Transition Metal-Based Polyoxometalates for Oxygen Electrode Bifunctional Electrocatalysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis and Characterization of Five POMs Electrocatalysts
2.2. Electrocatalysis with Transition Metal POMs
3. Results
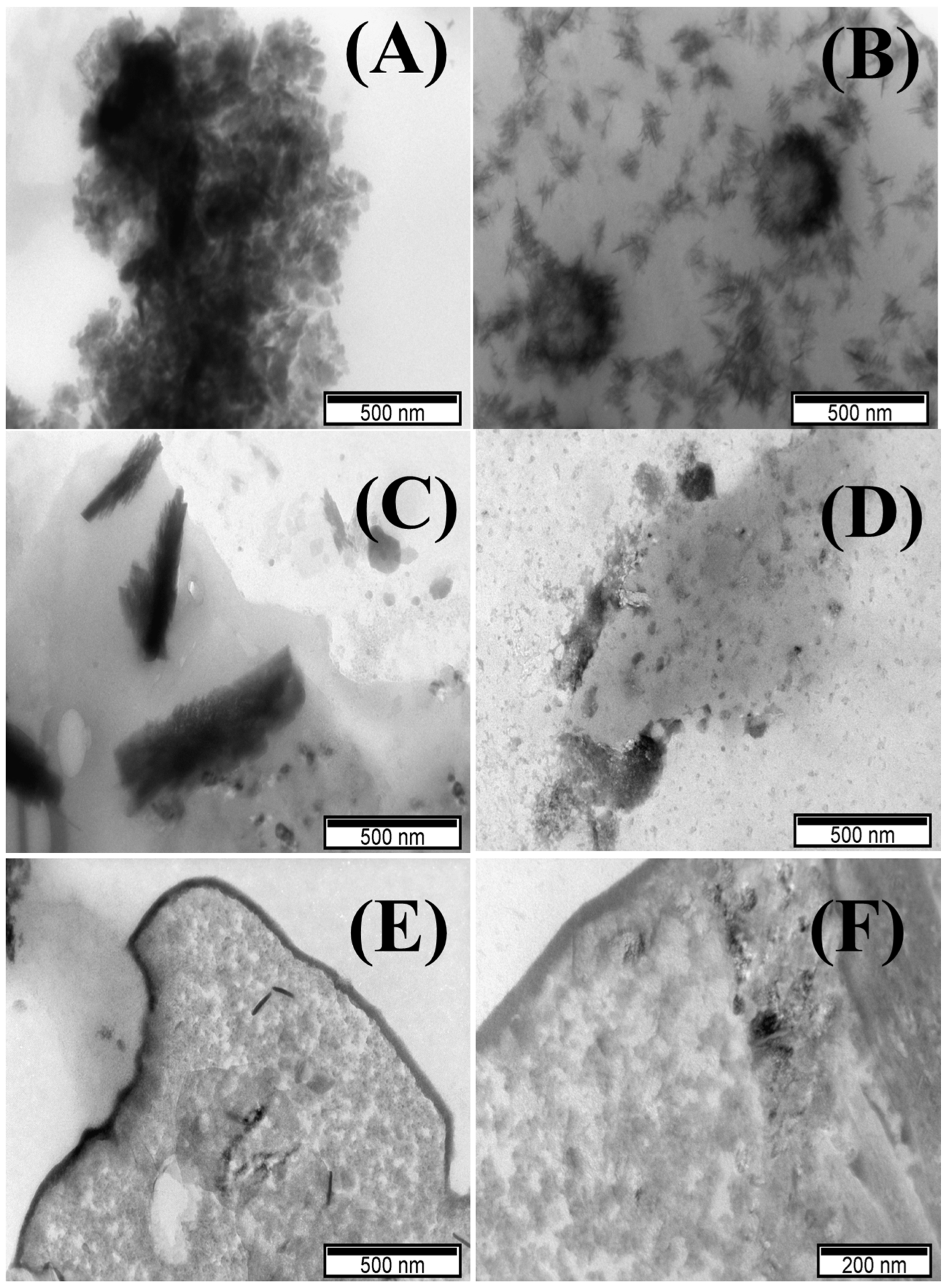
3.1. Characterization of TM-POMs
3.2. Catalysis of OER Using TM-POMs
3.3. Catalysis of ORR Using TM-POMs
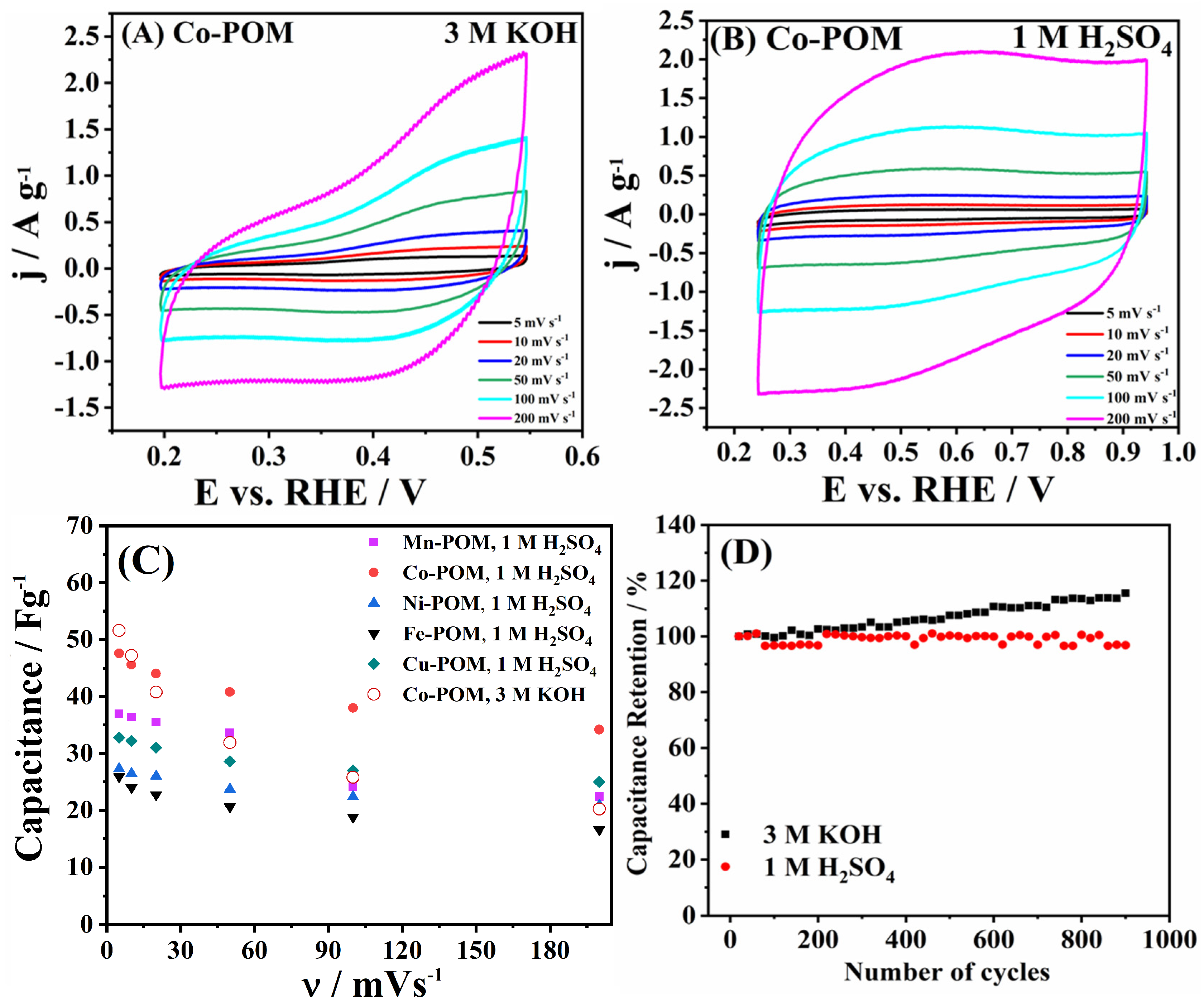
3.4. Capacitance and Impedance Study
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ling, W.; Wang, H.; Chen, Z.; Ji, Z.; Wang, J.; Wei, J.; Huang, Y. Intrinsic Structure Modification of Electrode Materials for Aqueous Metal-Ion and Metal-Air Batteries. Adv. Funct. Mater. 2021, 31, 2006855. [Google Scholar] [CrossRef]
- Liu, Q.; Pan, Z.; Wang, E.; An, L.; Sun, G. Aqueous Metal-Air Batteries: Fundamentals and Applications. Energy Storage Mater. 2020, 27, 478–505. [Google Scholar] [CrossRef]
- Zhang, J.; Yang, H.; Liu, B. Coordination Engineering of Single-Atom Catalysts for the Oxygen Reduction Reaction: A Review. Adv. Energy Mater. 2021, 11, 2002473. [Google Scholar] [CrossRef]
- Zhou, W.; Su, H.; Cheng, W.; Li, Y.; Jiang, J.; Liu, M.; Yu, F.; Wang, W.; Wei, S.; Liu, Q. Regulating the Scaling Relationship for High Catalytic Kinetics and Selectivity of the Oxygen Reduction Reaction. Nat. Commun. 2022, 13, 6414. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.; Du, L.; Yan, L.; Park, S.; Qiu, Y.; Sokolowski, J.; Wang, W.; Shao, Y. Oxygen Evolution Reaction in Alkaline Environment: Material Challenges and Solutions. Adv. Funct. Mater. 2022, 32, 2110036. [Google Scholar] [CrossRef]
- Chen, F.Y.; Wu, Z.Y.; Adler, Z.; Wang, H. Stability Challenges of Electrocatalytic Oxygen Evolution Reaction: From Mechanistic Understanding to Reactor Design. Joule 2021, 5, 1704–1731. [Google Scholar] [CrossRef]
