High-Performance Supercapacitors Based on Graphene/Activated Carbon Hybrid Electrodes Prepared via Dry Processing
Abstract
1. Introduction
2. Materials and Methods
3. Results
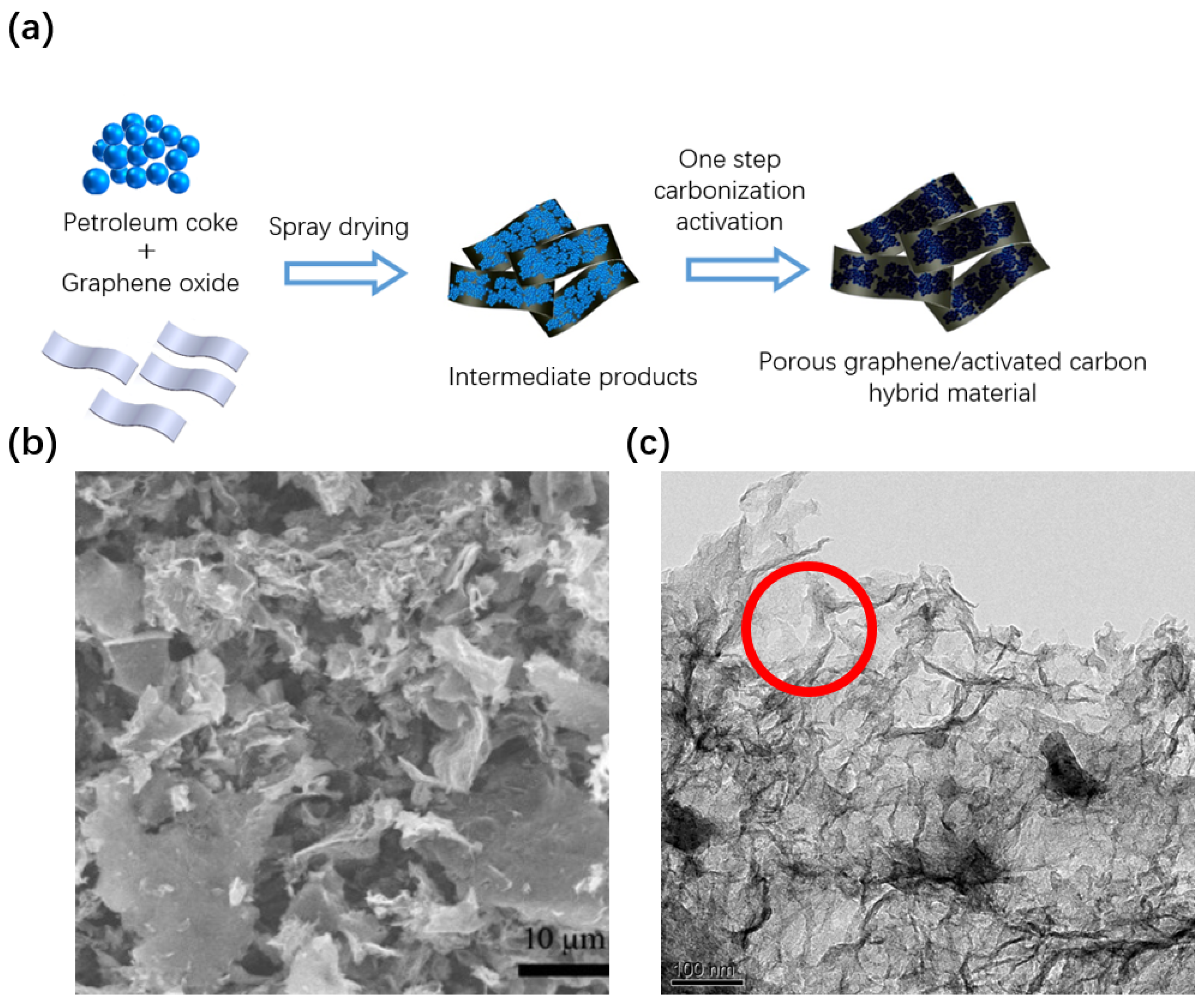
3.1. Preparation of Graphene/Activated Carbon Hybrid Electrode
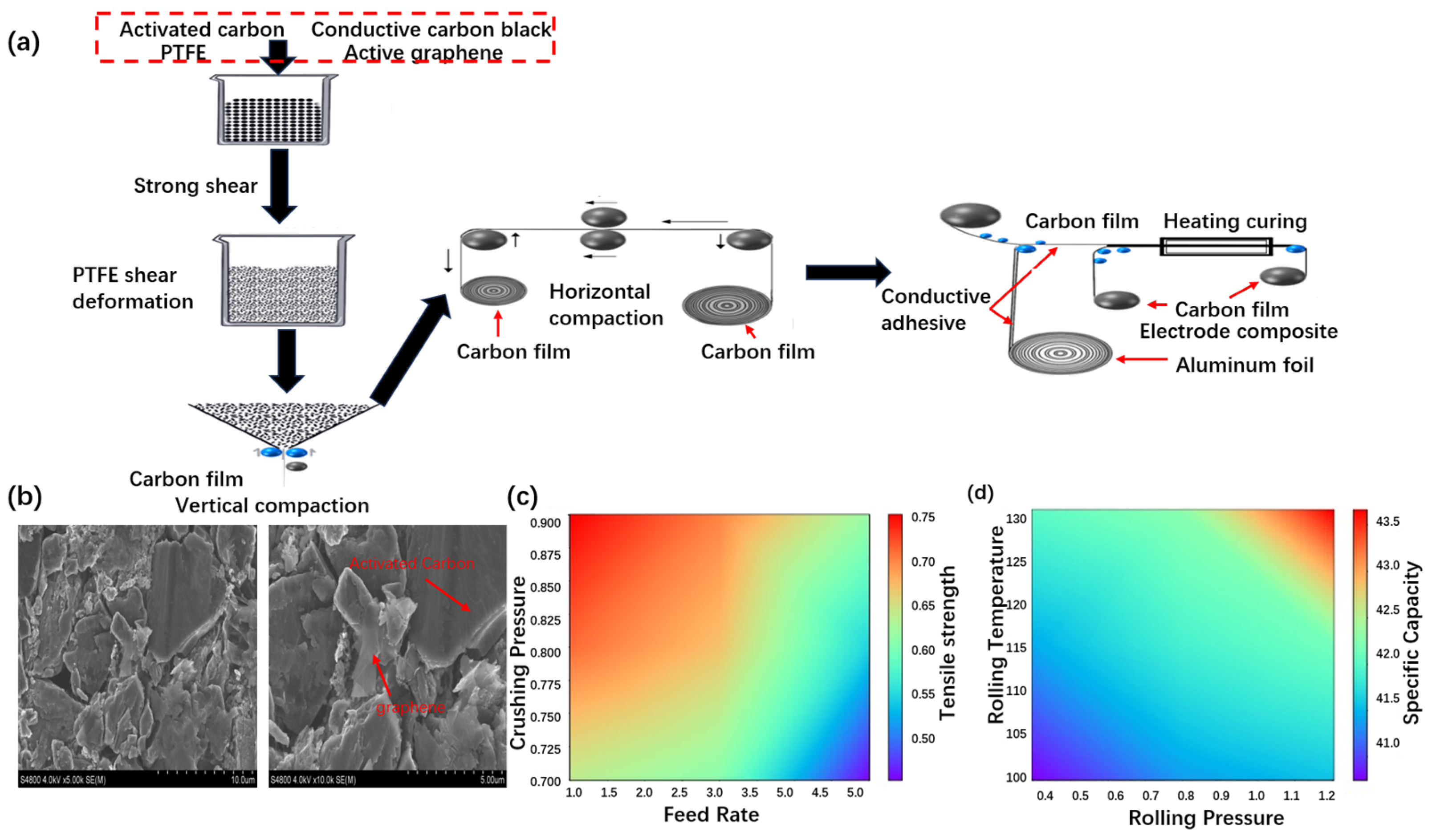
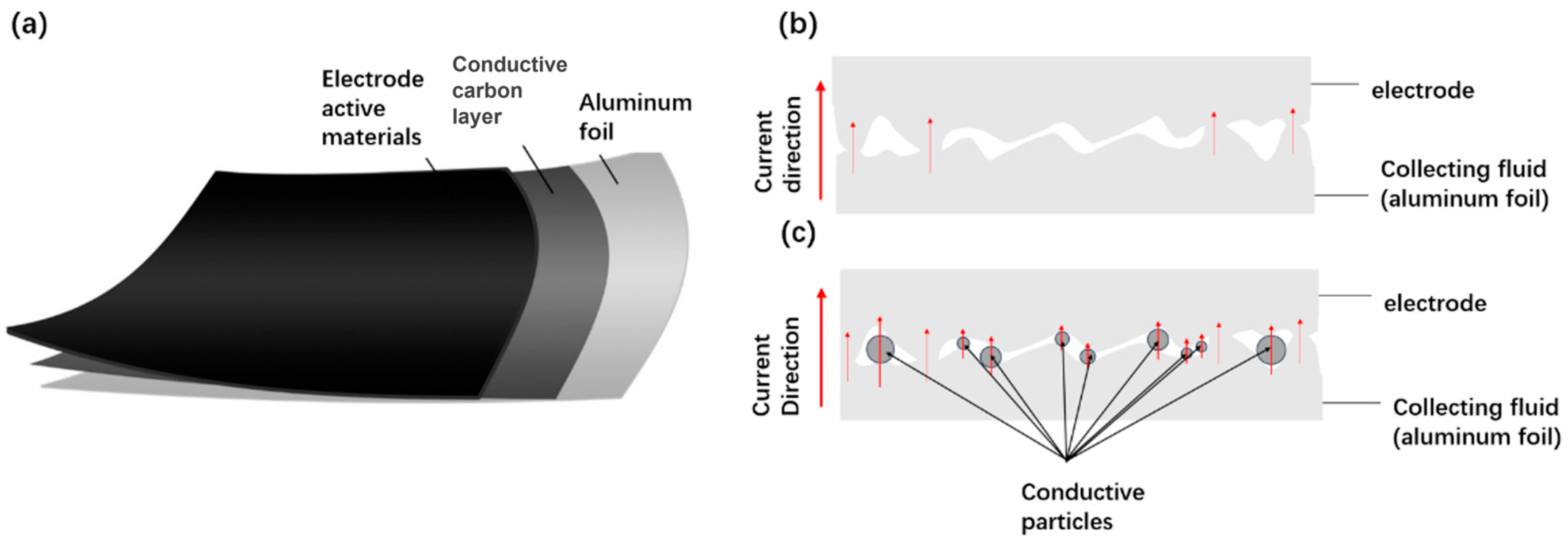
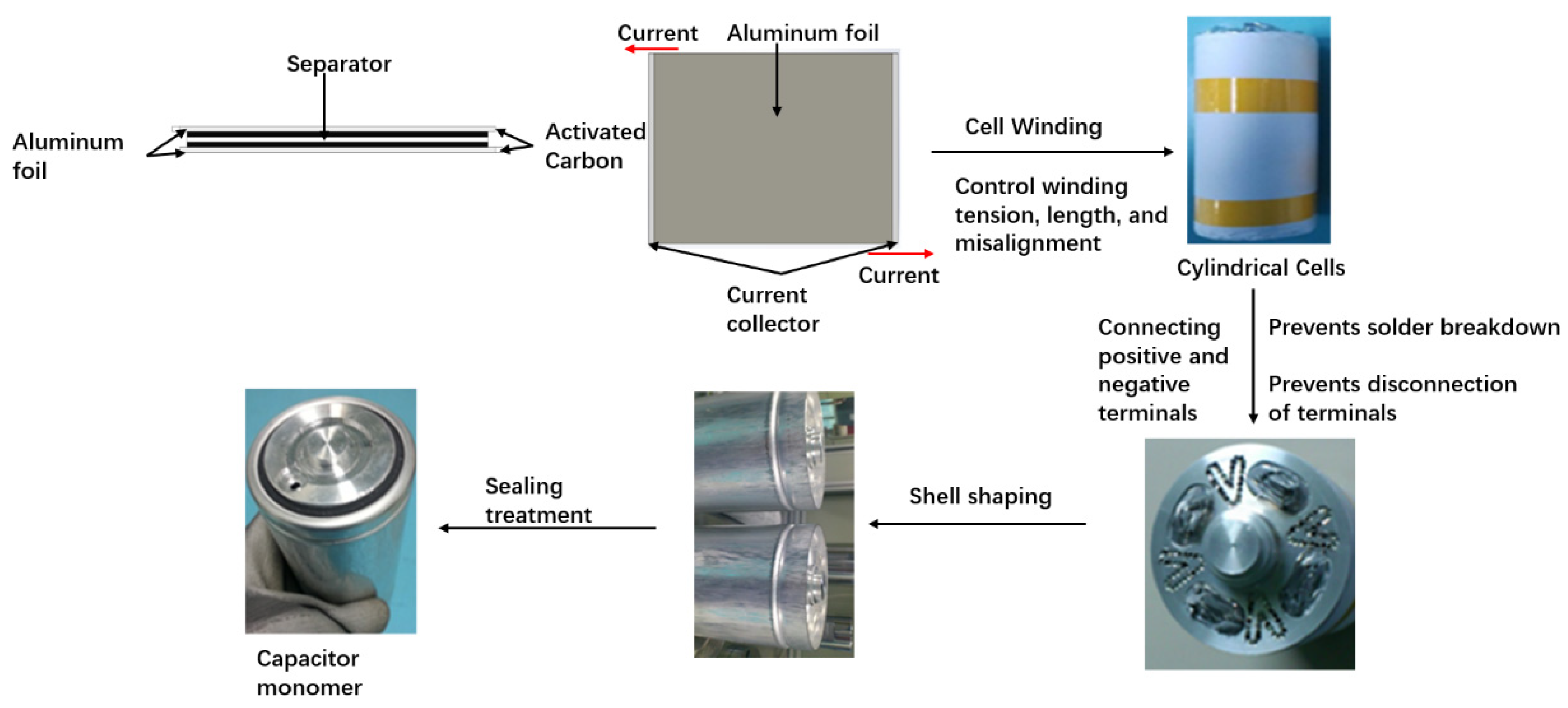
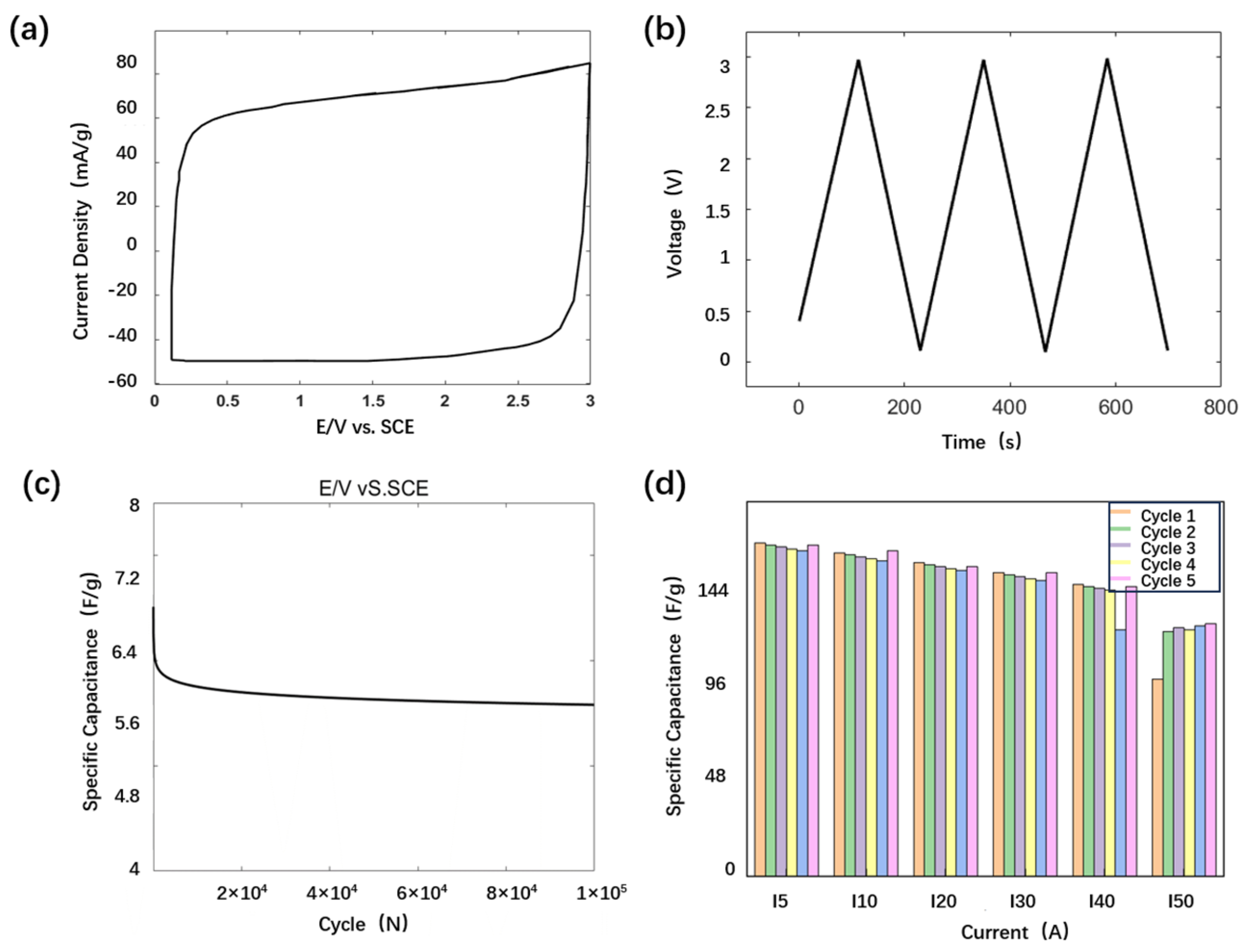
3.2. Fabrication of Collector-Optimized Supercapacitor
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Arunkumar, C.R.; Manthati, U.B.; Punna, S. Supercapacitor-based transient power supply for DC microgrid applications. Electr. Eng. 2022, 104, 463–472. [Google Scholar] [CrossRef]
- Fernando, J.; Kularatna, N.; Silva, S.; Thotabaddadurage, S.S. Supercapacitor assisted surge absorber technique: High performance transient surge protectors for consumer electronics. IEEE Power Electron. Mag. 2022, 9, 48–60. [Google Scholar] [CrossRef]
- Luo, L.; Lan, Y.; Zhang, Q.; Deng, J.; Luo, L.; Zeng, Q.; Gao, H.; Zhao, W. A review on biomass-derived activated carbon as electrode materials for energy storage supercapacitors. J. Energy Storage 2022, 55, 105839. [Google Scholar] [CrossRef]
- Jiang, Y.; Li, J.; Jiang, Z.; Shi, M.; Sheng, R.; Liu, Z.; Zhang, S.; Cao, Y.; Wei, T.; Fan, Z. Large-surface-area activated carbon with high density by electrostatic densification for supercapacitor electrodes. Carbon 2021, 175, 281–288. [Google Scholar] [CrossRef]
