Experimental Research on Thermal-Venting Characteristics of the Failure 280 Ah LiFePO4 Battery: Atmospheric Pressure Impacts and Safety Assessment
Abstract
:1. Introduction
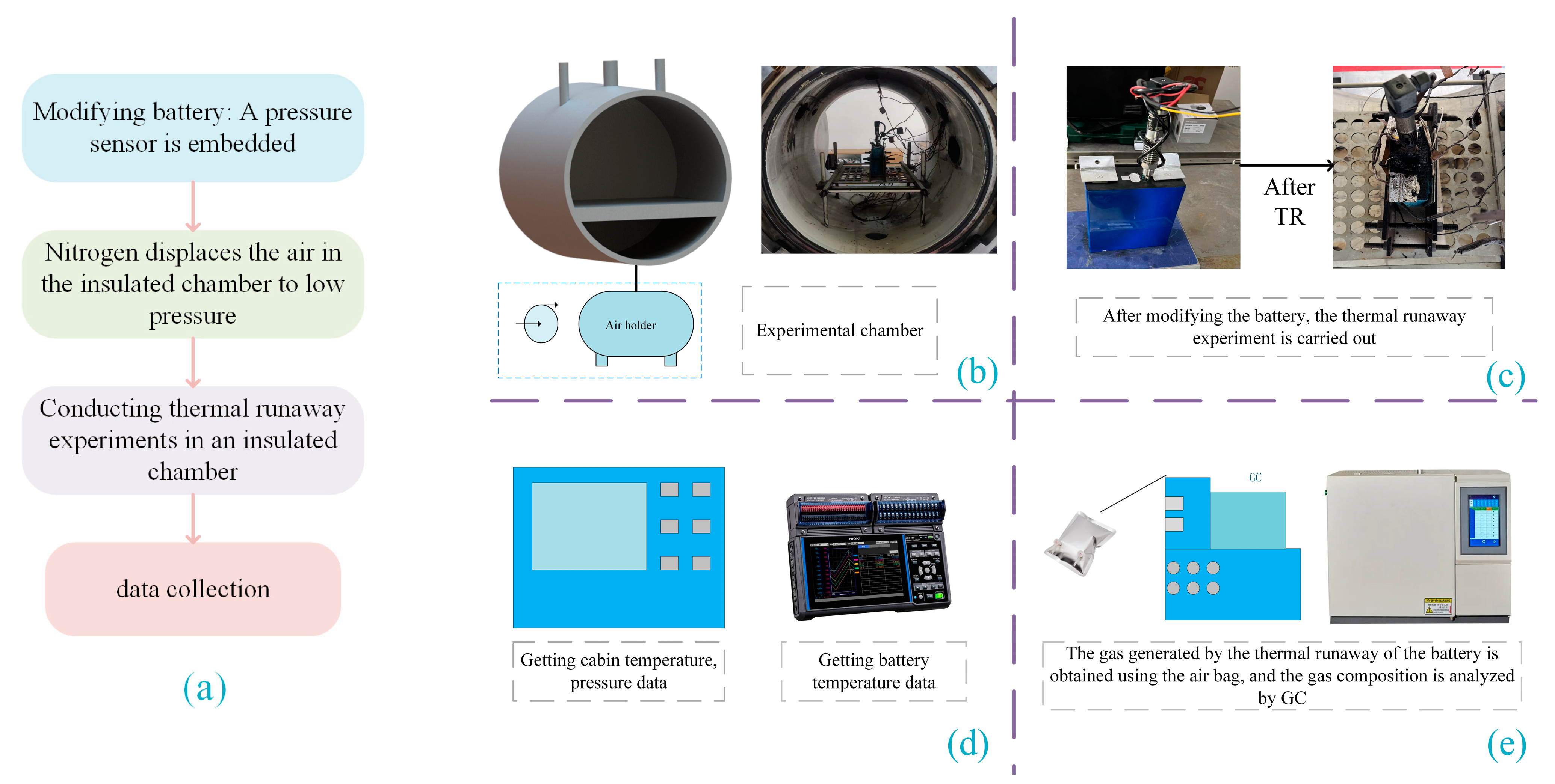
2. Thermal Runaway Experimental Equipment and Methods
2.1. Methodology and Procedure
