The Suppression Effect of Water Mist Released at Different Stages on Lithium-Ion Battery Flame Temperature, Heat Release, and Heat Radiation
Abstract
1. Introduction
2. Experimental Settings
2.1. Battery Sample
2.2. Experimental Setup
2.3. Case Setting
3. Experimental Results and Analyses
3.1. Analysis of the Temperatures of Battery Surface and TR Flame
3.2. Analysis of the Effect of WM on TR Flame
3.3. Analysis of the Heat Cooling of TR Flame and Specific Cooling Capacity of WM
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Nomenclature
| A | Flame surface area, m2 |
| ALIB | Area of battery upper surface, m2 |
| c | Specific heat capacity of air, J/(kg·K) |
| d | Combustion chamber diameter, m |
| h | Convective heat transfer coefficient, W/(m2·K) |
| Li | Distance between i temperature measurement point and battery’s upper surface, m |
| mWM | WM consumption for flame extinguishing, kg |
| Nu | Nusselt number |
| PE,i | Heat radiation power of each measurement points on the surface of the battery, W |
| Pr | Prandtl number |
| (Prf/Prw)0.25 | Physical property correction factor |
| Q | Heat release of battery flame, J |
| QE | Heat radiation of flame to the battery, J |
| Qm | Specific cooling capacity of WM, kJ/kg |
| ΔQ | Difference in heat release, J |
| ΔQE | Differences in flame heat radiation to battery’s upper surface, J |
| q | Heat flux density of battery flame, W/m2 |
| qm | WM mass flow, kg/s |
| Re | Reynolds number |
| Ta | Ambient temperature, °C |
| TF,i | Flame temperature at i position, K |
| Tf,i | Flame temperature at i position, °C |
| te | Time of SV opening, s |
| tSV | Time of TR ending, s |
| v | Gas flow rate, m/s |
| Abbreviations | |
| LIB | Lithium-ion Battery |
| LFP | Lithium Iron Phosphate |
| NCA | Nickel Cobalt Aluminum Ternary Lithium |
| NCM | Nickel Cobalt Manganese Ternary Lithium |
| SEI | Solid Electrolyte Interface |
| SOC | State of Charge |
| SOH | State of Health |
| SV | Safety Valve |
| TR | Thermal Runaway |
| WM | Water Mist |
| Greek | |
| λ | Thermal conductivity of air, W/(m·K) |
| ε | Surface emissivity of the aluminum battery surface |
| α | Thermal diffusion coefficient, m2/s |
| η | Proportion of heat radiation cooling |
| μ | Dynamic viscosity of air, m2/s |
| ρ | Density of air, kg/m3 |
| σ | Stefan–Boltzmann constant |
| τWM | Release duration for WM to extinguish the flame, s |
References
