Extraction Strategies from Black Alloy Leachate: A Comparative Study of Solvent Extractants
Abstract
:1. Introduction
2. Materials and Methods
3. Results
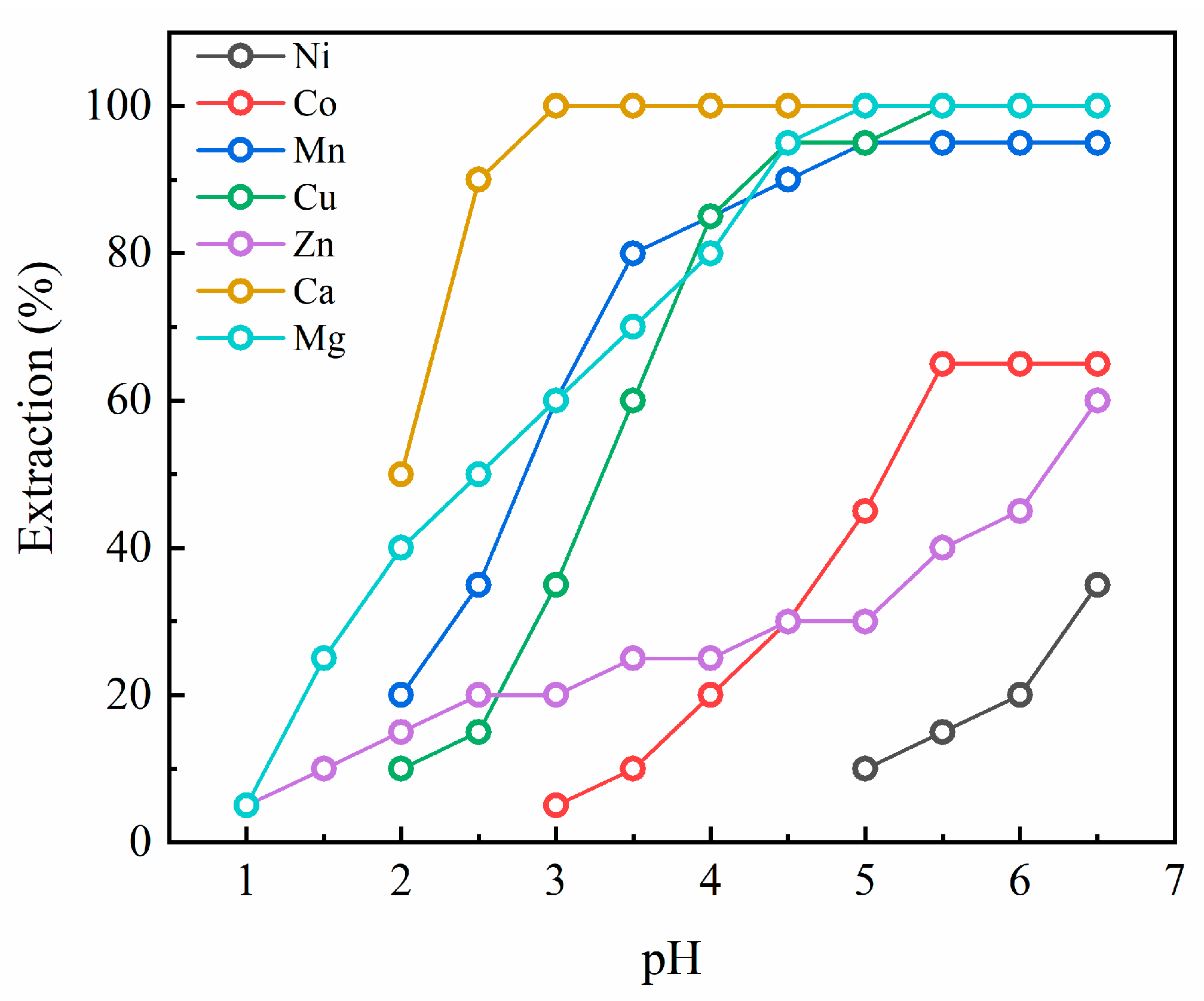
3.1. Extraction pH Isotherms for D2EHPA
3.2. Extraction pH Isotherms for Cyanex 272
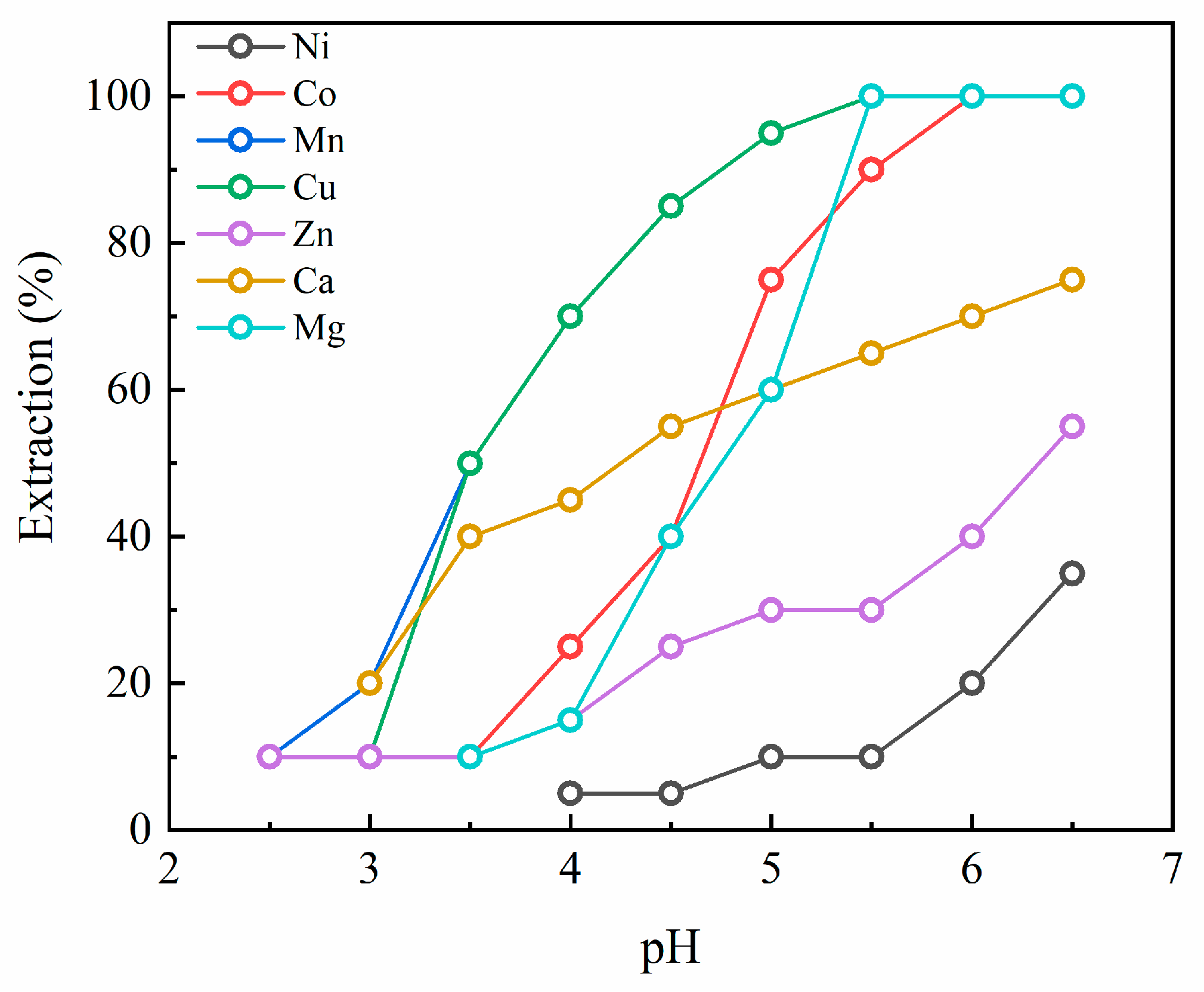
3.3. Extraction pH Isotherms for PC88A
3.4. Extraction pH Isotherms for Versatic Acid 10
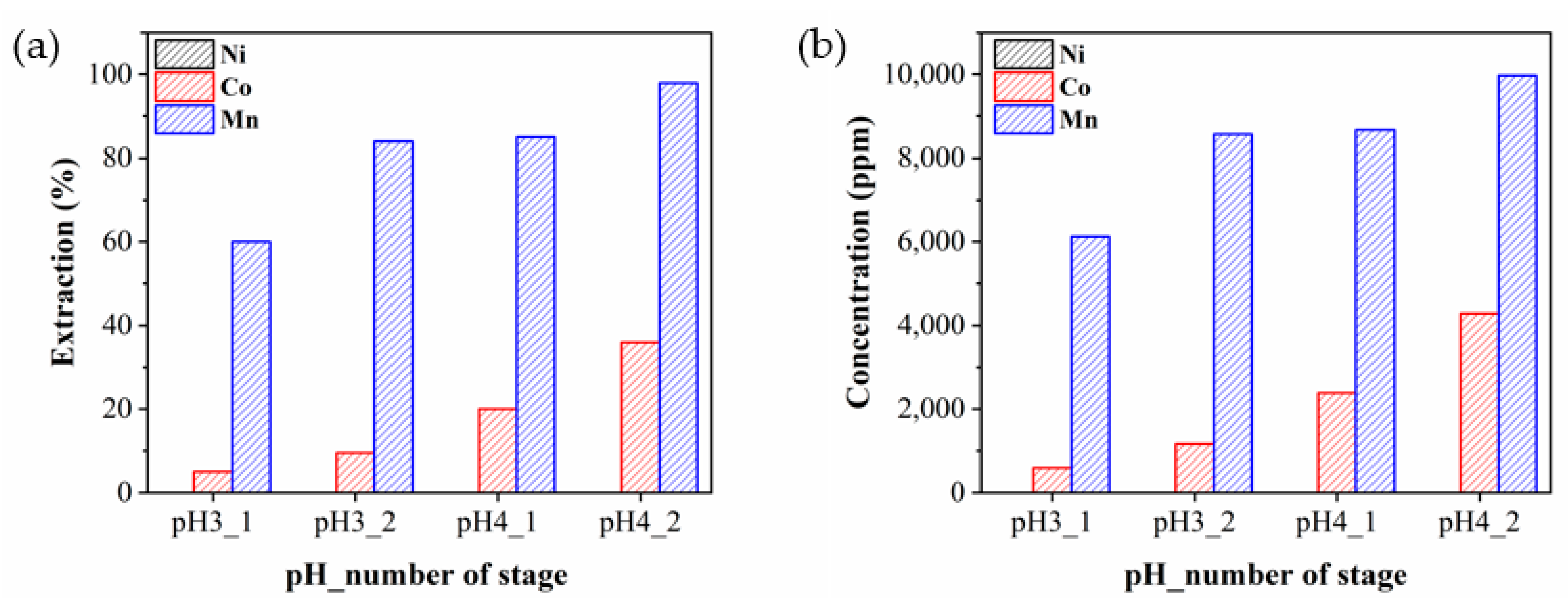
3.5. Extraction Behaviors of the Metals
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kim, H.C.; Lee, S.; Wallington, T.J. Cradle-to-Gate and Use-Phase Carbon Footprint of a Commercial Plug-in Hybrid Electric Vehicle Lithium-Ion Battery. Environ. Sci. Technol. 2023, 57, 11834–11842. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.; Li, J.; Singh, N. Recycling of Spent Lithium-Ion Battery: A Critical Review. Crit. Rev. Environ. Sci. Technol. 2014, 44, 1129–1165. [Google Scholar] [CrossRef]
- Kim, S.; Bang, J.; Yoo, J.; Shin, Y.; Bae, J.; Jeong, J.; Kim, K.; Dong, P.; Kwon, K. A comprehensive review on the pretreatment process in lithium-ion battery recycling. J. Clean. Prod. 2021, 294, 126329. [Google Scholar] [CrossRef]
- Yao, Y.; Zhu, M.; Zhao, Z.; Tong, B.; Fan, Y.; Hua, Z. Hydrometallurgical Processes for Recycling Spent Lithium-Ion Batteries: A Critical Review. ACS Sustain. Chem. Eng. 2018, 6, 13611–13627. [Google Scholar] [CrossRef]
- Jung, J.C.-Y.; Sui, P.-C.; Zhang, J. A review of recycling spent lithium-ion battery cathode materials using hydrometallurgical treatments. J. Energy Storage 2021, 35, 102217. [Google Scholar] [CrossRef]
