Electrochemical Studies of Metal Phthalocyanines as Alternative Cathodes for Aqueous Zinc Batteries in “Water-in-Salt” Electrolytes
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Materials Characterizations
2.3. Electrode Preparation
2.4. Electrolyte Preparation
2.5. Battery Assembly and Testing
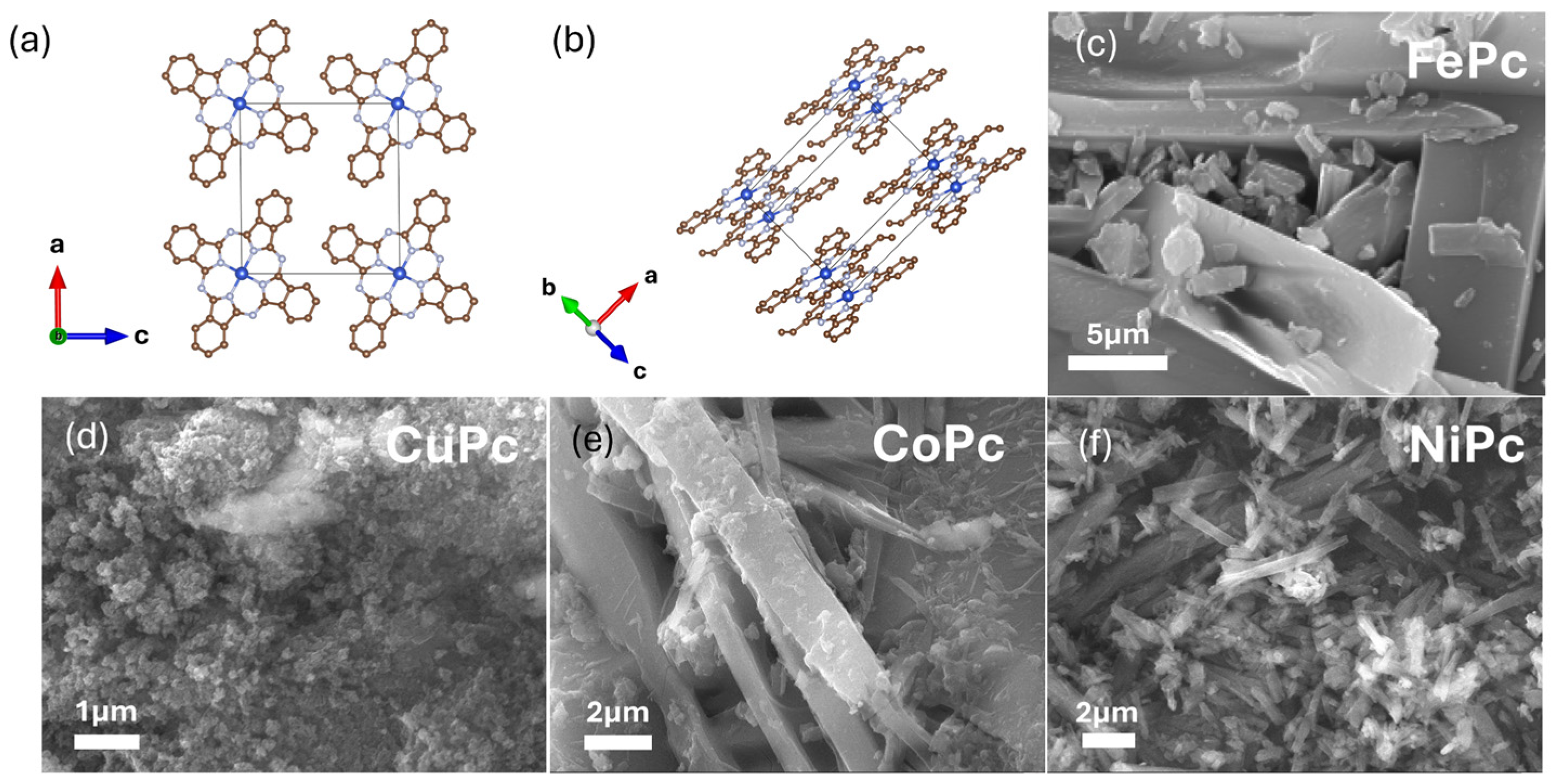
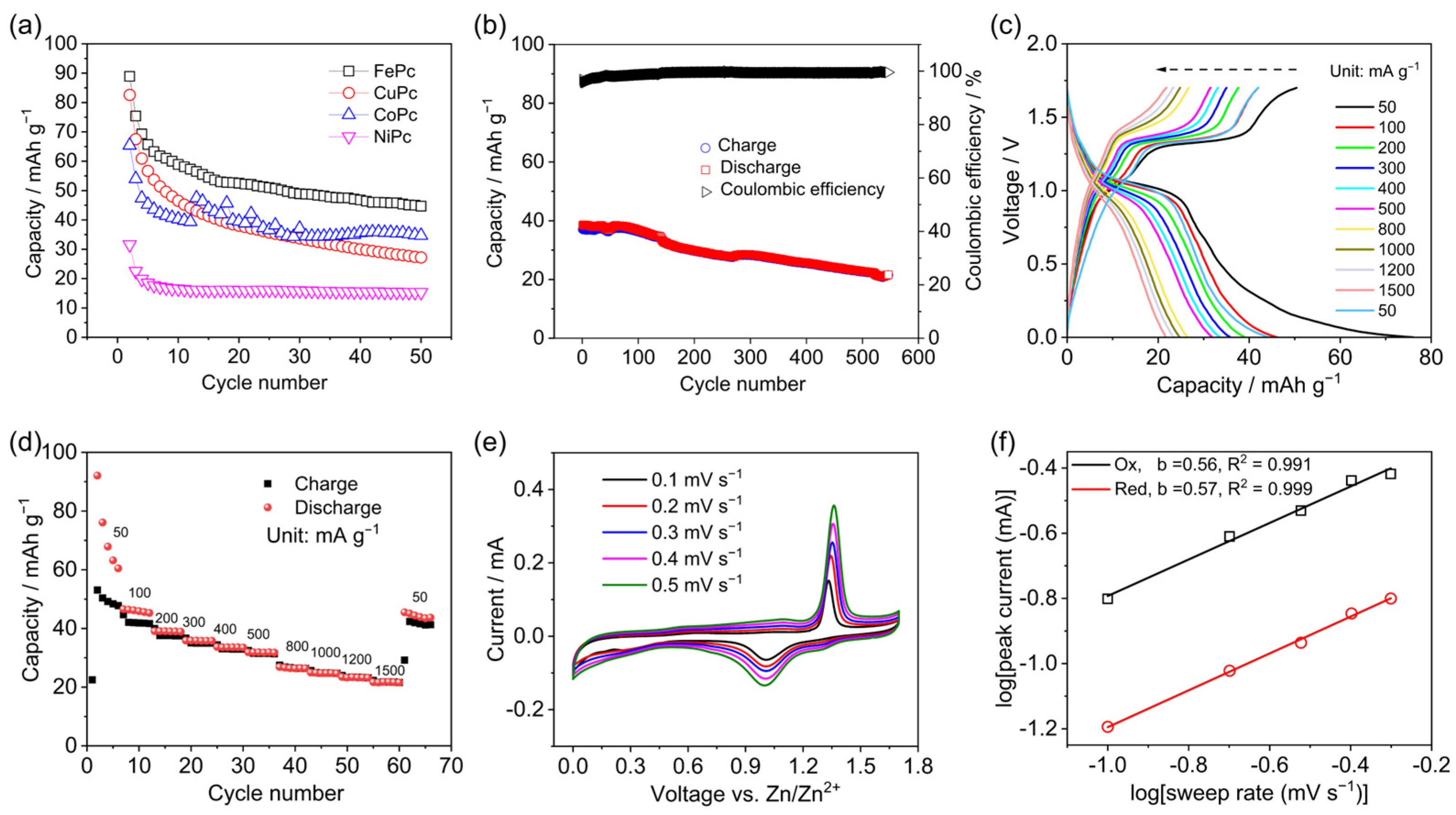
3. Results
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ji, X. A paradigm of storage batteries. Energy Environ. Sci. 2019, 12, 3203–3224. [Google Scholar] [CrossRef]
- Yang, Y.; Okonkwo, E.G.; Huang, G.; Xu, S.; Sun, W.; He, Y. On the sustainability of lithium-ion battery industry—A review and perspective. Energy Storage Mater. 2021, 36, 186–212. [Google Scholar] [CrossRef]
- Liang, Y.; Yao, Y. Designing modern aqueous batteries. Nat. Rev. Mater. 2023, 8, 109–122. [Google Scholar] [CrossRef]
- Dong, C.; Xu, F.; Chen, L.; Chen, Z.; Cao, Y. Design Strategies for High-Voltage Aqueous Batteries. Small Struct. 2021, 2, 2100001. [Google Scholar] [CrossRef]
- Chang, S.; Qiu, S.; Katiyar, S.; Florez Gomez, J.F.; Feng, Z.; Wu, X. Research Progress on Iron-Based Materials for Aqueous Sodium-Ion Batteries. Batteries 2023, 9, 349. [Google Scholar] [CrossRef]
- Wang, Y.; Yi, J.; Xia, Y. Recent Progress in Aqueous Lithium-Ion Batteries. Adv. Energy Mater. 2012, 2, 830–840. [Google Scholar] [CrossRef]
- Xing, Z.; Wang, S.; Yu, A.; Chen, Z. Aqueous intercalation-type electrode materials for grid-level energy storage: Beyond the limits of lithium and sodium. Nano Energy 2018, 50, 229–244. [Google Scholar] [CrossRef]
- Shangguan, M.; Wang, K.; Zhao, Y.; Xia, L. Tetraethylene Glycol Dimethyl Ether (TEGDME)-Water Hybrid Electrolytes Enable Excellent Cyclability in Aqueous Zn-Ion Batteries. Batteries 2023, 9, 462. [Google Scholar] [CrossRef]
- Ren, W.; Xiong, F.; Fan, Y.; Xiong, Y.; Jian, Z. Hierarchical Copper Sulfide Porous Nanocages for Rechargeable Multivalent-Ion Batteries. ACS Appl. Mater. Interfaces 2020, 12, 10471–10478. [Google Scholar] [CrossRef] [PubMed]
- Xue, T.; Fan, H.J. From aqueous Zn-ion battery to Zn-MnO2 flow battery: A brief story. J. Energy Chem. 2021, 54, 194–201. [Google Scholar] [CrossRef]
