Carbonaceous Materials as Anodes for Lithium-Ion and Sodium-Ion Batteries
Abstract
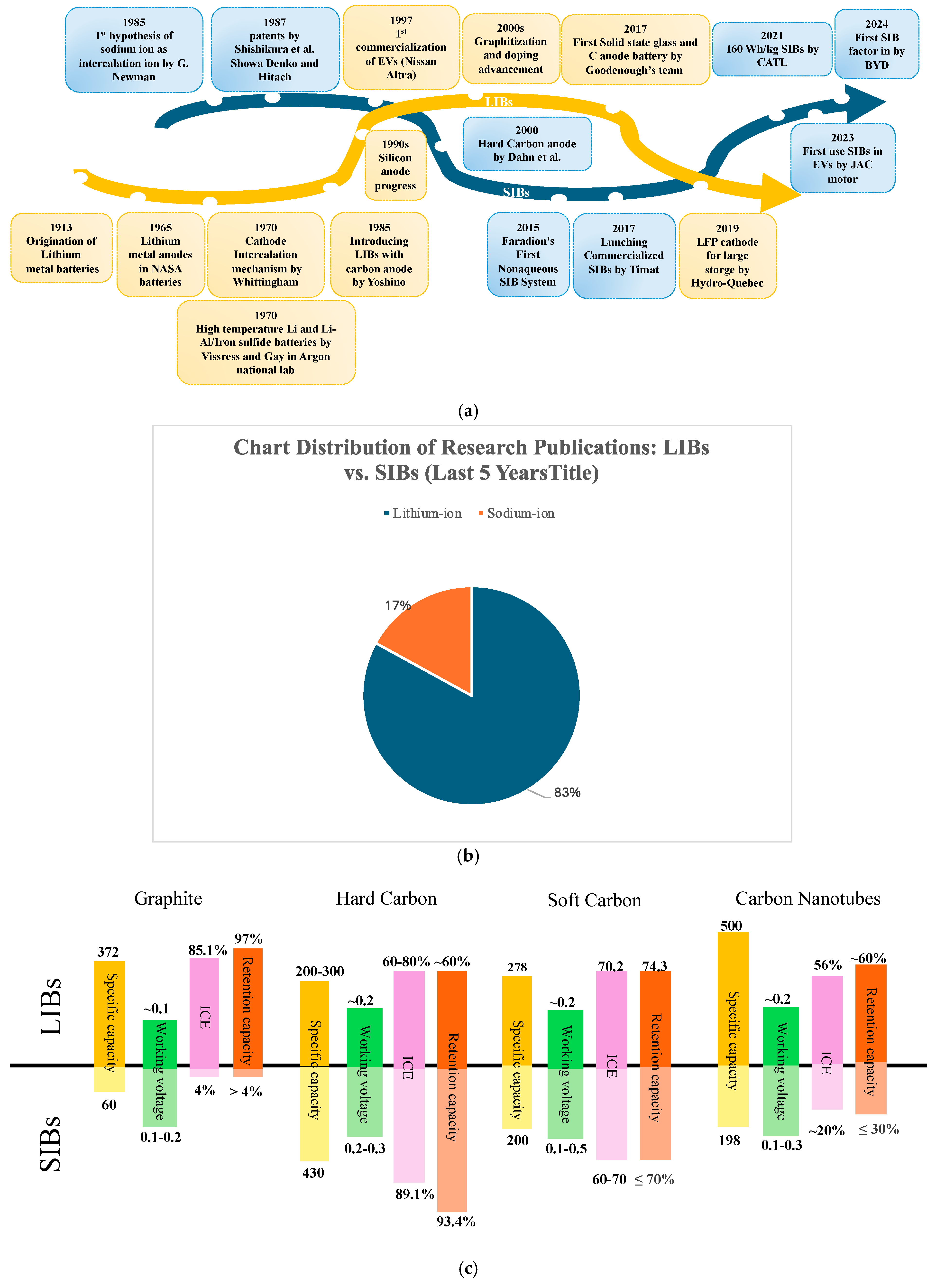
:1. Introduction
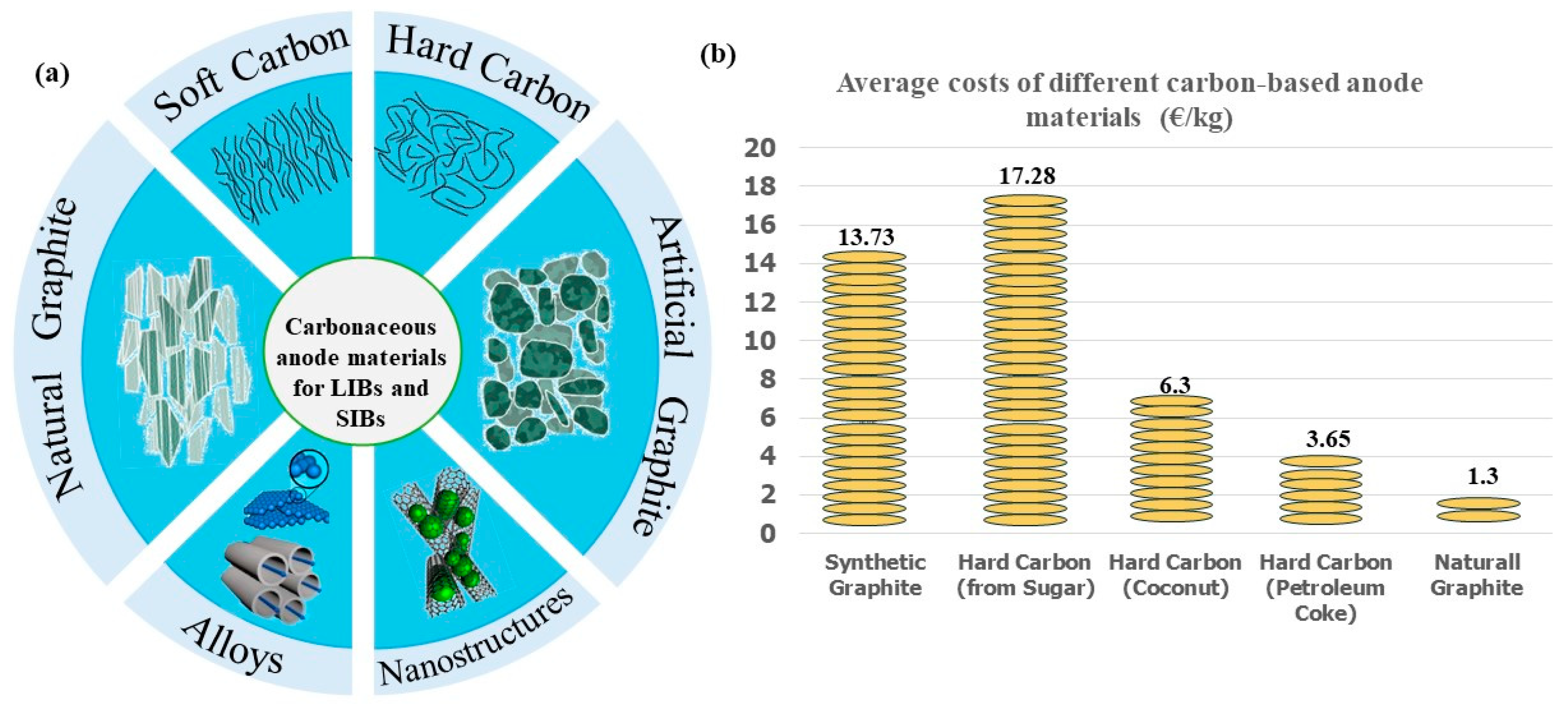
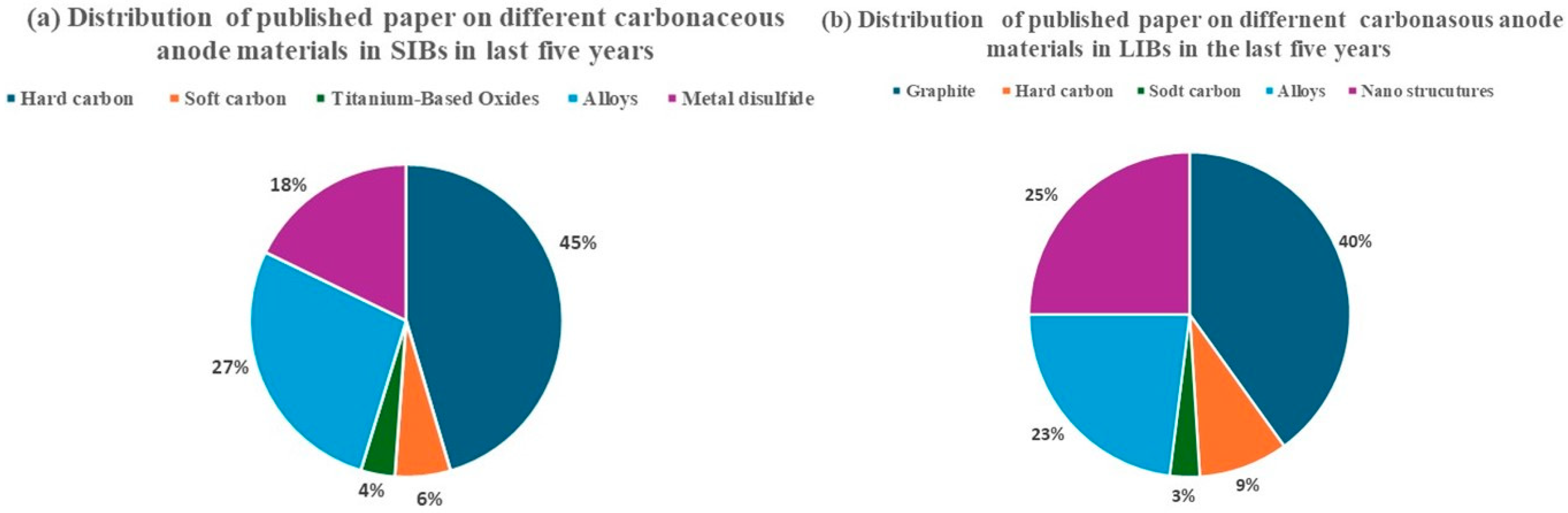
1.1. Anode Materials and Costs
1.2. Hard Carbon and Soft Carbon
2. Graphite
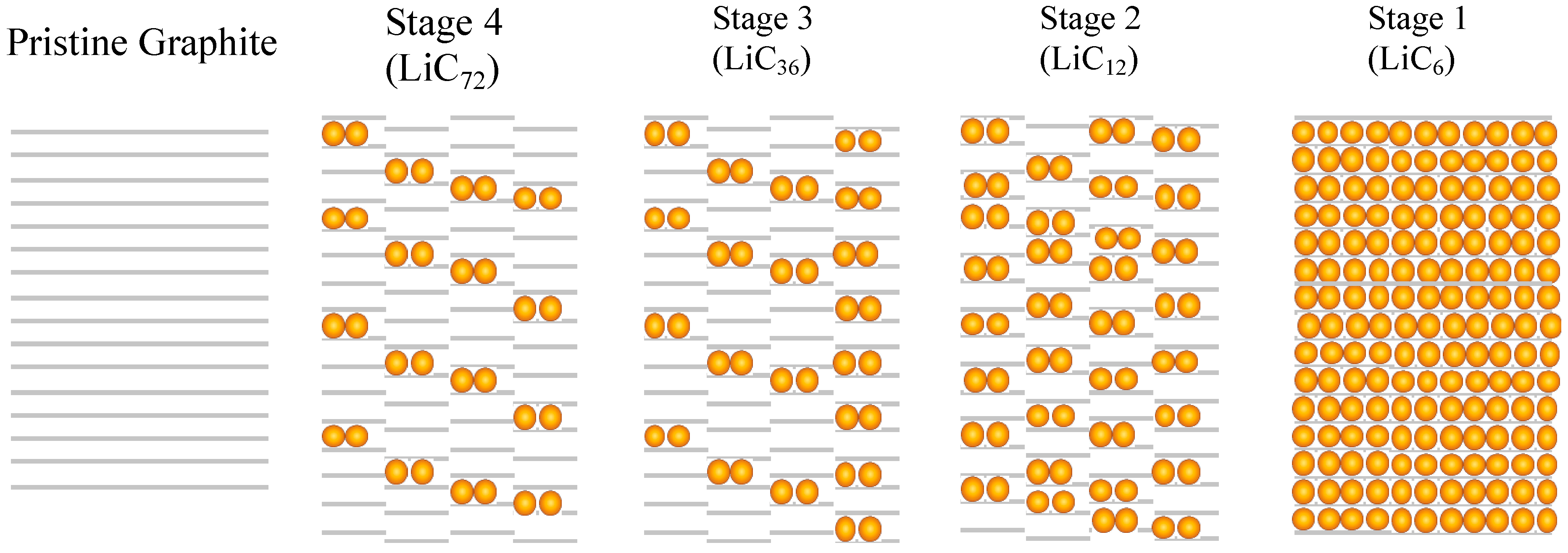
2.1. Graphite Intercalation Compounds
2.2. Artificial Graphite
2.2.1. Synthesis
2.2.2. Doping Carbon Materials
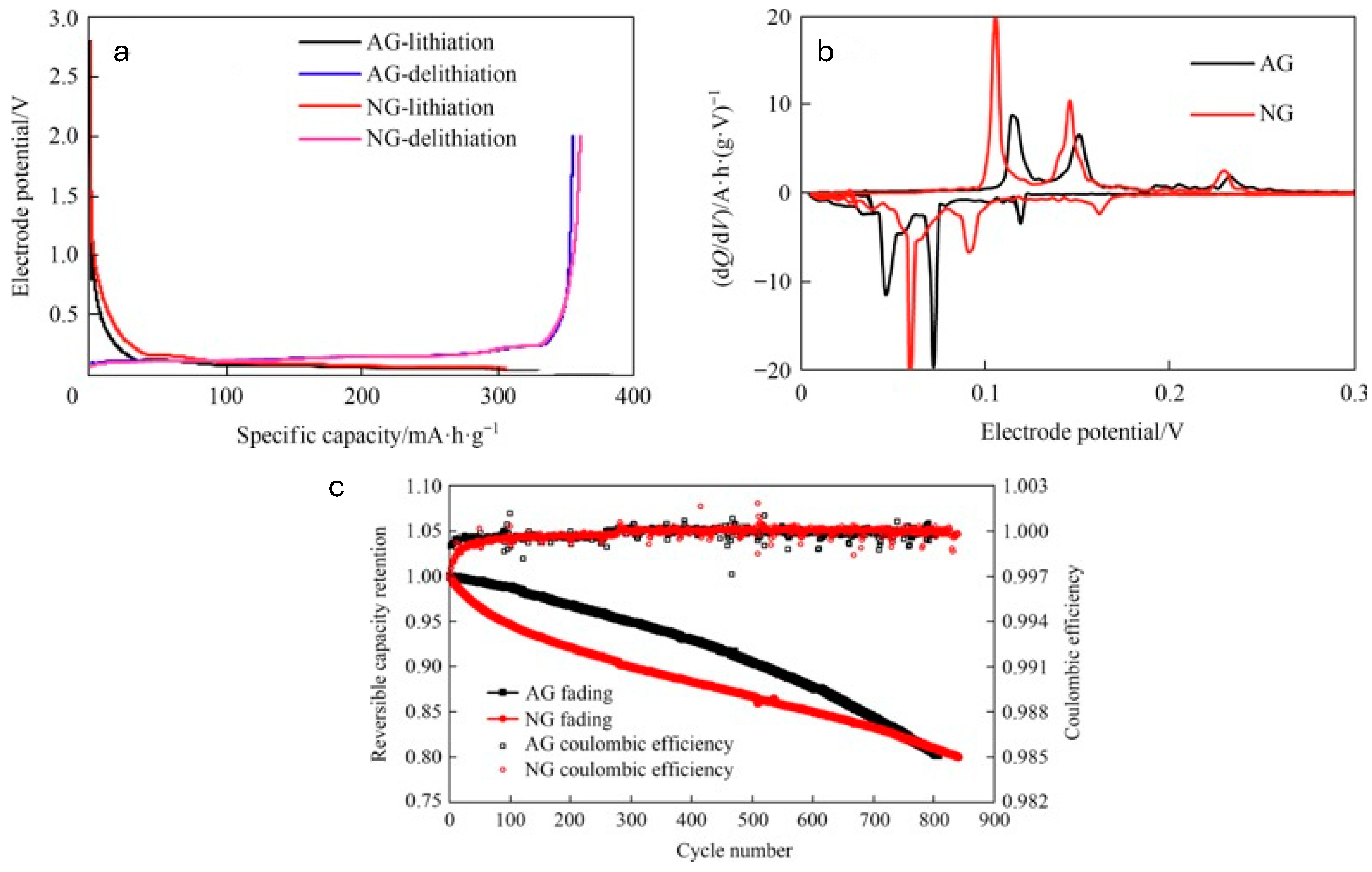
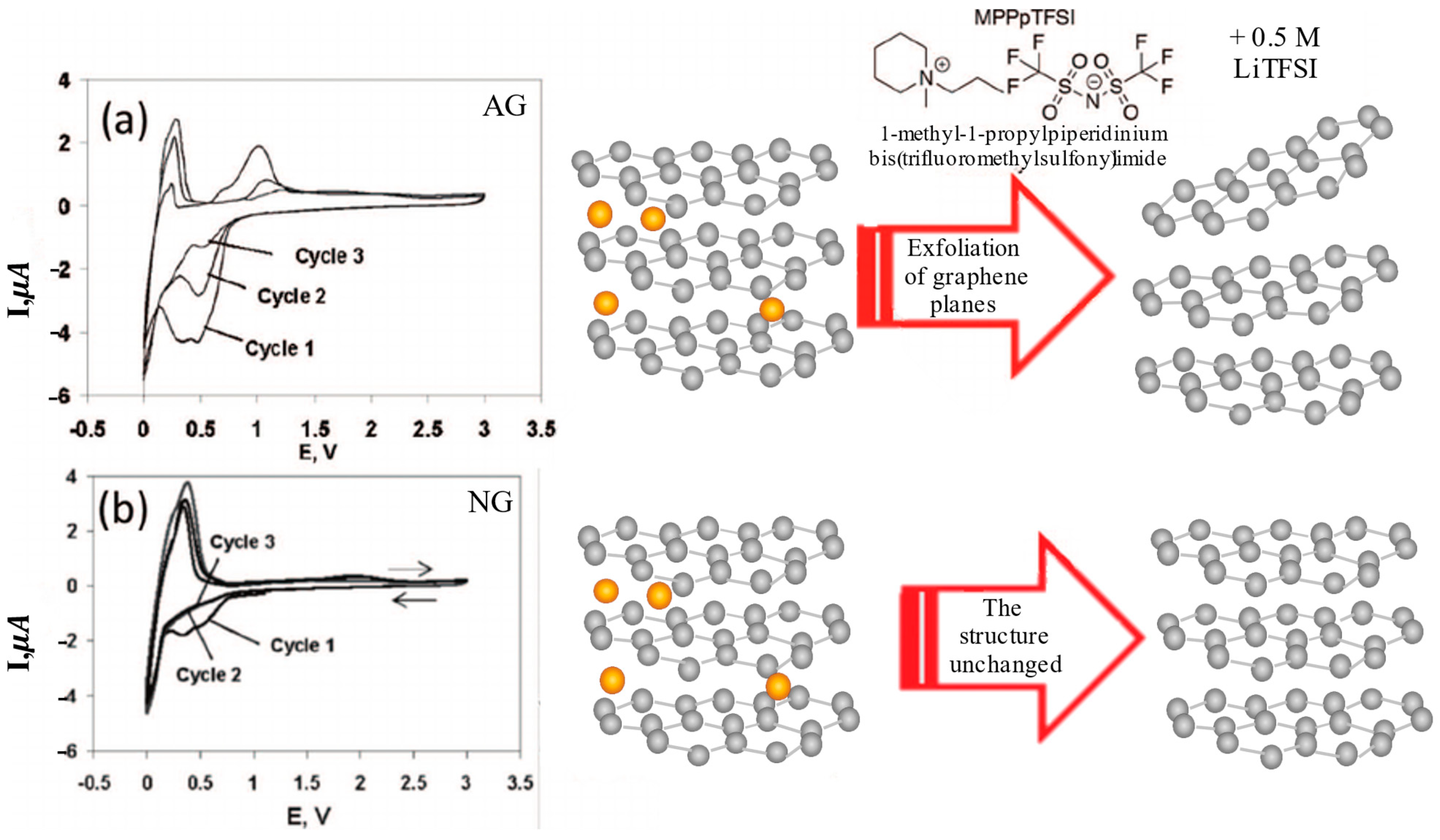
2.3. Comparative Analysis of Natural and Artificial Graphite
2.3.1. Structural Analysis
2.3.2. Electrochemical Behavior
2.4. Electrochemical Properties vs. Forms of Carbon
3. Lithium-Ion Batteries
3.1. Intercalation
3.2. Cycle Life and Calendar Life
3.3. Energy Density and Capacity
- (a)
- Coating
- (b)
- Nanostructuring techniques
- (c)
- Hybrid composites and alloys
3.4. Voltage (Cell Voltage) and SOC
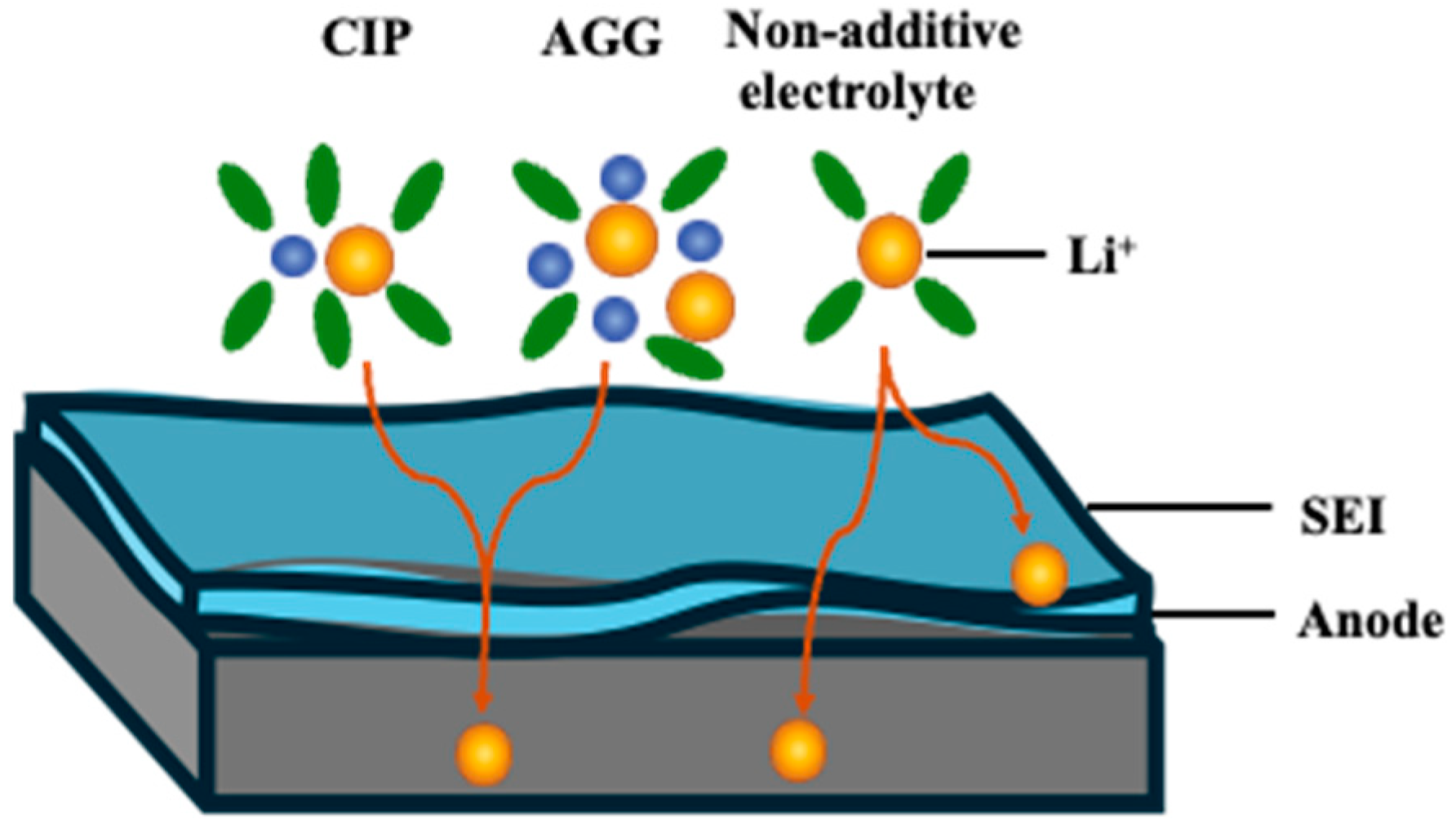
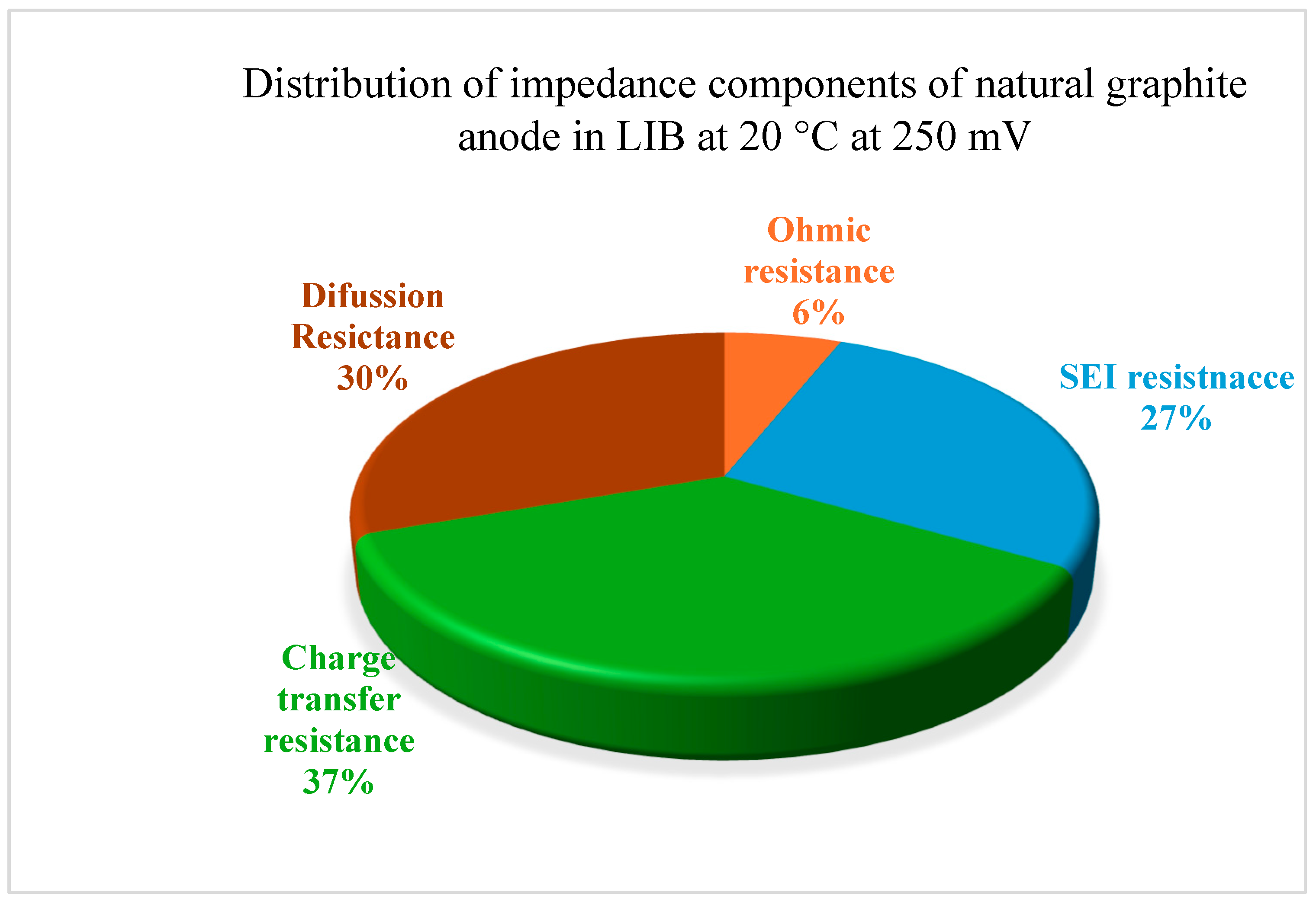
3.5. Internal Resistance and Impendence
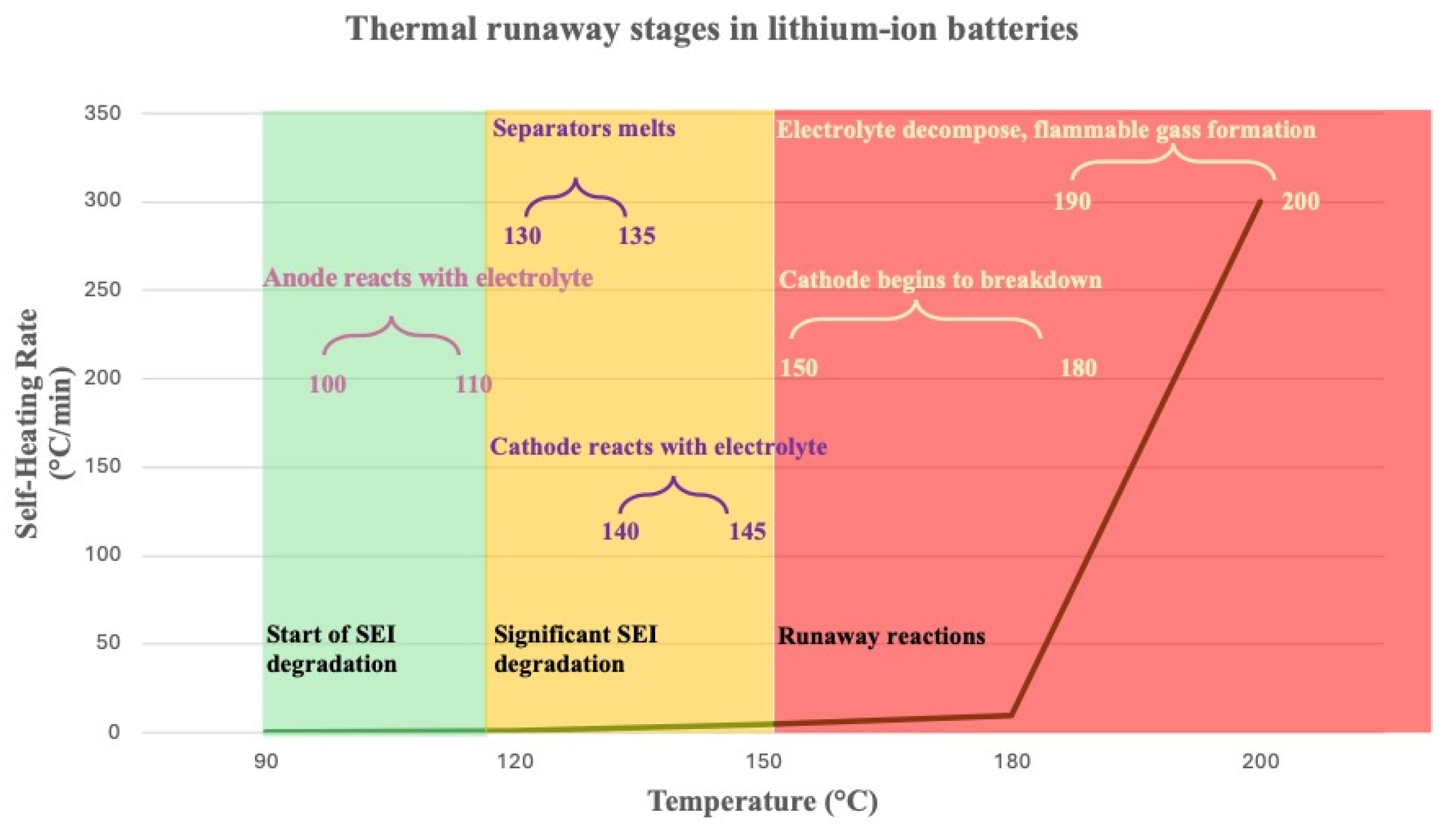
3.6. Safety
- (i)
- Material design technique: As discussed, the SEI layer plays an essential role in influencing the thermal runaway behavior of lithium-ion batteries. Therefore, one of the efficient strategies is to engineer a stable SEI layer with enhanced mechanical strength and chemical stability. Elemental doping and surface doping have been reported to be efficient in enhancing the thermal stability of these batteries [177,188,202,203,204]. These techniques can prevent side reactions by increasing temperature and maintaining structural integrity over extended cycles;
- (ii)
- Using machine learning (ML): Extensive datasets derived from electrolyte and anode properties can be effectively analyzed and comprehended by ML to maximize battery performance. Advanced models can simulate the dynamics of SEI formation under various conditions and provide insights into factors such as ion diffusion rates and reaction kinetics. Furthermore, ML anticipated the pattern of SEI decomposition as a function of time and facilitated the designing of materials that reduce heat generation and resist breakdown. This algorithm can be combined with battery management systems (BMSs) and provides insights that can effectively maximize the function of electrochemical parameters such as the SOC, C rates, ICE, and voltage limits to maintain SEI integrity [205,206,207].