- Li, N.; Liu, J.; Dong, B.X.; Lan, Y.Q. Polyoxometalate-Based Compounds for Photo- and Electrocatalytic Applications. Angew. Chem.-Int. Ed. 2020, 59, 20779–20793. [Google Scholar] [CrossRef] [PubMed]
- Gusmão, F.M.B.; Mladenović, D.; Radinović, K.; Santos, D.M.F.; Šljukić, B. Polyoxometalates as Electrocatalysts for Electrochemical Energy Conversion and Storage. Energies 2022, 15, 9021. [Google Scholar] [CrossRef]
- Zheng, Y.; Xu, X.; Chen, J.; Wang, Q. Surface O2− Regulation on POM Electrocatalyst to Achieve Accurate 2e/4e-ORR Control for H2O2 Production and Zn-Air Battery Assemble. Appl. Catal. B 2021, 285, 119788. [Google Scholar] [CrossRef]
- Song, Y.; Peng, Y.; Yao, S.; Zhang, P.; Wang, Y.; Gu, J.; Lu, T.; Zhang, Z. Co-POM@MOF-Derivatives with Trace Cobalt Content for Highly Efficient Oxygen Reduction. Chin. Chem. Lett. 2022, 33, 1047–1050. [Google Scholar] [CrossRef]
- Yin, D.; Wang, M.L.; Cao, Y.D.; Yang, X.; Ji, S.Y.; Hao, H.P.; Gao, G.G.; Fan, L.L.; Liu, H. Polyoxometalate@ZIF Induced CoWO4/WS2@C-N Nanoflower as a Highly Efficient Catalyst for Zn-Air Batteries. ACS Appl. Energy Mater. 2021, 4, 6892–6902. [Google Scholar] [CrossRef]
- Nagaiah, T.C.; Gupta, D.; Das Adhikary, S.; Kafle, A.; Mandal, D. Tuning Polyoxometalate Composites with Carbonaceous Materials towards Oxygen Bifunctional Activity. J. Mater. Chem. A Mater. 2021, 9, 9228–9237. [Google Scholar] [CrossRef]
- Yin, X.; Zhang, Z.; Yao, K.; Xu, X.; Wang, Y. Hydrophobic POM Electrocatalyst Achieves Low Voltage “Charge” in Zn-Air Battery Coupled with Bisphenol A Degradation. Chemistry 2021, 27, 8774–8781. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Yuan, L.; Wen, T.; Yu, J.; Xu, X. Enhanced Electrocatalytic Activity of POM-Derived CoMoS/FCP Heterostructures for Overall Water Splitting in Alkaline Media. Int. J. Electrochem. Sci. 2023, 18, 100076. [Google Scholar] [CrossRef]
