EIS Ageing Prediction of Lithium-Ion Batteries Depending on Charge Rates
Abstract
:1. Introduction
2. Materials and Methods
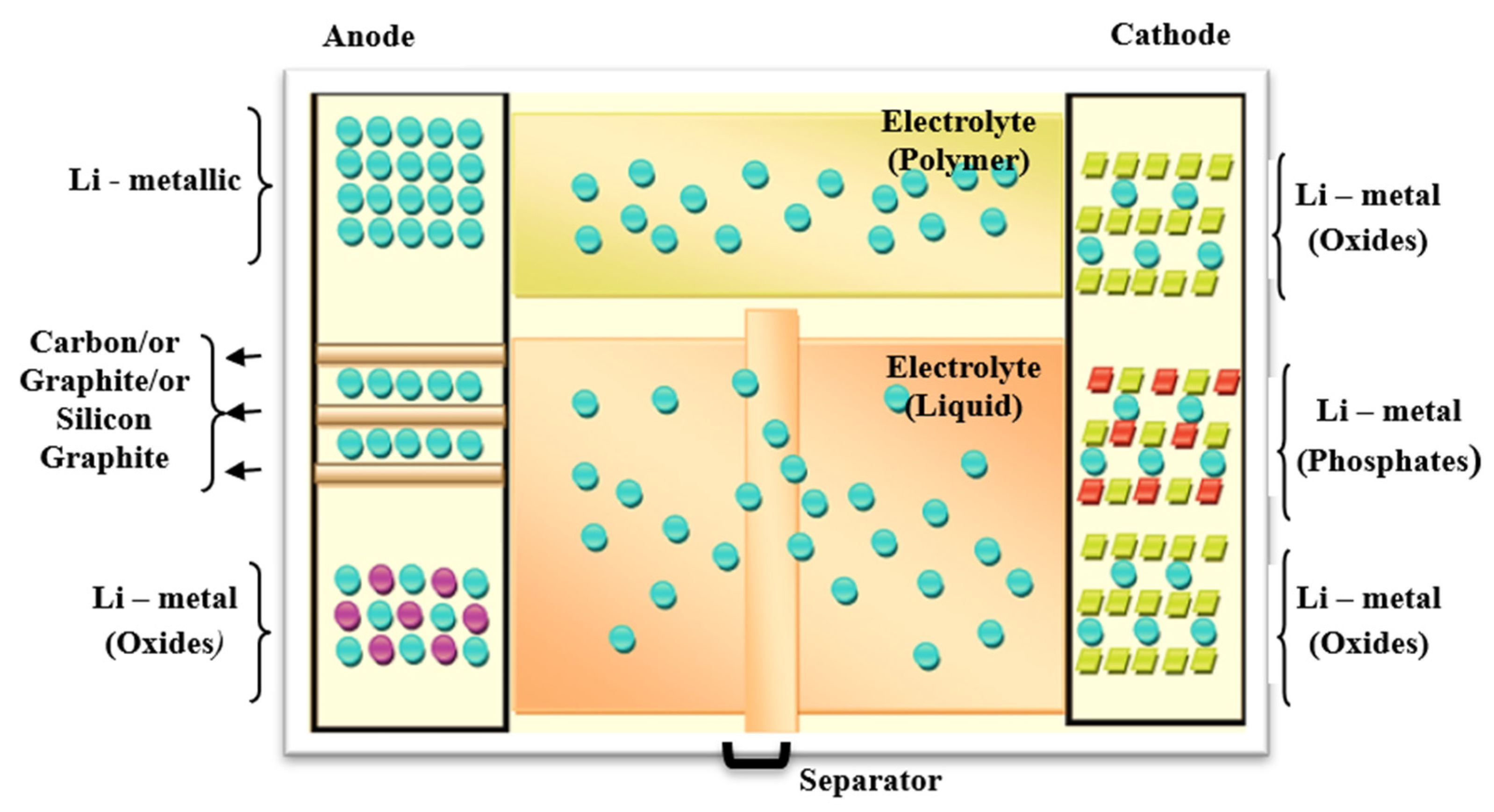
2.1. Lithium Batteries
2.2. Experimental Set-Up
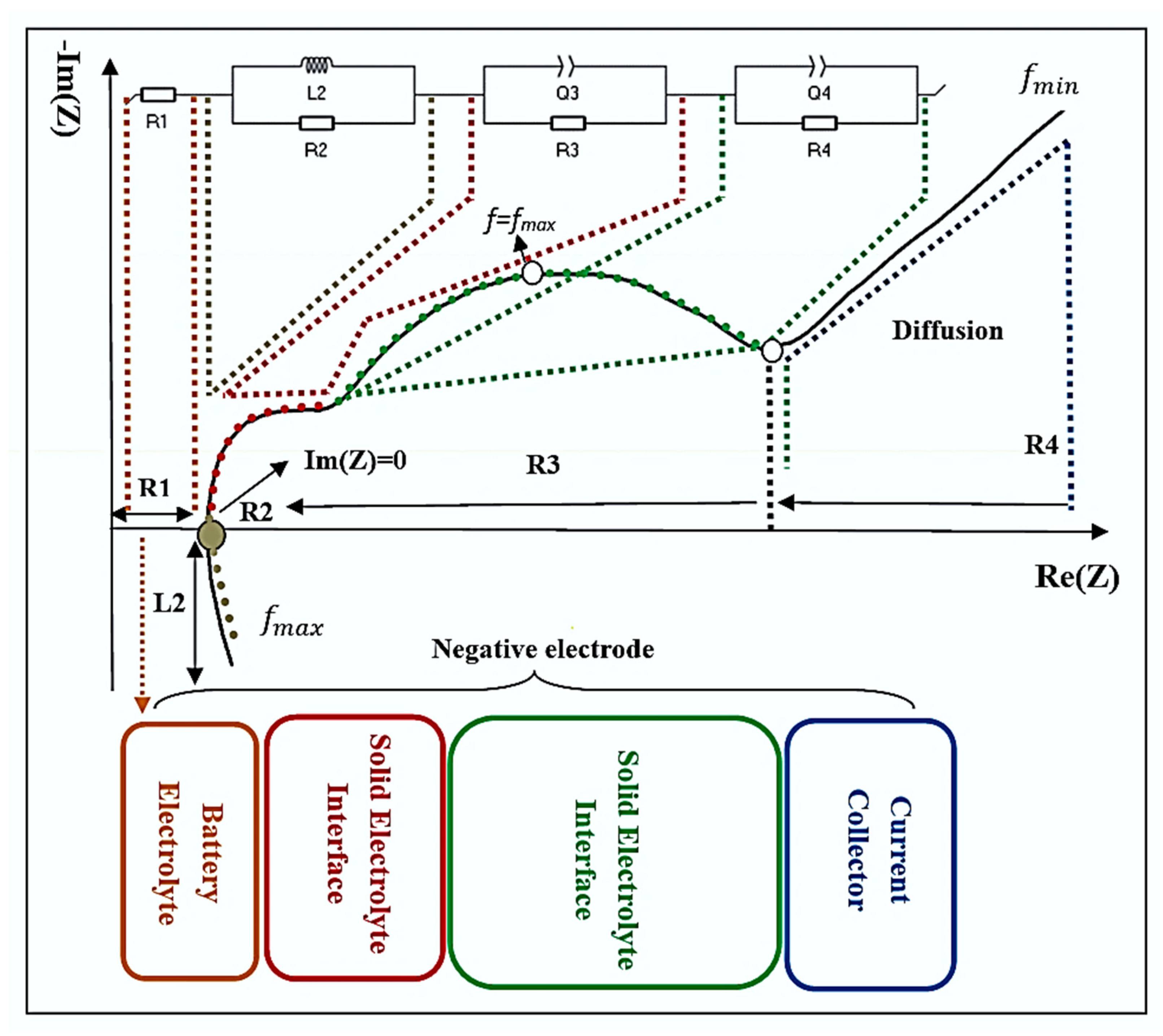
2.3. Electrochemical Impedance Spectroscopy (EIS)
2.4. Ageing Method Protocol
3. Results and Discussion
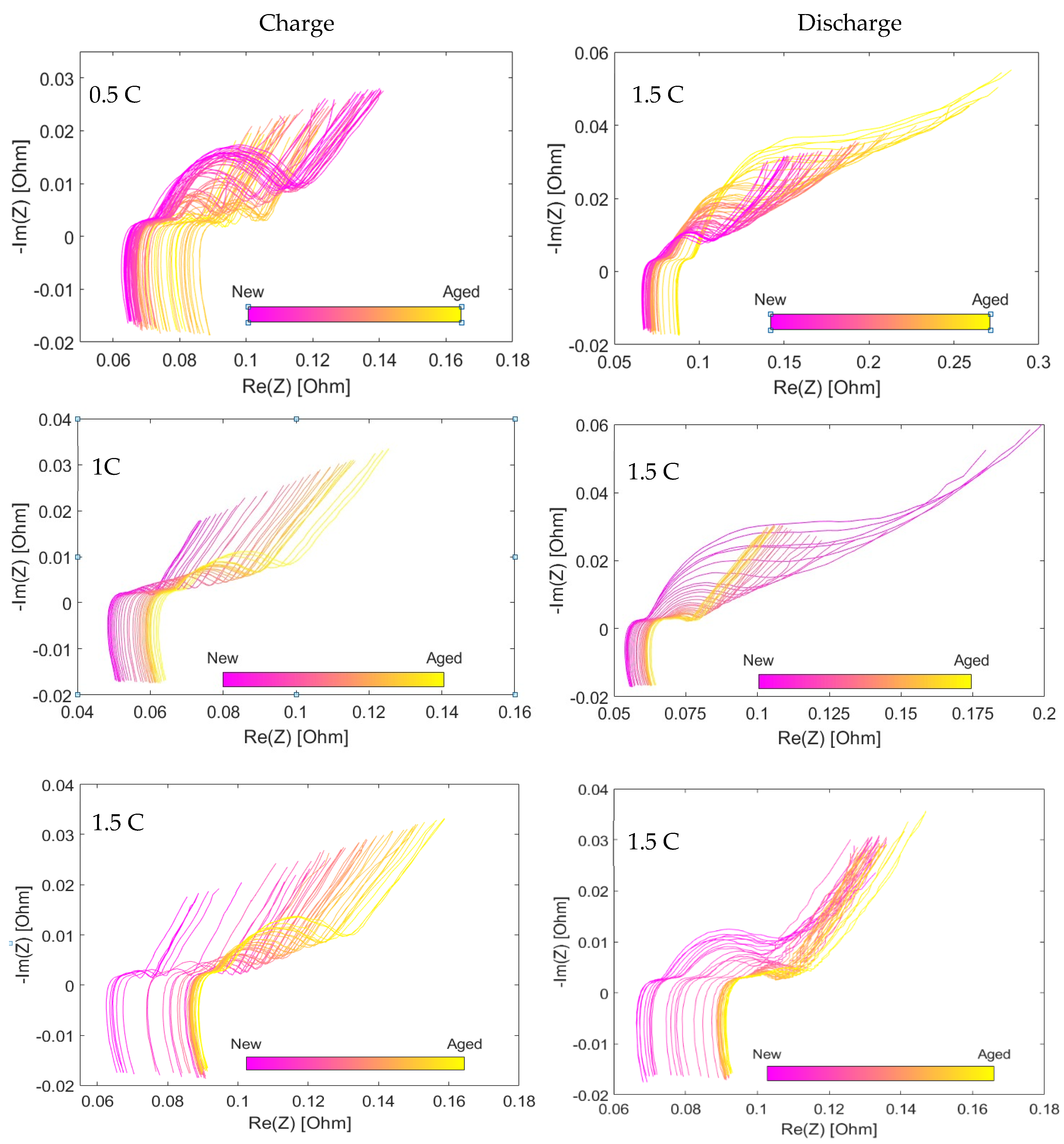
3.1. Dependence of Li-Ion Batteries Impedance on the SOH vs. Charge Rate
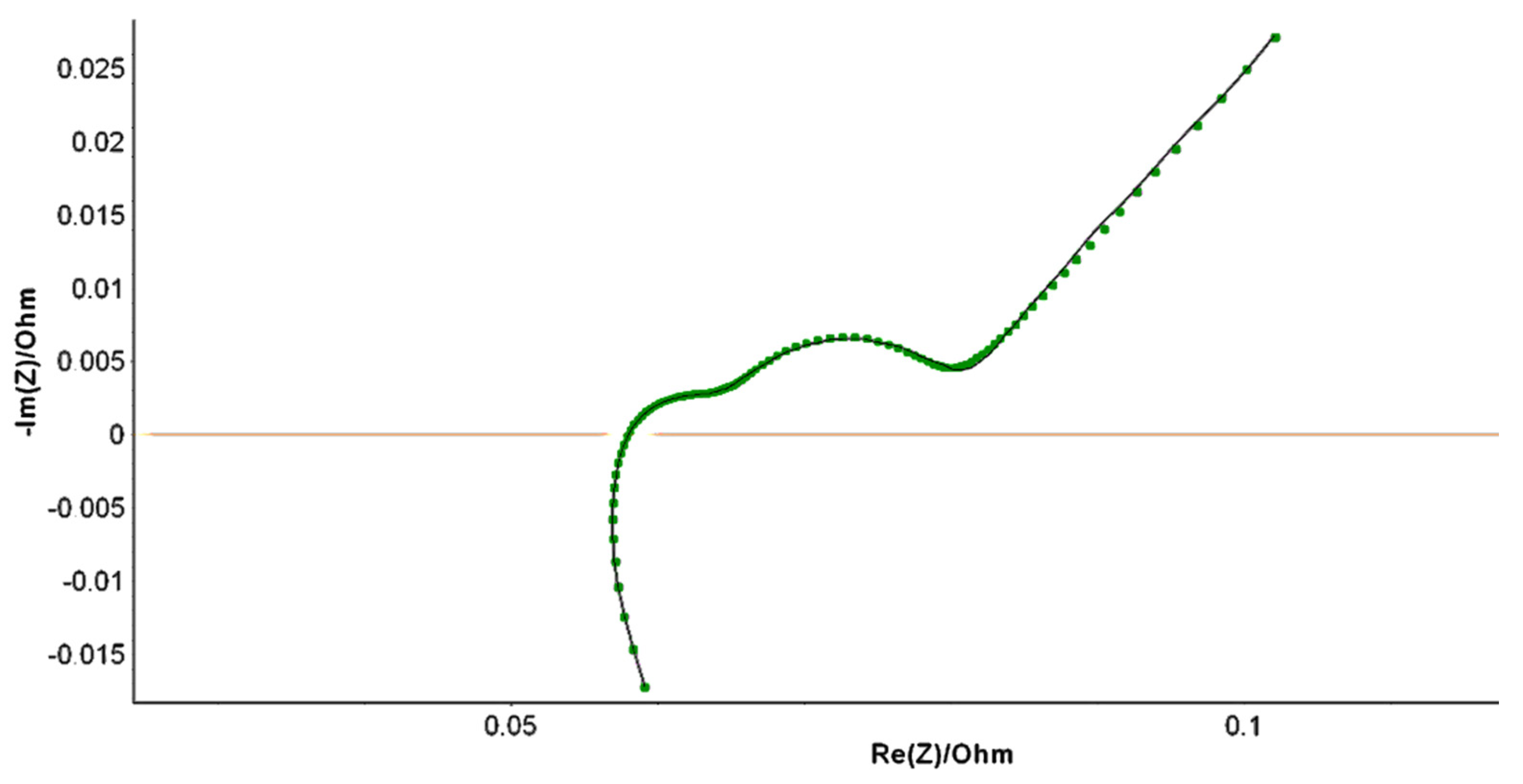
3.2. Variation of the Equivalent Electrical Circuit CPE-Q Parameter with SOH
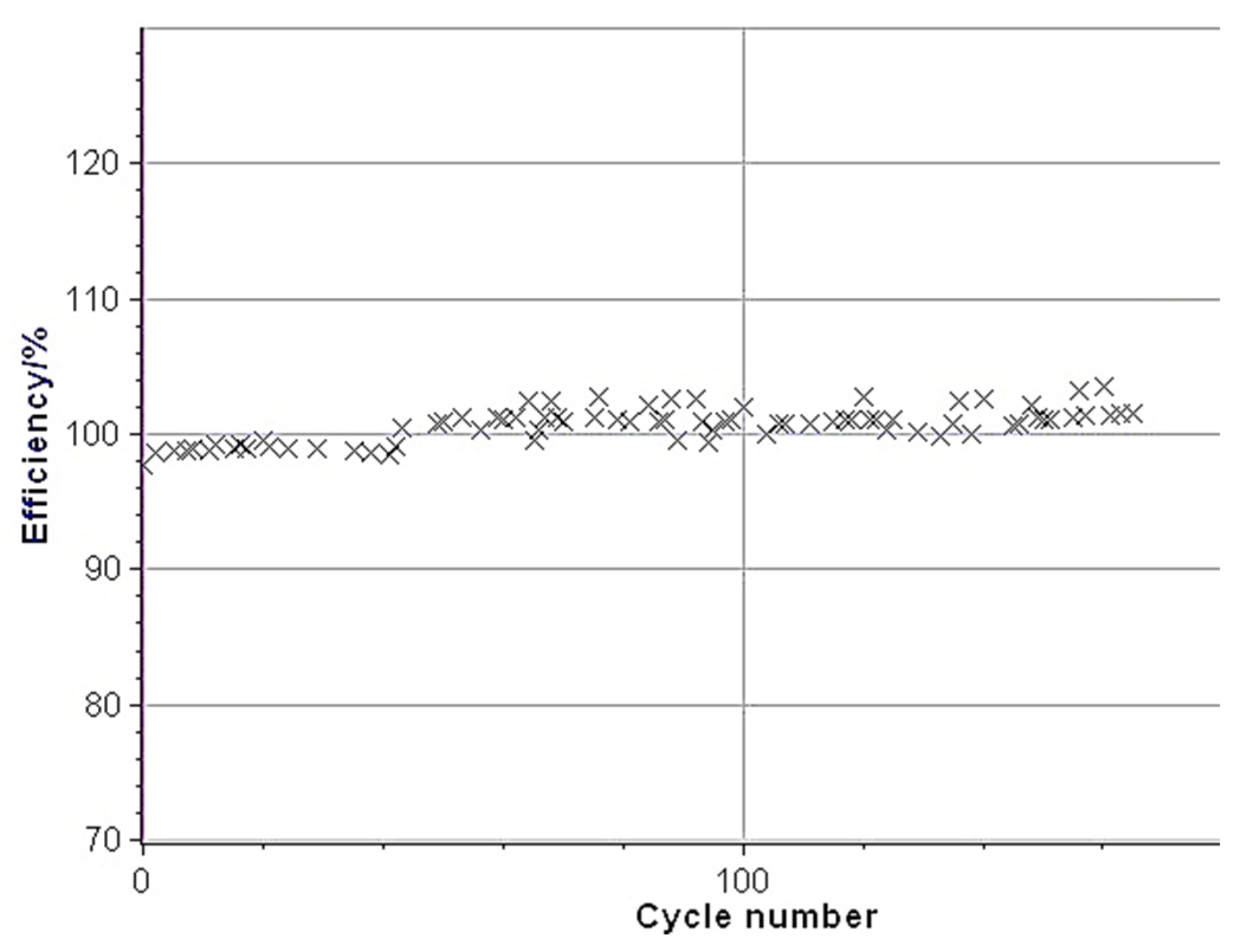
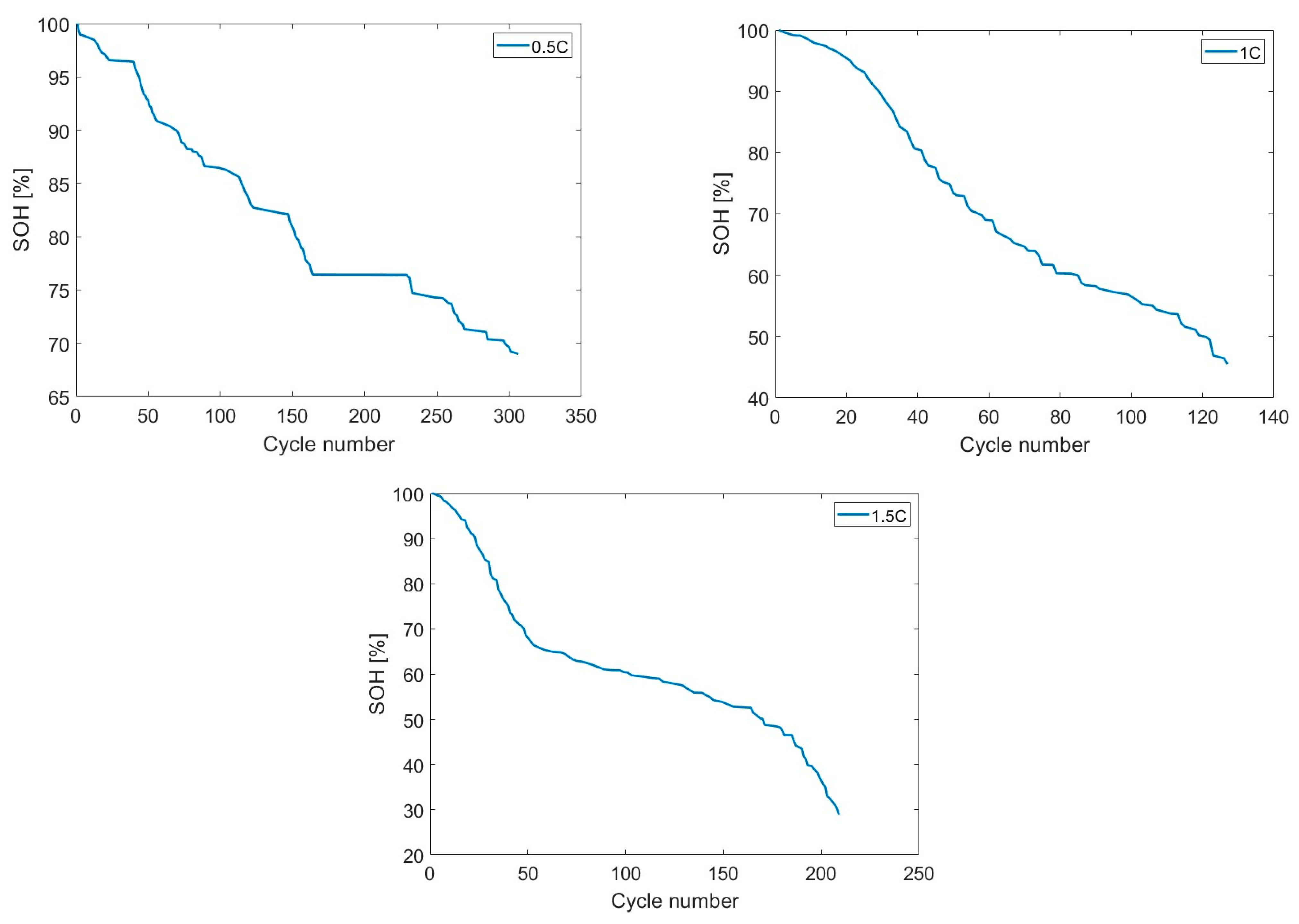
3.3. SOH Dependency on Charging Rate
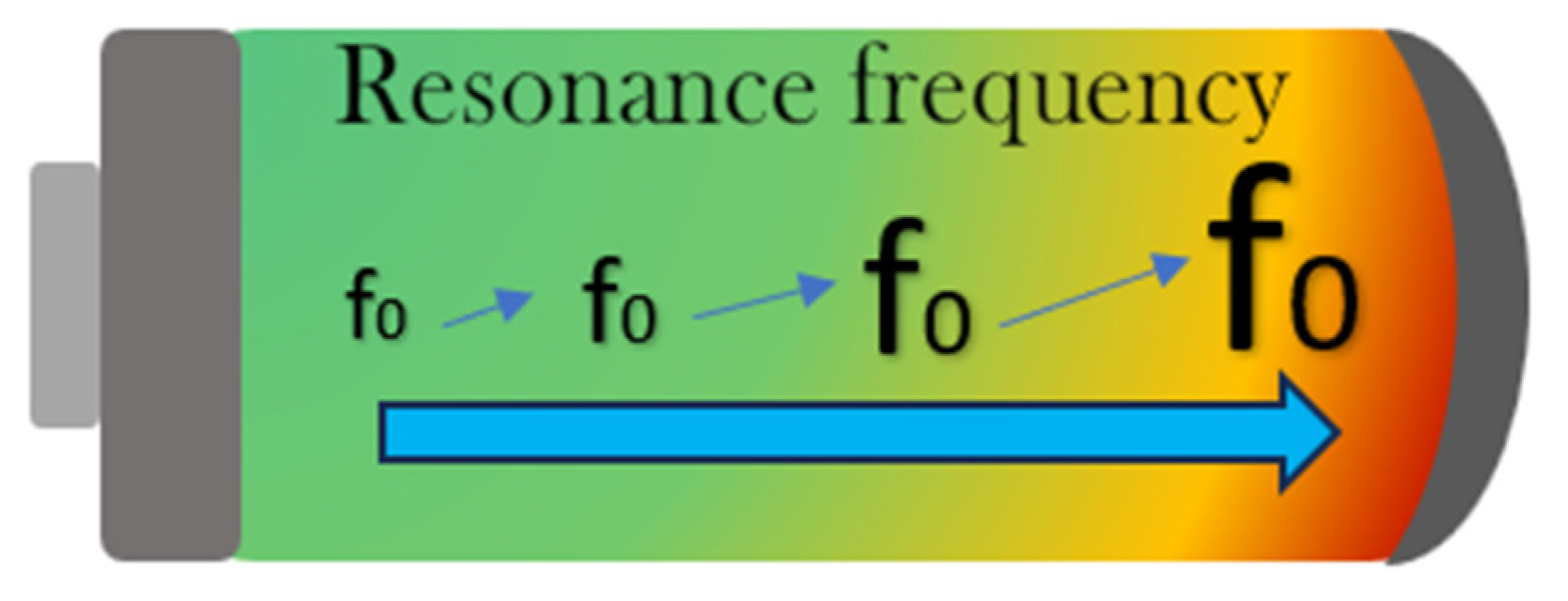
3.4. Resonance Frequency Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Nelson, P.; Bloom, I.; Amine, K.; Henriksen, G. Design modelling of lithium-ion battery performance. J. Power Sources 2002, 110, 437–444. [Google Scholar] [CrossRef]
- Eddahech, A.; Briat, O.; Vinassa, J.-M. Lithium-ion battery performance improvement based on capacity recovery exploitation. Electrochim. Acta 2013, 114, 750–757. [Google Scholar] [CrossRef]
- Yamada, M.; Watanabe, T.; Gunji, T.; Wu, J.; Matsumoto, F. Review of the Design of Current Collectors for Improving the Battery Performance in Lithium-Ion and Post-Lithium-Ion Batteries. Electrochem 2020, 1, 124–159. [Google Scholar] [CrossRef]
- van Ree, T. Electrolyte additives for improved lithium-ion battery performance and overcharge protection. Curr. Opin. Electrochem. 2020, 21, 22–30. [Google Scholar] [CrossRef]