- Nithya, V.D. A review on holey graphene electrode for supercapacitor. J. Energy Storage 2021, 44, 103380. [Google Scholar] [CrossRef]
- Yang, Z.; Tian, J.; Ye, Z.; Jin, Y.; Cui, C.; Xie, Q.; Wang, J.; Zhang, G.; Dong, Z.; Miao, Y.; et al. High energy and high power density supercapacitor with 3D Al foam-based thick graphene electrode: Fabrication and simulation. Energy Storage Mater. 2020, 33, 18–25. [Google Scholar] [CrossRef]
- Yan, Z.; Gao, Z.; Zhang, Z.; Dai, C.; Wei, W.; Shen, P.K. Graphene nanosphere as advanced electrode material to promote high performance symmetrical supercapacitor. Small 2021, 17, 2007915. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Q.; Liu, D.; Liu, B.; Zhou, T.; Zhou, J. Blotting paper-derived activated porous carbon/reduced graphene oxide composite electrodes for supercapacitor applications. Molecules 2019, 24, 4625. [Google Scholar] [CrossRef]
- Thalji, M.R.; Ali, G.A.; Liu, P.; Zhong, Y.L.; Chong, K.F. W18O49 nanowires-graphene nanocomposite for asymmetric supercapacitors employing AlCl3 aqueous electrolyte. Chem. Eng. J. 2021, 409, 128216. [Google Scholar] [CrossRef]
- Wang, J.; Li, Q.; Peng, C.; Shu, N.; Niu, L.; Zhu, Y. To increase electrochemical performance of electrode material by attaching activated carbon particles on reduced graphene oxide sheets for supercapacitor. J. Power Sources 2020, 450, 227611. [Google Scholar] [CrossRef]