- Hu, X.Y.; Liu, T.; Zhu, G.Q.; Cui, S.Q.; Huang, J.H.; Dong, X.T.; Guo, X.Y. Study on temperature heterogeneity and flame confrontation of LiFePO4 battery thermal runaway inhibition by water mist. Appl. Therm. Eng. 2024, 244, 122675. [Google Scholar] [CrossRef]
- Li, Q.; Yu, J.S.; Liu, G.Z.; Ma, X.G.; Si, W.; Hu, X.Y.; Zhu, G.Q.; Liu, T. Study on the Effectiveness of Water Mist on Suppressing Thermal Runaway in LiFePO4 Batteries. Crystals 2023, 13, 1346. [Google Scholar] [CrossRef]
- Liu, J.L.; Duan, Q.L.; Qi, K.X.; Liu, Y.J.; Sun, J.H.; Wang, Z.R.; Wang, Q.S. Capacity fading mechanisms and state of health prediction of commercial lithium-ion battery in total lifespan. J. Energy Storage 2022, 46, 103910. [Google Scholar] [CrossRef]
- Liu, T.; Huang, J.H.; Hu, X.Y.; Cui, S.Q.; Zhu, G.Q. Study on the variation of normalized heat and gas release of overcharge-induced thermal runaway in confined space. Appl. Therm. Eng. 2024, 243, 122636. [Google Scholar] [CrossRef]
- Zheng, Y.S.; Che, Y.H.; Hu, X.S.; Sui, X.; Stroe, D.I.; Teodorescu, R. Thermal state monitoring of lithium-ion batteries: Progress, challenges, and opportunities. Prog. Energy Combust. Sci. 2024, 100, 101120. [Google Scholar] [CrossRef]
- Al-Zareer, M.; Dincer, I.; Rosen, M.A. Comparative assessment of new liquid-to-vapor type battery cooling systems. Energy 2019, 188, 116010. [Google Scholar] [CrossRef]
- Feng, X.N.; He, X.M.; Ouyang, M.G.; Wang, L.; Lu, L.G.; Ren, D.S.; Santhanagopalan, S. A Coupled Electrochemical-Thermal Failure Model for Predicting the Thermal Runaway Behavior of Lithium-Ion Batteries. J. Electrochem. Soc. 2018, 165, A3748–A3765. [Google Scholar] [CrossRef]
- Wang, G.Q.; Kong, D.P.; Ping, P.; Wen, J.; He, X.Q.; Zhao, H.L.; He, X.; Peng, R.Q.; Zhang, Y.; Dai, X.Y. Revealing particle venting of lithium-ion batteries during thermal runaway: A multi-scale model toward multiphase process. Etransportation 2023, 16, 100237. [Google Scholar] [CrossRef]
- Cao, Y.F.; Wang, K.; Wang, Z.R.; Wang, J.L.; Yang, Y.; Xu, X.Y. Utilization of liquid nitrogen as efficient inhibitor upon thermal runaway of 18650 lithium ion battery in open space. Renew. Energy 2023, 206, 1097–1105. [Google Scholar] [CrossRef]
- Zhu, X.Q.; Wang, H.; Wang, X.; Gao, Y.F.; Allu, S.; Cakmak, E.; Wang, Z.P. Internal short circuit and failure mechanisms of lithium-ion pouch cells under mechanical indentation abuse conditions: An experimental study. J. Power Sources 2020, 455, 100237. [Google Scholar] [CrossRef]