- Masias, A.; Marcicki, J.; Paxton, W.A. Opportunities and Challenges of Lithium Ion Batteries in Automotive Applications. ACS Energy Lett. 2021, 6, 621–630. [Google Scholar] [CrossRef]
- Xiong, R.; Pan, Y.; Shen, W.; Li, H.; Sun, F. Lithium-ion battery aging mechanisms and diagnosis method for automotive applications: Recent advances and perspectives. Renew. Sustain. Energy Rev. 2020, 131, 110048. [Google Scholar] [CrossRef]
- Dai, Q.; Kelly, J.C.; Gaines, L.; Wang, M. Life Cycle Analysis of Lithium-Ion Batteries for Automotive Applications. Batteries 2019, 5, 48. [Google Scholar] [CrossRef]
- Kim, T.; Makwana, D.; Adhikaree, A.; Vagdoda, J.S.; Lee, Y. Cloud-Based Battery Condition Monitoring and Fault Diagnosis Platform for Large-Scale Lithium-Ion Battery Energy Storage Systems. Energies 2018, 11, 125. [Google Scholar] [CrossRef]
- Moyer, K.; Meng, C.; Marshall, B.; Assal, O.; Eaves, J.; Perez, D.; Karkkainen, R.; Roberson, L.; Pint, C.L. Carbon fiber reinforced structural lithium-ion battery composite: Multifunctional power integration for CubeSats. Energy Storage Mater. 2020, 24, 676–681. [Google Scholar] [CrossRef]
- Marsh, R.A.; Vukson, S.; Surampudi, S.; Ratnakumar, B.V.; Smart, M.C.; Manzo, M.; Dalton, P.J. Li ion batteries for aerospace applications. J. Power Sources 2001, 97–98, 25–27. [Google Scholar] [CrossRef]