- Li, X.; Chen, Z.; Yang, Y.; Liang, S.; Lu, B.; Zhou, J. The phosphate cathodes for aqueous zinc-ion batteries. Inorg. Chem. Front. 2022, 9, 3986–3998. [Google Scholar] [CrossRef]
- Li, S.; Wei, Z.; Yang, J.; Chen, G.; Zhi, C.; Li, H.; Liu, Z. A High-Energy Four-Electron Zinc Battery Enabled by Evoking Full Electrochemical Activity in Copper Sulfide Electrode. ACS Nano 2023, 17, 22478–22487. [Google Scholar] [CrossRef]
- Hao, J.; Yuan, L.; Zhu, Y.; Bai, X.; Ye, C.; Jiao, Y.; Qiao, S.-Z. Low-cost and Non-flammable Eutectic Electrolytes for Advanced Zn-I2 Batteries. Angew. Chem. Int. Ed. 2023, 62, e202310284. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Hao, J.; Zhu, Y.; Zhang, S.-J.; Zuo, P.; Zhao, X.; Jaroniec, M.; Qiao, S.-Z. Anti-Swelling Microporous Membrane for High-Capacity and Long-Life Zn−I2 Batteries. Angew. Chem. Int. Ed. 2025, 64, e202413703. [Google Scholar] [CrossRef]
- Zhang, S.-J.; Hao, J.; Wu, H.; Kao, C.-C.; Chen, Q.; Ye, C.; Qiao, S.-Z. Toward High-Energy-Density Aqueous Zinc–Iodine Batteries: Multielectron Pathways. ACS Nano 2024, 18, 28557–28574. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhao, J.; Hu, Q.; Hao, T.; Cao, H.; Huang, X.; Liu, Y.; Zhang, Y.; Lin, D.; Tang, Y.; et al. Prussian blue analogs cathodes for aqueous zinc ion batteries. Mater. Today Energy 2022, 29, 101095. [Google Scholar] [CrossRef]
- Nam, K.W.; Kim, H.; Beldjoudi, Y.; Kwon, T.-w.; Kim, D.J.; Stoddart, J.F. Redox-Active Phenanthrenequinone Triangles in Aqueous Rechargeable Zinc Batteries. J. Am. Chem. Soc. 2020, 142, 2541–2548. [Google Scholar] [CrossRef]
- Niu, B.; Wang, J.; Guo, Y.; Li, Z.; Yuan, C.; Ju, A.; Wang, X. Polymers for Aqueous Zinc-Ion Batteries: From Fundamental to Applications Across Core Components. Adv. Energy Mater. 2024, 14, 2303967. [Google Scholar] [CrossRef]
- Wu, F.; Wu, B.; Mu, Y.; Zhou, B.; Zhang, G.; Zeng, L. Metal-Organic Framework-Based Materials in Aqueous Zinc-Ion Batteries. Int. J. Mol. Sci. 2023, 24, 6041. [Google Scholar] [CrossRef]