- Al-Thyabat, S.; Nakamura, T.; Shibata, E.; Iizuka, A. Adaptation of minerals processing operations for lithium-ion (LiBs) and nickel metal hydride (NiMH) batteries recycling: Critical review. Miner. Eng. 2013, 45, 4–17. [Google Scholar] [CrossRef]
- Zhao, Y.; Yuan, X.; Jiang, L.; Wen, J.; Wang, H.; Guan, R.; Zhang, J.; Zeng, G. Regeneration and reutilization of cathode materials from spent lithium-ion batteries. Chem. Eng. J. 2020, 383, 123089. [Google Scholar] [CrossRef]
- Wang, J.; Chen, M.; Chen, H.; Luo, T.; Xu, Z. Leaching Study of Spent Li-ion Batteries. Procedia Environ. Sci. 2012, 16, 443–450. [Google Scholar] [CrossRef]
- Lee, C.K.; Rhee, K.-I. Reductive leaching of cathodic active materials from lithium ion battery wastes. Hydrometallurgy 2003, 68, 5–10. [Google Scholar] [CrossRef]
- Joulié, M.; Laucournet, R.; Billy, E. Hydrometallurgical process for the recovery of high value metals from spent lithium nickel cobalt aluminum oxide based lithium-ion batteries. J. Power Sources 2014, 247, 551–555. [Google Scholar] [CrossRef]
- Li, L.; Ge, J.; Chen, R.; Wu, F.; Chen, S.; Zhang, X. Environmental friendly leaching reagent for cobalt and lithium recovery from spent lithium-ion batteries. Waste Manag. 2010, 30, 2615–2621. [Google Scholar] [CrossRef] [PubMed]
- Vieceli, N.; Nogueira, C.A.; Guimarães, C.; Pereira, M.F.C.; Durão, F.O.; Margarido, F. Hydrometallurgical recycling of lithium-ion batteries by reductive leaching with sodium metabisulphite. Waste Manag. 2018, 71, 350–361. [Google Scholar] [CrossRef] [PubMed]