- Gautam, J.; Kannan, K.; Meshesha, M.M.; Dahal, B.; Subedi, S.; Ni, L.; Wei, Y.; Yang, B.L. Heterostructure of Polyoxometalate/Zinc-Iron-Oxide Nanoplates as an Outstanding Bifunctional Electrocatalyst for the Hydrogen and Oxygen Evolution Reaction. J. Colloid. Interface Sci. 2022, 618, 419–430. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.; Yang, D.H.; Han, B.H. Application of Polyoxometalate Derivatives in Rechargeable Batteries. J. Mater. Chem. A Mater. 2020, 8, 4593–4628. [Google Scholar] [CrossRef]
- Singh, G.; Das Adhikary, S.; Mandal, D. Physico- and Electrochemical Properties of First-Row Transition-Metal-Substituted Sandwich Polyoxometalates. Inorg. Chem. 2023, 62, 8551–8564. [Google Scholar] [CrossRef] [PubMed]
- Mir, S.; Yadollahi, B.; Omidyan, R. Theoretical Comparative Survey on the Structure and Electronic Properties of First Row Transition Metal Substituted Keggin Type Polyoxometalates. J. Solid. State Chem. 2022, 305, 122667. [Google Scholar] [CrossRef]
- Wang, Y.; Li, L.; Wang, S.; Dong, X.; Ding, C.; Mu, Y.; Cui, M.; Hu, T.; Meng, C.; Zhang, Y. Anion Structure Regulation of Cobalt Silicate Hydroxide Endowing Boosted Oxygen Evolution Reaction. Small 2024. [Google Scholar] [CrossRef]
- Mu, Y.; Wang, T.; Zhang, J.; Meng, C.; Zhang, Y.; Kou, Z. Single-Atom Catalysts: Advances and Challenges in Metal-Support Interactions for Enhanced Electrocatalysis. Electrochem. Energy Rev. 2022, 5, 145–186. [Google Scholar] [CrossRef]
- Clemente-Juan, J.M.; Coronado, E.; Galán-Mascarós, J.R.; Gómez-García, C.J.G. Increasing the Nuclearity of Magnetic Polyoxometalates. Syntheses, Structures, and Magnetic Properties of Salts of the Heteropoly Complexes [Ni3(H2O)3(PW10O39)H2O]7−, [Ni4(H2O)2(PW9O34)2]10−, and [Ni9(OH)3(H2O)6(HPO4)2(PW9O34)3]16−. Inorg. Chem. 1999, 38, 55–63. [Google Scholar] [CrossRef]