- Iakunkov, A.; Skrypnychuk, V.; Nordenström, A.; Shilayeva, E.A.; Korobov, M.; Prodana, M.; Enachescu, M.; Larsson, S.H.; Talyzin, A.V. Activated graphene as a material for supercapacitor electrodes: Effects of surface area, pore size distribution and hydrophilicity. Phys. Chem. Chem. Phys. 2019, 21, 17901–17912. [Google Scholar] [CrossRef] [PubMed]
- Ramadoss, A.; Kim, S.J. Improved activity of a graphene–TiO2 hybrid electrode in an electrochemical supercapacitor. Carbon 2013, 63, 434–445. [Google Scholar] [CrossRef]
- Costentin, C.; Savéant, J.M. Energy storage: Pseudocapacitance in prospect. Chem. Sci. 2019, 10, 5656–5666. [Google Scholar] [CrossRef] [PubMed]
- George, J.; Balachandran, M. Extrinsic pseudocapacitance: Tapering the borderline between pseudocapacitive and battery type electrode materials for energy storage applications. J. Energy Storage 2023, 74, 109292. [Google Scholar] [CrossRef]
- Aderyani, S.; Flouda, P.; Shah, S.A.; Green, M.J.; Lutkenhaus, J.L.; Ardebili, H. Simulation of cyclic voltammetry in structural supercapacitors with pseudocapacitance behavior. Electrochim. Acta 2021, 390, 138822. [Google Scholar] [CrossRef]
- Park, H.W.; Roh, K.C. Recent advances in and perspectives on pseudocapacitive materials for supercapacitors—A review. J. Power Sources 2023, 557, 232558. [Google Scholar] [CrossRef]