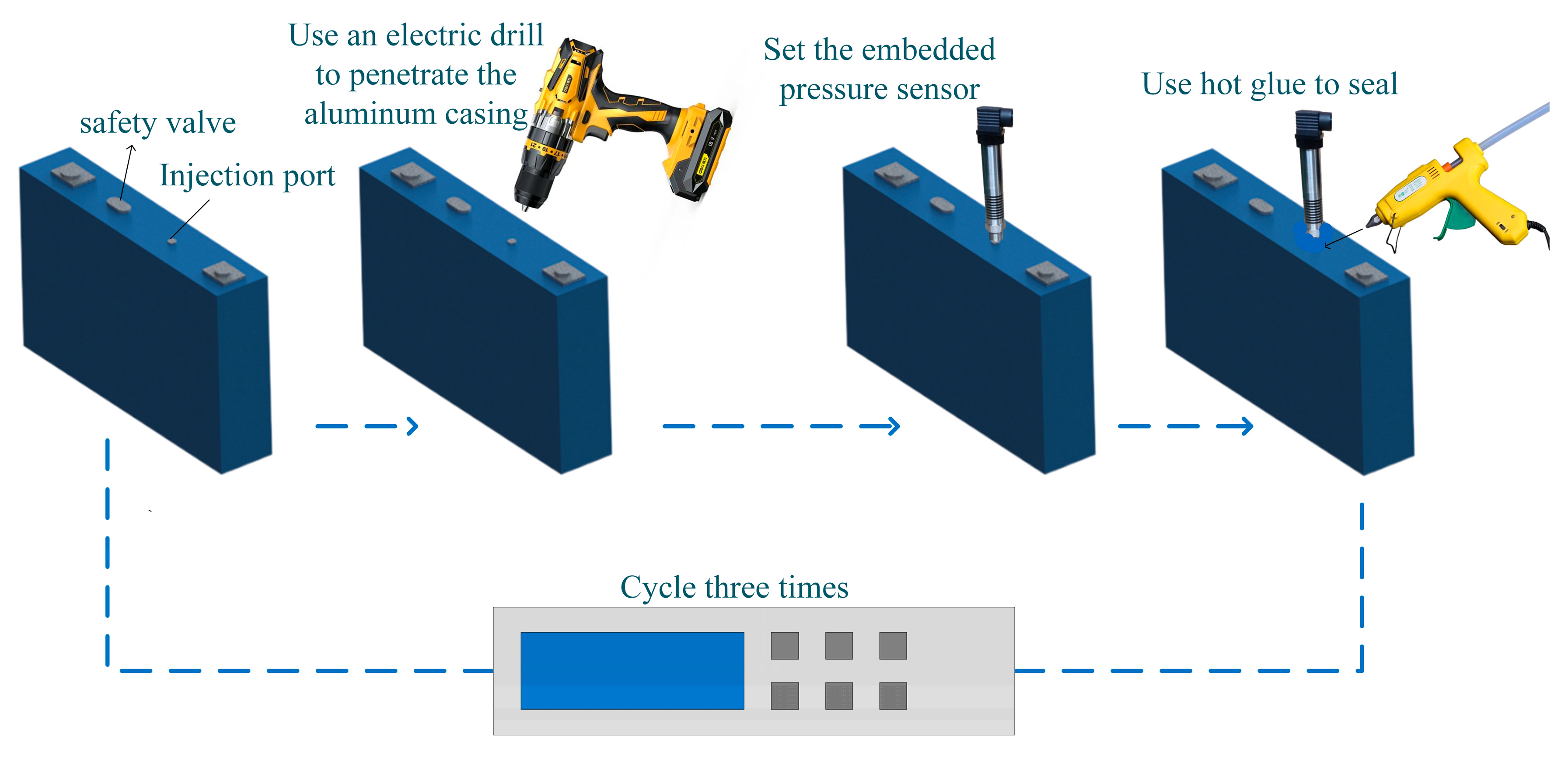
2.2. Battery with Embedded Sensor
2.3. Thermal Runaway Experiment
3. Thermal-Venting Characteristics at 40 kPa
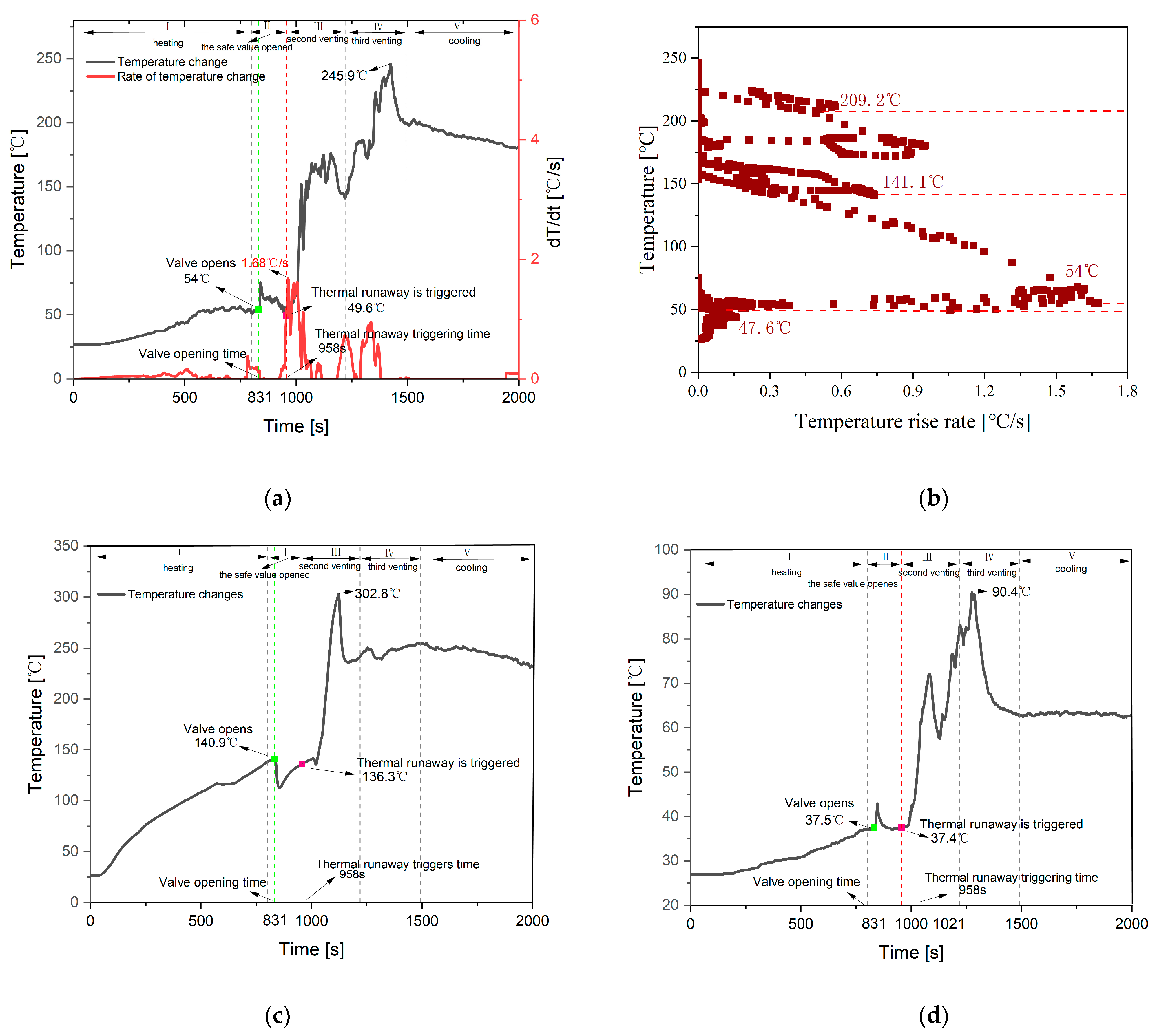
3.1. Feature Temperature
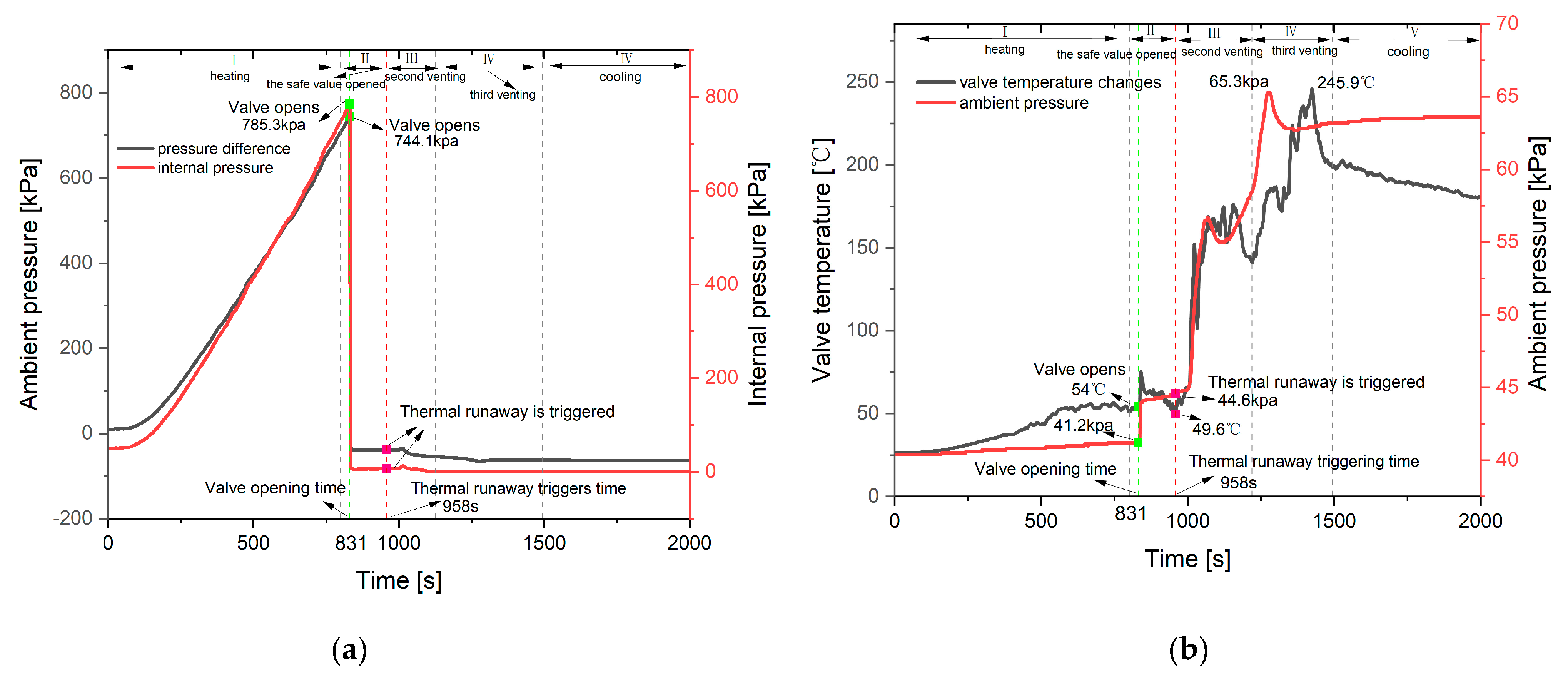
3.2. Internal and Chamber Pressure