- Yin, C.; Pan, C.; Liao, X.; Pan, Y.; Yuan, L. Coordinately Unsaturated Manganese-Based Metal–Organic Frameworks as a High-Performance Cathode for Aqueous Zinc-Ion Batteries. ACS Appl. Mater. Interfaces 2021, 13, 35837–35847. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.; Ali, A.; Asif, M.; Shim, J.; Park, G. Exploring innovative trends and advancements in rechargeable zinc-air batteries. Inorg. Chem. Commun. 2024, 170, 113288. [Google Scholar] [CrossRef]
- Gounden, D.; Nombona, N.; van Zyl, W.E. Recent advances in phthalocyanines for chemical sensor, non-linear optics (NLO) and energy storage applications. Coord. Chem. Rev. 2020, 420, 213359. [Google Scholar] [CrossRef]
- Ambily, S.; Menon, C.S. Electrical conductivity studies and optical absorption studies in copper phthalocyanine thin films. Solid State Commun. 1995, 94, 485–487. [Google Scholar] [CrossRef]
- Claessens, C.G.; Hahn, U.; Torres, T. Phthalocyanines: From outstanding electronic properties to emerging applications. Chem. Record 2008, 8, 75–97. [Google Scholar] [CrossRef] [PubMed]
- Otero Vélez, C.; Flores, S.Y.; Fonseca, L.F.; Piñero Cruz, D.M. Palladium Phthalocyanine Nanowire-Based Highly Sensitive Sensors for NO2(g) Detection. Sensors 2024, 24, 1819. [Google Scholar] [CrossRef]
- Zagal, J.H.; Griveau, S.; Silva, J.F.; Nyokong, T.; Bedioui, F. Metallophthalocyanine-based molecular materials as catalysts for electrochemical reactions. Coord. Chem. Rev. 2010, 254, 2755–2791. [Google Scholar] [CrossRef]
- Wang, H.-g.; Wu, Q.; Cheng, L.; Chen, L.; Li, M.; Zhu, G. Porphyrin- and phthalocyanine-based systems for rechargeable batteries. Energy Storage Mater. 2022, 52, 495–513. [Google Scholar] [CrossRef]
- Oni, J.; Ozoemena, K.I. Phthalocyanines in batteries and supercapacitors. J. Porphyr. Phthalocyanines 2012, 16, 754–760. [Google Scholar] [CrossRef]
- Zhao, J.; Zhou, M.; Chen, J.; Tao, L.; Zhang, Q.; Li, Z.; Zhong, S.; Fu, H.; Wang, H.; Wu, L. Phthalocyanine-based covalent organic frameworks as novel anode materials for high-performance lithium-ion/sodium-ion batteries. Chem. Eng. J. 2021, 425, 131630. [Google Scholar] [CrossRef]