- Liu, Q.; Wang, X. Polyoxometalate Clusters: Sub-Nanometer Building Blocks for Construction of Advanced Materials. Matter 2020, 2, 816–841. [Google Scholar] [CrossRef]
- Lafuente, M.; Pellejero, I.; Clemente, A.; Urbiztondo, M.A.; Mallada, R.; Reinoso, S.; Pina, M.P.; Gandía, L.M. In Situ Synthesis of SERS-Active Au@POM Nanostructures in a Microfluidic Device for Real-Time Detection of Water Pollutants. ACS Appl. Mater. Interfaces 2020, 12, 36458–36467. [Google Scholar] [CrossRef] [PubMed]
- Yokuş, Ö.A.; Kardaş, F.; Akyildirim, O.; Eren, T.; Atar, N.; Yola, M.L. Sensitive Voltammetric Sensor Based on Polyoxometalate/Reduced Graphene Oxide Nanomaterial: Application to the Simultaneous Determination of l-Tyrosine and l-Tryptophan. Sens. Actuators B Chem. 2016, 233, 47–54. [Google Scholar] [CrossRef]
- Rajkumar, T.; Rao, G.R. Investigation of Hybrid Molecular Material Prepared by Ionic Liquid and Polyoxometalate Anion. J. Chem. Sci. 2008, 120, 587–594. [Google Scholar] [CrossRef]
- Karimi, Z.; Mahjoub, A.R.; Harati, S.M. Polyoxometalate-Based Hybrid Mesostructured Catalysts for Green Epoxidation of Olefins. Inorg. Chim. Acta 2011, 376, 1–9. [Google Scholar] [CrossRef]
- Cuentas-Gallegos, A.K.; Martínez-Rosales, R.; Rincón, M.E.; Hirata, G.A.; Orozco, G. Design of Hybrid Materials Based on Carbon Nanotubes and Polyoxometalates. Opt. Mater. 2006, 29, 126–133. [Google Scholar] [CrossRef]
- Wu, C.; Pei, Z.; Lv, M.; Huang, D.; Wang, Y.; Yuan, S. Polypyrrole-Coated Low-Crystallinity Iron Oxide Grown on Carbon Cloth Enabling Enhanced Electrochemical Supercapacitor Performance. Molecules 2023, 28, 434. [Google Scholar] [CrossRef] [PubMed]
- Tarlani, A.; Abedini, M.; Nemati, A.; Khabaz, M.; Amini, M.M. Immobilization of Keggin and Preyssler Tungsten Heteropolyacids on Various Functionalized Silica. J. Colloid. Interface Sci. 2006, 303, 32–38. [Google Scholar] [CrossRef]