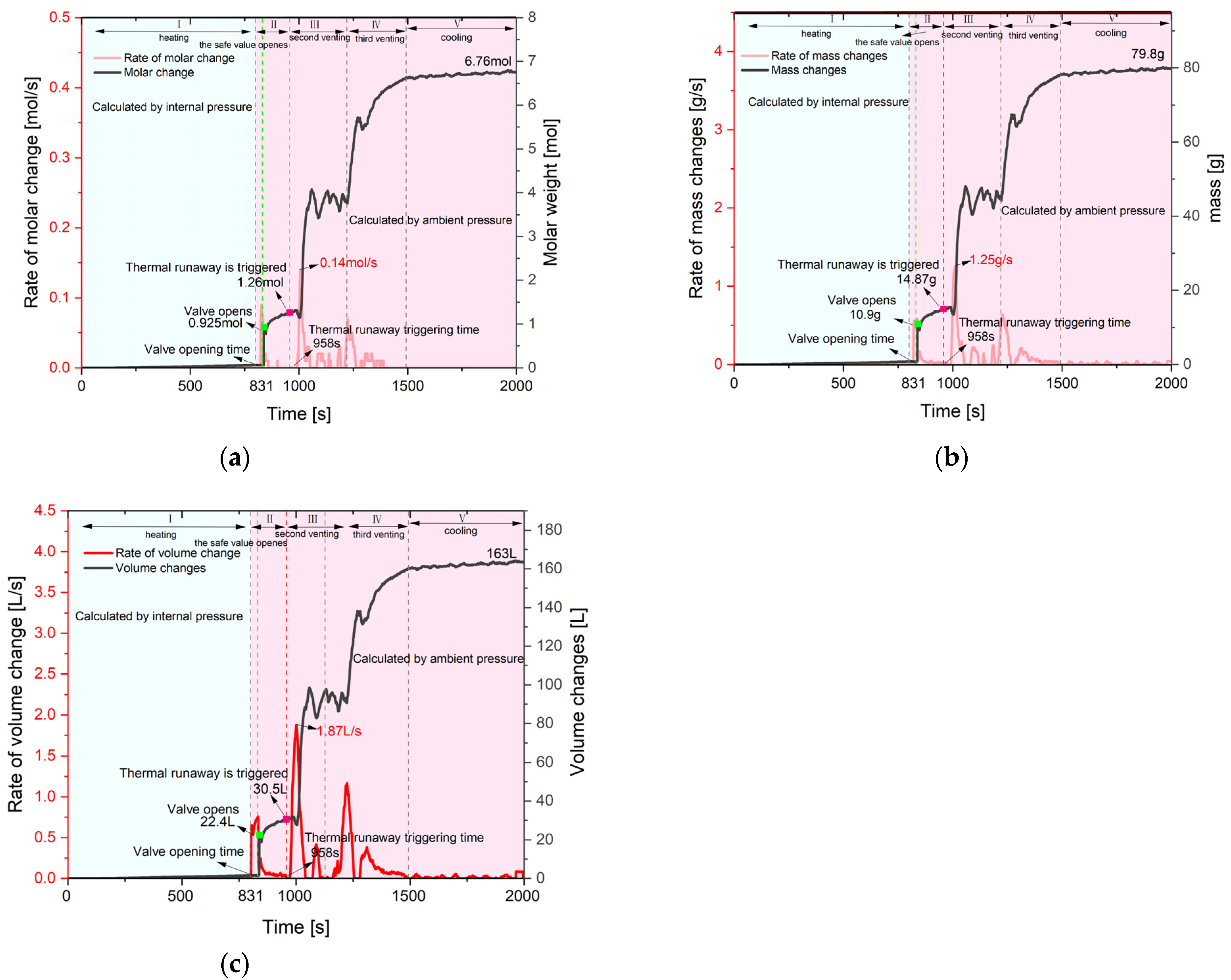
3.3. Venting Flow Characteristics
3.3.1. Component Identification
3.3.2. Flammability Analysis
3.3.3. Gas Content Calculation
3.3.4. Venting Flow Characteristics
4. Pressure Effect on Thermal Behavior
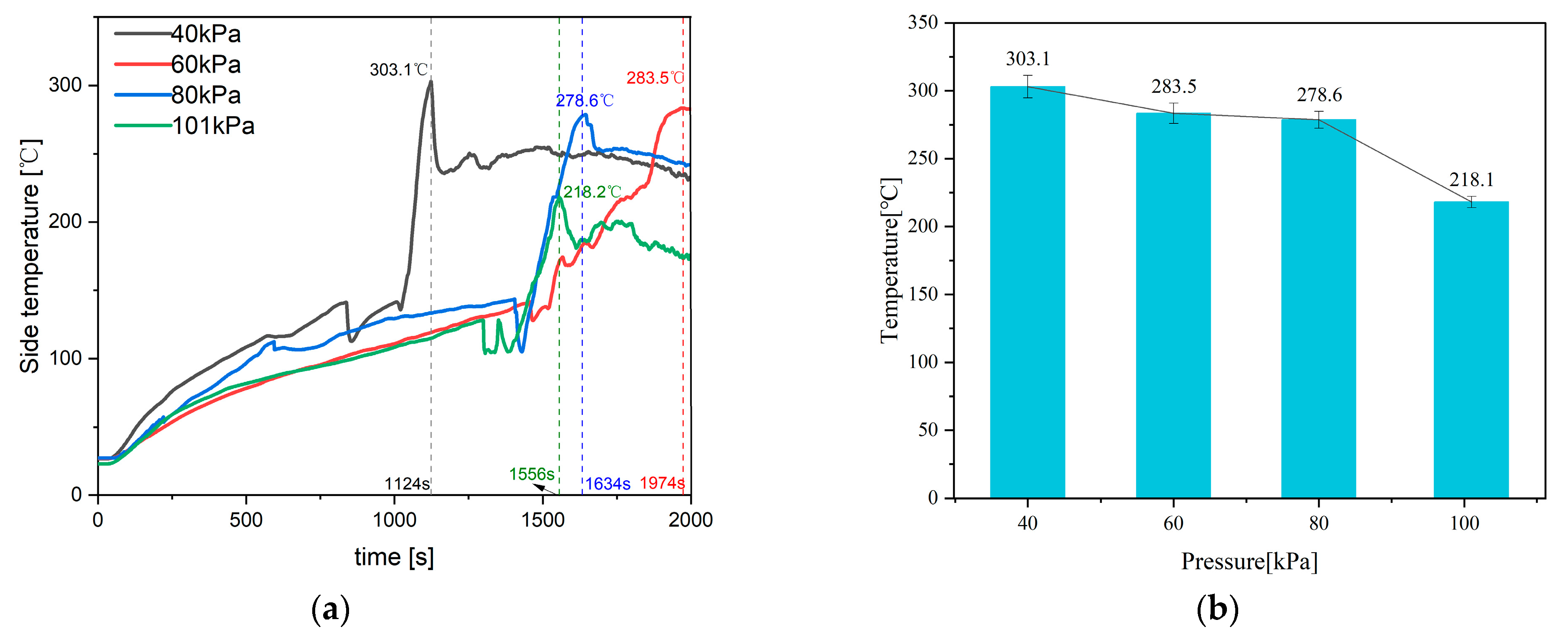
4.1. Feature Temperature