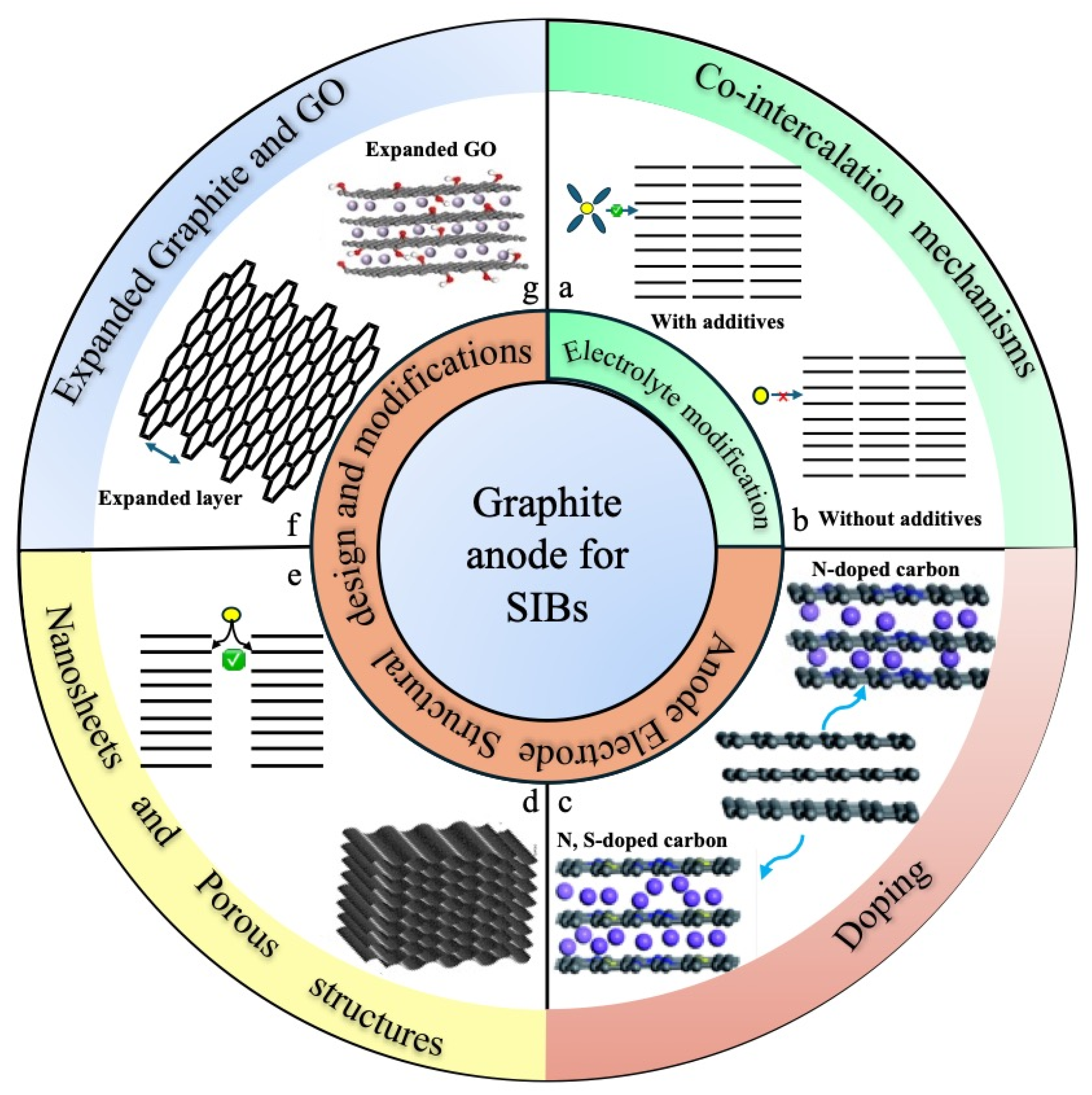
4. Sodium-Ion Batteries
4.1. Intercalation
4.2. Energy Density and Capacity
4.3. Safety
4.3.1. Thermal Runaway
4.3.2. Strategies to Enhance Safety Profile
- (i)
- Anode–electrolyte modification
- (ii)
- Coating
- (iii)
- Machine learning
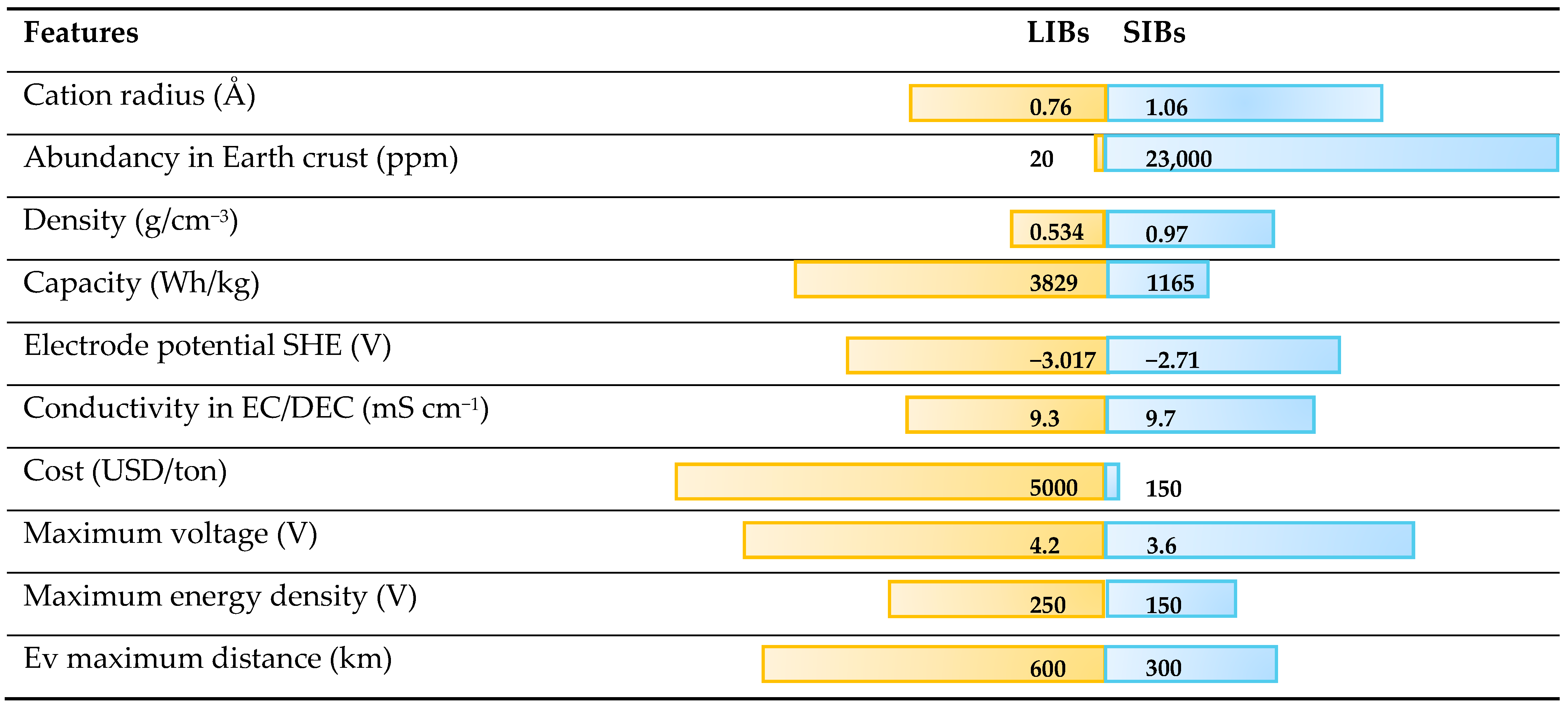
4.4. Comparison Between LIBs and SIBs
5. Conclusions and Perspectives
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, J.; Azam, W. Natural Resource Scarcity, Fossil Fuel Energy Consumption, and Total Greenhouse Gas Emissions in Top Emitting Countries. Geosci. Front. 2024, 15, 101757. [Google Scholar] [CrossRef]
- Zhang, L.; Li, X.; Yang, M.; Chen, W. High-Safety Separators for Lithium-Ion Batteries and Sodium-Ion Batteries: Advances and Perspective. Energy Storage Mater. 2021, 41, 522–545. [Google Scholar] [CrossRef]
- Wang, M.; Wang, Q.; Ding, X.; Wang, Y.; Xin, Y.; Singh, P.; Wu, F.; Gao, H. The Prospect and Challenges of Sodium-Ion Batteries for Low-Temperature Conditions. Interdiscip. Mater. 2022, 1, 373–395. [Google Scholar] [CrossRef]
- Kumar Prajapati, A.; Bhatnagar, A. A Review on Anode Materials for Lithium/Sodium-Ion Batteries. J. Energy Chem. 2023, 83, 509–540. [Google Scholar] [CrossRef]
- Xiong, K.; Qi, T.; Zhang, X. Advancements in Graphite Anodes for Lithium-Ion and Sodium-Ion Batteries: A Review. Electroanalysis 2025, 37, e202400318. [Google Scholar] [CrossRef]
- Liu, C.; Sun, J.; Zheng, P.; Jiang, L.; Liu, H.; Chai, J.; Liu, Q.; Liu, Z.; Zheng, Y.; Rui, X. Recent Advances of Non-Lithium Metal Anode Materials for Solid-State Lithium-Ion Batteries. J. Mater. Chem. A 2022, 10, 16761–16778. [Google Scholar] [CrossRef]
- He, W.; Guo, W.; Wu, H.; Lin, L.; Liu, Q.; Han, X.; Xie, Q.; Liu, P.; Zheng, H.; Wang, L.; et al. Challenges and Recent Advances in High Capacity Li-Rich Cathode Materials for High Energy Density Lithium-Ion Batteries. Adv. Mater. 2021, 33, 2005937. [Google Scholar] [CrossRef]
- Olabi, A.G.; Abbas, Q.; Shinde, P.A.; Abdelkareem, M.A. Rechargeable Batteries: Technological Advancement, Challenges, Current and Emerging Applications. Energy 2023, 266, 126408. [Google Scholar] [CrossRef]
- Nzereogu, P.U.; Omah, A.D.; Ezema, F.I.; Iwuoha, E.I.; Nwanya, A.C. Anode Materials for Lithium-Ion Batteries: A Review. Appl. Surf. Sci. Adv. 2022, 9, 100233. [Google Scholar] [CrossRef]
- Chen, Y.; Kang, Y.; Zhao, Y.; Wang, L.; Liu, J.; Li, Y.; Liang, Z.; He, X.; Li, X.; Tavajohi, N.; et al. A Review of Lithium-Ion Battery Safety Concerns: The Issues, Strategies, and Testing Standards. J. Energy Chem. 2021, 59, 83–99. [Google Scholar] [CrossRef]
- Monteiro, I.F.; Silva, M.M.; Fidalgo-Marijuan, A.; Gonçalves, R.; Costa, C.M.; Lanceros-Mendez, S. Improving Lithium-Ion Battery Safety Through Separators with Thermal Shutdown Characteristics Induced by Thermal Expansion Microspheres. J. Power Sources 2025, 631, 236311. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, H.; Qi, S.; Wu, D.; Huang, J.; Li, X.; Wang, C.; Ma, J. Structural Regulation Chemistry of Lithium Ion Solvation for Lithium Batteries. EcoMat 2022, 4, e12200. [Google Scholar] [CrossRef]
- Qiao, S.; Zhou, Q.; Ma, M.; Liu, H.K.; Dou, S.X.; Chong, S. Advanced Anode Materials for Rechargeable Sodium-Ion Batteries. ACS Nano 2023, 17, 11220–11252. [Google Scholar] [CrossRef] [PubMed]
- Deng, L.; Wei, T.; Liu, J.; Zhan, L.; Chen, W.; Cao, J. Recent Developments of Carbon-Based Anode Materials for Flexible Lithium-Ion Batteries. Crystals 2022, 12, 1279. [Google Scholar] [CrossRef]
- Sarfraz, N.; Kanwal, N.; Ali, M.; Ali, K.; Hasnain, A.; Ashraf, M.; Ayaz, M.; Ifthikar, J.; Ali, S.; Hendi, A.; et al. Materials Advancements in Solid-State Inorganic Electrolytes for Highly Anticipated All Solid Li-Ion Batteries. Energy Storage Mater. 2024, 71, 103619. [Google Scholar] [CrossRef]
- Xu, G.; Jiang, M.; Li, J.; Xuan, X.; Li, J.; Lu, T.; Pan, L. Machine Learning-Accelerated Discovery and Design of Electrode Materials and Electrolytes for Lithium Ion Batteries. Energy Storage Mater. 2024, 72, 103710. [Google Scholar] [CrossRef]
- Zhou, G.; Niu, C.; Kong, Y.; Wei, Z.; Wang, J.; Huang, Q.; Lu, H.; Zhang, Q. Research on Stimulation Responsive Electrolytes from the Perspective of Thermal Runaway in Lithium-Ion Batteries: A Review. Fuel 2024, 368, 131599. [Google Scholar] [CrossRef]
- Ma, J.; Li, Z. Computational Design of Inorganic Solid-State Electrolyte Materials for Lithium-Ion Batteries. Acc. Mater. Res. 2024, 5, 523–532. [Google Scholar] [CrossRef]
- Majid, A.; Najam, U.; Ahmad, S.; Alkhedher, M. On the Prospects of Using B4C3 as a Potential Electrode Material for Lithium-Ion Batteries. Mater. Sci. Semicond. Process. 2024, 176, 108320. [Google Scholar] [CrossRef]
- Zhao, L.; Hu, Z.; Lai, W.; Tao, Y.; Peng, J.; Miao, Z.; Wang, Y.; Chou, S.; Liu, H.; Dou, S. Hard Carbon Anodes: Fundamental Understanding and Commercial Perspectives for Na-Ion Batteries beyond Li-Ion and K-Ion Counterparts. Adv. Energy Mater. 2021, 11, 2002704. [Google Scholar] [CrossRef]
- Reddy, M.V.; Mauger, A.; Julien, C.M.; Paolella, A.; Zaghib, K. Brief History of Early Lithium-Battery Development. Materials 2020, 13, 1884. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Wu, M.; Wang, Y.; Zaghib, K. Insights into Pseudographite-Structured Hard Carbon with Stabilized Performance for High Energy K-Ion Storage. J. Power Sources 2019, 444, 227310. [Google Scholar] [CrossRef]
- Yu, P.; Tang, W.; Wu, F.-F.; Zhang, C.; Luo, H.-Y.; Liu, H.; Wang, Z.-G. Recent Progress in Plant-Derived Hard Carbon Anode Materials for Sodium-Ion Batteries: A Review. Rare Met. 2020, 39, 1019–1033. [Google Scholar] [CrossRef]
- Jara, A.D.; Betemariam, A.; Woldetinsae, G.; Kim, J.Y. Purification, Application and Current Market Trend of Natural Graphite: A Review. Int. J. Min. Sci. Technol. 2019, 29, 671–689. [Google Scholar] [CrossRef]
- Dresselhaus, M.S.; Dresselhaus, G. Intercalation Compounds of Graphite. Adv. Phys. 2002, 51, 1–186. [Google Scholar] [CrossRef]
- Chang, H.; Wu, Y.-R.; Han, X.; Yi, T.-F. Recent Developments in Advanced Anode Materials for Lithium-Ion Batteries. Energy Mater. 2022, 1, 100003. [Google Scholar] [CrossRef]
- Thauer, E.; Ottmann, A.; Schneider, P.; Möller, L.; Deeg, L.; Zeus, R.; Wilhelmi, F.; Schlestein, L.; Neef, C.; Ghunaim, R.; et al. Filled Carbon Nanotubes as Anode Materials for Lithium-Ion Batteries. Molecules 2020, 25, 1064. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Vasileiadis, A.; Zhou, Q.; Lu, Y.; Meng, Q.; Li, Y.; Ombrini, P.; Zhao, J.; Chen, Z.; Niu, Y.; et al. Author Correction: Origin of Fast Charging in Hard Carbon Anodes. Nat. Energy 2024, 9, 357. [Google Scholar] [CrossRef]
- Yu, P.; Li, Z.; Zhang, D.; Xiong, Q.; Yu, J.; Zhi, C. Hierarchical Yolk-Shell Silicon/Carbon Anode Materials Enhanced by Vertical Graphene Sheets for Commercial Lithium-Ion Battery Applications. Adv. Funct. Mater. 2025, 35, 2413081. [Google Scholar] [CrossRef]
- Peters, J.F.; Peña Cruz, A.; Weil, M. Exploring the Economic Potential of Sodium-Ion Batteries. Batteries 2019, 5, 10. [Google Scholar] [CrossRef]
- Vaalma, C.; Buchholz, D.; Weil, M.; Passerini, S. A Cost and Resource Analysis of Sodium-Ion Batteries. Nat. Rev. Mater. 2018, 3, 18013. [Google Scholar] [CrossRef]
- Ding, J.; Ji, D.; Yue, Y.; Smedskjaer, M.M. Amorphous Materials for Lithium-Ion and Post-Lithium-Ion Batteries. Small 2024, 20, 2304270. [Google Scholar] [CrossRef]
- Liu, C.-F.; Liu, Y.-C.; Yi, T.-Y.; Hu, C.-C. Carbon Materials for High-Voltage Supercapacitors. Carbon 2019, 145, 529–548. [Google Scholar] [CrossRef]
- Kim, M.I.; Cho, J.H.; Hwang, J.U.; Bai, B.C.; Im, J.S. Preparation of High-Crystallinity Synthetic Graphite from Hard Carbon-Based Carbon Black. Appl. Phys. A 2021, 127, 156. [Google Scholar] [CrossRef]
- Xia, P.; Qin, Z.; Jing, S.; Li, S.; Peng, X.; Yuan, L.; Lu, S.; Zhang, Y.; Fan, H. Polymer Derived Mesoporous Hard Carbon Nanospheres as High-Performance Anode Materials for Potassium-Ion Batteries. Colloids Surf. Physicochem. Eng. Asp. 2024, 701, 134807. [Google Scholar] [CrossRef]
- Alvira, D.; Antorán, D.; Manyà, J.J. Plant-Derived Hard Carbon as Anode for Sodium-Ion Batteries: A Comprehensive Review to Guide Interdisciplinary Research. Chem. Eng. J. 2022, 447, 137468. [Google Scholar] [CrossRef]