- Krause, F.C.; Ruiz, J.P.; Jones, S.C.; Brandon, E.J.; Darcy, E.C.; Iannello, C.J.; Bugga, R.V. Performance of Commercial Li-Ion Cells for Future NASA Missions and Aerospace Applications. J. Electrochem. Soc. 2021, 168, 040504. [Google Scholar] [CrossRef]
- European Comission. Lithium Ion Battery Value Chain and Related Opportunities for Europe. Available online: https://core.ac.uk/download/pdf/154760177.pdf (accessed on 8 July 2024).
- Wang, Y.; Li, H.; He, P.; Hosono, E.; Zhou, H. Nano active materials for lithium-ion batteries. Nanoscale 2010, 2, 1294–1305. [Google Scholar] [CrossRef]
- Mekonnen, Y.; Sundararajan, A.; Sarwat, A.I. A review of cathode and anode materials for lithium-ion batteries. In Proceedings of the IEEE SoutheastCon 2016 Conference, Norfolk, VA, USA, 30 March–3 April 2016. [Google Scholar]
- Khalifa, H.; El-Safty, S.A.; Reda, A.; Selim, M.M.; Shenashen, M.A. One-dimensional hierarchical anode/cathode materials engineering for high-performance lithium ion batteries. Energy Storage Mater. 2021, 37, 363–377. [Google Scholar] [CrossRef]
- Hassoun, J.; Bonaccorso, F.; Agostini, M.; Angelucci, M.; Betti, M.G.; Cingolanti, R.; Gemmi, M.; Mariani, C.; Panero, S.; Pellegrini, V.; et al. An advanced Lithium-Ion Battery Based on a Graphene Anode and a Lithium Iron Phosphate Cathode. Nano Lett. 2014, 14, 4901–4906. [Google Scholar] [CrossRef] [PubMed]
- Lagadec, M.F.; Zahn, R.; Wood, V. Characterization and performance evaluation of lithium-ion battery separators. Nat. Energy 2019, 4, 16–25. [Google Scholar] [CrossRef]
- Yuan, M.; Liu, K. Rational design on separators and liquid electrolytes for safer lithium-ion batteries. J. Energy Chem. 2020, 43, 58–70. [Google Scholar] [CrossRef]