- Hu, Y.R.; Dong, X.L.; Zhuang, H.K.; Yan, D.; Hou, L.; Li, W.C. Introducing Electrochemically Active Oxygen Species to Boost the Pseudocapacitance of Carbon-based Supercapacitor. ChemElectroChem 2021, 8, 3073–3079. [Google Scholar] [CrossRef]
- Bokhari, S.W.; Siddique, A.H.; Sherrell, P.C.; Yue, X.; Karumbaiah, K.M.; Wei, S.; Ellis, A.V.; Gao, W. Advances in graphene-based supercapacitor electrodes. Energy Rep. 2020, 6, 2768–2784. [Google Scholar] [CrossRef]
- Down, M.P.; Rowley-Neale, S.J.; Smith, G.C.; Banks, C.E. Fabrication of graphene oxide supercapacitor devices. ACS Appl. Energy Mater. 2018, 1, 707–714. [Google Scholar] [CrossRef]
- Zhang, S.; Pan, N. Supercapacitors performance evaluation. Adv. Energy Mater. 2015, 5, 1401401. [Google Scholar] [CrossRef]
- Ke, Q.; Wang, J. Graphene-based materials for supercapacitor electrodes—A review. J. Mater. 2016, 2, 37–54. [Google Scholar] [CrossRef]
- Li, Z.J.; Yang, B.C.; Zhang, S.R.; Zhao, C.M. Graphene oxide with improved electrical conductivity for supercapacitor electrodes. Appl. Surf. Sci. 2012, 258, 3726–3731. [Google Scholar] [CrossRef]
- Zhang, H.; Yang, D.; Lau, A.; Ma, T.; Lin, H.; Jia, B. Hybridized graphene for supercapacitors: Beyond the limitation of pure graphene. Small 2021, 17, 2007311. [Google Scholar] [CrossRef] [PubMed]