- Li, X.; Ding, C.; Liang, Q.; Hu, J.; Xu, L.; Li, Y.; Liu, Y.; Gao, Y. Progress in Hard Carbons for Sodium-Ion Batteries: Microstructure, Sodium Storage Mechanism and Initial Coulombic Efficiency. J. Energy Storage 2024, 98, 112986. [Google Scholar] [CrossRef]
- Wang, K.; Xu, Y.; Li, Y.; Dravid, V.; Wu, J.; Huang, Y. Sodium Storage in Hard Carbon with Curved Graphene Platelets as the Basic Structural Units. J. Mater. Chem. A 2019, 7, 3327–3335. [Google Scholar] [CrossRef]
- Ren, N.; Wang, L.; He, X.; Zhang, L.; Dong, J.; Chen, F.; Xiao, J.; Pan, B.; Chen, C. High ICE Hard Carbon Anodes for Lithium-Ion Batteries Enabled by a High Work Function. ACS Appl. Mater. Interfaces 2021, 13, 46813–46820. [Google Scholar] [CrossRef]
- Zheng, H.; Zeng, J.; Wan, X.; Song, X.; Peng, C.; Wang, J.; Sun, L.; Wang, H.; Zhu, M.; Liu, J. ICE Optimization Strategies of Hard Carbon Anode for Sodium-Ion Batteries: From the Perspective of Material Synthesis. Mater. Futur. 2024, 3, 032102. [Google Scholar] [CrossRef]
- Chu, Y.; Zhang, J.; Zhang, Y.; Li, Q.; Jia, Y.; Dong, X.; Xiao, J.; Tao, Y.; Yang, Q. Reconfiguring Hard Carbons with Emerging Sodium-Ion Batteries: A Perspective. Adv. Mater. 2023, 35, 2212186. [Google Scholar] [CrossRef]
- Guo, X.; Xue, Y.; Zhou, H.; Weng, Y.; Zhou, J. Achieving Slope-Reigned Na-Ion Storage in Carbon Nanofibers by Constructing Defect-Rich Texture by a Cu-Activation Strategy. ACS Appl. Mater. Interfaces 2020, 12, 2407–2416. [Google Scholar] [CrossRef]
- Chen, D.; Zhang, W.; Luo, K.; Song, Y.; Zhong, Y.; Liu, Y.; Wang, G.; Zhong, B.; Wu, Z.; Guo, X. Hard Carbon for Sodium Storage: Mechanism and Optimization Strategies Toward Commercialization. Energy Environ. Sci. 2021, 14, 2244–2262. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, W.; Lu, S.; Xiang, Y. Carbon anode materials: A detailed comparison between Na-ion and K-ion batteries. Adv. Energy Mater. 2021, 11, 2003640. [Google Scholar] [CrossRef]
- Li, X.; Zhao, H.; Zhang, C.; Xing, B.; Zhang, C.; Zhou, C. One-Pot Fabrication of Pitch-Derived Soft Carbon with Hierarchical Porous Structure and Rich Sp2 Carbon for Sodium-Ion Battery. J. Mater. Sci. Mater. Electron. 2021, 32, 21944–21956. [Google Scholar] [CrossRef]
- Ghosh, S.; Zaid, M.; Dutta, J.; Parvin, M.; Martha, S.K. Soft Carbon in Non-Aqueous Rechargeable Batteries: A Review of Its Synthesis, Carbonization Mechanism, Characterization, and Multifarious Applications. Energy Adv. 2024, 3, 1167–1195. [Google Scholar] [CrossRef]
- Tang, Z.; Zhou, S.; Huang, Y.; Wang, H.; Zhang, R.; Wang, Q.; Sun, D.; Tang, Y.; Wang, H. Improving the Initial Coulombic Efficiency of Carbonaceous Materials for Li/Na-Ion Batteries: Origins, Solutions, and Perspectives. Electrochem. Energy Rev. 2023, 6, 8. [Google Scholar] [CrossRef]
- Onnerud, P.; Shi, J.; Chamberlain, R.; Singh, S.K.; Barnett, B.; Lampe-Onnerud, C. Benchmarking Graphite Materials Used as Anodes in Lithium-Ion Batteries. Available online: https://www.google.com.hk/url?sa=t&source=web&rct=j&opi=89978449&url=https://www.electrochem.org/dl/ma/201/pdfs/0122.pdf&ved=2ahUKEwjR9J3msJuMAxXCsVYBHXOhM4UQFnoECBYQAQ&usg=AOvVaw3Ilqnglzs8KQhQvMsmzNRf (accessed on 20 February 2025).
- Ma, C.; Fan, Q.; Dirican, M.; Song, Y.; Zhang, X.; Shi, J. Porous Carbon Nanosheets Derived from Expanded Graphite for Supercapacitors and Sodium-Ion Batteries. J. Mater. Sci. 2020, 55, 16323–16333. [Google Scholar] [CrossRef]
- GRAPHITE MARKET. Available online: https://westwaterresources.net/minerals-portfolio/graphite-market/ (accessed on 20 February 2025).
- Mine Production of Graphite Worldwide from 2010 to 2024 (in 1000 Metric Tons). Available online: https://www.statista.com/statistics/1005851/global-graphite-production/ (accessed on 20 February 2025).
- U.S. Geological Survey. Mineral Commodity Summaries 2024. Available online: https://pubs.usgs.gov/publication/mcs2024 (accessed on 20 February 2025).
- Qiao, Y.; Zhao, H.; Shen, Y.; Li, L.; Rao, Z.; Shao, G.; Lei, Y. Recycling of Graphite Anode from Spent Lithium-Ion Batteries: Advances and Perspectives. EcoMat 2023, 5, e12321. [Google Scholar] [CrossRef]
- Meng, Y.; Li, J.; Gu, S.; Fu, Y.; Wang, Z.; Liu, J.; Gong, X. Li-Ion Complex Enhances Interfacial Lowest Unoccupied Molecular Orbital for Stable Solid Electrolyte Interface of Natural Graphite Anode. Electrochim. Acta 2023, 449, 142262. [Google Scholar] [CrossRef]
- Shen, Y.; Shen, X.; Yang, M.; Qian, J.; Cao, Y.; Yang, H.; Luo, Y.; Ai, X. Achieving Desirable Initial Coulombic Efficiencies and Full Capacity Utilization of Li-Ion Batteries by Chemical Prelithiation of Graphite Anode. Adv. Funct. Mater. 2021, 31, 2101181. [Google Scholar] [CrossRef]
- Asenbauer, J.; Eisenmann, T.; Kuenzel, M.; Kazzazi, A.; Chen, Z.; Bresser, D. The Success Story of Graphite as a Lithium-Ion Anode Material—Fundamentals, Remaining Challenges, and Recent Developments Including Silicon (Oxide) Composites. Sustain. Energy Fuels 2020, 4, 5387–5416. [Google Scholar] [CrossRef]
- Gallego, N.C.; Contescu, C.I.; Meyer, H.M.; Howe, J.Y.; Meisner, R.A.; Payzant, E.A.; Lance, M.J.; Yoon, S.Y.; Denlinger, M.; Wood, D.L. Advanced Surface and Microstructural Characterization of Natural Graphite Anodes for Lithium Ion Batteries. Carbon 2014, 72, 393–401. [Google Scholar] [CrossRef]
- Yang, Y.; Zou, Y.-C.; Woods, C.R.; Shi, Y.; Yin, J.; Xu, S.; Ozdemir, S.; Taniguchi, T.; Watanabe, K.; Geim, A.K.; et al. Stacking Order in Graphite Films Controlled by van Der Waals Technology. Nano Lett. 2019, 19, 8526–8532. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Zhao, C.; Wu, H.; Li, L.; Zhang, C. Progress, Challenge and Perspective of Graphite-Based Anode Materials for Lithium Batteries: A Review. J. Energy Storage 2024, 81, 110409. [Google Scholar] [CrossRef]
- Li, Y.; Lu, Y.; Adelhelm, P.; Titirici, M.-M.; Hu, Y.-S. Intercalation Chemistry of Graphite: Alkali Metal Ions and Beyond. Chem. Soc. Rev. 2019, 48, 4655–4687. [Google Scholar] [CrossRef]
- Kim, Y.; Jeong, E.H.; Kim, B.S.; Park, J.D. Comparative Study on the Rheological Properties of Natural and Synthetic Graphite-Based Anode Slurries for Lithium-Ion Batteries. Korea-Aust. Rheol. J. 2024, 36, 25–32. [Google Scholar] [CrossRef]
- Zhao, Y.; Fu, Y.; Meng, Y.; Wang, Z.; Liu, J.; Gong, X. Challenges and Strategies of Lithium-Ion Mass Transfer in Natural Graphite Anode. Chem. Eng. J. 2024, 480, 148047. [Google Scholar] [CrossRef]
- Hupp, T.R.; Lewis, I.C.; Criscione, J.M.; Reddy, R.L.; Fulgenzi, C.F.; Page, D.J.; Fisher, F.F.; Dzermejko, A.J.; Hedge, J.B. Graphite, Artificial. In Kirk-Othmer Encyclopedia of Chemical Technology; Kirk-Othmer, Ed.; Wiley: Hoboken, NJ, USA, 2003; ISBN 978-0-471-48494-3. [Google Scholar]
- Chen, Z.; Li, Y.; Wang, L.; Wang, Y.; Chai, J.; Du, J.; Li, Q.; Rui, Y.; Jiang, L.; Tang, B. A Comprehensive Review of Various Carbonaceous Materials for Anodes in Lithium-Ion Batteries. Dalton Trans. 2024, 53, 4900–4921. [Google Scholar] [CrossRef]
- Wang, S.; Li, Y.; Wang, L.; Qiao, Y.; Xu, J.; Li, J.; Zhang, S. PVA Generated Carbon-Coated Natural Graphite Anode Material for Enhanced Performances of Lithium-Ion Batteries. Ionics 2024, 30, 6845–6853. [Google Scholar] [CrossRef]
- Yoshio, M.; Wang, H.; Fukuda, K. Spherical Carbon-Coated Natural Graphite as a Lithium-Ion Battery-Anode Material. Angew. Chem. Int. Ed. 2003, 42, 4203–4206. [Google Scholar] [CrossRef]
- Fischer, S.; Doose, S.; Müller, J.; Höfels, C.; Kwade, A. Impact of Spheroidization of Natural Graphite on Fast-Charging Capability of Anodes for LIB. Batteries 2023, 9, 305. [Google Scholar] [CrossRef]
- Qi, H.; Shi, X.; Liu, Z.; Yan, Z.; Sun, Z. In Situ Etched Graphite Felt Modified with CuFe2O4/Cu2O/Cu Catalyst Derived from CuFe PBA for the Efficient Removal of Sulfamethoxazole Through a Heterogeneous Electro-Fenton Process. Appl. Catal. B Environ. 2023, 331, 122722. [Google Scholar] [CrossRef]
- Lund, S.; Kauppila, J.; Sirkiä, S.; Palosaari, J.; Eklund, O.; Latonen, R.-M.; Smått, J.-H.; Peltonen, J.; Lindfors, T. Fast High-Shear Exfoliation of Natural Flake Graphite with Temperature Control and High Yield. Carbon 2021, 174, 123–131. [Google Scholar] [CrossRef]
- Soldatos, J. (Ed.) Artificial Intelligence In Manufacturing: Enabling Intelligent, Flexible and Cost-Effective Production Through AI; Springer: Cham, Switzerland, 2024; Available online: https://library.oapen.org/handle/20.500.12657/87623 (accessed on 20 February 2025).