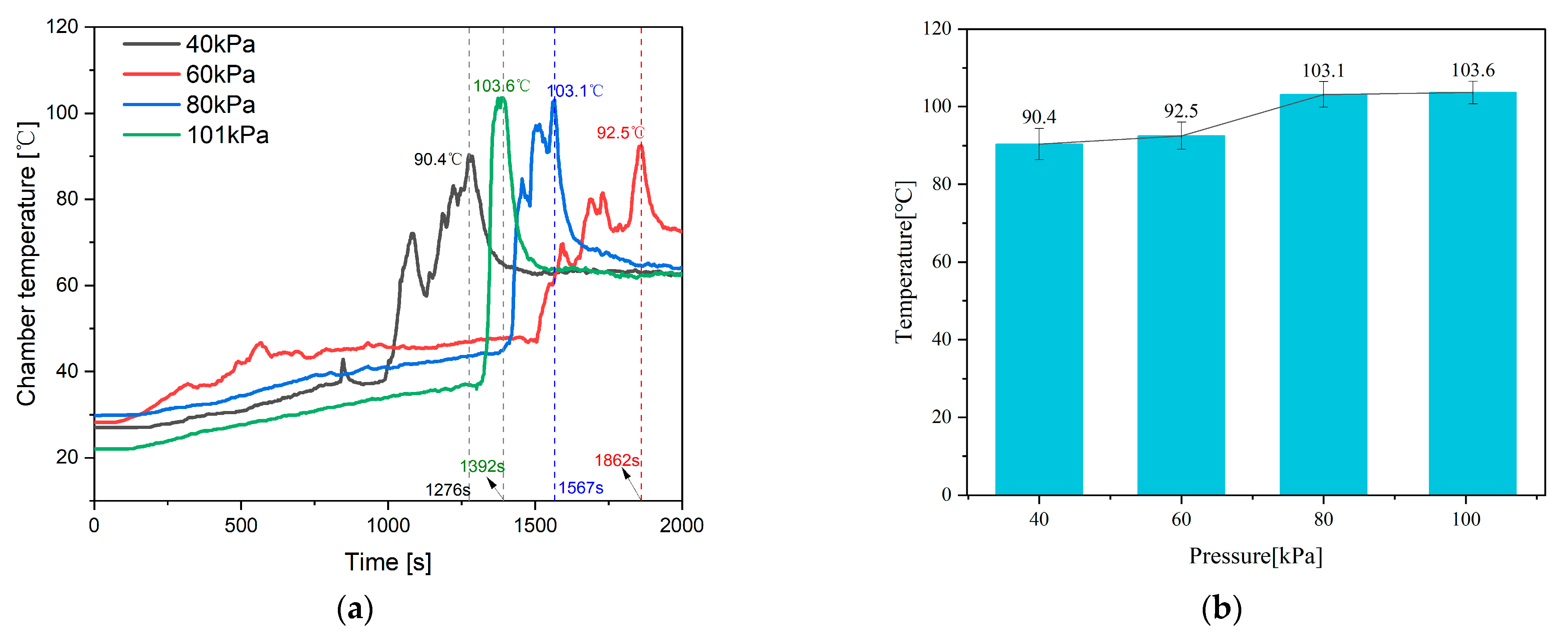
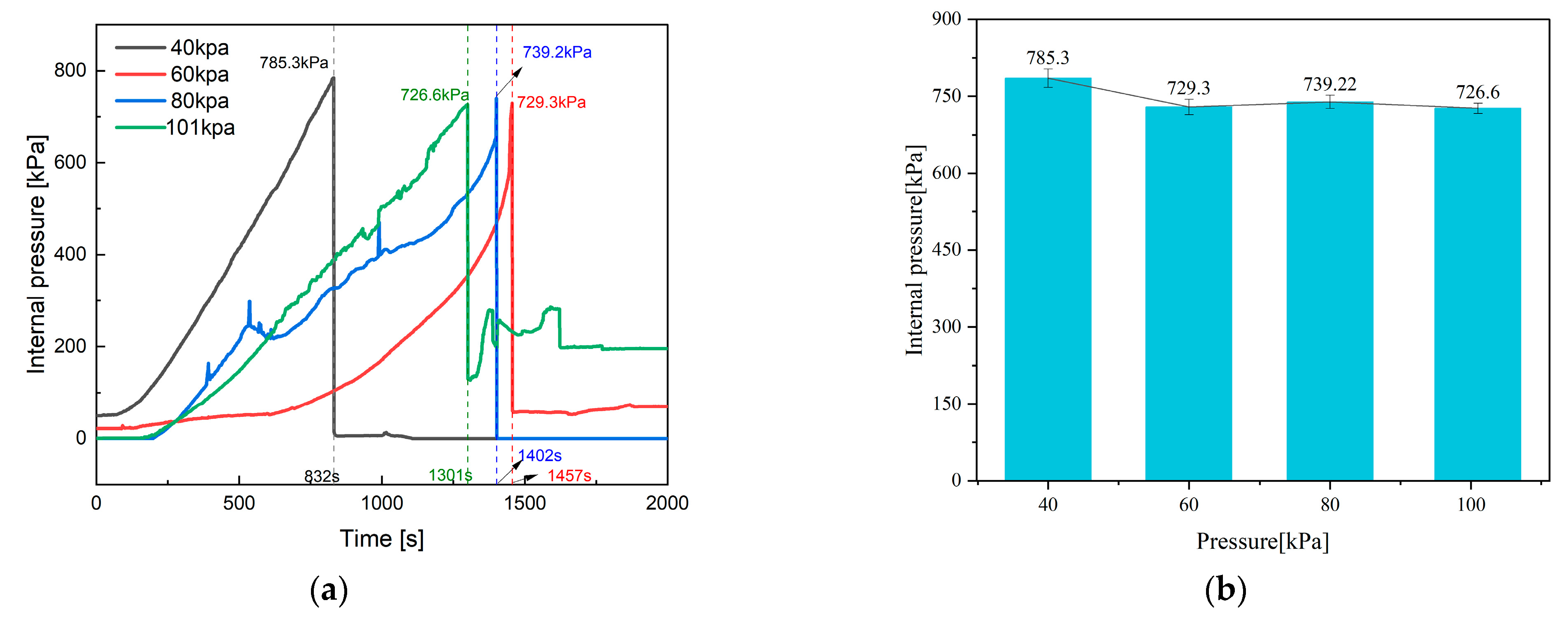
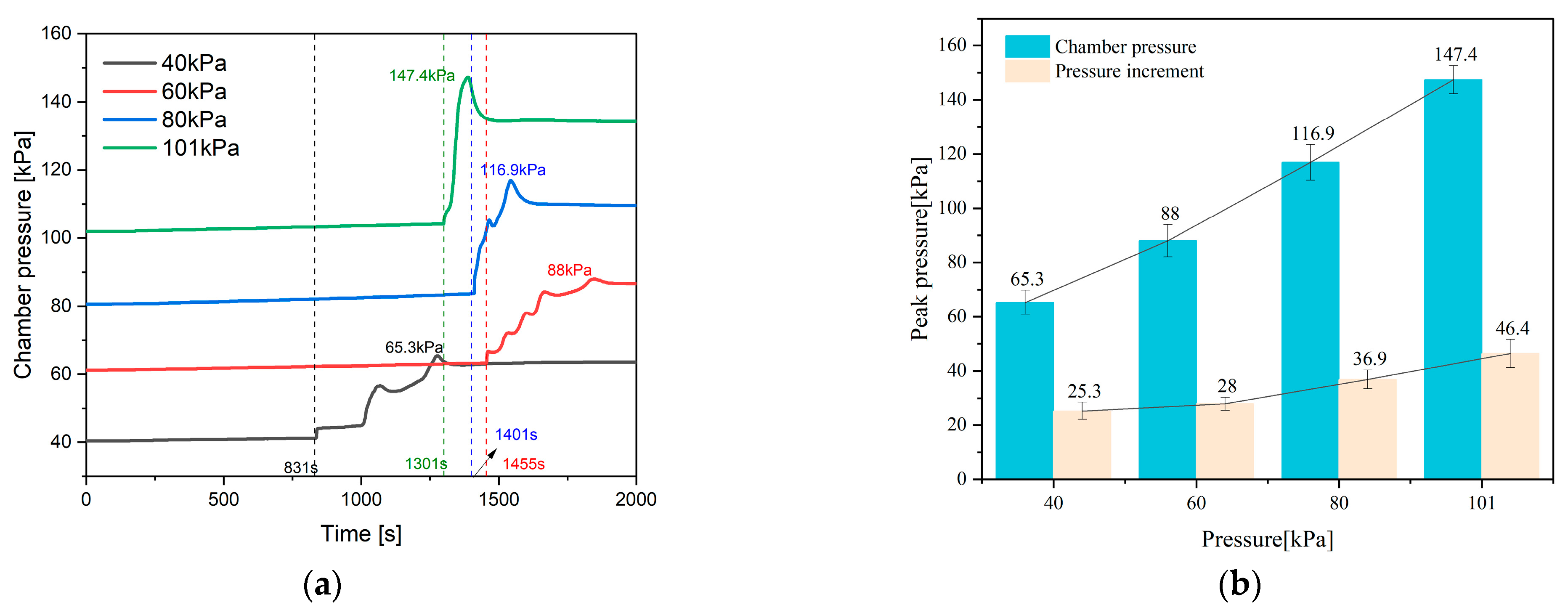
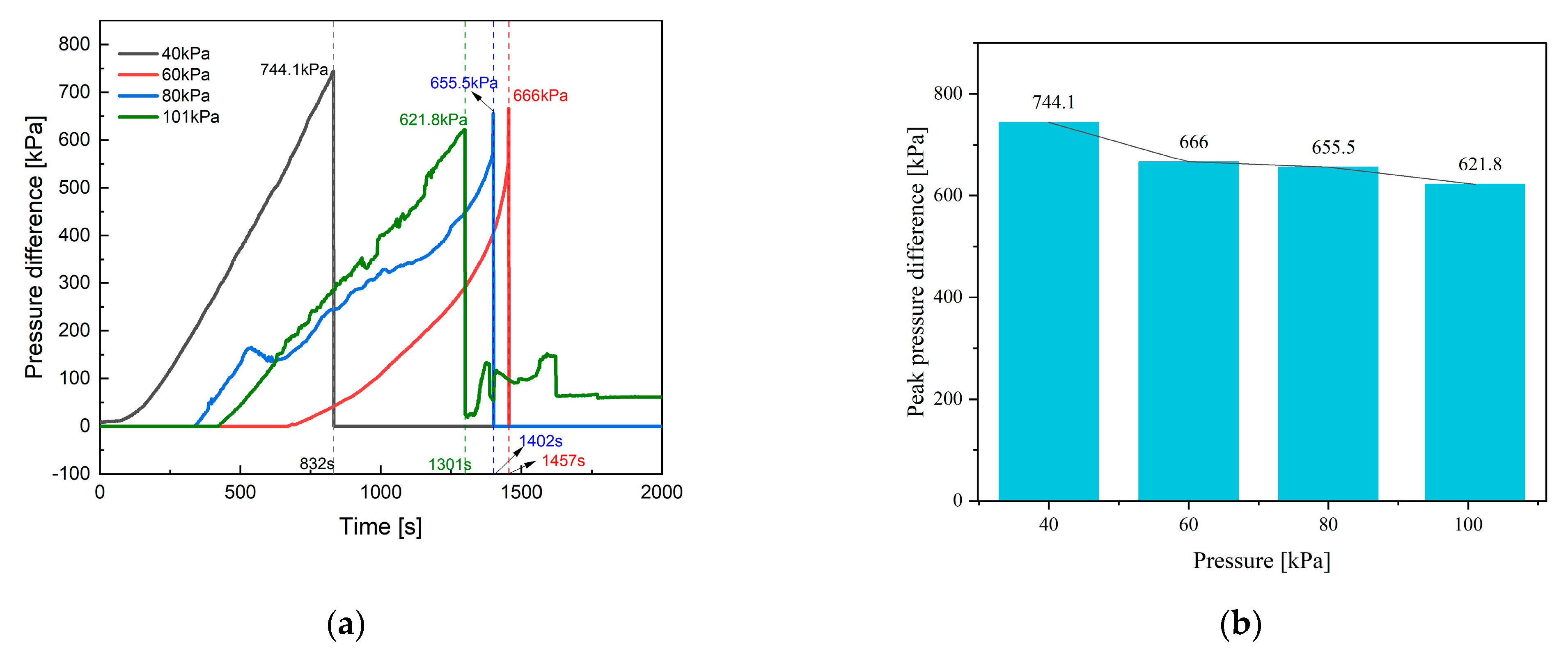
4.2. Internal and Chamber Pressure
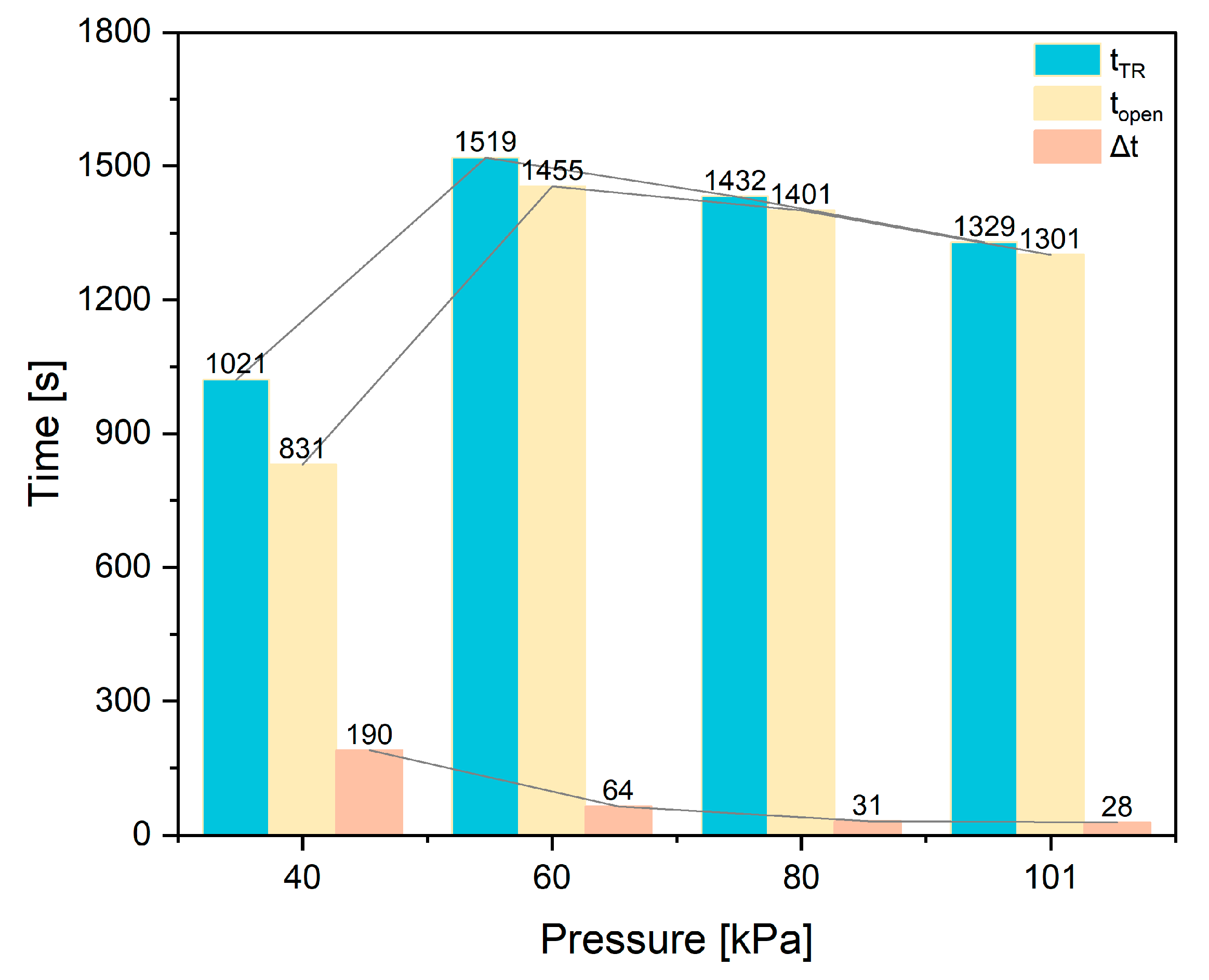
4.3. Feature Time
5. Pressure Effect on Venting Behavior
