Addition of a Polar, Porous Phase-Inversion-PVDF Membrane to Lithium–Sulfur Cells (LSBs) Already with a Microporous Polypropylene Separator Enhances the Battery Performance
Abstract
:1. Introduction
| 1st voltage plateau | 3 S8 + 6 Li+ + 6 e− ↔ 3 Li2S8 |
| sulfur utilization | 3 Li2S8 +2 Li+ + 2 e− ↔ 4 Li2S6 |
| 4 Li2S6 + 4 Li++ 4 e− ↔ 6 Li2S4 | |
| 2nd voltage plateau | 6 Li2S4 + 12 Li+ + 12 e− ↔ 12 Li2S2 |
| polysulfide conversion | 12 Li2S2 + 24 Li+ + 24 e− ↔ 24 Li2S |
2. Experimental
2.1. Materials
2.2. PVDF Membrane Fabrication by Phase Inversion
2.3. Film Characterization
2.4. Membrane Porosity and Electrolyte Uptake
2.5. Electrodes and Electrochemical Characterization
3. Results and Discussion
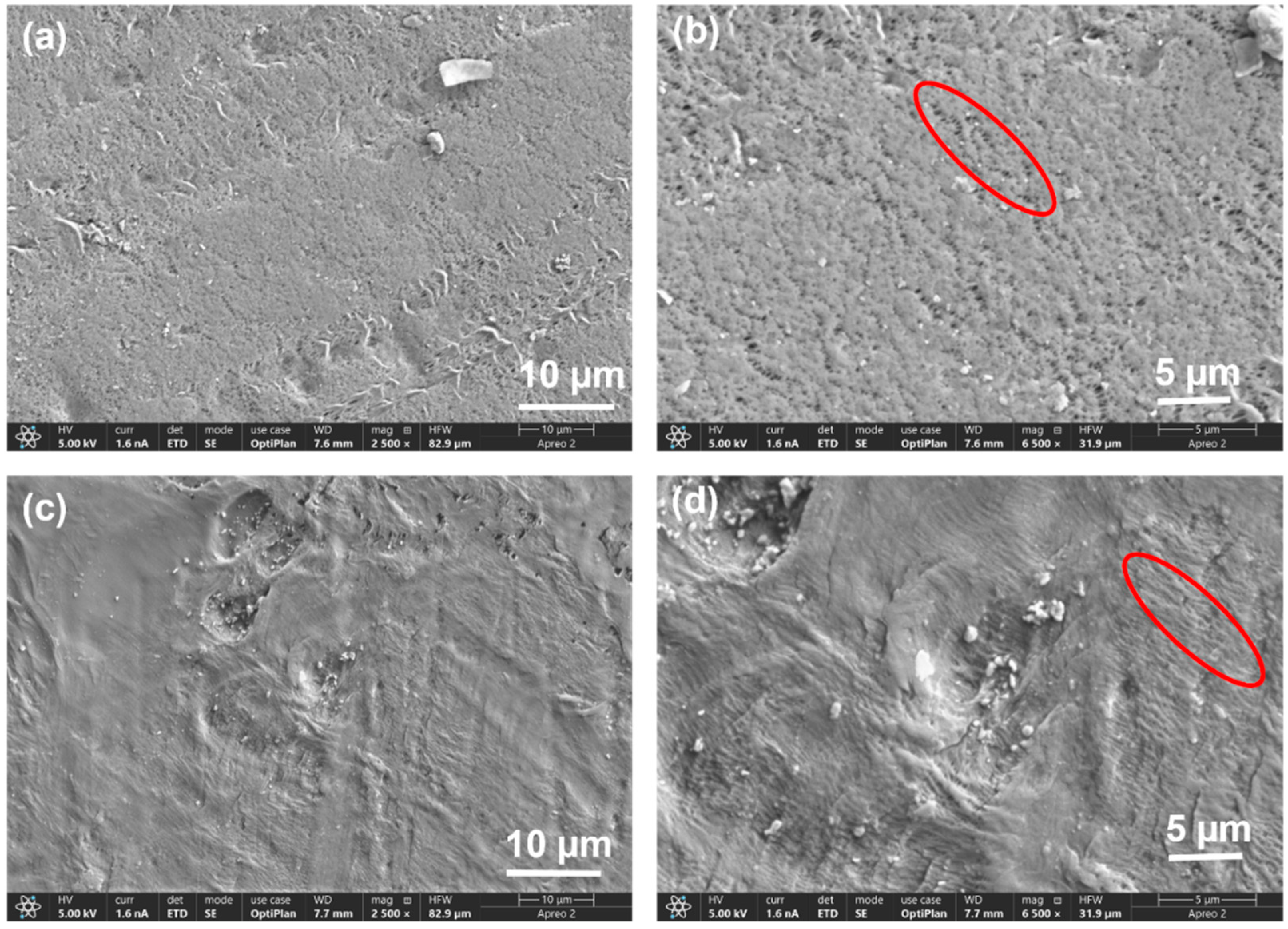
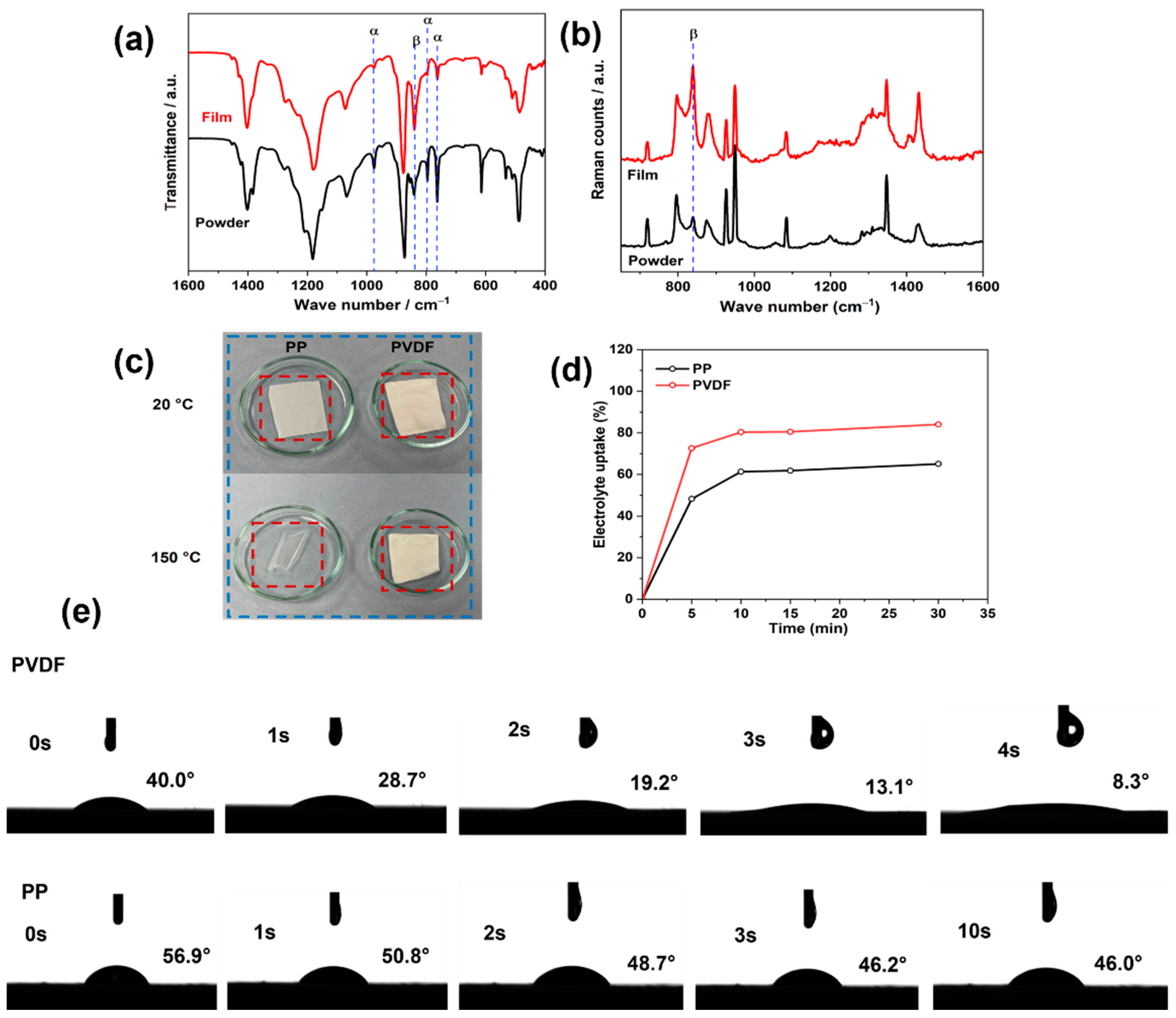
3.1. Characterization of the PVDF Membrane
3.2. Visual Studies of Polysulfide Permeation through Separator Membranes
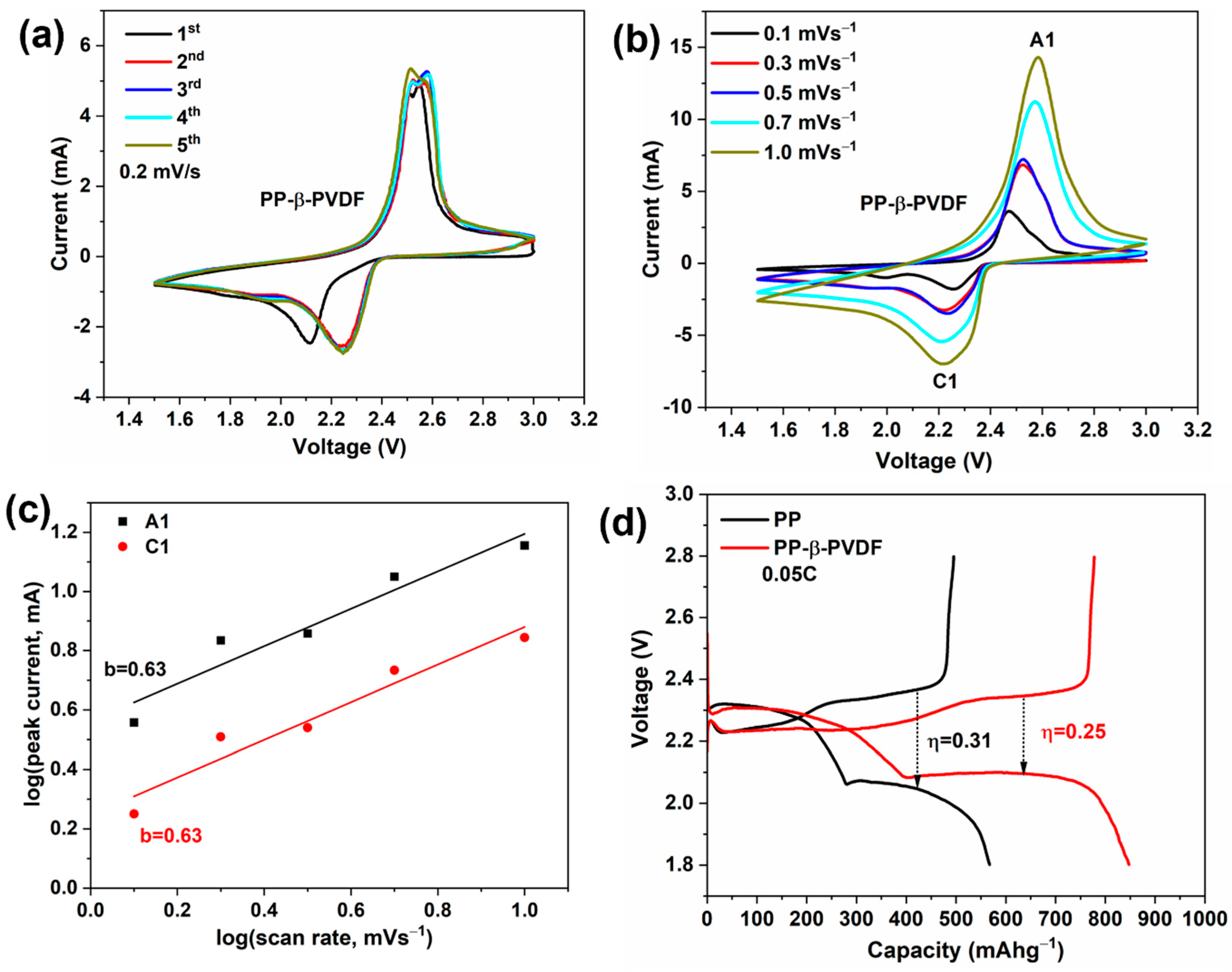
3.3. CV Traces and the First GDC Cycle
3.4. Detailed Electrochemical Cycling Studies of LSBs with the Hybrid Separator Combination and with PP Only
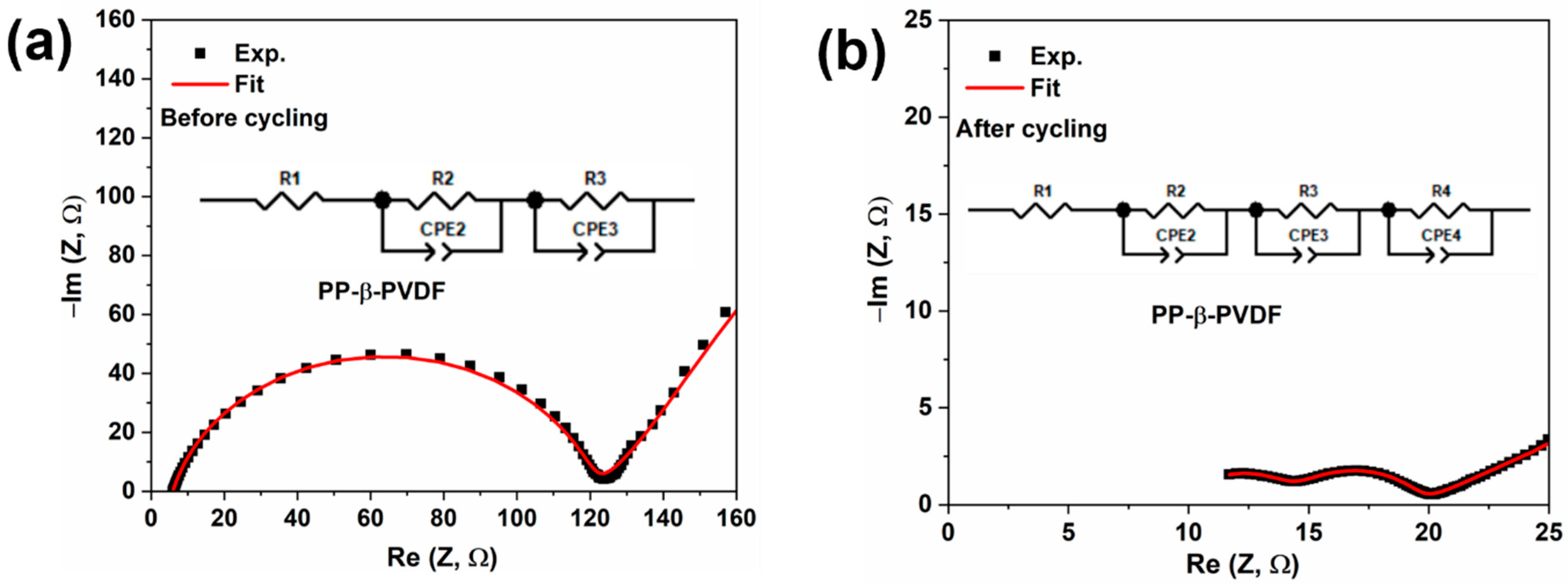
3.5. EIS Investigation of Cells before and after 100 Cycles
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Armand, M.; Tarascon, J.-M. Building Better Batteries. Nature 2008, 451, 652–657. [Google Scholar]
- Slater, M.D.; Kim, D.; Lee, E.; Johnson, C.S. Sodium-Ion Batteries. Adv. Funct. Mater. 2013, 23, 947–958. [Google Scholar]