- Singh, G.; Adhikary, S.D.; Mandal, D. Stabilization and Activation of Polyoxometalate over Poly(Vinyl Butylimidazolium) Cations towards Electrocatalytic Water Oxidation in Alkaline Media. Chem. Commun. 2023, 59, 4774–4777. [Google Scholar] [CrossRef]
- Zhao, Y.; Gao, D.; Biskupek, J.; Kaiser, U.; Liu, R.; Streb, C. Polyoxometalate-Assisted Synthesis of Amorphous Zeolitic Imidazolate for Efficient Electrocatalytic Oxygen Evolution. Results Chem. 2022, 4, 100568. [Google Scholar] [CrossRef]
- Shang, W.; Wang, Y.; Jiang, Y.; Wu, M.; Zeng, M.; Wang, P.; Qiu, L.; Jia, Z. Nanocomposite: Co4-Substituted Polyoxometalate@β-FeOOH as High-Performance Electrocatalysts for Oxygen Evolution Reaction in Alkaline Conditions. Appl. Catal. A Gen. 2022, 644, 118810. [Google Scholar] [CrossRef]
- Kang, Q.; Lai, D.; Su, M.; Xiong, B.; Tang, W.; Lu, Q.; Gao, F. Tailored Dodecahedral Polyoxometalates Nanoframes with in Situ Encapsulated Co, N, C for Oxygen Evolution Reaction. Chem. Eng. J. 2022, 430, 133116. [Google Scholar] [CrossRef]
- Marques, I.S.; Jarrais, B.; Mbomekallé, S.M.; Teillout, A.L.; De Oliveira, P.; Freire, C.; Fernandes, D.M. Synergetic Effects of Mixed-Metal Polyoxometalates@Carbon-Based Composites as Electrocatalysts for the Oxygen Reduction and the Oxygen Evolution Reactions. Catalysts 2022, 12, 440. [Google Scholar] [CrossRef]
- Mladenović, D.; Daş, E.; Santos, D.M.F.; Yurtcan, A.B.; Miljanić, Š.; Šljukić, B. Boosting Oxygen Electrode Kinetics by Addition of Cost-Effective Transition Metals (Ni, Fe, Cu) to Platinum on Graphene Nanoplatelets. J. Alloys Compd. 2022, 905, 164156. [Google Scholar] [CrossRef]
- Milikić, J.; Stojanović, S.; Damjanović-Vasilić, L.; Vasilić, R.; Šljukić, B. Efficient Bifunctional Cerium-Zeolite Electrocatalysts for Oxygen Evolution and Oxygen Reduction Reactions in Alkaline Media. Synth. Met. 2023, 292, 117231. [Google Scholar] [CrossRef]