5.1. Gas Content
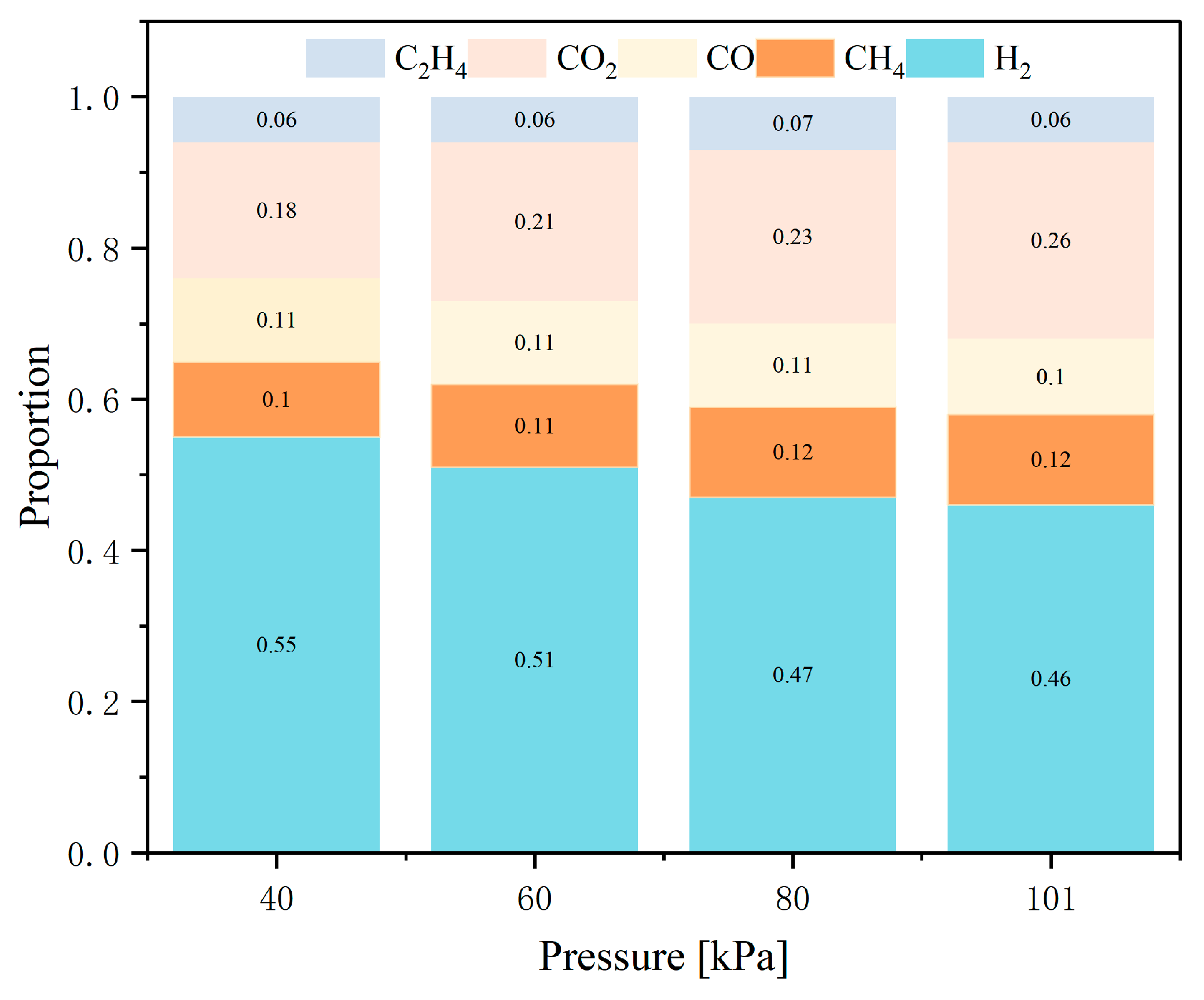
5.2. Gas Composition and Proportion
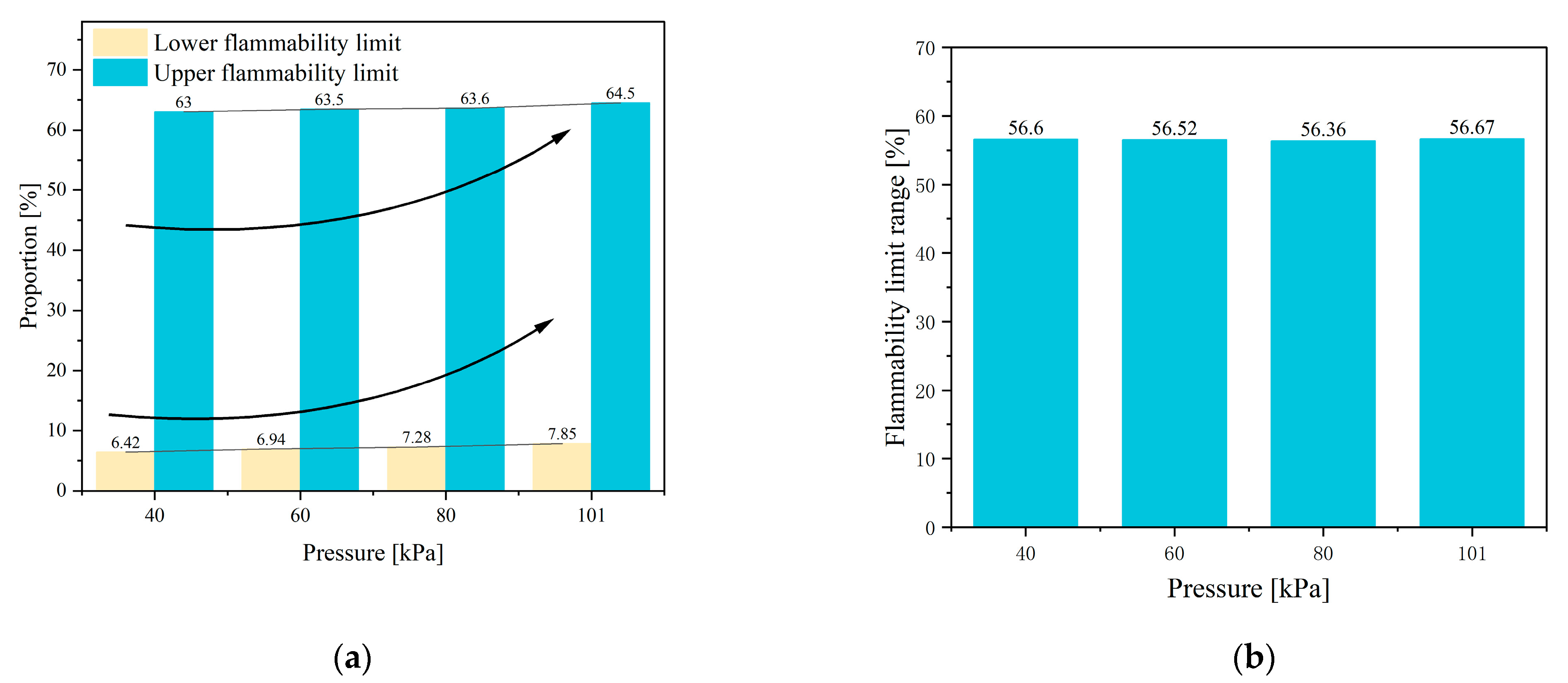
5.3. Flammability Characteristic
6. Safety Assessment
6.1. Methdology
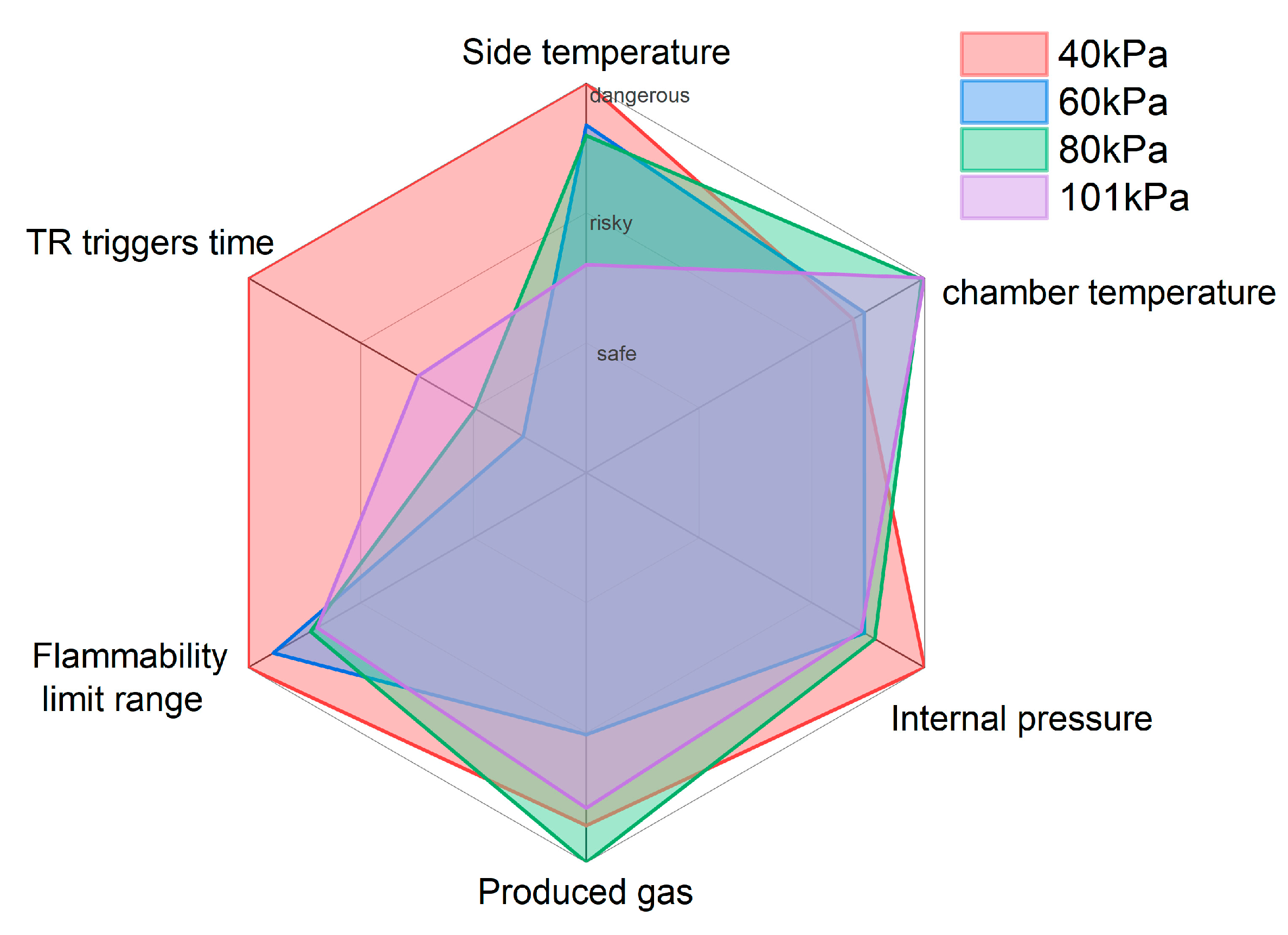
6.2. Six Dimensional Radar Evaluation Results
6.3. Sequence Analysis
7. Conclusions
- The paper measures the TR characteristics at 40 kPa using temperature and pressure sensors. The results indicate that venting occurs twice during the TR process, with corresponding Tv of 163.3 °C and 245.9 °C, and corresponding chamber pressures of 55.3 kPa and 65.3 kPa.