- Aurbach, D.; Lu, Z.; Schechter, A.; Gofer, Y.; Gizbar, H.; Turgeman, R.; Cohen, Y.; Moshkovich, M.; Levi, E. Prototype Systems for Rechargeable Magnesium Batteries. Nature 2000, 407, 724–727. [Google Scholar]
- Ponrouch, A.; Frontera, C.; Bardé, F.; Palacín, M.R. Towards a Calcium-Based Rechargeable Battery. Nat. Mater. 2016, 15, 169–172. [Google Scholar]
- Jayaprakash, N.; Das, S.K.; Archer, L.A. The Rechargeable Aluminum-Ion Battery. Chem. Commun. 2011, 47, 12610–12612. [Google Scholar]
- Mohammad, I.; Witter, R.; Fichtner, M.; Anji Reddy, M. Room-Temperature, Rechargeable Solid-State Fluoride-Ion Batteries. ACS Appl. Energy Mater. 2018, 1, 4766–4775. [Google Scholar]
- Pang, Q.; Liang, X.; Kwok, C.Y.; Nazar, L.F. Advances in Lithium–Sulfur Batteries Based on Multifunctional Cathodes and Electrolytes. Nat. Energy 2016, 1, 16132. [Google Scholar]
- Tarascon, J.-M.; Armand, M. Issues and Challenges Facing Rechargeable Lithium Batteries. Nature 2001, 414, 359–367. [Google Scholar] [PubMed]
- Fu, C.; Guo, J. Challenges and Current Development of Sulfur Cathode in Lithium–Sulfur Battery. Curr. Opin. Chem. Eng. 2016, 13, 53–62. [Google Scholar]
- Kolosnitsyn, V.S.; Kuzmina, E.V.; Karaseva, E.V. On the Reasons for Low Sulphur Utilization in the Lithium–Sulphur Batteries. J. Power Sources 2015, 274, 203–210. [Google Scholar]
- Manthiram, A.; Fu, Y.; Su, Y.-S. Challenges and Prospects of Lithium–Sulfur Batteries. Acc. Chem. Res. 2013, 46, 1125–1134. [Google Scholar]