- Luiso, S.; Fedkiw, P. Lithium-ion battery separators: Recent developments and state of art. Current Opinion in Electrochemistry 2020, 20, 99–107. [Google Scholar] [CrossRef]
- Bajolle, H.; Lagadic, M.; Louvet, N. The future of lithium-ion batteries: Exploring expert conceptions, market trends, and price scenarios. Energy Res. Soc. Sci. 2022, 93, 102850. [Google Scholar] [CrossRef]
- Hadouchi, M.; Koketsu, T.; Hu, Z.; Ma, J. The origin of fast-charging lithium iron phosphate for batteries. Battery Energy 2022, 1, 20210010. [Google Scholar] [CrossRef]
- Ioakimidis, C.S.; Murillo-Marrodan, A.; Bagheri, A.; Thomas, D.; Genikmsakis, K.N. Life Cycle Assessment of a Lithium Iron Phosphate (LFP) Electric Vehicle Battery in Second Life Application Scenarios. Sustainability 2019, 11, 2527. [Google Scholar] [CrossRef]
- Barker, J.; Batteries, L.-I.R.; Buschow, K.H.J.; Cahn, R.W.; Flemings, M.C.; Ilschner, B.; Kramer, E.J.; Mahajan, S.; Veyssière, P. Encyclopedia of Materials: Science and Technology; Elsevier: Amsterdam, The Netherlands, 2010; pp. 1–6. ISBN 9780080431529. [Google Scholar]
- Li, J.; Lin, C.; Weng, M.; Qiu, Y.; Chen, P.; Yang, K.; Huang, W.; Hong, Y.; Li, J.; Zhang, M.; et al. Structural origin of the high-voltage instability of lithium cobalt oxide. Nat. Nanotechnol. 2021, 16, 599–605. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Wang, B.; Zhang, X.; Zhao, S.; Zhang, Z.; Yu, H. Reviving the lithium-manganese-based-layered oxide cathodes for lithium-ion batteries. Matter 2021, 4, 1511–1527. [Google Scholar] [CrossRef]