- Milikić, J.; Knežević, S.; Stojadinović, S.; Alsaiari, M.; Harraz, F.A.; Santos, D.M.F.; Šljukić, B. Facile Synthesis of Low-Cost Copper-Silver and Cobalt-Silver Alloy Nanoparticles on Reduced Graphene Oxide as Efficient Electrocatalysts for Oxygen Reduction Reaction in Alkaline Media. Nanomaterials 2022, 12, 2657. [Google Scholar] [CrossRef] [PubMed]
- Milikić, J.; Knežević, S.; Ognjanović, M.; Stanković, D.; Rakočević, L.; Šljukić, B. Template-Based Synthesis of Co3O4 and Co3O4/SnO2 Bifunctional Catalysts with Enhanced Electrocatalytic Properties for Reversible Oxygen Evolution and Reduction Reaction. Int. J. Hydrogen Energy 2023, 48, 27568–27581. [Google Scholar] [CrossRef]
- Fernandes, D.M.; Novais, H.C.; Bacsa, R.; Serp, P.; Bachiller-Baeza, B.; Rodríguez-Ramos, I.; Guerrero-Ruiz, A.; Freire, C. Polyoxotungstate@Carbon Nanocomposites As Oxygen Reduction Reaction (ORR) Electrocatalysts. Langmuir 2018, 34, 6376–6387. [Google Scholar] [CrossRef]
- Liu, R.; Cao, K.; Clark, A.H.; Lu, P.; Anjass, M.; Biskupek, J.; Kaiser, U.; Zhang, G.; Streb, C. Top-down Synthesis of Polyoxometalate-like Sub-Nanometer Molybdenum-Oxo Clusters as High-Performance Electrocatalysts. Chem. Sci. 2020, 11, 1043–1051. [Google Scholar] [CrossRef]
- Ensafi, A.A.; Heydari-Soureshjani, E.; Rezaei, B. [PW11MO39]5− Decorated on Ru-Reduced Graphene Oxide Nanosheets, Characterizations and Application as a High Performance Storage Energy and Oxygen Reduction Reaction. Chem. Eng. J. 2017, 330, 1109–1118. [Google Scholar] [CrossRef]
- Gezović, A.; Mišurović, J.; Milovanović, B.; Etinski, M.; Krstić, J.; Grudić, V.; Dominko, R.; Mentus, S.; Vujković, M.J. High Al-Ion Storage of Vine Shoots-Derived Activated Carbon: New Concept for Affordable and Sustainable Supercapacitors. J. Power Sources 2022, 538, 231561. [Google Scholar] [CrossRef]