- Li, Y.; Shapter, J.G.; Cheng, H.; Xu, G.; Gao, G. Recent Progress in Sulfur Cathodes for Application to Lithium–Sulfur Batteries. Particuology 2021, 58, 1–15. [Google Scholar]
- Lee, J.; Choi, W. Surface Modification of Sulfur Cathodes with PEDOT:PSS Conducting Polymer in Lithium-Sulfur Batteries. J. Electrochem. Soc. 2015, 162, A935. [Google Scholar]
- Liu, Y.; Elias, Y.; Meng, J.; Aurbach, D.; Zou, R.; Xia, D.; Pang, Q. Electrolyte Solutions Design for Lithium-Sulfur Batteries. Joule 2021, 5, 2323–2364. [Google Scholar]
- Chen, W.; Lei, T.; Wu, C.; Deng, M.; Gong, C.; Hu, K.; Ma, Y.; Dai, L.; Lv, W.; He, W.; et al. Designing Safe Electrolyte Systems for a High-Stability Lithium–Sulfur Battery. Adv. Energy Mater. 2018, 8, 1702348. [Google Scholar]
- Xiong, X.; Yan, W.; You, C.; Zhu, Y.; Chen, Y.; Fu, L.; Zhang, Y.; Yu, N.; Wu, Y. Methods to Improve Lithium Metal Anode for Li-S Batteries. Front. Chem. 2019, 7, 827. [Google Scholar]
- Han, D.-D.; Liu, S.; Liu, Y.-T.; Zhang, Z.; Li, G.-R.; Gao, X.-P. Lithiophilic Gel Polymer Electrolyte to Stabilize the Lithium Anode for a Quasi-Solid-State Lithium–Sulfur Battery. J. Mater. Chem. A 2018, 6, 18627–18634. [Google Scholar]
- Li, J.; Xiao, Z.; Chen, A.; Zhang, W.; Zhu, D.; Jin, Y.; Mao, Q.; Wang, G.; He, J.; Xia, Y. Functionally Modified Polyolefin-Based Separators for Lithium-Sulfur Batteries: Progress and Prospects. Front. Energy Res. 2020, 8, 593640. [Google Scholar] [CrossRef]
