaws, micro EVs, or HEVs—in the conversion of vintage cars weighing up to 750 kg (Citroen 2CV, Renault 4, Suzuki Alto, Trabant 601, etc.). The LESMS with an NCA type cell is recommended for mini urban EVs, and if there is a need for fast charging from charging stations, it can be assembled with a cooling system.
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BMS | Battery management system |
| C | Capacity of cells |
| CC method | Coulomb counting method |
| ECU | Electronic control unit |
| EV/HEV | Electric vehicle/hybrid electric vehicle |
| GUI | Graphical user interface |
| LFP | Lithium–iron–phosphate |
| LESMS | Lithium-ion energy storage and management system |
| NCA | Lithium–cobalt–nickel–aluminum oxide |
| NMC | Nickel–manganese–cobalt |
| NCA | Nickel–cobalt–aluminum |
| OCV | Open-circuit voltage |
| SOC | State of charge |
| SOH | State of health |
References
- Saldaña, G.; Martín, J.I.S.; Zamora, I.; Asensio, F.J.; Oñederra, O. Analysis of the Current Electric Battery Models for Electric Vehicle Simulation. Energies 2019, 12, 2750. [Google Scholar] [CrossRef]
- Ribeiro, P.J.G.; Dias, G.; Mendes, J.F.G. Public Transport Decarbonization: An Exploratory Approach to Bus Electrification. World Electr. Veh. J. 2024, 15, 81. [Google Scholar] [CrossRef]
- Poggio, A.E.; Balest, J.; Zubaryeva, A.; Sparber, W. Monitored Data and Social Perceptions Analysis of Battery Electric and Hydrogen Fuelled Buses in Urban and Suburban Areas. J. Energy Storage 2023, 72, 108411. [Google Scholar] [CrossRef]
- Velev, B. Comparative analysis of lithium-ion batteries for EV/HEV applications. Ind. 4.0 2018, 3, 73–76. [Google Scholar]
- Blomgren, G.E. The Development and Future of Lithium Ion Batteries. J. Electrochem. Soc. 2016, 164, A5019–A5025. [Google Scholar] [CrossRef]
- Rangarajan, S.S.; Sunddararaj, S.P.; Sudhakar, A.; Shiva, C.K.; Subramaniam, U.; Collins, E.R.; Senjyu, T. Lithium-Ion Batteries—The Crux of Electric Vehicles with Opportunities and Challenges. Clean Technol. 2022, 4, 908–930. [Google Scholar] [CrossRef]
- Lu, L.; Han, X.; Li, J.; Hua, J.; Ouyang, M. A review on the key issues for lithium-ion battery management in electric vehicles. J. Power Sources 2013, 226, 272–288. [Google Scholar] [CrossRef]
- Elmahallawy, M.; Elfouly, T.; Alouani, A.; Massoud, A.M. A Comprehensive Review of Lithium-Ion Batteries Modeling, and State of Health and Remaining Useful Lifetime Prediction. IEEE Access 2022, 10, 119040–119070. [Google Scholar] [CrossRef]
- Yang, X.; Adair, K.R.; Gao, X.; Sun, X. Recent advances and perspectives on thin electrolytes for high-energy-density solid-state lithium batteries. Energy Environ. Sci. 2020, 14, 643–671. [Google Scholar] [CrossRef]
- Aichberger, C.; Jungmeier, G. Environmental Life Cycle Impacts of Automotive Batteries Based on a Literature Review. Energies 2020, 13, 6345. [Google Scholar] [CrossRef]
- Preger, Y.; Barkholtz, H.M.; Fresquez, A.; Campbell, D.L.; Juba, B.W.; Romàn-Kustas, J.; Ferreira, S.R.; Chalamala, B.R. Degradation of Commercial Lithium-Ion Cells as a Function of Chemistry and Cycling Conditions. J. Electrochem. Soc. 2020, 167, 120532. [Google Scholar] [CrossRef]
- Tran, M.-K.; DaCosta, A.; Mevawalla, A.; Panchal, S.; Fowler, M. Comparative Study of Equivalent Circuit Models Performance in Four Common Lithium-Ion Batteries: LFP, NMC, LMO, NCA. Batteries 2021, 7, 51. [Google Scholar] [CrossRef]
- Reddy, M.V.; Mauger, A.; Julien, C.M.; Paolella, A.; Zaghib, K. Brief History of Early Lithium-Battery Development. Materials 2020, 13, 1884. [Google Scholar] [CrossRef] [PubMed]