- Hang, M.N.; Gunsolus, I.L.; Wayland, H.; Melby, E.S.; Mensch, A.C.; Hurley, K.R.; Pedersen, J.A.; Haynes, C.L.; Hamers, R.J. Impact of Nanoscale Lithium Nickel Manganese Cobalt Oxide (NMC) on the Bacterium Shewanella oneidensis MR-1. Chem. Mater. 2016, 28, 1092–1100. [Google Scholar] [CrossRef]
- Pant, D.; Dolker, T. Green and facile method for the recovery of spent Lithium Nickel Manganese Cobalt Oxide (NMC) Based Lithium Ion Batteries. Waste Manag. 2017, 60, 689–695. [Google Scholar] [CrossRef]
- Vadivel, S.; Phattharasupakun, N.; Wutthiprom, J.; Duangdangchote, S.; Sawanphruk, M. High-Performance Li-Ion Batteries Using Nickel-Rich Lithium Nickel Cobalt Aluminium Oxide-Nanocarbon Core-Shell Cathode: In Operando X-ray Diffraction. ACS Appl. Mater. Interfaces 2019, 11, 30719–30727. [Google Scholar] [CrossRef] [PubMed]
- Brand, M.; Glaser, S.; Geder, J.; Menacher, S.; Obpacher, S.; Jossen, A.; Quinger, D. Electrical safety of commercial Li-ion cells based on NMC and NCA technology compared to LFP technology. World Electr. Veh. J. 2013, 6, 572–580. [Google Scholar] [CrossRef]
- Fell, C.R.; Sun, L.; Hallac, P.B.; Metz, B.; Sisk, B. Investigation of the Gas Generation in Lithium Titanate Anode Based Lithium Ion Batteries. J. Electrochem. Soc. 2015, 162, A1916. [Google Scholar] [CrossRef]
- Assefa, T.A.; Suzana, A.F.; Wu, L.; Koch, R.J.; Li, L.; Cha, W.; Harder, R.J.; Bozin, E.S.; Wang, F.; Robinson, I.K. Imaging the Phase Transformation in Single Particles of the Lithium Titanate Anode for Lithium-Ion Batteries. ACS Appl. Energy Mater. 2021, 4, 111–118. [Google Scholar] [CrossRef]