- Pavlin, N.; Hribernik, S.; Kapun, G.; Talian, S.D.; Njel, C.; Dedryvère, R.; Dominko, R. The Role of Cellulose Based Separator in Lithium Sulfur Batteries. J. Electrochem. Soc. 2018, 166, A5237. [Google Scholar]
- Shi, S.; Takeuchi, S.; Alexander, G.V.; Hamann, T.; O’Neill, J.; Dura, J.A.; Wachsman, E.D. High Sulfur Loading and Capacity Retention in Bilayer Garnet Sulfurized-Polyacrylonitrile/Lithium-Metal Batteries with Gel Polymer Electrolytes. Adv. Energy Mater. 2023, 13, 2301656. [Google Scholar]
- Ding, B.; Wang, J.; Fan, Z.; Chen, S.; Lin, Q.; Lu, X.; Dou, H.; Kumar Nanjundan, A.; Yushin, G.; Zhang, X.; et al. Solid-State Lithium–Sulfur Batteries: Advances, Challenges and Perspectives. Mater. Today 2020, 40, 114–131. [Google Scholar]
- Wang, Q.; Wen, Z.; Jin, J.; Guo, J.; Huang, X.; Yang, J.; Chen, C. A Gel-Ceramic Multi-Layer Electrolyte for Long-Life Lithium Sulfur Batteries. Chem. Commun. 2016, 52, 1637–1640. [Google Scholar]
- Wang, M.; Emre, A.E.; Kim, J.-Y.; Huang, Y.; Liu, L.; Cecen, V.; Huang, Y.; Kotov, N.A. Multifactorial Engineering of Biomimetic Membranes for Batteries with Multiple High-Performance Parameters. Nat. Commun. 2022, 13, 278. [Google Scholar] [PubMed]
- Pei, H.; Yang, C.; Wu, Q.; Zhou, X.; Xie, X.; Hwang, B.; Ye, Y. Ion-Selective Aramid Nanofiber-Based Janus Separators Fabricated by a Dry-Wet Phase Inversion Approach for Lithium–Sulfur Batteries. J. Mater. Chem. A 2022, 10, 5317–5327. [Google Scholar]
- Li, Z.; Tang, L.; Liu, X.; Song, T.; Xu, Q.; Liu, H.; Wang, Y. A Polar TiO/MWCNT Coating on a Separator Significantly Suppress the Shuttle Effect in a Lithium-Sulfur Battery. Electrochim. Acta 2019, 310, 1–12. [Google Scholar]