- Dunn, J.B.; Gaines, L.; Sullivan, J.; Wang, M.Q. Impact of Recycling on Cradle-to-Gate Energy Consumption and Greenhouse Gas Emissions of Automotive Lithium-Ion Batteries. Environ. Sci. Technol. 2012, 46, 12704–12710. [Google Scholar] [CrossRef] [PubMed]
- Barik, S.P.; Prabaharan, G.; Kumar, B. An innovative approach to recover the metal values from spent lithium-ion batteries. Waste Manag. 2016, 51, 222–226. [Google Scholar] [CrossRef] [PubMed]
- Brückner, L.; Frank, J.; Elwert, T. Industrial Recycling of Lithium-Ion Batteries—A Critical Review of Metallurgical Process Routes. Metals 2020, 10, 1107. [Google Scholar] [CrossRef]
- Ruismäki, R.; Rinne, T.; Dańczak, A.; Taskinen, P.; Serna-Guerrero, R.; Jokilaakso, A. Integrating Flotation and Pyrometallurgy for Recovering Graphite and Valuable Metals from Battery Scrap. Metals 2020, 10, 680. [Google Scholar] [CrossRef]
- Li, Y.; Zeng, L.; Du, J.; Zhang, G.; Cao, Z.; Wu, S. Improving Extraction Performance of D2EHPA for Impurities Removal from Spent Lithium-Ion Batteries Leaching Solution by TPC[4]. ACS Sustain. Chem. Eng. 2022, 10, 4312–4322. [Google Scholar] [CrossRef]
- Gaines, L. The future of automotive lithium-ion battery recycling: Charting a sustainable course. Sustain. Mater. Technol. 2014, 1–2, 2–7. [Google Scholar] [CrossRef]
- Makuza, B.; Tian, Q.; Guo, X.; Chattopadhyay, K.; Yu, D. Pyrometallurgical options for recycling spent lithium-ion batteries: A comprehensive review. J. Power Sources 2021, 491, 229622. [Google Scholar] [CrossRef]
- Zheng, X.; Zhu, Z.; Lin, X.; Zhang, Y.; He, Y.; Cao, H.; Sun, Z. A Mini-Review on Metal Recycling from Spent Lithium Ion Batteries. Engineering 2018, 4, 361–370. [Google Scholar] [CrossRef]