- Gao, T.F.; Wang, Z.R.; Chen, S.C.; Guo, L.S. Hazardous characteristics of charge and discharge of lithium-ion batteries under adiabatic environment and hot environment. Int. J. Heat Mass Transf. 2019, 141, 419–431. [Google Scholar] [CrossRef]
- Mao, B.B.; Liu, C.Q.; Yang, K.; Li, S.; Liu, P.J.; Zhang, M.J.; Meng, X.D.; Gao, F.; Duan, Q.L.; Wang, Q.S.; et al. Thermal runaway and fire behaviors of a 300 Ah lithium ion battery with LiFePO4 as cathode. Renew. Sustain. Energy Rev. 2021, 139, 110717. [Google Scholar] [CrossRef]
- Wang, Z.; Ouyang, D.X.; Chen, M.Y.; Wang, X.H.; Zhang, Z.; Wang, J. Fire behavior of lithium-ion battery with different states of charge induced by high incident heat fluxes. J. Therm. Anal. Calorim. 2019, 136, 2239–2247. [Google Scholar] [CrossRef]
- Han, Z.; Zhao, L.; Zhao, J.; Xu, G.; Liu, H.; Chen, M. An Experimental Study on the Thermal Runaway Propagation of Cycling Aged Lithium-Ion Battery Modules. Fire 2024, 7, 119. [Google Scholar] [CrossRef]
- Wang, B.X.; Zhou, Z.Z.; Li, L.; Peng, Y.; Cao, J.D.; Yang, L.Z.; Cao, B. Experimental study on thermal runaway and its propagation of large format prismatic lithium-ion batteries. J. Energy Storage 2022, 55, 105550. [Google Scholar] [CrossRef]
- Feng, X.N.; Ouyang, M.G.; Liu, X.; Lu, L.G.; Xia, Y.; He, X.M. Thermal runaway mechanism of lithium ion battery for electric vehicles: A review. Energy Storage Mater. 2018, 10, 246–267. [Google Scholar] [CrossRef]
- Wang, Q.S.; Ping, P.; Zhao, X.J.; Chu, G.Q.; Sun, J.H.; Chen, C.H. Thermal runaway caused fire and explosion of lithium ion battery. J. Power Sources 2012, 208, 210–224. [Google Scholar] [CrossRef]
- Ghiji, M.; Novozhilov, V.; Moinuddin, K.; Joseph, P.; Burch, I.; Suendermann, B.; Gamble, G. A Review of Lithium-Ion Battery Fire Suppression. Energies 2020, 13, 5117. [Google Scholar] [CrossRef]
- Golubkov, A.W.; Fuchs, D.; Wagner, J.; Wiltsche, H.; Stangl, C.; Fauler, G.; Voitic, G.; Thaler, A.; Hacker, V. Thermal-runaway experiments on consumer Li-ion batteries with metal-oxide and olivin-type cathodes. Rsc Adv. 2014, 4, 3633–3642. [Google Scholar] [CrossRef]
- Zhong, G.B.; Mao, B.B.; Wang, C.; Jiang, L.; Xu, K.Q.; Sun, J.H.; Wang, Q.S. Thermal runaway and fire behavior investigation of lithium ion batteries using modified cone calorimeter. J. Therm. Anal. Calorim. 2019, 135, 2879–2889. [Google Scholar] [CrossRef]