- Song, S.; Shi, L.; Lu, S.; Pang, Y.; Wang, Y.; Zhu, M.; Ding, D.; Ding, S. A New Polysulfide Blocker—Poly(Acrylic Acid) Modified Separator for Improved Performance of Lithium-Sulfur Battery. J. Membr. Sci. 2018, 563, 277–283. [Google Scholar]
- Kim, D.H.; Lee, Y.-H.; Song, Y.B.; Kwak, H.; Lee, S.-Y.; Jung, Y.S. Thin and Flexible Solid Electrolyte Membranes with Ultrahigh Thermal Stability Derived from Solution-Processable Li Argyrodites for All-Solid-State Li-Ion Batteries. ACS Energy Lett. 2020, 5, 718–727. [Google Scholar]
- Peng, H.-J.; Wang, D.-W.; Huang, J.-Q.; Cheng, X.-B.; Yuan, Z.; Wei, F.; Zhang, Q. Janus Separator of Polypropylene-Supported Cellular Graphene Framework for Sulfur Cathodes with High Utilization in Lithium–Sulfur Batteries. Adv. Sci. 2016, 3, 1500268. [Google Scholar]
- Wang, L.; Liu, J.; Haller, S.; Wang, Y.; Xia, Y. A Scalable Hybrid Separator for a High Performance Lithium–Sulfur Battery. Chem. Commun. 2015, 51, 6996–6999. [Google Scholar]
- Kundu, M.; Costa, C.M.; Dias, J.; Maceiras, A.; Vilas, J.L.; Lanceros-Méndez, S. On the Relevance of the Polar β-Phase of Poly(vinylidene fluoride) for High Performance Lithium-Ion Battery Separators. J. Phys. Chem. C 2017, 121, 26216–26225. [Google Scholar]
- Ribeiro, C.; Costa, C.M.; Correia, D.M.; Nunes-Pereira, J.; Oliveira, J.; Martins, P.; Gonçalves, R.; Cardoso, V.F.; Lanceros-Méndez, S. Electroactive Poly(vinylidene fluoride)-Based Structures for Advanced Applications. Nat. Protoc. 2018, 13, 681–704. [Google Scholar]