- Wang, H.-g.; Wang, H.; Si, Z.; Li, Q.; Wu, Q.; Shao, Q.; Wu, L.; Liu, Y.; Wang, Y.; Song, S.; et al. A Bipolar and Self-Polymerized Phthalocyanine Complex for Fast and Tunable Energy Storage in Dual-Ion Batteries. Angew. Chem. Int. Ed. 2019, 58, 10204–10208. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Xu, Y.; Cao, M.; Zhu, C.; Liu, X.; Li, Y.; Zhong, S. A stable 2D nano-columnar sandwich layered phthalocyanine negative electrode for lithium-ion batteries. J. Power Sources 2019, 426, 169–177. [Google Scholar] [CrossRef]
- Hwang, B.; Cheong, J.Y.; Matteini, P.; Yun, T.G. Highly efficient phthalocyanine based aqueous Zn-ion flexible-batteries. Mater. Lett. 2022, 306, 130954. [Google Scholar] [CrossRef]
- Yang, C.; Suo, L.; Borodin, O.; Wang, F.; Sun, W.; Gao, T.; Fan, X.; Hou, S.; Ma, Z.; Amine, K.; et al. Unique aqueous Li-ion/sulfur chemistry with high energy density and reversibility. Proc. Natl. Acad. Sci. USA 2017, 114, 6197–6202. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Xu, Y.; Zhang, C.; Leonard, D.P.; Markir, A.; Lu, J.; Ji, X. Reverse Dual-Ion Battery via a ZnCl2 Water-in-Salt Electrolyte. J. Am. Chem. Soc. 2019, 141, 6338–6344. [Google Scholar] [CrossRef]
- Suo, L.; Borodin, O.; Gao, T.; Olguin, M.; Ho, J.; Fan, X.; Luo, C.; Wang, C.; Xu, K. “Water-in-salt” electrolyte enables high-voltage aqueous lithium-ion chemistries. Science 2015, 350, 938–943. [Google Scholar] [CrossRef]
- Qiu, S.; Xu, Y.; Li, X.; Sandstrom, S.K.; Wu, X.; Ji, X. Reinforced potassium and ammonium storage of the polyimide anode in acetate-based water-in-salt electrolytes. Electrochem. Commun. 2021, 122, 106880. [Google Scholar] [CrossRef]
- Zhang, C.; Holoubek, J.; Wu, X.; Daniyar, A.; Zhu, L.; Chen, C.; Leonard, D.P.; Rodríguez-Pérez, I.A.; Jiang, J.-X.; Fang, C.; et al. A ZnCl2 water-in-salt electrolyte for a reversible Zn metal anode. Chem. Commun. 2018, 54, 14097–14099. [Google Scholar] [CrossRef]
- Chao, D.; Zhou, W.; Xie, F.; Ye, C.; Li, H.; Jaroniec, M.; Qiao, S.-Z. Roadmap for advanced aqueous batteries: From design of materials to applications. Sci. Adv. 2020, 6, eaba4098. [Google Scholar] [CrossRef]
- Harris, D.C. Quantitative Chemical Analysis; Macmillan: New York, NY, USA, 2010. [Google Scholar]