- Feng, L.; Jiang, L.H.; Liu, J.L.; Wang, Z.Y.; Wei, Z.S.; Wang, Q.S. Dynamic overcharge investigations of lithium ion batteries with different state of health. J. Power Sources 2021, 507, 230262. [Google Scholar] [CrossRef]
- Liu, P.J.; Li, S.; Jin, K.Q.; Fu, W.D.; Wang, C.D.; Jia, Z.Z.; Jiang, L.H.; Wang, Q.S. Thermal Runaway and Fire Behaviors of Lithium Iron Phosphate Battery Induced by Overheating and Overcharging. Fire Technol. 2023, 59, 1051–1072. [Google Scholar] [CrossRef]
- Wei, D.; Zhang, M.Q.; Zhu, L.P.; Chen, H.; Huang, W.S.; Yao, J.; Yuan, Z.C.; Xu, C.S.; Feng, X.N. Study on Thermal Runaway Behavior of Li-Ion Batteries Using Different Abuse Methods. Batteries 2022, 8, 201. [Google Scholar] [CrossRef]
- Tao, C.F.; Zhu, Y.H.; Liu, Z.Q.; Li, R.; Chen, Z.Y.; Gong, L.L.; Liu, J.H. The experimental investigation of thermal runaway characteristics of lithium battery under different nitrogen concentrations. J. Therm. Anal. Calorim. 2023, 148, 12097–12107. [Google Scholar] [CrossRef]
- Li, Y.W.; Jiang, L.H.; Huang, Z.H.; Jia, Z.Z.; Qin, P.; Wang, Q.S. Pressure Effect on the Thermal Runaway Behaviors of Lithium-Ion Battery in Confined Space. Fire Technol. 2023, 59, 1137–1155. [Google Scholar] [CrossRef]
- Qiu, Y.S.; Jiang, F.M. A review on passive and active strategies of enhancing the safety of lithium-ion batteries. Int. J. Heat Mass Transf. 2022, 184, 122288. [Google Scholar] [CrossRef]
- Yuan, S.; Chang, C.Y.; Yan, S.S.; Zhou, P.; Qian, X.M.; Yuan, M.Q.; Liu, K. A review of fire-extinguishing agent on suppressing lithium-ion batteries fire. J. Energy Chem. 2021, 62, 262–280. [Google Scholar] [CrossRef]
- Zhang, L.; Li, Y.Q.; Duan, Q.L.; Chen, M.; Xu, J.J.; Zhao, C.P.; Sun, J.H.; Wang, Q.S. Experimental study on the synergistic effect of gas extinguishing agents and water mist on suppressing lithium-ion battery fires. J. Energy Storage 2020, 32, 101801. [Google Scholar] [CrossRef]
- Sun, H.L.; Zhang, L.; Duan, Q.L.; Wang, S.Y.; Sun, S.J.; Sun, J.H.; Wang, Q.S. Experimental study on suppressing thermal runaway propagation of lithium-ion batteries in confined space by various fire extinguishing agents. Process Saf. Environ. Prot. 2022, 167, 299–307. [Google Scholar] [CrossRef]