- Zequine, C.; Bhoyate, S.; de Souza, F.; Arukula, R.; Kahol, P.K.; Gupta, R.K. Recent advancements and key challenges of graphene for flexible supercapacitors. In Adapting 2D Nanomaterials for Advanced Applications; American Chemical Society: Washington, DC, USA, 2020; pp. 49–77. [Google Scholar]
- Pameté, E.; Köps, L.; Kreth, F.A.; Pohlmann, S.; Varzi, A.; Brousse, T.; Balducci, A.; Presser, V. The many deaths of supercapacitors: Degradation, aging, and performance fading. Adv. Energy Mater. 2023, 13, 2301008. [Google Scholar] [CrossRef]
- Kurzweil, P.; Schottenbauer, J.; Schell, C. Past, present and future of electrochemical capacitors: Pseudocapacitance, aging mechanisms and service life estimation. J. Energy Storage 2021, 35, 102311. [Google Scholar] [CrossRef]
- Pegel, H.; Wycisk, D.; Scheible, A.; Tendera, L.; Latz, A.; Sauer, D.U. Fast-charging performance and optimal thermal management of large-format full-tab cylindrical lithium-ion cells under varying environmental conditions. J. Power Sources 2023, 556, 232408. [Google Scholar] [CrossRef]
- Zhang, J.; Zhao, Y.; Zang, X.; Wang, B.; Ma, F. Application of three-dimensional porous graphene in high-magnification supercapacitors. Energy Eng. 2020, 58–64. [Google Scholar] [CrossRef]
- Balducci, A.; Dugas, R.; Taberna, P.L.; Simon, P.; Plée, D.; Mastragostino, M.; Passerini, S. High temperature carbon-carbon supercapacitor using ionic liquid as electrolyte. J. Power Sources 2007, 165, 922–927. [Google Scholar] [CrossRef]