- Baggio, A.R.; Machado, D.F.S.; Carvalho-Silva, V.H.; Paterno, L.G.; de Oliveira, H.C.B. Rovibrational spectroscopic constants of the interaction between ammonia and metallo-phthalocyanines: A theoretical protocol for ammonia sensor design. Phys. Chem. Chem. Phys. 2017, 19, 10843–10853. [Google Scholar] [CrossRef]
- Augustyn, V.; Come, J.; Lowe, M.A.; Kim, J.W.; Taberna, P.-L.; Tolbert, S.H.; Abruña, H.D.; Simon, P.; Dunn, B. High-rate electrochemical energy storage through Li+ intercalation pseudocapacitance. Nat. Mater. 2013, 12, 518–522. [Google Scholar] [CrossRef]
- Augustyn, V.; Simon, P.; Dunn, B. Pseudocapacitive oxide materials for high-rate electrochemical energy storage. Energy Environ. Sci. 2014, 7, 1597–1614. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hou, W.; Araujo-Correa, A.E.; Qiu, S.; Velez, C.O.; Acosta-Tejada, Y.D.; Feliz-Hernández, L.N.; González-Nieves, K.; Morell, G.; Piñero Cruz, D.M.; Wu, X. Electrochemical Studies of Metal Phthalocyanines as Alternative Cathodes for Aqueous Zinc Batteries in “Water-in-Salt” Electrolytes. Batteries 2025, 11, 88. https://doi.org/10.3390/batteries11030088
Hou W, Araujo-Correa AE, Qiu S, Velez CO, Acosta-Tejada YD, Feliz-Hernández LN, González-Nieves K, Morell G, Piñero Cruz DM, Wu X. Electrochemical Studies of Metal Phthalocyanines as Alternative Cathodes for Aqueous Zinc Batteries in “Water-in-Salt” Electrolytes. Batteries. 2025; 11(3):88. https://doi.org/10.3390/batteries11030088
Chicago/Turabian StyleHou, Wentao, Andres Eduardo Araujo-Correa, Shen Qiu, Crystal Otero Velez, Yamna D. Acosta-Tejada, Lexis N. Feliz-Hernández, Karilys González-Nieves, Gerardo Morell, Dalice M. Piñero Cruz, and Xianyong Wu. 2025. "Electrochemical Studies of Metal Phthalocyanines as Alternative Cathodes for Aqueous Zinc Batteries in “Water-in-Salt” Electrolytes" Batteries 11, no. 3: 88. https://doi.org/10.3390/batteries11030088
APA StyleHou, W., Araujo-Correa, A. E., Qiu, S., Velez, C. O., Acosta-Tejada, Y. D., Feliz-Hernández, L. N., González-Nieves, K., Morell, G., Piñero Cruz, D. M., & Wu, X. (2025). Electrochemical Studies of Metal Phthalocyanines as Alternative Cathodes for Aqueous Zinc Batteries in “Water-in-Salt” Electrolytes. Batteries, 11(3), 88. https://doi.org/10.3390/batteries11030088