- Zhao, J.C.; Xue, F.; Fu, Y.Y.; Cheng, Y.; Yang, H.; Lu, S. A comparative study on the thermal runaway inhibition of 18650 lithium-ion batteries by different fire extinguishing agents. Iscience 2021, 24, 102854. [Google Scholar] [CrossRef]
- Hill, D. Considerations for ESS Fire Safety; DNVGL: Byrum, Norway, 2017. [Google Scholar]
- Tang, W.; Yuan, L.M.; Thomas, R.; Soles, J. Comparison of Fire Suppression Techniques on Lithium-Ion Battery Pack Fires. Min. Metall. Explor. 2023, 40, 1081–1087. [Google Scholar] [CrossRef]
- Liu, T.; Tao, C.F.; Wang, X.S. Cooling control effect of water mist on thermal runaway propagation in lithium ion battery modules. Appl. Energy 2020, 267, 115087. [Google Scholar] [CrossRef]
- Zhang, T.W.; Liu, H.; Song, J.W.; Wang, B.; Wang, Y.; Shuai, X.C.; Guo, Z.D. Synergistic inhibition effect on lithium-ion batteries during thermal runaway by N2-twin-fluid liquid mist. Case Stud. Therm. Eng. 2022, 37, 102269. [Google Scholar] [CrossRef]
- Li, L.X.; Chen, Z.; Lu, Y.; Zang, P.J.; Zhan, W.; Cheng, Y.H. Study on the suppression of thermal runaway of lithium-ion battery by water mist with different additives. Energy Sources Part A-Recovery Util. Environ. Eff. 2023, 45, 11349–11362. [Google Scholar] [CrossRef]
- Zhang, L.; Duan, Q.L.; Xu, J.J.; Meng, X.D.; Sun, J.H.; Wang, Q.S. Experimental investigation on suppression of thermal runaway propagation of lithium-ion battery by intermittent spray. J. Energy Storage 2023, 58, 106434. [Google Scholar] [CrossRef]
- Mei, J.; Shi, G.Q.; Liu, H.; Wang, Z.; Chen, M.Y. Experimental study on the effect of passive retardation method for thermal runaway mitigation of lithium-ion battery. Appl. Therm. Eng. 2023, 230, 120861. [Google Scholar] [CrossRef]
- Lönnermark, A. TOXFIRE-Fire Characteristics and Smoke Gas Analysis in under-Ventilated Large-Scale Combustion Experiments. Tests in the ISO 9705 Room. 1996. Available online: https://www.diva-portal.org/smash/get/diva2:962010/FULLTEXT01.pdf (accessed on 6 June 2024).
- Diaz, F.; Wang, Y.; Weyhe, R.; Friedrich, B. Gas generation measurement and evaluation during mechanical processing and thermal treatment of spent Li-ion batteries. Waste Manag. 2019, 84, 102–111. [Google Scholar] [CrossRef]
- Lee, J.-H.; Hong, S.-H.; Lee, H.-S.; Park, M.-W. Study on Gas-Generating Property of Lithium-Ion Batteries. Fire Sci. Eng. 2021, 35, 1–8. [Google Scholar] [CrossRef]
- Incropera, F.P.; DeWitt, D.P.; Bergman, T.L.; Lavine, A.S. Fundamentals of Heat and Mass Transfer; Wiley: New York, NY, USA, 1996; Volume 6. [Google Scholar]