- Fu, S.; Liu, W.; Luo, W.; Zhang, Z.; Zhang, M.; Wu, L.; Luo, C.; Lv, T.; Xie, J. State of charge estimation of lithium-ion phosphate battery based on weighted multi-innovation cubature Kalman filter. J. Energy Storage 2022, 50, 104175. [Google Scholar] [CrossRef]
- Plett, G. Extended kalman filtering for battery management systems of LiPB-based HEV battery packs part 2 State and parameter estimation. J. Power Sources 2004, 134, 277–292. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, Z.; Chen, Z. Smart-Leader-Based Distributed Charging Control of Battery Energy Storage Systems Considering SoC Balance. Batteries 2023, 9, 18. [Google Scholar] [CrossRef]
- Hassan, M.; Jawad, M.; Saleem, N.; Raza, A.; Zaidi, K.; Rafiq, N. Solar Power Assisted Passive and Active Cell Balancing System: A Comprehensive Analysis. In Proceedings of the 2021 International Conference on Frontiers of Information Technology, FIT, Islamabad, Pakistan, 13–14 December 2021; pp. 224–229. [Google Scholar] [CrossRef]
- LFP Battery in Your Next EV? Tesla, Ford Rivian Say Yes. (n.d.). Available online: https://www.recurrentauto.com/research/lfp-battery-in-your-next-ev-tesla-and-others-say-yes (accessed on 13 April 2024).
- Premchand, M.; Gudey, S.K. Electric vehicle operation modes with reactive power support using SMC in distribution generation. J. Energy Syst. 2020, 4, 96–120. [Google Scholar] [CrossRef]
- Sepúlveda, F.J.; Montero, I.; Barrena, F.; Miranda, M.T.; Arranz, J.I. Design and Energy Analysis of Photovoltaic-Battery Prototype Considering Different Voltage Levels. Batteries 2023, 9, 16. [Google Scholar] [CrossRef]
- Velev, B.G.; Ivanov, I.S.; Kamenov, V.V. Experimental Study and Thermal Analysis of Cooling Systems for Brushless Motors with Double Stator and Axial Gap. SJEE 2021, 18, 333–349. [Google Scholar] [CrossRef]
- Geisbauer, C.; Woehrl, K.; Mittmann, C.; Schweiger, H.-G. Review-Review of Safety Aspects of Calendar Aged Lithium Ion Batteries. J. Electrochem. Soc. 2020, 167, 090523. [Google Scholar] [CrossRef]
- Whitlock, R. Lithium-Ion Battery Pack Prices Increase due to Rising Costs of Materials and Components. Available online: https://www.renewableenergymagazine.com/storage/lithiumion-battery-pack-prices-increase-due-to-20221206 (accessed on 6 December 2022).
- Samsung INR18650-30Q 3000 mAh 20 A. (n.d.). Available online: https://www.dcpower.eu/accu-rechargeable/lithium-ion-batteries/accu-industrial/samsung-sdi/samsung-inr18650-30q (accessed on 13 April 2024).
- Available online: http://www.batteryexpert.org/produkt/samsung-inr18650-35e-3500mah-8a/ (accessed on 10 March 2024).
- Nickel Fuse 2P-6P Wide Continuous Roll by the Foot! 18650 Cell Level F—Battery Hookup. (n.d.). Available online: https://batteryhookup.com/products/nickel-fuse-2p-wide-continuous-roll-by-the-foot-18650-cell-level-fusing (accessed on 13 April 2024).
- Xing, Y.; Ma, E.W.M.; Tsui, K.L.; Pecht, M. Battery Management Systems in Electric and Hybrid Vehicles. Energies 2011, 4, 1840–1857. [Google Scholar] [CrossRef]
- Wang, M.; Le, A.V.; Noelle, D.J.; Shi, Y.; Yoon, H.; Zhang, M.; Meng, Y.S.; Qiao, Y. Effects of electrode pattern on thermal runaway of lithium-ion battery. Int. J. Damage Mech. 2018, 27, 74–81. [Google Scholar] [CrossRef]
- Winston WB-LYP100AHA—3.3 V/100 Ah Tall LiFeYPO4 Lithium—LiFeYPO4. (n.d.). Available online: https://masori.de/en/products/wb-lyp100aha-3-3v-100ah-tall-lifeypo4 (accessed on 13 April 2024).