- Weimer, A.W. (Ed.) Carbide, Nitride and Boride Materials Synthesis and Processing, 1st ed.; Chapman & Hall: London, UK; Weinheim, Germany, 1997; ISBN 978-0-412-54060-8. [Google Scholar]
- Lee, S.-M.; Kang, D.-S.; Roh, J.-S. Bulk Graphite: Materials and Manufacturing Process. Carbon Lett. 2015, 16, 135–146. [Google Scholar] [CrossRef]
- Hwang, J.U.; Cho, J.H.; Lee, J.D.; Im, J.S. Characteristics of an Artificial Graphite Anode Material for Rapid Charging: Manufactured with Different Coke Particle Sizes. J. Mater. Sci. Mater. Electron. 2022, 33, 20095–20105. [Google Scholar] [CrossRef]
- Ōya, A.; Marsh, H. Phenomena of Catalytic Graphitization. J. Mater. Sci. 1982, 17, 309–322. [Google Scholar] [CrossRef]
- Kulkarni, S.; Huang, T.-Y.; Thapaliya, B.P.; Luo, H.; Dai, S.; Zhao, F. Prospective Life Cycle Assessment of Synthetic Graphite Manufactured via Electrochemical Graphitization. ACS Sustain. Chem. Eng. 2022, 10, 13607–13618. [Google Scholar] [CrossRef]
- Jin, X.; He, R.; Dai, S. Electrochemical Graphitization: An Efficient Conversion of Amorphous Carbons to Nanostructured Graphites. Chem.–Eur. J. 2017, 23, 11455–11459. [Google Scholar] [CrossRef]
- Mohamed, A.M.A.; Dong, S.; Elhefnawey, M.; Dong, G.; Gao, Y.; Zhu, K.; Cao, D. A Comparison of the Electrochemical Performance of Graphitized Coal Prepared by High-Temperature Heating and Flash Joule Heating as an Anode Material for Lithium and Potassium Ion Batteries. Chem. Phys. Lett. 2023, 815, 140362. [Google Scholar] [CrossRef]
- Xing, B.; Zhang, C.; Cao, Y.; Huang, G.; Liu, Q.; Zhang, C.; Chen, Z.; Yi, G.; Chen, L.; Yu, J. Preparation of Synthetic Graphite from Bituminous Coal as Anode Materials for High Performance Lithium-Ion Batteries. Fuel Process. Technol. 2018, 172, 162–171. [Google Scholar] [CrossRef]
- Shi, M.; Song, C.; Tai, Z.; Zou, K.; Duan, Y.; Dai, X.; Sun, J.; Chen, Y.; Liu, Y. Coal-Derived Synthetic Graphite with High Specific Capacity and Excellent Cyclic Stability as Anode Material for Lithium-Ion Batteries. Fuel 2021, 292, 120250. [Google Scholar] [CrossRef]
- Wang, T.; Wang, Y.; Cheng, G.; Ma, C.; Liu, X.; Wang, J.; Qiao, W.; Ling, L. Catalytic Graphitization of Anthracite as an Anode for Lithium-Ion Batteries. Energy Fuels 2020, 34, 8911–8918. [Google Scholar] [CrossRef]
- Thapaliya, B.P.; Luo, H.; Halstenberg, P.; Meyer, H.M.; Dunlap, J.R.; Dai, S. Low-Cost Transformation of Biomass-Derived Carbon to High-Performing Nano-Graphite via Low-Temperature Electrochemical Graphitization. ACS Appl. Mater. Interfaces 2021, 13, 4393–4401. [Google Scholar] [CrossRef]
- Liu, M.; Shi, H.; Guo, L.; Fang, Z.; Chen, D.; Li, W.; Deng, B.; Li, W.; Du, K.; Yin, H.; et al. Enhanced Graphitization of CO2-Derived Carbon Anodes via Joule Heating Reformation for High-Performance Lithium-Ion Batteries. Carbon 2025, 232, 119781. [Google Scholar] [CrossRef]
- AbdelHamid, A.A.; Mendoza-Garcia, A.; Ying, J.Y. Advances in and Prospects of Nanomaterials’ Morphological Control for Lithium Rechargeable Batteries. Nano Energy 2022, 93, 106860. [Google Scholar] [CrossRef]
- Xia, Q.; Liu, H.; Zhao, X.S. Surface Engineering of Anode Materials for Improving Sodium-Ion Storage Performance. J. Mater. Chem. A 2022, 10, 3889–3904. [Google Scholar] [CrossRef]
- Cheng, Q.; Yuge, R.; Nakahara, K.; Tamura, N.; Miyamoto, S. KOH Etched Graphite for Fast Chargeable Lithium-Ion Batteries. J. Power Sources 2015, 284, 258–263. [Google Scholar] [CrossRef]
- Yuan, Y.; Chen, Z.; Yu, H.; Zhang, X.; Liu, T.; Xia, M.; Zheng, R.; Shui, M.; Shu, J. Heteroatom-Doped Carbon-Based Materials for Lithium and Sodium Ion Batteries. Energy Storage Mater. 2020, 32, 65–90. [Google Scholar] [CrossRef]
- Yang, M.; Zhou, Z. Recent Breakthroughs in Supercapacitors Boosted by Nitrogen-Rich Porous Carbon Materials. Adv. Sci. 2017, 4, 1600408. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.J.; Lim, J.; Kim, S.O. Nitrogen Dopants in Carbon Nanomaterials: Defects or a New Opportunity? Small Methods 2017, 1, 1600014. [Google Scholar] [CrossRef]
- Li, Y.; Chen, M.; Liu, B.; Zhang, Y.; Liang, X.; Xia, X. Heteroatom Doping: An Effective Way to Boost Sodium Ion Storage. Adv. Energy Mater. 2020, 10, 2000927. [Google Scholar] [CrossRef]
- Wang, L.; Yang, Z.; Nie, H.; Gu, C.; Hua, W.; Xu, X.; Chen, X.; Chen, Y.; Huang, S. A Lightweight Multifunctional Interlayer of Sulfur–Nitrogen Dual-Doped Graphene for Ultrafast, Long-Life Lithium–Sulfur Batteries. J. Mater. Chem. A 2016, 4, 15343–15352. [Google Scholar] [CrossRef]
- Zhang, T.; Li, C.; Wang, F.; Noori, A.; Mousavi, M.F.; Xia, X.; Zhang, Y. Recent Advances in Carbon Anodes for Sodium-Ion Batteries. Chem. Rec. 2022, 22, e202200083. [Google Scholar] [CrossRef] [PubMed]
- Inagaki, M.; Toyoda, M.; Soneda, Y.; Morishita, T. Nitrogen-Doped Carbon Materials. Carbon 2018, 132, 104–140. [Google Scholar] [CrossRef]
- Kim, D.-Y.; Li, O.L.; Kang, J. Novel Synthesis of Highly Phosphorus-Doped Carbon as an Ultrahigh-Rate Anode for Sodium Ion Batteries. Carbon 2020, 168, 448–457. [Google Scholar] [CrossRef]
- Matthews, P.D.; King, T.C.; Glass, H.; Magusin, P.C.M.M.; Tustin, G.J.; Brown, P.A.C.; Cormack, J.A.; García-Rodríguez, R.; Leskes, M.; Dutton, S.E.; et al. Synthesis and Extensive Characterisation of Phosphorus Doped Graphite. RSC Adv. 2016, 6, 62140–62145. [Google Scholar] [CrossRef]
- Groult, H.; Nakajima, T.; Perrigaud, L.; Ohzawa, Y.; Yashiro, H.; Komaba, S.; Kumagai, N. Surface-Fluorinated Graphite Anode Materials for Li-Ion Batteries. J. Fluor. Chem. 2005, 126, 1111–1116. [Google Scholar] [CrossRef]
- Divya, M.L.; Natarajan, S.; Aravindan, V. Graphene from Spent Lithium-Ion Batteries. Batter. Supercaps 2022, 5, e202200046. [Google Scholar] [CrossRef]
- Li, Y.; Guo, W.; Stroe, D.-I.; Zhao, H.; Kjær Kristensen, P.; Rosgaard Jensen, L.; Pedersen, K.; Gurevich, L. Evolution of Aging Mechanisms and Performance Degradation of Lithium-Ion Battery from Moderate to Severe Capacity Loss Scenarios. Chem. Eng. J. 2024, 498, 155588. [Google Scholar] [CrossRef]
- Synthetic versus Natural Graphite Debate Rages on: 2023 Preview. Available online: https://www.fastmarkets.com/insights/synthetic-versus-natural-graphite-debate/#:~:text=Total%20apparent%20demand%20for%20natural,1.28%20million%20tonnes%20in%202022 (accessed on 20 February 2025).
- Pérez, B.; Echeberria, J. Influence of Abrasives and Graphite on Processing and Properties of Sintered Metallic Friction Materials. Heliyon 2019, 5, e02311. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Xu, Z.-L.; Kang, K. Solvated Ion Intercalation in Graphite: Sodium and Beyond. Front. Chem. 2020, 8, 432. [Google Scholar] [CrossRef]
- Niu, S.; Zhu, G.; Wu, K.; Zheng, H. The Feasibility for Natural Graphite to Replace Artificial Graphite in Organic Electrolyte with Different Film-Forming Additives. Chin. J. Chem. Eng. 2023, 56, 58–69. [Google Scholar] [CrossRef]
- Erickson, E.M.; Markevich, E.; Salitra, G.; Sharon, D.; Hirshberg, D.; De La Llave, E.; Shterenberg, I.; Rosenman, A.; Frimer, A.; Aurbach, D. Review—Development of Advanced Rechargeable Batteries: A Continuous Challenge in the Choice of Suitable Electrolyte Solutions. J. Electrochem. Soc. 2015, 162, A2424–A2438. [Google Scholar] [CrossRef]
- Rey, I.; Vallejo, C.; Santiago, G.; Iturrondobeitia, M.; Lizundia, E. Environmental Impacts of Graphite Recycling from Spent Lithium-Ion Batteries Based on Life Cycle Assessment. ACS Sustain. Chem. Eng. 2021, 9, 14488–14501. [Google Scholar] [CrossRef]
- Zhang, J.; Liang, C.; Dunn, J.B. Graphite Flows in the U.S.: Insights into a Key Ingredient of Energy Transition. Environ. Sci. Technol. 2023, 57, 3402–3414. [Google Scholar] [CrossRef]
- Esteve-Adell, I.; Porcel-Valenzuela, M.; Zubizarreta, L.; Gil-Agustí, M.; García-Pellicer, M.; Quijano-Lopez, A. Influence of the Specific Surface Area of Graphene Nanoplatelets on the Capacity of Lithium-Ion Batteries. Front. Chem. 2022, 10, 807980. [Google Scholar] [CrossRef]
- Liu, Z.; Shi, Y.; Yang, Q.; Shen, H.; Fan, Q.; Nie, H. Effects of Crystal Structure and Electronic Properties on Lithium Storage Performance of Artificial Graphite. RSC Adv. 2023, 13, 29923–29930. [Google Scholar] [CrossRef]
- Pati, S.K.; Hwang, Y.; Lee, H.-M.; Kim, B.-J.; Park, S. Porous Activated Carbon Derived from Petroleum Coke as a High-Performance Anodic Electrode Material for Supercapacitors. Carbon Lett. 2024, 34, 153–162. [Google Scholar] [CrossRef]
- Yue, J.; Zhu, Y.; Lv, J.; Wang, Y.; Cheng, J.; Zhao, X. Application and Research Progress of Coating Pitch in Anode Materials for Lithium-Ion Batteries. Chem. Eng. Sci. 2024, 297, 120302. [Google Scholar] [CrossRef]
- Liang, C.; Chen, Y.; Wu, M.; Wang, K.; Zhang, W.; Gan, Y.; Huang, H.; Chen, J.; Xia, Y.; Zhang, J.; et al. Green Synthesis of Graphite from CO2 Without Graphitization Process of Amorphous Carbon. Nat. Commun. 2021, 12, 119. [Google Scholar] [CrossRef] [PubMed]
- Kamal, A.S.; Othman, R.; Jabarullah, N.H. Preparation and Synthesis of Synthetic Graphite from Biomass Waste: A Review. Syst. Rev. Pharm. 2020, 11, 881–894. [Google Scholar]
- Eftekhari, A. Lithium-Ion Batteries with High Rate Capabilities. ACS Sustain. Chem. Eng. 2017, 5, 2799–2816. [Google Scholar] [CrossRef]
- Li, Y.; Fan, Z.; Li, S.; Zhao, Y.; Li, Z.; Xu, C.; Dou, H.; Zhang, X. A Successive Intercalation-Deposition Mechanism Induced by Hard Carbon for Hybrid Lithium-Ion/Lithium Metal Batteries. J. Energy Chem. 2025, in press. [Google Scholar] [CrossRef]
- Xu, X.; Han, X.; Lu, L.; Wang, F.; Yang, M.; Liu, X.; Wu, Y.; Tang, S.; Hou, Y.; Hou, J.; et al. Challenges and Opportunities Toward Long-Life Lithium-Ion Batteries. J. Power Sources 2024, 603, 234445. [Google Scholar] [CrossRef]
- Yang, C.; Jiang, Z.; Chen, X.; Luo, W.; Zhou, T.; Yang, J. Lithium Metal Based Battery Systems with Ultra-High Energy Density Beyond 500 W h Kg−1. Chem. Commun. 2024, 60, 10245–10264. [Google Scholar] [CrossRef]
- Placke, T.; Kloepsch, R.; Dühnen, S.; Winter, M. Lithium Ion, Lithium Metal, and Alternative Rechargeable Battery Technologies: The Odyssey for High Energy Density. J. Solid State Electrochem. 2017, 21, 1939–1964. [Google Scholar] [CrossRef]
- IEA. Energy Technology Perspectives 2023; IEA: Paris, France, 2023. [Google Scholar]
- Hofmann, U.; Rüdorff, W. The Formation of Salts from Graphite by Strong Acids. Trans. Faraday Soc. 1938, 34, 1017–1021. [Google Scholar] [CrossRef]
- Besenhard, J.O. The Electrochemical Preparation and Properties of Ionic Alkali Metal-and NR4-Graphite Intercalation Compounds in Organic Electrolytes. Carbon 1976, 14, 111–115. [Google Scholar] [CrossRef]
- Rüdorff, W.; Stumpp, E.; Spriessler, W.; Siecke, F.W. Reactions of Graphite with Metal Chlorides. Angew. Chem. Int. Ed. Engl. 1963, 2, 67–73. [Google Scholar] [CrossRef]
- Oka, H.; Makimura, Y.; Uyama, T.; Nonaka, T.; Kondo, Y.; Okuda, C. Changes in the Stage Structure of Li-Intercalated Graphite Electrode at Elevated Temperatures. J. Power Sources 2021, 482, 228926. [Google Scholar] [CrossRef]
- Gavilán-Arriazu, E.M.; Pinto, O.A.; López De Mishima, B.A.; Barraco, D.E.; Oviedo, O.A.; Leiva, E.P.M. The Kinetic Origin of the Daumas-Hérold Model for the Li-Ion/Graphite Intercalation System. Electrochem. Commun. 2018, 93, 133–137. [Google Scholar] [CrossRef]
- Yoda, S.; Ishihara, K. The Advent of Battery-Based Societies and the Global Environment in the 21st Century. J. Power Sources 1999, 81–82, 162–169. [Google Scholar] [CrossRef]
- Edge, J.S.; O’Kane, S.; Prosser, R.; Kirkaldy, N.D.; Patel, A.N.; Hales, A.; Ghosh, A.; Ai, W.; Chen, J.; Yang, J.; et al. Lithium Ion Battery Degradation: What You Need to Know. Phys. Chem. Chem. Phys. 2021, 23, 8200–8221. [Google Scholar] [CrossRef]
- Wright, R.B.; Motloch, C.G.; Belt, J.R.; Christophersen, J.P.; Ho, C.D.; Richardson, R.A.; Bloom, I.; Jones, S.A.; Battaglia, V.S.; Henriksen, G.L.; et al. Calendar- and Cycle-Life Studies of Advanced Technology Development Program Generation 1 Lithium-Ion Batteries. J. Power Sources 2002, 110, 445–470. [Google Scholar] [CrossRef]