- Martins, P.; Lopes, A.C.; Lanceros-Mendez, S. Electroactive Phases of Poly(vinylidene fluoride): Determination, Processing and Applications. Prog. Polym. Sci. 2014, 39, 683–706. [Google Scholar]
- Pai, J.-Y.; Hsieh, C.-T.; Lee, C.-H.; Wang, J.-A.; Ku, H.-Y.; Huang, C.-L.; Hardwick, L.J.; Hu, C.-C. Engineering of Electrospun Polyimide Separators for Electrical Double-Layer Capacitors and Lithium-Ion Cells. J. Power Sources 2021, 482, 229054. [Google Scholar]
- Zhang, F.; Ma, X.; Cao, C.; Li, J.; Zhu, Y. Poly(vinylidene fluoride)/SiO2 Composite Membranes Prepared by Electrospinning and Their Excellent Properties for Nonwoven Separators for Lithium-Ion Batteries. J. Power Sources 2014, 251, 423–431. [Google Scholar]
- Lindström, H.; Södergren, S.; Solbrand, A.; Rensmo, H.; Hjelm, J.; Hagfeldt, A.; Lindquist, S.-E. Li+ Ion Insertion in TiO2 (Anatase). 2. Voltammetry on Nanoporous Films. J. Phys. Chem. B 1997, 101, 7717–7722. [Google Scholar]
- Flory, P.J. Thermodynamics of High Polymer Solutions. J. Chem. Phys. 1941, 9, 660. [Google Scholar]
- Huggins, M.L. Solutions of Long Chain Compounds. J. Chem. Phys. 1941, 9, 440. [Google Scholar]
- Saxena, P.; Shukla, P. A comprehensive review on fundamental properties and applications of poly(vinylidene fluoride) (PVDF). Adv. Compos. Hybrid Mater. 2021, 4, 8–26. [Google Scholar]