- Meshram, P.; Pandey, B.D.; Mankhand, T.R. Extraction of lithium from primary and secondary sources by pre-treatment, leaching and separation: A comprehensive review. Hydrometallurgy 2014, 150, 192–208. [Google Scholar] [CrossRef]
- Georgi-Maschler, T.; Friedrich, B.; Weyhe, R.; Heegn, H.; Rutz, M. Development of a recycling process for Li-ion batteries. J. Power Sources 2012, 207, 173–182. [Google Scholar] [CrossRef]
- Lei, S.; Sun, W.; Yang, Y. Solvent extraction for recycling of spent lithium-ion batteries. J. Hazard. Mater. 2022, 424, 127654. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Chen, J.; Li, H.; Li, K. An integrated process for the separation and recovery of valuable metals from the spent LiNi0.5Co0.2Mn0.3O2 cathode materials. Sep. Purif. Technol. 2020, 245, 116869. [Google Scholar] [CrossRef]
- Tang, Y.-C.; Wang, J.-Z.; Shen, Y.-H. Separation of Valuable Metals in The Recycling of Lithium Batteries via Solvent Extraction. Minerals 2023, 13, 285. [Google Scholar] [CrossRef]
- Swain, B.; Jeong, J.; Lee, J.-C.; Lee, G.-H.; Sohn, J.-S. Hydrometallurgical process for recovery of cobalt from waste cathodic active material generated during manufacturing of lithium ion batteries. J. Power Sources 2007, 167, 536–544. [Google Scholar] [CrossRef]
- Sethurajan, M.; van Hullebusch, E.D.; Fontana, D.; Akcil, A.; Deveci, H.; Batinic, B.; Leal, J.P.; Gasche, T.A.; Kucuker, M.A.; Kuchta, K.; et al. Recent advances on hydrometallurgical recovery of critical and precious elements from end of life electronic wastes—A review. Crit. Rev. Environ. Sci. Technol. 2019, 49, 212–275. [Google Scholar] [CrossRef]