- At 40 kPa, gas chromatography analysis reveals that the highest proportion of H2 in the mixed gas is 55%, followed by CO2 at 18%, and then CO and CH4 at 11% and 10%, respectively. The LEL and UEL are calculated using the Le Chatelier formula and are found to be 6.42% and 63%, respectively.
- The paper further investigates the effects of 40 kPa, 60 kPa, 80 kPa, and 101 kPa on the TR characteristics of the battery. It is found that chamber pressure significantly affects the peak of Ts, Tc, topen, tTR, Δt, gas composition, LEL, and UEL. Conversely, pressure has minimal impact on the internal pressure and gas generation rate of the battery.
- A six-dimensional radar chart analysis method is proposed to evaluate the danger of TR under different pressures. The results show that the most dangerous pressure is at 40 kPa, followed by 80 kPa and 101 kPa, while the 60 kPa is relatively safer.
- At low pressure, it is found that topen and tTR are much shorter than that of the battery at normal pressure. Δt is significantly longer than that of the battery under normal pressure. It is recommended that designers use the opening sound of the safety valve or the gas concentration to catch the opening action quickly and take action at longer time intervals.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wang, Q.; Mao, B.; Stoliarov, S.I.; Sun, J. A review of lithium ion battery failure mechanisms and fire prevention strategies. Prog. Energy Combust. Sci. 2019, 73, 95–131. [Google Scholar] [CrossRef]
- Jia, Z.; Wang, S.; Qin, P.; Li, C.; Song, L.; Cheng, Z.; Jin, K.; Sun, J.; Wang, Q. Comparative investigation of the thermal runaway and gas venting behaviors of large-format LiFePO4 batteries caused by overcharging and overheating. J. Energy Storage 2023, 61, 106791. [Google Scholar] [CrossRef]
- Peng, Y.; Yang, L.; Ju, X.; Liao, B.; Ye, K.; Li, L.; Cao, B.; Ni, Y. A comprehensive investigation on the thermal and toxic hazards of large format lithium-ion batteries with LiFePO4 cathode. J. Hazard. Mater. 2020, 381, 120916. [Google Scholar] [CrossRef] [PubMed]
- Gyatso, N.; Li, Y.; Gao, Z.; Wang, Q.; Li, S.; Yin, Q.; Chen, J.; Jin, P.; Liu, Z.; Ma, Z.; et al. Wind power performance assessment at high plateau region: A case study of the wind farm field test on the Qinghai-Tibet plateau. Appl. Energy 2023, 336, 120789. [Google Scholar] [CrossRef]
- Ren, D.; Feng, X.; Liu, L.; Hsu, H.; Lu, L.; Wang, L.; He, X.; Ouyang, M. Investigating the relationship between internal short circuit and thermal runaway of lithium-ion batteries under thermal abuse condition. Energy Storage Mater. 2021, 34, 563–573. [Google Scholar] [CrossRef]
- Zhu, N.; Wang, X.; Chen, M.; Huang, Q.; Ding, C.; Wang, J. Study on the Combustion Behaviors and Thermal Stability of Aging Lithium-Ion Batteries with Different States of Charge at Low Pressure. PROCESS Saf. Environ. Prot. 2023, 174, 391–402. [Google Scholar] [CrossRef]
- Zhang, Q.; Liu, T.; Wang, Q. Experimental study on the influence of different heating methods on thermal runaway of lithium-ion battery. J. Energy Storage 2021, 42, 103063. [Google Scholar] [CrossRef]
- Feng, X.; Ouyang, M.; Liu, X.; Lu, L.; Xia, Y.; He, X. Thermal runaway mechanism of lithium ion battery for electric vehicles: A review. Energy Storage Mater. 2018, 10, 246–267. [Google Scholar] [CrossRef]
- Tran, M.-K.; Mevawalla, A.; Aziz, A.; Panchal, S.; Xie, Y.; Fowler, M. A Review of Lithium-Ion Battery Thermal Runaway Modeling and Diagnosis Approaches. Processes 2022, 10, 1192. [Google Scholar] [CrossRef]
- Li, Y.; Feng, X.; Ren, D.; Ouyang, M.; Lu, L.; Han, X. Thermal Runaway Triggered by Plated Lithium on the Anode after Fast Charging. ACS Appl. Mater. Interfaces 2019, 11, 46839–46850. [Google Scholar] [CrossRef]
- Guo, Q.; Zhang, J.; Zhou, C.; Huang, Z.; Han, D. Thermal Runaway Behaviors and Kinetics of NCM Lithium-Ion Batteries at Different Heat Dissipation Conditions. J. Electrochem. Soc. 2023, 170, 080507. [Google Scholar] [CrossRef]
- Xu, X.; Sun, X.; Zhao, L.; Li, R.; Tang, W. Research on thermal runaway characteristics of NCM lithium-ion battery under thermal-electrical coupling abuse. Ionics 2022, 28, 5449–5467. [Google Scholar] [CrossRef]
- Pastor, J.V.; García, A.; Monsalve-Serrano, J.; Golke, D. Analysis of the aging effects on the thermal runaway characteristics of Lithium-Ion cells through stepwise reactions. Appl. Therm. Eng. 2023, 230, 120685. [Google Scholar] [CrossRef]
- Li, Q.; Yang, C.; Santhanagopalan, S.; Smith, K.; Lamb, J.; Steele, L.A.; Torres-Castro, L. Numerical investigation of thermal runaway mitigation through a passive thermal management system. J. Power Sources 2019, 429, 80–88. [Google Scholar] [CrossRef]
- Qian, F.; Wang, H.; Li, M.; Li, C.; Shen, H.; Wang, J.; Li, Y.; Ouyang, M. Thermal Runaway Vent Gases from High-Capacity Energy Storage LiFePO4 Lithium Iron. Energies 2023, 16, 3485. [Google Scholar] [CrossRef]
- Chen, S.; Wang, Z.; Wang, J.; Tong, X.; Yan, W. Lower explosion limit of the vented gases from Li-ion batteries thermal runaway in high temperature condition. J. Loss Prev. Process. Ind. 2020, 63, 103992. [Google Scholar] [CrossRef]
- Zhang, Q.; Niu, J.; Yang, J.; Liu, T.; Bao, F.; Wang, Q. In-situ explosion limit analysis and hazards research of vent gas from lithium-ion battery thermal runaway. J. Energy Storage 2022, 56, 106146. [Google Scholar] [CrossRef]
- Baird, A.R.; Archibald, E.J.; Marr, K.C.; Ezekoye, O.A. Explosion hazards from lithium-ion battery vent gas. J. Power Sources 2020, 446, 227257. [Google Scholar] [CrossRef]
- Zhang, F.; Feng, X.; Xu, C.; Jiang, F.; Ouyang, M. Thermal runaway front in failure propagation of long-shape lithium-ion battery. Int. J. Heat Mass Transf. 2022, 182, 121928. [Google Scholar] [CrossRef]
- Huang, Q.; Weng, J.; Ouyang, D.; Chen, M.; Wang, X.; Wang, J. Comparative studies on the combustion characteristics of electrolytes and carbonate mixed solvents with flame retardant additives under low pressures. Case Stud. Therm. Eng. 2023, 43, 102810. [Google Scholar] [CrossRef]
- Zou, K.; Chen, X.; Ding, Z.; Gu, J.; Lu, S. Jet behavior of prismatic lithium-ion batteries during thermal runaway. Appl. Therm. Eng. 2020, 179, 115745. [Google Scholar] [CrossRef]
- Kang, R.; Jia, C.; Zhao, J.; Zhao, L.; Zhang, J. Effects of capacity on the thermal runaway and gas venting behaviors of large-format lithium iron phosphate batteries induced by overcharge. J. Energy Storage 2024, 87, 111523. [Google Scholar] [CrossRef]