- De Hoog, J.; Timmermans, J.-M.; Ioan-Stroe, D.; Swierczynski, M.; Jaguemont, J.; Goutam, S.; Omar, N.; Van Mierlo, J.; Van Den Bossche, P. Combined Cycling and Calendar Capacity Fade Modeling of a Nickel-Manganese-Cobalt Oxide Cell with Real-Life Profile Validation. Appl. Energy 2017, 200, 47–61. [Google Scholar] [CrossRef]
- Feinauer, M.; Wohlfahrt-Mehrens, M.; Hölzle, M.; Waldmann, T. Temperature-Driven Path Dependence in Li-Ion Battery Cyclic Aging. J. Power Sources 2024, 594, 233948. [Google Scholar] [CrossRef]
- Liu, Q.; Rao, A.M.; Han, X.; Lu, B. Artificial SEI for Superhigh-Performance K-Graphite Anode. Adv. Sci. 2021, 8, 2003639. [Google Scholar] [CrossRef]
- Tzeng, Y.; Jhan, C.-Y.; Sung, S.-H.; Chiou, Y.-Y. Effects of Crystalline Diamond Nanoparticles on Silicon Thin Films as an Anode for a Lithium-Ion Battery. Batteries 2024, 10, 321. [Google Scholar] [CrossRef]
- Sagar, R.U.R.; Mahmood, N.; Stadler, F.J.; Anwar, T.; Navale, S.T.; Shehzad, K.; Du, B. High Capacity Retention Anode Material for Lithium Ion Battery. Electrochim. Acta 2016, 211, 156–163. [Google Scholar] [CrossRef]
- Liang, B.; Liu, Y.; Xu, Y. Silicon-Based Materials as High Capacity Anodes for Next Generation Lithium Ion Batteries. J. Power Sources 2014, 267, 469–490. [Google Scholar] [CrossRef]
- Song, T.; Jeon, Y.; Paik, U. Si Nanotubes Array Sheathed with SiN/SiOxNy Layer as an Anode Material for Lithium Ion Batteries. J. Electroceramics 2014, 32, 66–71. [Google Scholar] [CrossRef]
- Shen, Y.; Qian, J.; Yang, H.; Zhong, F.; Ai, X. Chemically Prelithiated Hard-Carbon Anode for High Power and High Capacity Li-Ion Batteries. Small 2020, 16, 1907602. [Google Scholar] [CrossRef]
- Liu, Y.; Li, X.; Guo, H.; Wang, Z.; Peng, W.; Yang, Y.; Liang, R. Effect of Carbon Nanotube on the Electrochemical Performance of C-LiFePO4/Graphite Battery. J. Power Sources 2008, 184, 522–526. [Google Scholar] [CrossRef]
- An, S.J.; Li, J.; Daniel, C.; Mohanty, D.; Nagpure, S.; Wood, D.L. The State of Understanding of the Lithium-Ion-Battery Graphite Solid Electrolyte Interphase (SEI) and Its Relationship to Formation Cycling. Carbon 2016, 105, 52–76. [Google Scholar] [CrossRef]
- Blomgren, G.E. Liquid Electrolytes for Lithium and Lithium-Ion Batteries. J. Power Sources 2003, 119–121, 326–329. [Google Scholar] [CrossRef]
- Huang, Y.; Yang, H.; Gao, Y.; Chen, G.; Li, Y.; Shi, L.; Zhang, D. Mechanism and Solutions of Lithium Dendrite Growth in Lithium Metal Batteries. Mater. Chem. Front. 2024, 8, 1282–1299. [Google Scholar] [CrossRef]
- Ong, M.T.; Verners, O.; Draeger, E.W.; Van Duin, A.C.T.; Lordi, V.; Pask, J.E. Lithium Ion Solvation and Diffusion in Bulk Organic Electrolytes from First-Principles and Classical Reactive Molecular Dynamics. J. Phys. Chem. B 2015, 119, 1535–1545. [Google Scholar] [CrossRef]
- Li, S.; Luo, Z.; Li, L.; Hu, J.; Zou, G.; Hou, H.; Ji, X. Recent Progress on Electrolyte Additives for Stable Lithium Metal Anode. Energy Storage Mater. 2020, 32, 306–319. [Google Scholar] [CrossRef]
- Dou, F.; Shi, L.; Chen, G.; Zhang, D. Silicon/Carbon Composite Anode Materials for Lithium-Ion Batteries. Electrochem. Energy Rev. 2019, 2, 149–198. [Google Scholar] [CrossRef]
- Mei, Y.; He, Y.; Zhu, H.; Ma, Z.; Pu, Y.; Chen, Z.; Li, P.; He, L.; Wang, W.; Tang, H. Recent Advances in the Structural Design of Silicon/Carbon Anodes for Lithium Ion Batteries: A Review. Coatings 2023, 13, 436. [Google Scholar] [CrossRef]
- Yang, Z.; Shen, J.; Archer, L.A. An In Situ Method of Creating Metal Oxide–Carbon Composites and Their Application as Anode Materials for Lithium-Ion Batteries. J. Mater. Chem. 2011, 21, 11092. [Google Scholar] [CrossRef]
- Putois, F. Market for Nickel-Cadmium Batteries. J. Power Sources 1995, 57, 67–70. [Google Scholar] [CrossRef]
- Nishi, Y. Lithium Ion Secondary Batteries; Past 10 Years and the Future. J. Power Sources 2001, 100, 101–106. [Google Scholar] [CrossRef]
- De, S.; Northrop, P.W.C.; Ramadesigan, V.; Subramanian, V.R. Model-Based Simultaneous Optimization of Multiple Design Parameters for Lithium-Ion Batteries for Maximization of Energy Density. J. Power Sources 2013, 227, 161–170. [Google Scholar] [CrossRef]
- Goodenough, J.B.; Kim, Y. Challenges for Rechargeable Li Batteries. Chem. Mater. 2010, 22, 587–603. [Google Scholar] [CrossRef]
- Goriparti, S.; Miele, E.; De Angelis, F.; Di Fabrizio, E.; Proietti Zaccaria, R.; Capiglia, C. Review on Recent Progress of Nanostructured Anode Materials for Li-Ion Batteries. J. Power Sources 2014, 257, 421–443. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, Y.; Zhang, B.; Yang, D.; Zhou, K.; Huang, Y.; Wang, F.; Duan, J.; Wang, X.; Dong, P.; et al. Enhanced Electrochemical Performance of a Cost-Effective Sm2O3-Coated Spinel LiNi0.5Mn1.5O4 Cathode for High-Voltage Lithium-Ion Batteries. J. Power Sources 2024, 614, 235008. [Google Scholar] [CrossRef]
- Zhu, C.; Shen, X.; Gao, Z.; Li, Y.; Wu, X.; Zhao, J.; Zhou, P.; Zhuo, S.; Zhou, J. Enhancing Electrochemical Performance of Fluorinated Graphite by Polydopamine−Derived Nitrogen−Doped Carbon Coating. Electrochim. Acta 2022, 425, 140718. [Google Scholar] [CrossRef]
- Wang, F.; Lin, S.; Lu, X.; Hong, R.; Liu, H. Poly-Dopamine Carbon-Coated Stable Silicon/Graphene/CNT Composite as Anode for Lithium Ion Batteries. Electrochim. Acta 2022, 404, 139708. [Google Scholar] [CrossRef]
- Liu, P.; Peng, J.; He, L.; Yang, J.; Tang, Y.; Zhou, K.; Xie, Z.; Wang, X. Amorphous Carbon Coating Enabling Waste Graphite to Reuse as High-Performance Anode of Lithium-Ion Battery. ACS Appl. Energy Mater. 2025, 8, 442–451. [Google Scholar] [CrossRef]
- Hafiz, W.; Du, B.; Zhang, J.; Xiao, M.; Meng, Y.; Zhu, F. Enhancing the Performance of Lithium-Ion Batteries with NiCo2S4/C-Hollow Sphere Nanocomposites. J. Mater. Sci. Mater. Electron. 2024, 35, 1987. [Google Scholar] [CrossRef]
- Asasian-Kolur, N.; Sharifian, S.; Haddadi, B.; Jordan, C.; Harasek, M. Ordered Porous Carbon Preparation by Hard Templating Approach for Hydrogen Adsorption Application. Biomass Convers. Biorefinery 2024, 14, 18381–18416. [Google Scholar] [CrossRef]
- Li, Z.; Ottmann, A.; Zhang, T.; Sun, Q.; Meyer, H.-P.; Vaynzof, Y.; Xiang, J.; Klingeler, R. Preparation of Hierarchical C@MoS2 @C Sandwiched Hollow Spheres for Lithium Ion Batteries. J. Mater. Chem. A 2017, 5, 3987–3994. [Google Scholar] [CrossRef]
- Chuenchom, L.; Kraehnert, R.; Smarsly, B.M. Recent Progress in Soft-Templating of Porous Carbon Materials. Soft Matter 2012, 8, 10801. [Google Scholar] [CrossRef]
- Li, C.; Li, Q.; Kaneti, Y.V.; Hou, D.; Yamauchi, Y.; Mai, Y. Self-Assembly of Block Copolymers Towards Mesoporous Materials for Energy Storage and Conversion Systems. Chem. Soc. Rev. 2020, 49, 4681–4736. [Google Scholar] [CrossRef]
- Feyzi, E.; Madikere Raghunatha Reddy, A.K.; Li, X.; Deng, S.; Nanda, J.; Zaghib, K. A Comprehensive Review of Silicon Anodes for High-Energy Lithium-Ion Batteries: Challenges, Latest Developments, and Perspectives. Energy 2024, 5, 100176. [Google Scholar] [CrossRef]
- Ji, H.; Xu, X.; Li, X.; Li, K.; Yuan, L.; Han, Z.; Tang, K. A Low-Cost Si@C Composite for Lithium-Ion Batteries Anode Materials Synthesized via Freeze-Drying Process Using Kerf Loss Si Waste. Ionics 2024, 30, 2585–2599. [Google Scholar] [CrossRef]
- Poetke, S.; Hippauf, F.; Baasner, A.; Dörfler, S.; Althues, H.; Kaskel, S. Nanostructured Si−C Composites as High-Capacity Anode Material for All-Solid-State Lithium-Ion Batteries. Batter. Supercaps 2021, 4, 1323–1334. [Google Scholar] [CrossRef]
- Park, I.; Lee, H.; Chae, O.B. Synthesis Methods of Si/C Composite Materials for Lithium-Ion Batteries. Batteries 2024, 10, 381. [Google Scholar] [CrossRef]
- Xin, F.; Whittingham, M.S. Challenges and Development of Tin-Based Anode with High Volumetric Capacity for Li-Ion Batteries. Electrochem. Energy Rev. 2020, 3, 643–655. [Google Scholar] [CrossRef]
- Fang, G.; Liu, W.; Kaneko, S.; Xia, B.; Sun, H.; Zheng, J.; Li, D. Preparation, Microstructure, and Electrochemical Properties of Sn-Co-C Anode Materials Using Composited Carbon Sources. J. Solid State Electrochem. 2013, 17, 2521–2529. [Google Scholar] [CrossRef]
- Zheng, H.; Qu, Q.; Zhang, L.; Liu, G.; Battaglia, V.S. Hard Carbon: A Promising Lithium-Ion Battery Anode for High Temperature Applications with Ionic Electrolyte. RSC Adv. 2012, 2, 4904. [Google Scholar] [CrossRef]
- Matsunaga, T.; Takagi, S.; Shimoda, K.; Okazaki, K.; Ishikawa, Y.; Yonemura, M.; Ukyo, Y.; Fukunaga, T.; Matsubara, E. Comprehensive Elucidation of Crystal Structures of Lithium-Intercalated Graphite. Carbon 2019, 142, 513–517. [Google Scholar] [CrossRef]
- Liu, X.; Si, Y.; Li, K.; Xu, Y.; Zhao, Z.; Li, C.; Fu, Y.; Li, D. Exploring Sodium Storage Mechanism of Topological Insulator Bi2Te3 Nanosheets Encapsulated in Conductive Polymer. Energy Storage Mater. 2021, 41, 255–263. [Google Scholar] [CrossRef]
- Park, S.-H.; Kim, H.J.; Lee, J.; Jeong, Y.K.; Choi, J.W.; Lee, H. Mussel-Inspired Polydopamine Coating for Enhanced Thermal Stability and Rate Performance of Graphite Anodes in Li-Ion Batteries. ACS Appl. Mater. Interfaces 2016, 8, 13973–13981. [Google Scholar] [CrossRef]
- Du, J.; Wang, W.; Jia, H.; Li, T.; Song, K. High-Rate Soft Carbon Anode for Lithium Storage: From Modified Pitch Molecular Structure to Ordered Carbon Microcrystals. Ionics 2024, 31, 151–163. [Google Scholar] [CrossRef]
- Zuo, X.; Zhu, J.; Müller-Buschbaum, P.; Cheng, Y.-J. Silicon Based Lithium-Ion Battery Anodes: A Chronicle Perspective Review. Nano Energy 2017, 31, 113–143. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, X.; Yan, F.; Chan, H.L.W.; Ding, F. Mechanism of Boron and Nitrogen In Situ Doping during Graphene Chemical Vapor Deposition Growth. Carbon 2016, 98, 633–637. [Google Scholar] [CrossRef]
- Daigle, J.-C.; Barray, F.; Gagnon, C.; Clément, D.; Hovington, P.; Demers, H.; Guerfi, A.; Zaghib, K. Amphiphilic Latex as a Water-Based Binder for LiFePO4 Cathode. J. Power Sources 2019, 415, 172–178. [Google Scholar] [CrossRef]
- Chitraningrum, N.; Gunawan, F.; Farma, R.; Subyakto, S.; Subhan, A.; Fudholi, A.; Rajani, A.; Apriyani, I.; Manurung, K.S.; Ramadhan, F.A.; et al. Nitrogen-Doped Activated Carbon Derived from Oil Palm Empty Fruit Bunch (OPEFB) for Sustainable Lithium-Ion Battery. Biomass Convers. Biorefinery 2024. [Google Scholar] [CrossRef]
- Geng, H.; Zhou, Q.; Pan, Y.; Gu, H.; Zheng, J. Preparation of Fluorine-Doped, Carbon-Encapsulated Hollow Fe3O4 Spheres as an Efficient Anode Material for Li-Ion Batteries. Nanoscale 2014, 6, 3889. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhang, C.; Liu, Z.; Wang, L.; Han, P.; Xu, H.; Zhang, K.; Dong, S.; Yao, J.; Cui, G. Nitrogen-Doped Graphene Nanosheets with Excellent Lithium Storage Properties. J. Mater. Chem. 2011, 21, 5430. [Google Scholar] [CrossRef]
- Kim, K.-J.; Lee, T.-S.; Kim, H.-G.; Lim, S.-H.; Lee, S.-M. A Hard Carbon/Microcrystalline Graphite/Carbon Composite with a Core-Shell Structure as Novel Anode Materials for Lithium-Ion Batteries. Electrochim. Acta 2014, 135, 27–34. [Google Scholar] [CrossRef]
- Rodríguez, E.; Cameán, I.; García, R.; García, A.B. Graphitized Boron-Doped Carbon Foams: Performance as Anodes in Lithium-Ion Batteries. Electrochim. Acta 2011, 56, 5090–5094. [Google Scholar] [CrossRef]
- Wang, C.; Zhao, H.; Wang, J.; Wang, J.; Lv, P. Electrochemical Performance of Modified Artificial Graphite as Anode Material for Lithium Ion Batteries. Ionics 2013, 19, 221–226. [Google Scholar] [CrossRef]
- Xu, J.-L.; Zhang, X.; Miao, Y.-X.; Wen, M.-X.; Yan, W.-J.; Lu, P.; Wang, Z.-R.; Sun, Q. In-Situ Plantation of Fe3O4@C Nanoparticles on Reduced Graphene Oxide Nanosheet as High-Performance Anode for Lithium/Sodium-Ion Batteries. Appl. Surf. Sci. 2021, 546, 149163. [Google Scholar] [CrossRef]
- Feng, T.; Xu, Y.; Zhang, Z.; Du, X.; Sun, X.; Xiong, L.; Rodriguez, R.; Holze, R. Low-Cost Al2O3 Coating Layer As a Preformed SEI on Natural Graphite Powder To Improve Coulombic Efficiency and High-Rate Cycling Stability of Lithium-Ion Batteries. ACS Appl. Mater. Interfaces 2016, 8, 6512–6519. [Google Scholar] [CrossRef]