- Hibms Smart Bms Active Balancer|Smart Bms 4s Lifepo4 Bluetooth—Smart Bms Balancer—Aliexpress. (n.d.). Available online: https://www.aliexpress.com/i/1005004603727710.html (accessed on 13 April 2024).
- Zhu, W. A Smart Battery Management System for Large Format Lithium Ion Cells. Ph.D. Thesis, University of Toledo, Toledo, OH, USA, 2011. [Google Scholar]
- Babu, P.S.; Ilango, K. Comparative Analysis of Passive and Active Cell Balancing of Li Ion Batteries. In Proceedings of the 2022 3rd International Conference on Intelligent Computing, Instrumentation and Control Technologies: Computational Intelligence for Smart Systems, ICICICT 2022, Kannur, India, 11–12 August 2022; pp. 711–716. [Google Scholar] [CrossRef]
- Ng, K.S.; Moo, C.-S.; Chen, Y.-P.; Hsieh, Y.-C. Enhanced coulomb counting method for estimating state-of-charge and state-of-health of lithium-ion batteries. Appl. Energy 2009, 86, 1506–1511. [Google Scholar] [CrossRef]
- Çarkit, T.; Alçi, M. Comparison of the performances of heuristic optimization algorithms PSO, ABC and GA for parameter estimation in the discharge processes of Li-NMC battery. J. Energy Syst. 2022, 6, 387–400. [Google Scholar] [CrossRef]
- Docimo, D.J. Estimation and balancing of multi-state differences between lithium-ion cells within a battery pack. J. Energy Storage 2022, 50, 104264. [Google Scholar] [CrossRef]
- Aimo, C.; Schmidhalter, I.; Aguirre, P. Multi-objective optimization of a lithium cell design operating in a cyclic process. J. Energy Storage 2023, 57, 106256. [Google Scholar] [CrossRef]
- Wentker, M.; Greenwood, M.; Leker, J. A Bottom-Up Approach to Lithium-Ion Battery Cost Modeling with a Focus on Cathode Active Materials. Energies 2019, 12, 504. [Google Scholar] [CrossRef]


| Cell Chemistry | Capacity C [Ah] | Dimensions of One Cell [mm] | Nominal Voltage [V] | Weight [kg] | Energy [Wh] |
|---|---|---|---|---|---|
| LFP | 100 | 150 × 180 × 70 | 3.3 | 3 | 330 |
| NCA | 91 (26p) | 18 × 65 | 3.65 | 1.3 | 332 |
| Cell Chemistry | Capacity @ 1C [Ah] | Series Connections | Nominal Total | Energy [kWh] | Total Cell Only Weight [kg] |
|---|---|---|---|---|---|
| LFP | 100 | 24 | 79.2 | 7.92 | 72 |
| NCA | 91 (26p) | 26 | 96.4 | 8 | 34.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Velev, B.; Djudzhev, B.; Dimitrov, V.; Hinov, N. Comparative Analysis of Lithium-Ion Batteries for Urban Electric/Hybrid Electric Vehicles. Batteries 2024, 10, 186. https://doi.org/10.3390/batteries10060186
Velev B, Djudzhev B, Dimitrov V, Hinov N. Comparative Analysis of Lithium-Ion Batteries for Urban Electric/Hybrid Electric Vehicles. Batteries. 2024; 10(6):186. https://doi.org/10.3390/batteries10060186
Chicago/Turabian StyleVelev, Boris, Bozhidar Djudzhev, Vladimir Dimitrov, and Nikolay Hinov. 2024. "Comparative Analysis of Lithium-Ion Batteries for Urban Electric/Hybrid Electric Vehicles" Batteries 10, no. 6: 186. https://doi.org/10.3390/batteries10060186
APA StyleVelev, B., Djudzhev, B., Dimitrov, V., & Hinov, N. (2024). Comparative Analysis of Lithium-Ion Batteries for Urban Electric/Hybrid Electric Vehicles. Batteries, 10(6), 186. https://doi.org/10.3390/batteries10060186