- Gandara, M.; Mladenović, D.; Oliveira Martins, M.d.J.; Rakocevic, L.; Kruszynski de Assis, J.M.; Šljukić, B.; Sarmento Gonçalves, E. MAX Phase (Nb4AlC3) For Electrocatalysis Applications. Small 2024. [Google Scholar] [CrossRef] [PubMed]
- Milikić, J.; Nastasić, A.; Rakočević, L.; Radinović, K.; Stojadinović, S.; Stanković, D.; Šljukić, B. FeM/RGO (M = Ni and Cu) as Bifunctional Oxygen Electrode. Fuel 2024, 368, 131654. [Google Scholar] [CrossRef]




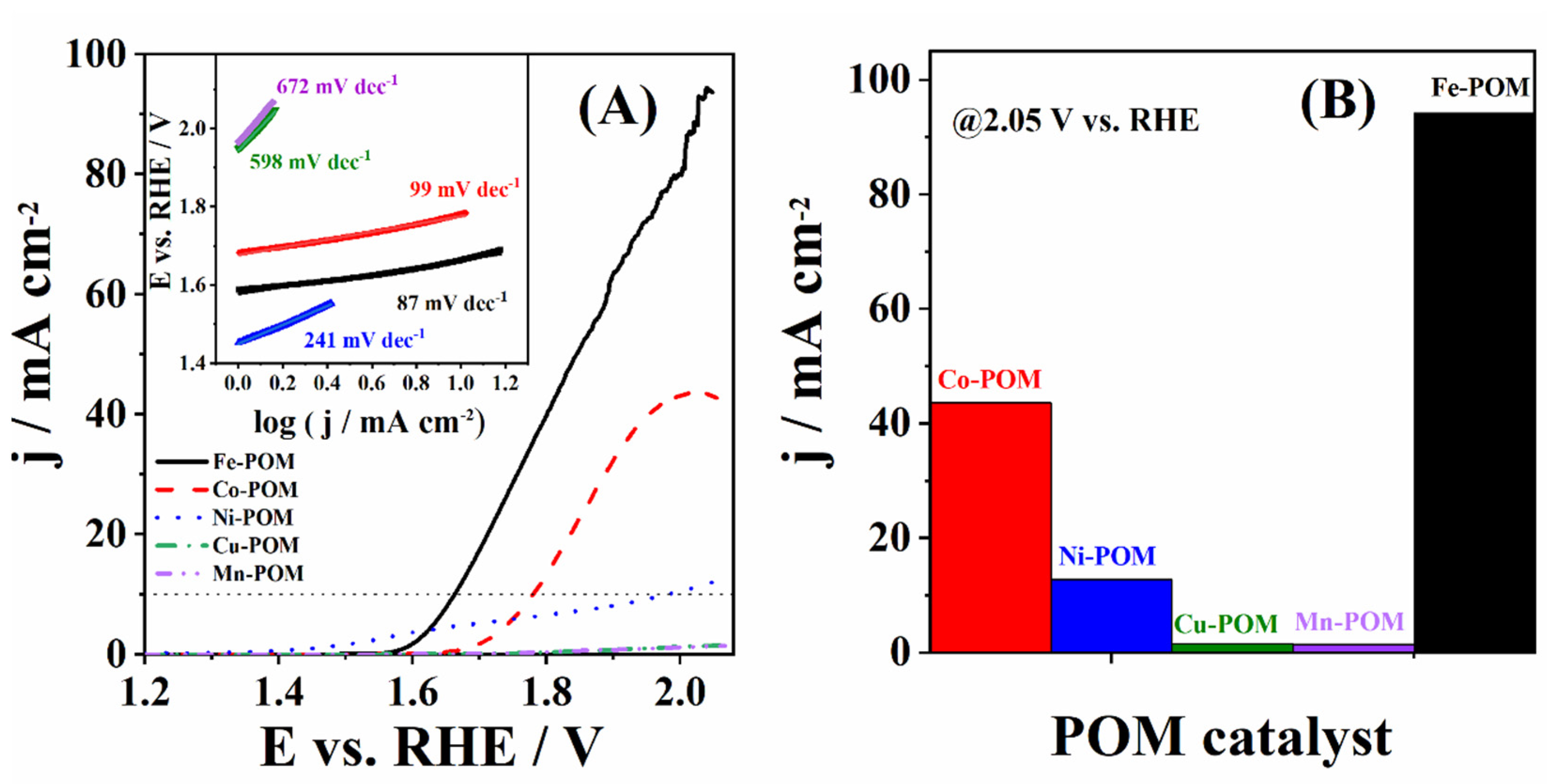


| Catalyst | Eonset/V | η10/mV | j400/mA cm−2 | b/mV dec−1 | Source |
|---|---|---|---|---|---|
| Fe-POM | 1.588 | 434 | 4.78 | 87 | This work |
| Co-POM | 1.683 | 551 | 0.14 | 99 | This work |
| Ni-POM | 1.453 | 752 | 4.13 | 241 | This work |
| Cu-POM | 1.948 | – | 0.04 | 598 | This work |
| Mn-POM | 1.966 | – | 0.03 | 672 | This work |
| ([PVIM][V-Co4]) | – | 430 | – | – | [30] |
| PNiW11@amZIF | – | 375 | – | 69 | [31] |
| PW12@amZIF | – | 423 | – | 86 | [31] |
| PW12@FeOOH-P | – | 230 | – | 79 | [32] |
| PMo12@FeOOH-P | – | 235 | – | 123 | [32] |
| SiW12@FeOOH-P | – | 260 | – | 126 | [32] |
| FeOOH-P | – | 290 | – | 202 | [32] |
| CNCP-0 | – | 310 | – | 105.8 | [33] |
| CNCP-1 | – | 293 | – | 88.2 | [33] |
| CNCP-12 | – | 269 | – | 79.5 | [33] |
| IrO2 | – | – | – | 90.2 | [33] |
| Fe4@MWCNT_N6 | – | 580 | – | 102 | [34] |
| NiP4Mo6 | – | 250 | – | 73 | [9] |
| Electrocatalysts | Eonset/V | E1/2/V | Epp/V | b/mV dec−1 | n | Source |
|---|---|---|---|---|---|---|
| Fe-POM | 0.695 | 0.580 | 0.632 | 85 | 2.8–2.9 | This work |
| Co-POM | 0.655 | 0.552 | 0.558 | 109 | 3.8–3.9 | This work |
| Ni-POM | 0.639 | 0.533 | 0.494 | 144 | 2.3 | This work |
| Cu-POM | 0.695 | 0.580 | 0.529 | 94 | 2.4–2.5 | This work |
| Mn-POM | 0.694 | 0.556 | 0.622 | 107 | 2.6–2.9 | This work |
| NiP4Mo6 | – | – | – | 106 | 3.80–3.85 | [9] |
| S-NiP4Mo6 | – | – | – | 98 | 2.08–2.15 | [9] |