- Wang, Z.; Jiang, X.; Ke, W.; Wang, W.; Zhang, S.; Zhou, B. Effect of lithium-ion battery diameter on thermal runaway propagation rate under one-dimensional linear arrangement. Therm. Sci. Eng. Prog. 2022, 31, 101301. [Google Scholar] [CrossRef]
- Liu, Y.; Niu, H.; Liu, J.; Huang, X. Layer-to-layer thermal runaway propagation of open-circuit cylindrical li-ion batteries: Effect of ambient pressure. J. Energy Storage 2022, 55, 105709. [Google Scholar] [CrossRef]
- Li, Y.; Jiang, L.; Huang, Z.; Jia, Z.; Qin, P.; Wang, Q. Pressure Effect on the Thermal Runaway Behaviors of Lithium-Ion Battery in Confined Space. Fire Technol. 2023, 59, 1137–1155. [Google Scholar] [CrossRef]
- Liu, Q.; Zhu, Q.; Zhu, W.; Yi, X.; Han, X. Thermal Runaway Characteristics of 18650 NCM Lithium-ion Batteries under the Different Initial Pressures. Electrochemistry 2022, 90, 087004. [Google Scholar] [CrossRef]
- Ding, C.; Zhu, N.; Yu, J.; Li, Y.; Sun, X.; Liu, C.; Huang, Q.; Wang, J. Experimental investigation of environmental pressure effects on thermal runaway properties of 21700 lithium-ion batteries with high energy density. Case Stud. Therm. Eng. 2022, 38, 102349. [Google Scholar] [CrossRef]
- Sun, Q.; Liu, H.; Zhi, M.; Chen, X.; Lv, P.; He, Y. Thermal characteristics of thermal runaway for pouch lithium-ion battery with different state of charges under various ambient pressures. J. Power Sources 2022, 527, 231175. [Google Scholar] [CrossRef]
- Zhao, J.; Feng, X.; Tran, M.-K.; Fowler, M.; Ouyang, M.; Burke, A.F. Battery safety: Fault diagnosis from laboratory to real world. J. Power Sources 2024, 598, 234111. [Google Scholar] [CrossRef]
- Qiu, M.; Liu, J.; Cong, B.; Cui, Y. Research Progress in Thermal Runaway Vent Gas Characteristics of Li-Ion Battery. Batteries 2023, 9, 411. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, H.; Wang, Y.; Li, C.; Liu, Y.; Ouyang, M. Thermal abusive experimental research on the large-format lithium-ion battery using a buried dual-sensor. J. Energy Storage 2021, 33, 102156. [Google Scholar] [CrossRef]
- Song, L.; Zheng, Y.; Xiao, Z.; Wang, C.; Long, T. Review on Thermal Runaway of Lithium-Ion Batteries for Electric Vehicles. J. Electron. Mater. 2022, 51, 30–46. [Google Scholar] [CrossRef]
- Sun, Y.; Jin, Y.; Jiang, Z.; Li, L. A review of mitigation strategies for li-ion battery thermal runaway. Eng. Fail. Anal. 2023, 149, 107259. [Google Scholar] [CrossRef]
- Shen, H.; Wang, H.; Li, M.; Li, C.; Zhang, Y.; Li, Y.; Yang, X.; Feng, X.; Ouyang, M. Thermal Runaway Characteristics and Gas Composition Analysis of Lithium-Ion Batteries with Different LFP and NCM Cathode Materials under Inert Atmosphere. Electronics 2023, 12, 1603. [Google Scholar] [CrossRef]
- Ohsaki, T.; Kishi, T.; Kuboki, T.; Takami, N.; Shimura, N.; Sato, Y.; Sekino, M.; Satoh, A. Overcharge reaction of lithium-ion batteries. J. Power Sources 2005, 146, 97–100. [Google Scholar] [CrossRef]
- Wang, H.; Du, Z.; Liu, L.; Zhang, Z.; Hao, J.; Wang, Q.; Wang, S. Study on the Thermal Runaway and Its Propagation of Lithium-Ion Batteries Under Low Pressure. Fire Technol. 2020, 56, 2427–2440. [Google Scholar] [CrossRef]
- Yu, R.; Liu, J.; Liang, W.; Law, C.K.; Wang, H.; Ouyang, M. On flammability limits of battery vent gas: Role of diffusion, radiation and chemical kinetics. Combust. Flame 2023, 249, 112631. [Google Scholar] [CrossRef]



| Parameters | Value |
|---|---|
| Weight | 5.34 ± 0.3 kg |
| End of charge voltage | 3.65 V |
| End of discharge voltage | 2.5 V |
| Specific heat | 1029.49 J/(kg∙°C) |
| Stage | Ⅰ | Ⅱ | Ⅲ | Ⅳ | Ⅴ |
|---|---|---|---|---|---|
| Time (s) | 0–800 | 800–958 | 958–1220 | 1220–1493 | 1493–2000 |
| Basic features | 1. Heating period. 2. Tv and Tc increase slowly. | 1. The safety valve opens. 2. Tv increases dramatically, and the first crest occurs, respectively. | 1. TR is triggered. 2. Tv and Tc increase dramatically, and the second crest occurs, respectively. | 1. The second venting occurs. 2. Tv and Tc decrease after the third peak. | 1. Cooling stage. 2. The temperature drops. |
| Temperature range (°C) | Tv | 26.6–54 °C | 49.6–74.6 °C | 49.6–176.2 °C | 141.8–245.9 °C |
| Ts | 112.7–138.4 °C | 112.7–140.9 °C | 136.3–302.8 °C | 242.1–254.5 °C | |
| Tc | 27–37 °C | 37–42.9 °C | 37.4–63.1 °C | 63–90.4 °C |
| Parameter | LEL (%) | UEL (%) |
|---|---|---|
| H2 | 4 | 75 |
| CO | 12.5 | 74 |
| CH4 | 5 | 15 |
| C2H4 | 2.7 | 36 |
| Mixed gas | 6.42 | 63 |
| Flammability limit range | 56.6 | |
| Parameter | 40 | 60 | 80 | 101 | |
|---|---|---|---|---|---|
| The first peak | Value (mol/s) | 0.09 | 0.06 | 0.1 | 0.38 |
| Time (s) | 831 | 1456 | 1411 | 1336 | |
| The second peak | Value (mol/s) | 0.14 | 0.1 | 0.06 | \ |
| Time (s) | 1012 | 1638 | 1455 | \ | |
| The third peak | Value (mol/s) | 0.07 | 0.04 | 0.06 | \ |
| Time (s) | 1228 | 1778 | 1520 | \ | |
| Parameter | 40 kPa | 60 kPa | 80 kPa | 101 kPa |
|---|---|---|---|---|
| Side temperature [°C] | 100 | 93.5 | 91.9 | 72 |
| Chamber temperature [°C] | 87.3 | 89.3 | 99.5 | 100 |
| Internal pressure [kPa] | 100 | 92.9 | 94.1 | 92.5 |
| Gas content [mol] | 94.3 | 80.3 | 100 | 91.6 |
| Lower flammable limit [%] | 100 | 92 | 86.6 | 77.7 |
| TR triggers time [s] | 100 | 51.2 | 59.7 | 69.8 |
| Average scores | 96.9 | 83.2 | 88.6 | 83.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Wang, Y.; Zhao, J.; Li, H.; Xu, C.; Li, Y.; Wang, H.; Lu, L.; Dai, F.; Yu, R.; et al. Experimental Research on Thermal-Venting Characteristics of the Failure 280 Ah LiFePO4 Battery: Atmospheric Pressure Impacts and Safety Assessment. Batteries 2024, 10, 270. https://doi.org/10.3390/batteries10080270
Wang Y, Wang Y, Zhao J, Li H, Xu C, Li Y, Wang H, Lu L, Dai F, Yu R, et al. Experimental Research on Thermal-Venting Characteristics of the Failure 280 Ah LiFePO4 Battery: Atmospheric Pressure Impacts and Safety Assessment. Batteries. 2024; 10(8):270. https://doi.org/10.3390/batteries10080270
Chicago/Turabian StyleWang, Yu, Yan Wang, Jingyuan Zhao, Hongxu Li, Chengshan Xu, Yalun Li, Hewu Wang, Languang Lu, Feng Dai, Ruiguang Yu, and et al. 2024. "Experimental Research on Thermal-Venting Characteristics of the Failure 280 Ah LiFePO4 Battery: Atmospheric Pressure Impacts and Safety Assessment" Batteries 10, no. 8: 270. https://doi.org/10.3390/batteries10080270