- Cui, Z.; Wang, L.; Li, Q.; Wang, K. A Comprehensive Review on the State of Charge Estimation for Lithium-Ion Battery Based on Neural Network. Int. J. Energy Res. 2022, 46, 5423–5440. [Google Scholar] [CrossRef]
- Lee, S.; Kim, J.; Lee, J.; Cho, B.H. State-of-Charge and Capacity Estimation of Lithium-Ion Battery Using a New Open-Circuit Voltage Versus State-of-Charge. J. Power Sources 2008, 185, 1367–1373. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, L.; Cheng, J.; Zhou, J.; Wang, S. A State of Charge Estimation Method of Lithium-Ion Battery Based on Fused Open Circuit Voltage Curve. Appl. Sci. 2020, 10, 1264. [Google Scholar] [CrossRef]
- Dahn, J.R.; Zheng, T.; Liu, Y.; Xue, J.S. Mechanisms for Lithium Insertion in Carbonaceous Materials. Science 1995, 270, 590–593. [Google Scholar] [CrossRef]
- Stevens, D.A.; Dahn, J.R. High Capacity Anode Materials for Rechargeable Sodium-Ion Batteries. J. Electrochem. Soc. 2000, 147, 1271. [Google Scholar] [CrossRef]
- Lou, T.T.; Zhang, W.G.; Guo, H.Y.; Wang, J.S. The Internal Resistance Characteristics of Lithium-Ion Battery Based on HPPC Method. Adv. Mater. Res. 2012, 455–456, 246–251. [Google Scholar] [CrossRef]
- Stolz, L.; Winter, M.; Kasnatscheew, J. Practical Relevance of Charge Transfer Resistance at the Li Metal Electrode|electrolyte Interface in Batteries? J. Solid State Electrochem. 2024. [Google Scholar] [CrossRef]
- Wang, X.; Wei, X.; Dai, H. Estimation of State of Health of Lithium-Ion Batteries Based on Charge Transfer Resistance Considering Different Temperature and State of Charge. J. Energy Storage 2019, 21, 618–631. [Google Scholar] [CrossRef]
- Eom, K.; Jung, J.; Lee, J.T.; Lair, V.; Joshi, T.; Lee, S.W.; Lin, Z.; Fuller, T.F. Improved Stability of Nano-Sn Electrode with High-Quality Nano-SEI Formation for Lithium Ion Battery. Nano Energy 2015, 12, 314–321. [Google Scholar] [CrossRef]
- Huang, Q.-A.; Shen, Y.; Huang, Y.; Zhang, L.; Zhang, J. Impedance Characteristics and Diagnoses of Automotive Lithium-Ion Batteries at 7.5% to 93.0% State of Charge. Electrochim. Acta 2016, 219, 751–765. [Google Scholar] [CrossRef]
- Ruggeri, I.; Martin, J.; Wohlfahrt-Mehrens, M.; Mancini, M. Interfacial Kinetics and Low-Temperature Behavior of Spheroidized Natural Graphite Particles as Anode for Li-Ion Batteries. J. Solid State Electrochem. 2022, 26, 73–83. [Google Scholar] [CrossRef]
- Nam, K.-H.; Hwa Chae, K.; Choi, J.-H.; Jeon, K.-J.; Park, C.-M. Superior Carbon Black: High-Performance Anode and Conducting Additive for Rechargeable Li- and Na-Ion Batteries. Chem. Eng. J. 2021, 417, 129242. [Google Scholar] [CrossRef]
- Kong, L.; Li, Y.; Feng, W. Strategies to Solve Lithium Battery Thermal Runaway: From Mechanism to Modification. Electrochem. Energy Rev. 2021, 4, 633–679. [Google Scholar] [CrossRef]
- Abraham, D.P.; Roth, E.P.; Kostecki, R.; McCarthy, K.; MacLaren, S.; Doughty, D.H. Diagnostic Examination of Thermally Abused High-Power Lithium-Ion Cells. J. Power Sources 2006, 161, 648–657. [Google Scholar] [CrossRef]
- Klein, E.J.; Carter, R.; Love, C.T. Accelerating Rate Calorimetry and Complementary Techniques to Characterize Battery Safety Hazards. J. Vis. Exp. 2021, 15, 60342. [Google Scholar] [CrossRef] [PubMed]
- Md Said, M.S.; Mohd Tohir, M.Z. Characterisation of Thermal Runaway Behaviour of Cylindrical Lithium-Ion Battery Using Accelerating Rate Calorimeter and Oven Heating. Case Stud. Therm. Eng. 2021, 28, 101474. [Google Scholar] [CrossRef]
- Yang, Y.; Chen, L.; Yang, L.; Du, X.; Yang, Y. Capacity Fade Characteristics of Lithium Iron Phosphate Cell during Dynamic Cycle. Energy 2020, 206, 118155. [Google Scholar] [CrossRef]
- Fan, J.; Tan, S. Studies on Charging Lithium-Ion Cells at Low Temperatures. J. Electrochem. Soc. 2006, 153, A1081. [Google Scholar] [CrossRef]
- Hausbrand, R.; Cherkashinin, G.; Ehrenberg, H.; Gröting, M.; Albe, K.; Hess, C.; Jaegermann, W. Fundamental Degradation Mechanisms of Layered Oxide Li-Ion Battery Cathode Materials: Methodology, Insights and Novel Approaches. Mater. Sci. Eng. B 2015, 192, 3–25. [Google Scholar] [CrossRef]
- Sharifi-Asl, S.; Lu, J.; Amine, K.; Shahbazian-Yassar, R. Oxygen Release Degradation in Li-Ion Battery Cathode Materials: Mechanisms and Mitigating Approaches. Adv. Energy Mater. 2019, 9, 1900551. [Google Scholar] [CrossRef]
- Kalnaus, S.; Wang, Y.; Turner, J.A. Mechanical Behavior and Failure Mechanisms of Li-Ion Battery Separators. J. Power Sources 2017, 348, 255–263. [Google Scholar] [CrossRef]
- Larsson, F.; Mellander, B.-E. Abuse by External Heating, Overcharge and Short Circuiting of Commercial Lithium-Ion Battery Cells. J. Electrochem. Soc. 2014, 161, A1611–A1617. [Google Scholar] [CrossRef]
- Lai, X.; Jin, C.; Yi, W.; Han, X.; Feng, X.; Zheng, Y.; Ouyang, M. Mechanism, Modeling, Detection, and Prevention of the Internal Short Circuit in Lithium-Ion Batteries: Recent Advances and Perspectives. Energy Storage Mater. 2021, 35, 470–499. [Google Scholar] [CrossRef]
- Saito, Y.; Takano, K.; Negishi, A. Thermal Behaviors of Lithium-Ion Cells during Overcharge. J. Power Sources 2001, 97–98, 693–696. [Google Scholar] [CrossRef]
- Yuge, R.; Tamura, N.; Manako, T.; Nakano, K.; Nakahara, K. High-Rate Charge/Discharge Properties of Li-Ion Battery Using Carbon-Coated Composites of Graphites, Vapor Grown Carbon Fibers, and Carbon Nanohorns. J. Power Sources 2014, 266, 471–474. [Google Scholar] [CrossRef]
- Jung, Y.S.; Cavanagh, A.S.; Riley, L.A.; Kang, S.; Dillon, A.C.; Groner, M.D.; George, S.M.; Lee, S. Ultrathin Direct Atomic Layer Deposition on Composite Electrodes for Highly Durable and Safe Li-Ion Batteries. Adv. Mater. 2010, 22, 2172–2176. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.; Zhou, C.; Zhou, H.; Wang, Z.; Ren, J. Synthesis of Alumina-Coated Natural Graphite for Highly Cycling Stability and Safety of Li-Ion Batteries. Chin. J. Chem. 2019, 37, 342–346. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, L.; Zhang, H.; Xu, H.; He, X. Materials Descriptors of Machine Learning to Boost Development of Lithium-Ion Batteries. Nano Converg. 2024, 11, 8. [Google Scholar] [CrossRef]
- Ghodake, A.; Sadakale, R.; Dhanvijay, M.; Mandhana, A.; Joshi, U. Integrating Thermal Mechanisms with Machine Learning for Accurate State of Health Estimation in Lithium-Ion Batteries. In Proceedings of the 12th International Conference on Soft Computing for Problem Solving; Pant, M., Deep, K., Nagar, A., Eds.; Lecture Notes in Networks and Systems; Springer Nature: Singapore, 2024; Volume 994, pp. 767–782. ISBN 978-981-97-3179-4. [Google Scholar]
- Valizadeh, A.; Amirhosseini, M.H. Machine Learning in Lithium-Ion Battery: Applications, Challenges, and Future Trends. SN Comput. Sci. 2024, 5, 717. [Google Scholar] [CrossRef]
- Darga, J.; Lamb, J.; Manthiram, A. Industrialization of Layered Oxide Cathodes for Lithium-Ion and Sodium-Ion Batteries: A Comparative Perspective. Energy Technol. 2020, 8, 2000723. [Google Scholar] [CrossRef]
- Wu, H.; Hao, J.; Jiang, Y.; Jiao, Y.; Liu, J.; Xu, X.; Davey, K.; Wang, C.; Qiao, S.-Z. Alkaline-Based Aqueous Sodium-Ion Batteries for Large-Scale Energy Storage. Nat. Commun. 2024, 15, 575. [Google Scholar] [CrossRef]
- Li, Y.; Lu, Y.; Zhao, C.; Hu, Y.-S.; Titirici, M.-M.; Li, H.; Huang, X.; Chen, L. Recent Advances of Electrode Materials for Low-Cost Sodium-Ion Batteries towards Practical Application for Grid Energy Storage. Energy Storage Mater. 2017, 7, 130–151. [Google Scholar] [CrossRef]
- Chang, X.; Yang, Z.; Liu, Y.; Chen, J.; Wu, M.; Li, L.; Chou, S.; Qiao, Y. The Guarantee of Large-Scale Energy Storage: Non-Flammable Organic Liquid Electrolytes for High-Safety Sodium Ion Batteries. Energy Storage Mater. 2024, 69, 103407. [Google Scholar] [CrossRef]
- Nobuhara, K.; Nakayama, H.; Nose, M.; Nakanishi, S.; Iba, H. First-Principles Study of Alkali Metal-Graphite Intercalation Compounds. J. Power Sources 2013, 243, 585–587. [Google Scholar] [CrossRef]
- Lyu, L.; Yi, Y.; Xu, Z. Graphite Co-Intercalation Chemistry in Sodium-Ion Batteries. Batter. Supercaps 2024, 8, e202400521. [Google Scholar] [CrossRef]
- Liu, Y.; Merinov, B.V.; Goddard, W.A. Origin of Low Sodium Capacity in Graphite and Generally Weak Substrate Binding of Na and Mg Among Alkali and Alkaline Earth Metals. Proc. Natl. Acad. Sci. USA 2016, 113, 3735–3739. [Google Scholar] [CrossRef] [PubMed]
- Jache, B.; Adelhelm, P. Use of Graphite as a Highly Reversible Electrode with Superior Cycle Life for Sodium-Ion Batteries by Making Use of Co-Intercalation Phenomena. Angew. Chem. Int. Ed. 2014, 53, 10169–10173. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Kim, H.; Ding, Z.; Lee, M.H.; Lim, K.; Yoon, G.; Kang, K. Recent Progress in Electrode Materials for Sodium-Ion Batteries. Adv. Energy Mater. 2016, 6, 1600943. [Google Scholar] [CrossRef]
- Kim, H.; Hong, J.; Yoon, G.; Kim, H.; Park, K.-Y.; Park, M.-S.; Yoon, W.-S.; Kang, K. Sodium Intercalation Chemistry in Graphite. Energy Environ. Sci. 2015, 8, 2963–2969. [Google Scholar] [CrossRef]
- Jache, B.; Binder, J.O.; Abe, T.; Adelhelm, P. A Comparative Study on the Impact of Different Glymes and Their Derivatives as Electrolyte Solvents for Graphite Co-Intercalation Electrodes in Lithium-Ion and Sodium-Ion Batteries. Phys. Chem. Chem. Phys. 2016, 18, 14299–14316. [Google Scholar] [CrossRef]
- Escher, I.; Freytag, A.I.; López Del Amo, J.M.; Adelhelm, P. Solid-State NMR Study on the Structure and Dynamics of Graphite Electrodes in Sodium-Ion Batteries with Solvent Co-Intercalation. Batter. Supercaps 2023, 6, e202200421. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, S.; Zhang, W.; Quadrelli, A.; Jarvis, S.; Chen, J.; Lu, H.; Mangayarkarasi, N.; Niu, Y.; Tao, J.; et al. Operando Nano-Mapping of Sodium-Diglyme Co-Intercalation and SEI Formation in Sodium Ion Batteries’ Graphene Anodes. Appl. Phys. Rev. 2024, 11, 021422. [Google Scholar] [CrossRef]
- Maibach, J.; Jeschull, F.; Brandell, D.; Edström, K.; Valvo, M. Surface Layer Evolution on Graphite During Electrochemical Sodium-Tetraglyme Co-Intercalation. ACS Appl. Mater. Interfaces 2017, 9, 12373–12381. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, A.; Wang, Z.; Bautista, S.P.; Weil, M.; Müller, F.; Löwe, R.; Schneider, L.; Mohsin, I.U.; Hanemann, T. Comprehensive Characterization of Propylene Carbonate Based Liquid Electrolyte Mixtures for Sodium-Ion Cells. Electrochim. Acta 2022, 403, 139670. [Google Scholar] [CrossRef]
- Parveen, S.; Sehrawat, P.; Hashmi, S.A. Triglyme-Based Solvate Ionic Liquid Gelled in a Polymer: A Novel Electrolyte Composition for Sodium Ion Battery. Mater. Today Commun. 2022, 31, 103392. [Google Scholar] [CrossRef]
- Goktas, M.; Akduman, B.; Huang, P.; Balducci, A.; Adelhelm, P. Temperature-Induced Activation of Graphite Co-Intercalation Reactions for Glymes and Crown Ethers in Sodium-Ion Batteries. J. Phys. Chem. C 2018, 122, 26816–26824. [Google Scholar] [CrossRef]
- Murali, A.S.; Susan Baji, D.; Nair, S.; Santhanagopalan, D. Vapour Phase Conversion of Metal Oxalates to Metal Phosphide Nanostructures and Their Use as Anode in Rechargeable Li, Na and K-Ion Batteries. Electrochim. Acta 2021, 388, 138643. [Google Scholar] [CrossRef]
- Wen, Y.; He, K.; Zhu, Y.; Han, F.; Xu, Y.; Matsuda, I.; Ishii, Y.; Cumings, J.; Wang, C. Expanded Graphite as Superior Anode for Sodium-Ion Batteries. Nat. Commun. 2014, 5, 4033. [Google Scholar] [CrossRef]
- Ding, J.; Zhou, X.; Gao, J.; Lei, Z. Activating Graphite with Defects and Oxygenic Functional Groups to Boost Sodium-Ion Storage. Nanoscale 2023, 15, 13760–13769. [Google Scholar] [CrossRef]
- Luo, P.; Zheng, C.; He, J.; Tu, X.; Sun, W.; Pan, H.; Zhou, Y.; Rui, X.; Zhang, B.; Huang, K. Structural Engineering in Graphite-Based Metal-Ion Batteries. Adv. Funct. Mater. 2022, 32, 2107277. [Google Scholar] [CrossRef]
- Hu, M.; Zhou, H.; Gan, X.; Yang, L.; Huang, Z.-H.; Wang, D.-W.; Kang, F.; Lv, R. Ultrahigh Rate Sodium Ion Storage with Nitrogen-Doped Expanded Graphite Oxide in Ether-Based Electrolyte. J. Mater. Chem. A 2018, 6, 1582–1589. [Google Scholar] [CrossRef]