| Co4(PW9)2@N-CNT | 0.90 | – | – | 89 | 2.73 | [39] |
| [Mo-oxo]n clusters | 0.75 | – | 0.62 | 109 | ~1.5 | [40] |
| Fe4@MWCNT_N6 | 0.81 | – | – | 35.4 | 2.9 | [34] |
| Ni4@MWCNT_N6 | 0.80 | – | – | 34.7 | 2.7 | [34] |
| Fe2Ni2@MWCNT_N6 | 0.80 | – | – | 37.9 | 3.2 | [34] |
| [PW11MO39]5@Ru-rGO (M = Co, Ni, Cu) | – | – | – | – | 3.9–4.2 | [41] |
| Electrocatalyst | Rs/Ω | Re/Ω | Rct/Ω | Qe/mF | Qdl/mF |
|---|---|---|---|---|---|
| Fe-POM | 5.27 | 0.15 | 3.35 | 3.20 × 10−3 | 7.40 × 10−4 |
| Co-POM | 50.49 | 1.36 | 15.04 | 3.25 × 10−4 | 1.93 × 10−2 |
| Ni-POM | 184.80 | 44.78 | 137.00 | 8.08 × 10−4 | 1.94 × 10−4 |
| Cu-POM | 31.49 | 22.08 | 67.28 | 4.72 × 10−4 | 6.57 × 10−4 |
| Mn-POM | 9.25 | 2.68 | 239.00 | 8.22 × 10−2 | 1.65 × 10−4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Milikić, J.; Gusmão, F.; Knežević, S.; Gavrilov, N.; Paul, A.; Santos, D.M.F.; Šljukić, B. Transition Metal-Based Polyoxometalates for Oxygen Electrode Bifunctional Electrocatalysis. Batteries 2024, 10, 197. https://doi.org/10.3390/batteries10060197
Milikić J, Gusmão F, Knežević S, Gavrilov N, Paul A, Santos DMF, Šljukić B. Transition Metal-Based Polyoxometalates for Oxygen Electrode Bifunctional Electrocatalysis. Batteries. 2024; 10(6):197. https://doi.org/10.3390/batteries10060197
Chicago/Turabian StyleMilikić, Jadranka, Filipe Gusmão, Sara Knežević, Nemanja Gavrilov, Anup Paul, Diogo M. F. Santos, and Biljana Šljukić. 2024. "Transition Metal-Based Polyoxometalates for Oxygen Electrode Bifunctional Electrocatalysis" Batteries 10, no. 6: 197. https://doi.org/10.3390/batteries10060197
APA StyleMilikić, J., Gusmão, F., Knežević, S., Gavrilov, N., Paul, A., Santos, D. M. F., & Šljukić, B. (2024). Transition Metal-Based Polyoxometalates for Oxygen Electrode Bifunctional Electrocatalysis. Batteries, 10(6), 197. https://doi.org/10.3390/batteries10060197