- Tan, S.; Ji, Y.J.; Zhang, Z.R.; Yang, Y. Recent Progress in Research on High-Voltage Electrolytes for Lithium-Ion Batteries. ChemPhysChem 2014, 15, 1956–1969. [Google Scholar] [CrossRef]
- Wang, C.; Yang, C.; Zheng, Z. Toward Practical High-Energy and High-Power Lithium Battery Anodes: Present and Future. Adv. Sci. 2022, 9, 2105213. [Google Scholar] [CrossRef]
- Xu, W.-T.; Peng, H.-J.; Huang, J.-Q.; Zhao, C.-Z.; Cheng, X.-B.; Zhang, Q. Towards Stable Lithium–Sulfur Batteries with a Low Self-Discharge Rate: Ion Diffusion Modulation and Anode Protection. ChemSusChem 2015, 8, 2892–2901. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Zou, P.; Bak, S.-M.; Wang, C.; Zhang, R.; Yao, L.; Du, Y.; Hu, E.; Lin, R.; Xin, H.L. Dual Passivation of Cathode and Anode through Electrode-Electrolyte Interface Engineering Enables Long-Lifespan Li Metal -SPAN Batteries. ACS Energy Lett. 2022, 7, 2866–2875. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, L.; Wang, Q.; Li, W.; Wu, B.; Jia, W.; Wang, Y.; Li, J.; Li, H. Long lifespan lithium metal anodes enabled by Al2O3 sputter coating. Energy Storage Mater. 2018, 10, 16–23. [Google Scholar] [CrossRef]
- AllCell Technologies LLC. A Comparison of Lead Acid to Lithium-Ion in Stationary Storage Applications. Available online: http://www.batterypoweronline.com/wp-content/uploads/2012/07/Lead-acid-white-paper.pdf (accessed on 8 July 2024).