- Han, R.; Tang, M.; Wang, D.; Zhang, S. Numerical analysis of the convective heat transfer coefficient effect on lithium battery thermal diffusion when considering temperature effect. Sci. Technol. Rev. 2023, 41, 104–112. [Google Scholar]
- Hu, Z. Experimental Investigation of Steel and Aluminum Alloy Surface Emissivity Characteristics; Henan Normal University: Xinxiang, China, 2010. [Google Scholar]

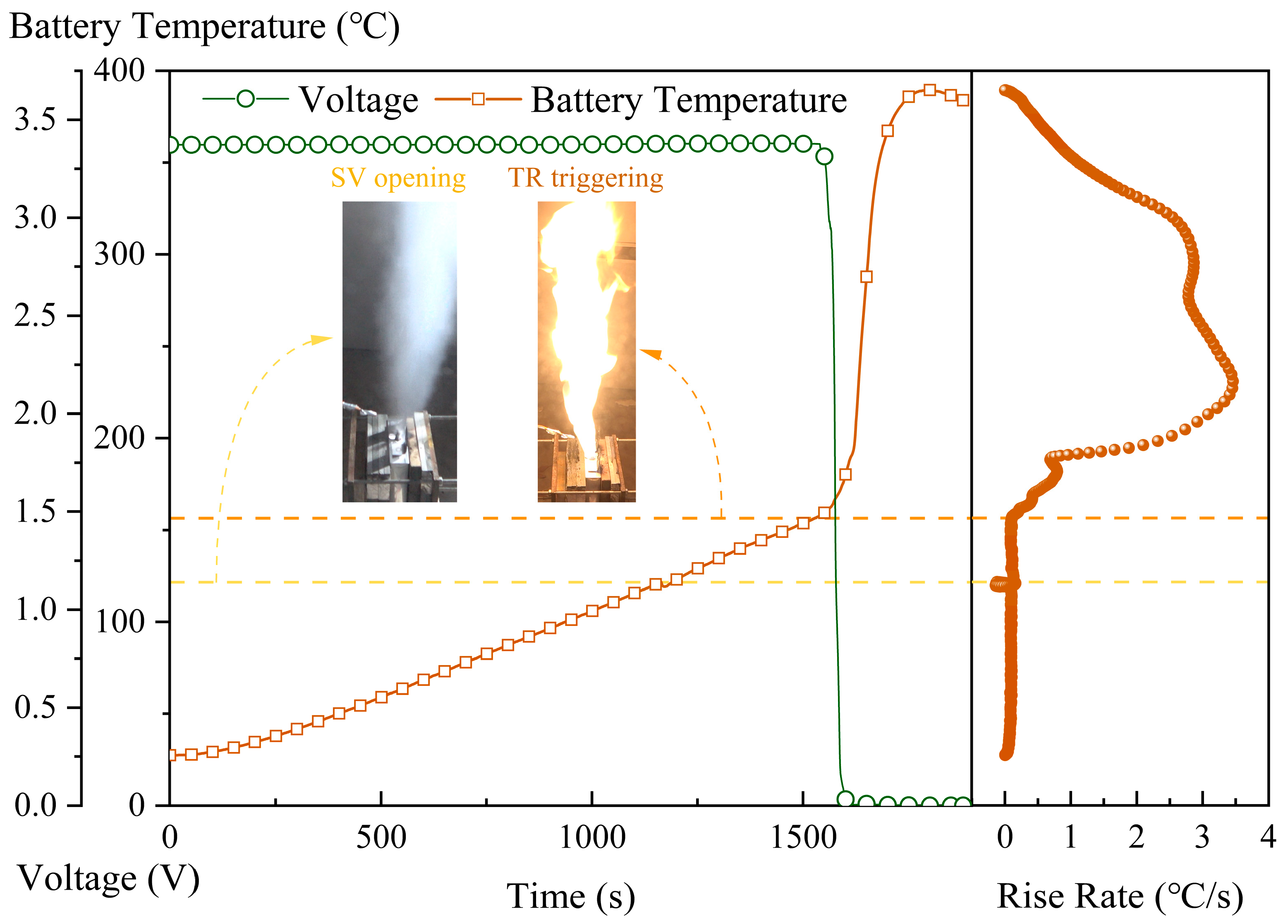

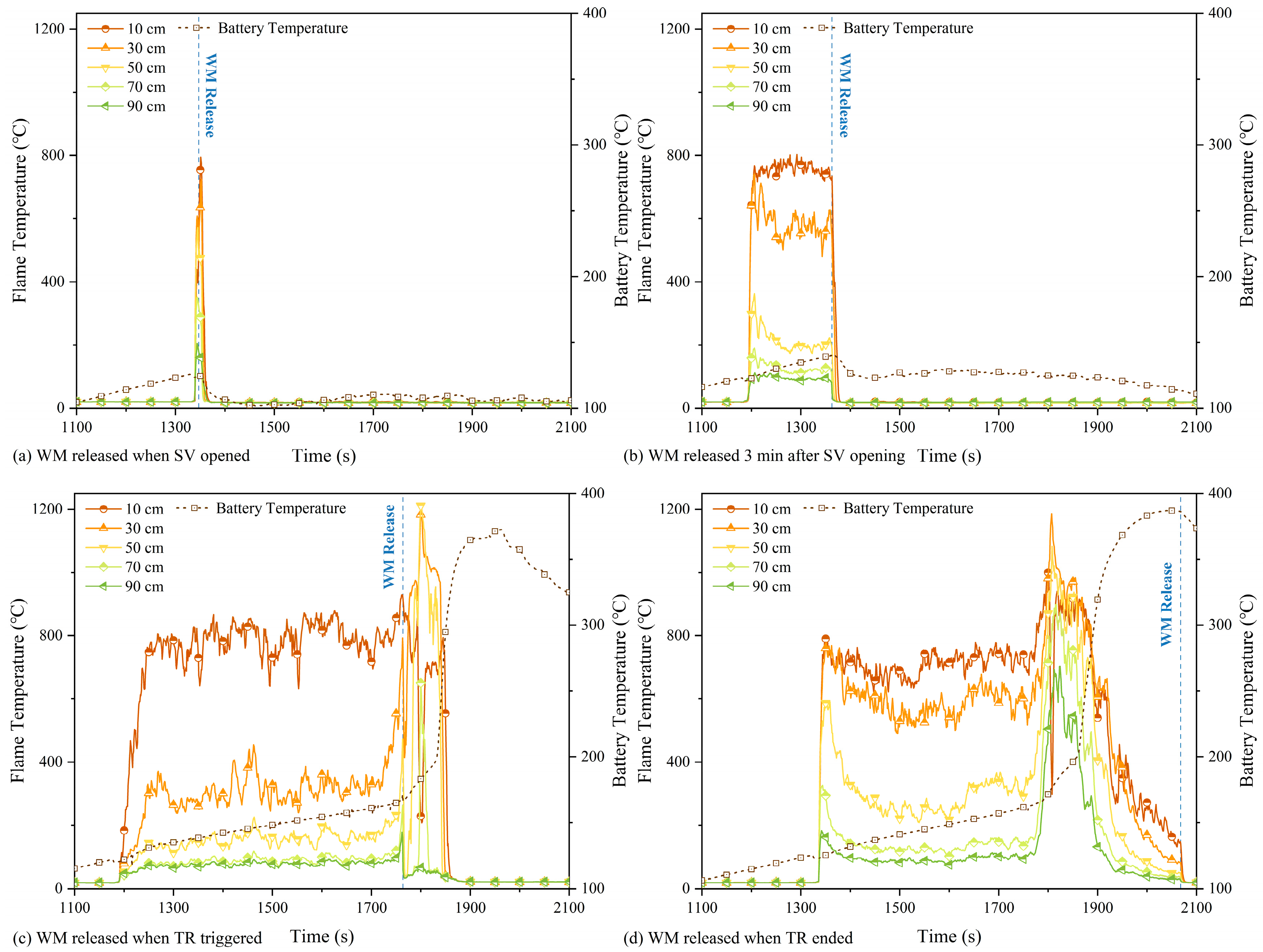

| No. | WM Release Temperature | Case Description |
|---|---|---|
| Case 1 | \ | No WM extinguishing. |
| Case 2 | 108 °C | WM released at SV opening. |
| Case 3 | 116 °C | WM released 3 min after SV opening. |
| Case 4 | 140 °C | WM released at TR triggering. |
| Case 5 | 400 °C | WM released after TR ended. |
| No. | ΔQ (kJ) | Qm (kJ/kg) |
|---|---|---|
| Case 2 | 11.6 | 1.8 × 10−3 |
| Case 3 | 14.9 | 1.7 × 10−3 |
| Case 4 | 54.4 | 2.8 × 10−3 |
| Case 5 | 37.1 | 5.9 × 10−3 |
| No. | ΔQE (kJ) | η |
|---|---|---|
| Case 2 | 5.83 | 76.7% |
| Case 3 | 4.27 | 88.4% |
| Case 4 | 26.31 | 58.5% |
| Case 5 | 1.94 | 73.8% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miao, B.; Lv, J.; Wang, Q.; Zhu, G.; Guo, C.; An, G.; Ou, J. The Suppression Effect of Water Mist Released at Different Stages on Lithium-Ion Battery Flame Temperature, Heat Release, and Heat Radiation. Batteries 2024, 10, 232. https://doi.org/10.3390/batteries10070232
Miao B, Lv J, Wang Q, Zhu G, Guo C, An G, Ou J. The Suppression Effect of Water Mist Released at Different Stages on Lithium-Ion Battery Flame Temperature, Heat Release, and Heat Radiation. Batteries. 2024; 10(7):232. https://doi.org/10.3390/batteries10070232
Chicago/Turabian StyleMiao, Bin, Jiangfeng Lv, Qingbiao Wang, Guanzhang Zhu, Changfang Guo, Guodong An, and Jianchun Ou. 2024. "The Suppression Effect of Water Mist Released at Different Stages on Lithium-Ion Battery Flame Temperature, Heat Release, and Heat Radiation" Batteries 10, no. 7: 232. https://doi.org/10.3390/batteries10070232
APA StyleMiao, B., Lv, J., Wang, Q., Zhu, G., Guo, C., An, G., & Ou, J. (2024). The Suppression Effect of Water Mist Released at Different Stages on Lithium-Ion Battery Flame Temperature, Heat Release, and Heat Radiation. Batteries, 10(7), 232. https://doi.org/10.3390/batteries10070232