- Liu, F.; Xue, L. Effect of moulding process on the performance of activated carbon electrodes for supercapacitors. Electron. Compon. Mater. 2017, 36, 25–28. [Google Scholar]
- Dai, X. Research and Application of Modified Aluminum Foil Current Collector and Interface Optimization by Electric Spark Method in Supercapacitors. Master’s Thesis, Jilin University, Changchun, China, 2020. [Google Scholar]
- Zhang, D.; Wu, Y.; Li, T.; Huang, Y.; Zhang, A.; Miao, M. High performance carbon nanotube yarn supercapacitors with a surface-oxidized copper current collector. ACS Appl. Mater. Interfaces 2015, 7, 25835–25842. [Google Scholar] [CrossRef]
- Chae, C.; Han, J.H.; Lee, S.S.; Choi, Y.; Kim, T.H.; Jeong, S. A printable metallic current collector for all-printed high-voltage micro-supercapacitors: Instantaneous surface passivation by flash-light-sintering reaction. Adv. Funct. Mater. 2020, 30, 2000715. [Google Scholar] [CrossRef]
- An, G.H.; Cha, S.N.; Ahn, H.J. Surface functionalization of the terraced surface-based current collector for a supercapacitor with an improved energy storage performance. Appl. Surf. Sci. 2019, 478, 435–440. [Google Scholar] [CrossRef]
- Yu, J.; Yu, C.; Guo, W.; Wang, Z.; Ding, Y.; Xie, Y.; Liu, K.; Wang, H.; Tan, X.; Huang, H.; et al. Insight into the effects of current collectors and in situ Ni leaching in high-voltage aqueous supercapacitors. Adv. Funct. Mater. 2022, 32, 2204609. [Google Scholar] [CrossRef]