- Iurilli, P.; Brivio, C.; Wood, V. On the use of electrochemical impedance spectroscopy to characterize and model the aging phenomena of lithium-ion batteries: A critical review. J. Power Sources 2021, 505, 229860. [Google Scholar] [CrossRef]
- Meddings, N.; Heinrich, M.; Overney, F.; Lee, J.-S.; Ruiz, V.; Napolitano, E.; Seitz, S.; Hinds, G.; Raccichini, R.; Gaberscek, M.; et al. Application of electrochemical impedance spectroscopy to commercial Li-ion cells: A review. J. Power Sources 2020, 480, 228742. [Google Scholar] [CrossRef]
- Andre, D.; Meiler, M.; Steiner, K.; Wimmer, C.; Soczka-Guth, T.; Sauer, D.U. Characterization of high-power lithium-ion batteries by electrochemical impedance spectroscopy. I. Experimental investigation. J. Power Sources 2011, 196, 5334–5341. [Google Scholar] [CrossRef]
- Martí-Florences, M.; Cecilia, A.; Costa-Castelló, R. Modelling and Estimation in Lithium-Ion Batteries: A Literature Review. Energies 2023, 16, 6846. [Google Scholar] [CrossRef]
- Sadhukhan, C.; Mitra, S.K.; Bhattacharyya, S.; Almatrafi, E.; Saleh, B.; Naskar, M.K. Modeling and simulation of high energy density lithium-ion battery for multiple fault detection. Sci. Rep. 2022, 12, 9800. [Google Scholar] [CrossRef] [PubMed]
- Porras-Hermoso, Á.; Pindado, S.; Cubas, J. Lithium-ion battery performance modeling based on the energy discharge level. Meas. Sci. Technol. 2018, 29, 117002. Available online: https://oa.upm.es/53065/1/measurement2018.pdf (accessed on 8 July 2024). [CrossRef]
- Wang, J.; Xiang, Y. Fast Modeling of the Capacity Degradation of Lithium-Ion Batteries via a Conditional Temporal Convolutional Encoder–Decoder. IEEE Trans. Transp. Electrif. 2022, 8, 1695–1709. [Google Scholar] [CrossRef]
- Lee, J.; Lee, J.H. Simultaneous extraction of intra- and inter-cycle features for predicting lithium-ion battery’s knees using convolutional and recurrent neural networks. Appl. Energy 2024, 356, 122399. [Google Scholar] [CrossRef]
- Wu, L.; Lyu, Z.; Huang, Z.; Zhang, C.; Wei, C. Physics-based battery SOC estimation methods: Recent advances and future perspectives. J. Energy Chem. 2024, 89, 27–40. [Google Scholar] [CrossRef]
- Máthé, L.; Grosu, I. Transport Through a Quantum Dot with Electron-Phonon Interaction. Mater. Today Proc. 2018, 5, 15878–15887. [Google Scholar] [CrossRef]
- Máthé, L.; Grosu, I. Nonequilibrium Kondo effect in a graphene-coupled quantum dot in the presence of a magnetic field. Beilstein J. Nanotechnol. 2020, 11, 225–239. [Google Scholar] [CrossRef] [PubMed]
- Choi, W.; Shin, H.-C.; Kim, J.M.; Choi, J.-Y.; Yoon, S. Modeling and Applications of Electrochemical Impedance Spectroscopy (EIS) for Lithium-ion Batteries. J. Electrochem. Sci. Technol. 2020, 11, 1–13. [Google Scholar] [CrossRef]
- Pastor-Fernández, C.; Widanage, W.D.; Marco, J.; Gama-Valdez, M.-Á.; Chouchelamane, G.H. Identification and quantification of ageing mechanisms in Lithium-ion batteries using the EIS technique. In Proceedings of the 2016 IEEE Transportation Electrification Conference and Expo (ITEC), Dearborn, MI, USA, 27–29 June 2016; pp. 1–6. [Google Scholar]
- Crescentini, M.; De Angelis, A.; Ramilli, R.; De Angelis, G.; Tartagni, M.; Moschitta, A.; Traverso, P.A.; Carbone, P. Online EIS and Diagnostics on Lithium-Ion Batteries by Means of Low-Power Integrated Sensing and Parametric Modeling. IEEE Trans. Instrum. Meas. 2021, 70, 1–11. [Google Scholar] [CrossRef]
- Itagaki, M.; Honda, K.; Hoshi, Y.; Shitanda, I. In-situ EIS to determine impedance spectra of lithium-ion rechargeable batteries during charge and discharge cycle. J. Electroanal. Chem. 2015, 737, 78–84. [Google Scholar] [CrossRef]
- Dai, S.; Chen, J.; Ren, Y.; Liu, Z.; Chen, J.; Li, C.; Zhang, X.; Zhang, X.; Zeng, T. Electrochemical Corrosion Behavior of the Copper Current Collector in the Electrolyte of Lithium-ion Batteries. Int. J. Electrochem. Sci. 2017, 12, 10589–10598. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, X.; Sun, X.; An, Y.; Song, S.; Li, C.; Wang, K.; Su, F.; Chen, C.-M.; Liu, F.; et al. Electrochemical impedance spectroscopy study of lithium-ion capacitors: Modeling and capacity fading mechanism. J. Power Sources 2021, 488, 229454. [Google Scholar] [CrossRef]
- Liu, T.; Cai, S.; Zhao, G.; Gao, Z.; Liu, S.; Li, H.; Chen, L.; Li, M.; Yang, X.; Guo, H. Recycling valuable cobalt from spent lithium ion batteries for controllably designing a novel sea-urchin-like cobalt nitride-graphene hybrid catalyst: Towards efficient overall water splitting. J. Energy Chem. 2021, 62, 440–450. [Google Scholar] [CrossRef]
- Dong, Q.; Zhou, Y.; Lian, J.; Li, L. A Fast Fuel Cell EIS Measurement Method Based on Square Wave Excitation and Modified Wavelets. J. Electrochem. Soc. 2023, 170, 044502. [Google Scholar] [CrossRef]
- Calborean, A.; Bruj, O.; Murariu, T.; Morari, C. Resonance frequency analysis of lead-acid cells: An EIS approach to predict the state-of-health. J. Energy Storage 2020, 27, 101143. [Google Scholar] [CrossRef]
- Calborean, A.; Murariu, T.; Morari, C. Optimized lead-acid grid architectures for automotive lead-acid batteries: An electrochemical analysis. Electrochim. Acta 2021, 372, 137880. [Google Scholar] [CrossRef]
- Panasonic. Lithium Ion NCR18650B. Available online: https://www.batteryspace.com/prod-specs/ncr18650b.pdf (accessed on 8 July 2024).
- Available online: https://www.ancoo-battery.com/en/product/NCR18650B.html (accessed on 8 July 2024).
- Andre, D.; Meiler, M.; Steiner, K.; Walz, H.; Soczka-Guth, T.; Sauer, D.U. Characterization of high-power lithium-ion batteries by electrochemical impedance spectroscopy. II: Modelling. J. Power Sources 2011, 196, 5349–5356. [Google Scholar] [CrossRef]
- Soto, F.A.; Marzouk, A.; El-Mellouhi, F.; Balbuena, P.B. Understanding Ionic Diffusion through SEI Components for Lithium-Ion and Sodium-Ion Batteries: Insights from First-Principles Calculations. Chem. Mater. 2018, 30, 3315–3322. [Google Scholar] [CrossRef]
- Grepow. What Is the SEI, and What Effect Does It Have on the Battery? | Battery Monday. Available online: https://www.grepow.com/blog/what-is-the-sei-and-what-effect-does-it-have-on-the-battery-battery-monday.html (accessed on 8 July 2024).