- Zhao, Z.; Zhang, B.; Zou, J.; Li, P.; Liu, Z.; Cheng, L.; Ou, X.; Zhang, J. Regeneration of high-capacity Ni-rich layered cathode material from spent lithium-ion batteries. J. Energy Storage 2022, 45, 103512. [Google Scholar] [CrossRef]
- Tran, T.T.; Moon, H.S.; Lee, M.S. Recovery of valuable metals from the hydrochloric leaching solution of reduction smelted metallic alloys from spent lithium-ion batteries. J. Chem. Technol. Biotechnol. 2022, 97, 1247–1258. [Google Scholar] [CrossRef]
- Beak, M.; Park, J.; Park, S.; Jeong, S.; Kang, J.; Choi, W.; Yoon, W.-S.; Kwon, K. Understanding the effect of nonmetallic impurities in regenerated cathode materials for lithium-ion battery recycling by tracking down impurity elements. J. Hazard. Mater. 2022, 425, 127907. [Google Scholar] [CrossRef]
- Kim, W.; Park, S.; Ko, G.; Lee, J.; Kwon, K. Optimizing pH conditions for impurity removal in closed-loop Li-ion battery recycling. Chem. Eng. J. 2023, 475, 146121. [Google Scholar] [CrossRef]
- Sole, K. Solvent extraction in the hydrometallurgical processing and purification of metals: Process design and selected applications. In Solvent Extraction and Liquid Membranes: Fundamentals and Applications in New Materials; CRC Press: Boca Raton, FL, USA; Taylor &Francis: Boca Raton, FL, USA, 2008; pp. 141–200. [Google Scholar]
- Keller, A.; Hlawitschka, M.W.; Bart, H.J. Application of saponified D2EHPA for the selective extraction of manganese from spend lithium-ion batteries. Chem. Eng. Process.–Process. Intensif. 2022, 171, 108552. [Google Scholar] [CrossRef]






| Metal Element | Black Mass [g/L] | Black Alloy 1 [g/L] | Black Alloy 2 [wt%] | This Study [g/L] |