- Ruan, D. Dynamic Double-Layer Capacitors—Principles, Manufacturing, and Applications; Science Press: Beijing, China, 2018. [Google Scholar]

| Number | Weight (g) | Capacity (F) | Specific Energy (Wh/Kg) | Internal Resistance (mΩ) | Power Density (kW/kg) | Capacitance Retention (%) |
|---|---|---|---|---|---|---|
| 1# | 543.5 | 2960 | 6.81 | 0.066 | 62.72 | 81.5% |
| 2# | 534.5 | 2902 | 6.79 | 0.057 | 73.85 | 80.3% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, S.; Wang, W.; Zhang, X.; Wang, X. High-Performance Supercapacitors Based on Graphene/Activated Carbon Hybrid Electrodes Prepared via Dry Processing. Batteries 2024, 10, 195. https://doi.org/10.3390/batteries10060195
Chen S, Wang W, Zhang X, Wang X. High-Performance Supercapacitors Based on Graphene/Activated Carbon Hybrid Electrodes Prepared via Dry Processing. Batteries. 2024; 10(6):195. https://doi.org/10.3390/batteries10060195
Chicago/Turabian StyleChen, Shengjun, Wenrui Wang, Xinyue Zhang, and Xiaofeng Wang. 2024. "High-Performance Supercapacitors Based on Graphene/Activated Carbon Hybrid Electrodes Prepared via Dry Processing" Batteries 10, no. 6: 195. https://doi.org/10.3390/batteries10060195
APA StyleChen, S., Wang, W., Zhang, X., & Wang, X. (2024). High-Performance Supercapacitors Based on Graphene/Activated Carbon Hybrid Electrodes Prepared via Dry Processing. Batteries, 10(6), 195. https://doi.org/10.3390/batteries10060195