- Liao, J.; Ye, Z. Nontrivial Effects of “Trivial” Parameters on the Performance of Lithium–Sulfur Batteries. Batteries 2018, 4, 22. [Google Scholar] [CrossRef]
- Mohammad, I.; Barter, L.D.J.; Stolojan, V.; Crean, C.; Slade, R.C.T. Electrospun polar-nanofiber PVDF separator for lithium–sulfur batteries with enhanced charge storage capacity and cycling durability. Energy Adv. 2024, 3, 626–635. [Google Scholar]
- Zhang, L.; Guo, J. Understanding the Reaction Mechanism of Lithium–Sulfur Batteries by In Situ/Operando X-ray Absorption Spectroscopy. Arab. J. Sci. Eng. 2019, 44, 6217–6229. [Google Scholar]
- Jin, Z.; Xie, K.; Hong, X.; Hu, Z.; Liu, X. Application of Lithiated Nafion Ionomer Film as Functional Separator for Lithium Sulfur Cells. J. Power Sources 2012, 218, 163–167. [Google Scholar]
- Michan, A.L.; Divitini, G.; Pell, A.J.; Leskes, M.; Ducati, C.; Grey, C.P. Solid Electrolyte Interphase Growth and Capacity Loss in Silicon Electrodes. J. Am. Chem. Soc. 2016, 138, 7918–7931. [Google Scholar] [PubMed]
- Ahn, W.; Kim, K.-B.; Jung, K.-N.; Shin, K.-H.; Jin, C.-S. Synthesis and Electrochemical Properties of a Sulfur-Multi Walled Carbon Nanotubes Composite as a Cathode Material for Lithium Sulfur Batteries. J. Power Sources 2012, 202, 394–399. [Google Scholar]
- Yuan, L.; Qiu, X.; Chen, L.; Zhu, W. New Insight into the Discharge Process of Sulfur Cathode by Electrochemical Impedance Spectroscopy. J. Power Sources 2009, 189, 127–132. [Google Scholar]