|---|---|---|---|---|
| Ni | 35.9 | 3.87 | 61.99 | 36.3 |
| Co | 11.9 | 12.01 | 12.9 | 12.4 |
| Mn | 10.2 | 0.19 | 1.58 | 10.96 |
| Al | 6.32 | 0.04 | - | - |
| Fe | 0.11 | 3.01 | 0.5 | - |
| Cu | 2.57 | 16.42 | 22.3 | 2.86 |
| Zn | 0.13 | 0.0025 | - | 0.17 |
| Ca | 0.12 | 0.0032 | - | 0.15 |
| Mg | 0.02 | 0.0045 | - | 0.03 |
| pH | D2EHPA | Cyanex272 | PC88A | |||
|---|---|---|---|---|---|---|
| βMn/Ni | βCo/Ni | βMn/Co | βNi/Co | βCo/Ni | βCo/Ni | |
| 4 | 4151 | 132.4 | 31.35 | 0.01 | 58.18 | 3.099 |
| 5 | 159.2 | 10.68 | 14.92 | 0.09 | 75.13 | 41.53 |
| pH | DNi | DCo | DMn | DCu |
|---|---|---|---|---|
| 4 | 0.06 | 0.09 | 0.002 | 1.33 |
| 5 | 0.12 | 0.15 | 0.06 | 294 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koo, N.; Kim, B.; Kim, H.-I.; Kwon, K. Extraction Strategies from Black Alloy Leachate: A Comparative Study of Solvent Extractants. Batteries 2024, 10, 221. https://doi.org/10.3390/batteries10070221
Koo N, Kim B, Kim H-I, Kwon K. Extraction Strategies from Black Alloy Leachate: A Comparative Study of Solvent Extractants. Batteries. 2024; 10(7):221. https://doi.org/10.3390/batteries10070221
Chicago/Turabian StyleKoo, Namho, Byungseon Kim, Hong-In Kim, and Kyungjung Kwon. 2024. "Extraction Strategies from Black Alloy Leachate: A Comparative Study of Solvent Extractants" Batteries 10, no. 7: 221. https://doi.org/10.3390/batteries10070221
APA StyleKoo, N., Kim, B., Kim, H.-I., & Kwon, K. (2024). Extraction Strategies from Black Alloy Leachate: A Comparative Study of Solvent Extractants. Batteries, 10(7), 221. https://doi.org/10.3390/batteries10070221