- Li, Y.; Luo, L.; Zhang, C.; Liu, H. State of Health Assessment for Lithium-Ion Batteries Using Incremental Energy Analysis and Bidirectional Long Short-Term Memory. World Electr. Veh. J. 2023, 14, 188. [Google Scholar] [CrossRef]





 | Rated Capacity at 25 °C | 3200 mAh |
| Nominal Capacity at 25 °C | Min. 3250 mAh | |
| Typ. 3350 mAh | ||
| Nominal Voltage | 3.6 V | |
| Charging Method | CC-CV | |
| Charging current | 1625 mA | |
| Charging voltage | 4.2 V | |
| Charging Time | 2 h | |
| Cathode material | Nichel Oxide-Based New Platform (NNP) |
| Battery Charge Rates | 0.5 C (B1) | 1 C (B2) | 1.5 C (B3) | |||
|---|---|---|---|---|---|---|
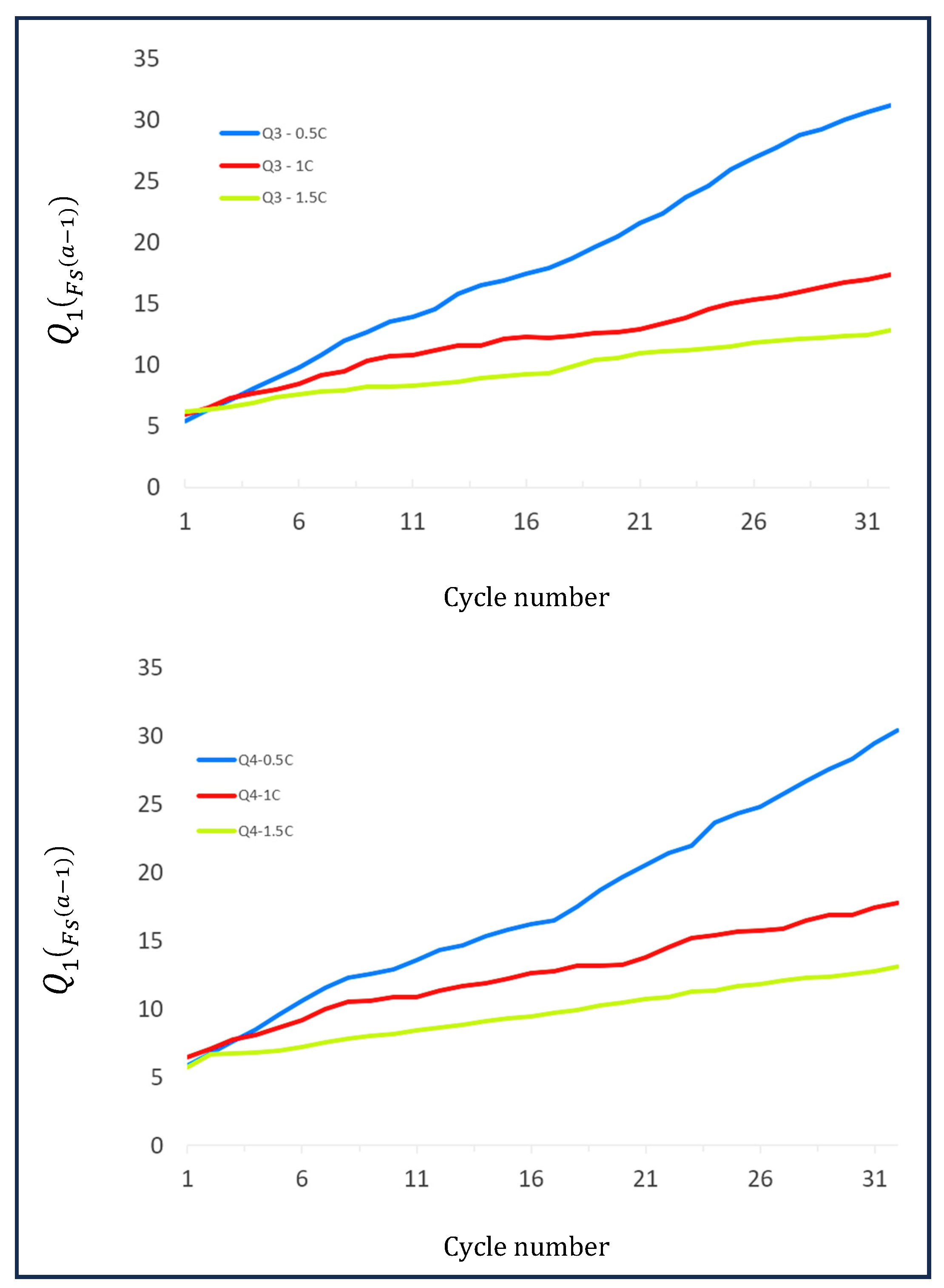
| ΔQ3 | Cycle 21 19.47 | Cycle 31 28.35 | Cycle 21 13.73 | Cycle 31 18.2 | Cycle 21 10.58 | Cycle 21 12.33 |
| ΔQ4 | Cycle 21 18.66 | Cycle 31 27.32 | Cycle 21 13.24 | Cycle 31 16.91 | Cycle 21 10.48 | Cycle 31 12.6 |
| Δcycle | 10 | 10 | 10 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bruj, O.; Calborean, A. EIS Ageing Prediction of Lithium-Ion Batteries Depending on Charge Rates. Batteries 2024, 10, 247. https://doi.org/10.3390/batteries10070247
Bruj O, Calborean A. EIS Ageing Prediction of Lithium-Ion Batteries Depending on Charge Rates. Batteries. 2024; 10(7):247. https://doi.org/10.3390/batteries10070247
Chicago/Turabian StyleBruj, Olivia, and Adrian Calborean. 2024. "EIS Ageing Prediction of Lithium-Ion Batteries Depending on Charge Rates" Batteries 10, no. 7: 247. https://doi.org/10.3390/batteries10070247
APA StyleBruj, O., & Calborean, A. (2024). EIS Ageing Prediction of Lithium-Ion Batteries Depending on Charge Rates. Batteries, 10(7), 247. https://doi.org/10.3390/batteries10070247