- Deng, Z.; Zhang, Z.; Lai, Y.; Liu, J.; Li, J.; Liu, Y. Electrochemical Impedance Spectroscopy Study of a Lithium/Sulfur Battery: Modeling and Analysis of Capacity Fading. J. Electrochem. Soc. 2013, 160, A553–A558. [Google Scholar]
- Cañas, N.A.; Hirose, K.; Pascucci, B.; Wagner, N.; Friedrich, K.A.; Hiesgen, R. Investigations of Lithium–Sulfur Batteries Using Electrochemical Impedance Spectroscopy. Electrochim. Acta 2013, 97, 42–51. [Google Scholar]
- Cheng, Q. Porous Graphene Sponge Additives for Lithium Ion Batteries with Excellent Rate Capability. Sci. Rep. 2017, 7, 925. [Google Scholar]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohammad, I.; Barter, L.D.J.; Crean, C.; Slade, R.C.T. Addition of a Polar, Porous Phase-Inversion-PVDF Membrane to Lithium–Sulfur Cells (LSBs) Already with a Microporous Polypropylene Separator Enhances the Battery Performance. Batteries 2024, 10, 293. https://doi.org/10.3390/batteries10080293
Mohammad I, Barter LDJ, Crean C, Slade RCT. Addition of a Polar, Porous Phase-Inversion-PVDF Membrane to Lithium–Sulfur Cells (LSBs) Already with a Microporous Polypropylene Separator Enhances the Battery Performance. Batteries. 2024; 10(8):293. https://doi.org/10.3390/batteries10080293
Chicago/Turabian StyleMohammad, Irshad, Luke D. J. Barter, Carol Crean, and Robert C. T. Slade. 2024. "Addition of a Polar, Porous Phase-Inversion-PVDF Membrane to Lithium–Sulfur Cells (LSBs) Already with a Microporous Polypropylene Separator Enhances the Battery Performance" Batteries 10, no. 8: 293. https://doi.org/10.3390/batteries10080293