- Liao, K.; Wang, H.; Wang, L.; Xu, D.; Wu, M.; Wang, R.; He, B.; Gong, Y.; Hu, X. A High-Energy Sodium-Ion Capacitor Enabled by a Nitrogen/Sulfur Co-Doped Hollow Carbon Nanofiber Anode and an Activated Carbon Cathode. Nanoscale Adv. 2019, 1, 746–756. [Google Scholar] [CrossRef]
- Bommier, C.; Surta, T.W.; Dolgos, M.; Ji, X. New Mechanistic Insights on Na-Ion Storage in Nongraphitizable Carbon. Nano Lett. 2015, 15, 5888–5892. [Google Scholar] [CrossRef] [PubMed]
- Zhong, B.; Liu, C.; Xiong, D.; Cai, J.; Li, J.; Li, D.; Cao, Z.; Song, B.; Deng, W.; Peng, H.; et al. Biomass-Derived Hard Carbon for Sodium-Ion Batteries: Basic Research and Industrial Application. ACS Nano 2024, 18, 16468–16488. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, A.; Janakiraman, S.; Biswas, K.; Venimadhav, A.; Srivastava, S.K.; Ghosh, S. Understanding the Improved Electrochemical Performance of Nitrogen-Doped Hard Carbons as an Anode for Sodium Ion Battery. Electrochim. Acta 2019, 317, 164–172. [Google Scholar] [CrossRef]
- Jin, Q.; Wang, K.; Feng, P.; Zhang, Z.; Cheng, S.; Jiang, K. Surface-Dominated Storage of Heteroatoms-Doping Hard Carbon for Sodium-Ion Batteries. Energy Storage Mater. 2020, 27, 43–50. [Google Scholar] [CrossRef]
- Doeff, M.M.; Ma, Y.; Visco, S.J.; De Jonghe, L.C. Electrochemical Insertion of Sodium into Carbon. J. Electrochem. Soc. 1993, 140, L169–L170. [Google Scholar] [CrossRef]
- Luo, W.; Jian, Z.; Xing, Z.; Wang, W.; Bommier, C.; Lerner, M.M.; Ji, X. Electrochemically Expandable Soft Carbon as Anodes for Na-Ion Batteries. ACS Cent. Sci. 2015, 1, 516–522. [Google Scholar] [CrossRef]
- Wan, X.; Li, Y.; Chen, S.; Duan, W.; Lei, W. Cathode Modification of Sodium-Ion Batteries for Improved Energy Density: A Review. Adv. Sustain. Syst. 2024, 8, 2400229. [Google Scholar] [CrossRef]
- Bommier, C.; Luo, W.; Gao, W.-Y.; Greaney, A.; Ma, S.; Ji, X. Predicting Capacity of Hard Carbon Anodes in Sodium-Ion Batteries Using Porosity Measurements. Carbon 2014, 76, 165–174. [Google Scholar] [CrossRef]
- Wang, K.; Sun, F.; Wang, H.; Wu, D.; Chao, Y.; Gao, J.; Zhao, G. Altering Thermal Transformation Pathway to Create Closed Pores in Coal-Derived Hard Carbon and Boosting of Na+ Plateau Storage for High-Performance Sodium-Ion Battery and Sodium-Ion Capacitor. Adv. Funct. Mater. 2022, 32, 2203725. [Google Scholar] [CrossRef]
- He, X.-X.; Zhao, J.-H.; Lai, W.-H.; Li, R.; Yang, Z.; Xu, C.; Dai, Y.; Gao, Y.; Liu, X.-H.; Li, L.; et al. Soft-Carbon-Coated, Free-Standing, Low-Defect, Hard-Carbon Anode To Achieve a 94% Initial Coulombic Efficiency for Sodium-Ion Batteries. ACS Appl. Mater. Interfaces 2021, 13, 44358–44368. [Google Scholar] [CrossRef]
- Zhu, Y.; Chen, M.; Li, Q.; Yuan, C.; Wang, C. A Porous Biomass-Derived Anode for High-Performance Sodium-Ion Batteries. Carbon 2018, 129, 695–701. [Google Scholar] [CrossRef]
- Zhao, Y.; Hu, Z.; Fan, C.; Gao, P.; Zhang, R.; Liu, Z.; Liu, J.; Liu, J. Novel Structural Design and Adsorption/Insertion Coordinating Quasi-Metallic Na Storage Mechanism toward High-Performance Hard Carbon Anode Derived from Carboxymethyl Cellulose. Small 2023, 19, 2303296. [Google Scholar] [CrossRef]
- Meng, Q.; Lu, Y.; Ding, F.; Zhang, Q.; Chen, L.; Hu, Y.-S. Tuning the Closed Pore Structure of Hard Carbons with the Highest Na Storage Capacity. ACS Energy Lett. 2019, 4, 2608–2612. [Google Scholar] [CrossRef]
- Fan, X.; Kong, X.; Zhang, P.; Wang, J. Research Progress on Hard Carbon Materials in Advanced Sodium-Ion Batteries. Energy Storage Mater. 2024, 69, 103386. [Google Scholar] [CrossRef]
- Liu, G.; Ouyang, M.; Lu, L.; Li, J.; Han, X. Analysis of the Heat Generation of Lithium-Ion Battery During Charging and Discharging Considering Different Influencing Factors. J. Therm. Anal. Calorim. 2014, 116, 1001–1010. [Google Scholar] [CrossRef]
- Liu, X.; Ren, D.; Hsu, H.; Feng, X.; Xu, G.-L.; Zhuang, M.; Gao, H.; Lu, L.; Han, X.; Chu, Z.; et al. Thermal Runaway of Lithium-Ion Batteries Without Internal Short Circuit. Joule 2018, 2, 2047–2064. [Google Scholar] [CrossRef]
- Qi, C.; Wang, H.; Li, M.; Li, C.; Li, Y.; Shi, C.; Wei, N.; Wang, Y.; Zhang, H. Research on the Thermal Runaway Behavior and Flammability Limits of Sodium-Ion and Lithium-Ion Batteries. Batteries 2025, 11, 24. [Google Scholar] [CrossRef]
- Zeng, G.; Liu, Y.; Gu, C.; Zhang, K.; An, Y.; Wei, C.; Feng, J.; Ni, J. A Nonflammable Fluorinated Carbonate Electrolyte for Sodium-Ion Batteries. Acta Phys.-Chim. Sin. 2020, 36, 1905006. [Google Scholar]
- Zhao, X.; Zhuang, Q.-C.; Shi, Y.-L.; Zhang, X.-X. Influence of Vinylene Carbonate as an Additive on the Electrochemical Performances of Graphite Electrode in Poly(Methyl Methacrylate) Gel Polymer Electrolytes. J. Appl. Electrochem. 2015, 45, 1013–1023. [Google Scholar] [CrossRef]
- Yang, Y.; Wu, C.; He, X.; Zhao, J.; Yang, Z.; Li, L.; Wu, X.; Li, L.; Chou, S. Boosting the Development of Hard Carbon for Sodium-Ion Batteries: Strategies to Optimize the Initial Coulombic Efficiency. Adv. Funct. Mater. 2024, 34, 2302277. [Google Scholar] [CrossRef]
- Wen, Y.; Wang, B.; Zeng, G.; Nogita, K.; Ye, D.; Wang, L. Electrochemical and Structural Study of Layered P2-Type Na2/3Ni1/3Mn2/3O2 as Cathode Material for Sodium-Ion Battery. Chem.–Asian J. 2015, 10, 661–666. [Google Scholar] [CrossRef]
- Deng, W.; Feng, X.; Xiao, Y.; Li, C. Layered Molybdenum (Oxy) Pyrophosphate (MoO2)2P2O7 as a Cathode Material for Sodium-Ion Batteries. ChemElectroChem 2018, 5, 1032–1036. [Google Scholar] [CrossRef]
- Dang, R.; Chen, M.; Li, Q.; Wu, K.; Lee, Y.L.; Hu, Z.; Xiao, X. Na+-Conductive Na2Ti3O7-Modified P2-Type Na2/3Ni1/3Mn2/3O2 via a Smart In Situ Coating Approach: Suppressing Na+/Vacancy Ordering and P2–O2 Phase Transition. ACS Appl. Mater. Interfaces 2019, 11, 856–864. [Google Scholar] [CrossRef] [PubMed]
- Kong, W.; Wang, H.; Sun, L.; Su, C.; Liu, X. Understanding the Synergic Roles of MgO Coating on the Cycling and Rate Performance of Na0.67Mn0.5Fe0.5O2 Cathode. Appl. Surf. Sci. 2019, 497, 143814. [Google Scholar] [CrossRef]
- Jo, J.H.; Choi, J.U.; Konarov, A.; Yashiro, H.; Yuan, S.; Shi, L.; Sun, Y.; Myung, S. Sodium-Ion Batteries: Building Effective Layered Cathode Materials with Long-Term Cycling by Modifying the Surface via Sodium Phosphate. Adv. Funct. Mater. 2018, 28, 1705968. [Google Scholar] [CrossRef]
- Sekine, S.; Hosaka, T.; Maejima, H.; Tatara, R.; Nakayama, M.; Komaba, S. Na[Mn0.36Ni0.44Ti0.15Fe0.05]O2 Predicted via Machine Learning for High Energy Na-Ion Batteries. J. Mater. Chem. A 2024, 12, 31103–31107. [Google Scholar] [CrossRef]
- Nekahi, A.; Dorri, M.; Rezaei, M.; Bouguern, M.D.; Madikere Raghunatha Reddy, A.K.; Li, X.; Deng, S.; Zaghib, K. Comparative Issues of Metal-Ion Batteries Toward Sustainable Energy Storage: Lithium vs. Sodium. Batteries 2024, 10, 279. [Google Scholar] [CrossRef]
- Hirsh, H.S.; Li, Y.; Tan, D.H.S.; Zhang, M.; Zhao, E.; Meng, Y.S. Sodium-Ion Batteries Paving the Way for Grid Energy Storage. Adv. Energy Mater. 2020, 10, 2001274. [Google Scholar] [CrossRef]
- IEA. Global EV Outlook 2023; International Energy Agency (IEA): Paris, France, 2023. [Google Scholar]
- Environmental Impacts of Lithium-Ion Batteries. Available online: https://www.instituteforenergyresearch.org/renewable/environmental-impacts-of-lithium-ion-batteries/ (accessed on 20 February 2025).
- Lilley, S. Sodium-ion Batteries: Inexpensive and Sustainable Energy Storage. Faraday Insights 2021, 11, 1–6. Available online: https://www.faraday.ac.uk/wp-content/uploads/2021/06/Faraday_Insights_11_FINAL.pdf (accessed on 20 February 2025).
- Islam, M.T.; Iyer-Raniga, U. Lithium-Ion Battery Recycling in the Circular Economy: A Review. Recycling 2022, 7, 33. [Google Scholar] [CrossRef]
- Subburam, G.; Ramachandran, K.; El-Khodary, S.A.; Zou, B.; Wang, J.; Wang, L.; Qiu, J.; Liu, X.; Ng, D.H.L.; Lian, J. Development of Porous Carbon Nanosheets from Polyvinyl Alcohol for Sodium-Ion Capacitors. Chem. Eng. J. 2021, 415, 129012. [Google Scholar] [CrossRef]
- Xiao, L.; Lu, H.; Fang, Y.; Sushko, M.L.; Cao, Y.; Ai, X.; Yang, H.; Liu, J. Low-Defect and Low-Porosity Hard Carbon with High Coulombic Efficiency and High Capacity for Practical Sodium Ion Battery Anode. Adv. Energy Mater. 2018, 8, 1703238. [Google Scholar] [CrossRef]
- Zhang, X.; Yang, J.; Ren, Z.; Xie, K.; Ye, Q.; Xu, F.; Liu, X. In-Situ Observation of Electrolyte-Dependent Interfacial Change of the Graphite Anode in Sodium-Ion Batteries by Atomic Force Microscopy. New Carbon Mater. 2022, 37, 371–379. [Google Scholar] [CrossRef]
- Pendashteh, A.; Orayech, B.; Suhard, H.; Jauregui, M.; Ajuria, J.; Silván, B.; Clarke, S.; Bonilla, F.; Saurel, D. Boosting the Performance of Soft Carbon Negative Electrode for High Power Na-Ion Batteries and Li-Ion Capacitors through a Rational Strategy of Structural and Morphological Manipulation. Energy Storage Mater. 2022, 46, 417–430. [Google Scholar] [CrossRef]
- Liu, K.; Liu, W.; Qiu, Y.; Kong, B.; Sun, Y.; Chen, Z.; Zhuo, D.; Lin, D.; Cui, Y. Electrospun Core-Shell Microfiber Separator with Thermal-Triggered Flame-Retardant Properties for Lithium-Ion Batteries. Sci. Adv. 2017, 3, e1601978. [Google Scholar] [CrossRef] [PubMed]
- Jian, Z.; Bommier, C.; Luo, L.; Li, Z.; Wang, W.; Wang, C.; Greaney, P.A.; Ji, X. Insights on the Mechanism of Na-Ion Storage in Soft Carbon Anode. Chem. Mater. 2017, 29, 2314–2320. [Google Scholar] [CrossRef]
- Evarts, E.C. Lithium Batteries: To the Limits of Lithium. Nature 2015, 526, S93–S95. [Google Scholar] [CrossRef]
- Jia, H.; Zou, L.; Gao, P.; Cao, X.; Zhao, W.; He, Y.; Engelhard, M.H.; Burton, S.D.; Wang, H.; Ren, X.; et al. High-Performance Silicon Anodes Enabled by Nonflammable Localized High-Concentration Electrolytes. Adv. Energy Mater. 2019, 9, 1900784. [Google Scholar] [CrossRef]
- Kubota, K.; Dahbi, M.; Hosaka, T.; Kumakura, S.; Komaba, S. Towards K-Ion and Na-Ion Batteries as “Beyond Li-Ion”. Chem. Rec. 2018, 18, 459–479. [Google Scholar] [CrossRef]
- Lewis, G.N.; Keyes, F.G. The potential of the lithium electrode. J. Am. Chem. Soc. 1913, 35, 340–344. [Google Scholar] [CrossRef]
- Li, Q.; Jiao, S.; Luo, L.; Ding, M.S.; Zheng, J.; Cartmell, S.S.; Wang, C.-M.; Xu, K.; Zhang, J.-G.; Xu, W. Wide-Temperature Electrolytes for Lithium-Ion Batteries. ACS Appl. Mater. Interfaces 2017, 9, 18826–18835. [Google Scholar] [CrossRef] [PubMed]
- Newman, G.H.; Klemann, L.P. Ambient Temperature Cycling of an Na-TiS2 Cell. J. Electrochem. Soc. 1980, 127, 2097–2099. [Google Scholar] [CrossRef]
- Paolella, A.; Faure, C.; Bertoni, G.; Marras, S.; Guerfi, A.; Darwiche, A.; Hovington, P.; Commarieu, B.; Wang, Z.; Prato, M.; et al. Light-Assisted Delithiation of Lithium Iron Phosphate Nanocrystals towards Photo-Rechargeable Lithium Ion Batteries. Nat. Commun. 2017, 8, 14643. [Google Scholar] [CrossRef]
- Rudola, A.; Rennie, A.J.R.; Heap, R.; Meysami, S.S.; Lowbridge, A.; Mazzali, F.; Sayers, R.; Wright, C.J.; Barker, J. Commercialisation of High Energy Density Sodium-Ion Batteries: Faradion’s Journey and Outlook. J. Mater. Chem. A 2021, 9, 8279–8302. [Google Scholar] [CrossRef]
- Sayahpour, B.; Hirsh, H.; Parab, S.; Nguyen, L.H.B.; Zhang, M.; Meng, Y.S. Perspective: Design of Cathode Materials for Sustainable Sodium-Ion Batteries. MRS Energy Sustain. 2022, 9, 183–197. [Google Scholar] [CrossRef]
- Song, L.; Li, S.; Wang, J.; Zhu, J.; Wang, Y.; Cai, X.; Zong, F.; Wang, H.; Cui, X.; Zhao, D. Building a Flexible and Highly Ionic Conductive Solid Electrolyte Interphase on the Surface of Si@C Anodes by Binary Electrolyte Additives. ACS Appl. Mater. Interfaces 2023, 15, 49727–49738. [Google Scholar] [CrossRef] [PubMed]
- Wentker, M.; Greenwood, M.; Leker, J. A Bottom-Up Approach to Lithium-Ion Battery Cost Modeling with a Focus on Cathode Active Materials. Energies 2019, 12, 504. [Google Scholar] [CrossRef]
- Whittingham, M.S. Electrical Energy Storage and Intercalation Chemistry. Science 1976, 192, 1126–1127. [Google Scholar] [CrossRef]
- Winter, M. The Solid Electrolyte Interphase—The Most Important and the Least Understood Solid Electrolyte in Rechargeable Li Batteries. Z. Für Phys. Chem. 2009, 223, 1395–1406. [Google Scholar] [CrossRef]
- Yabuuchi, N.; Hara, R.; Kubota, K.; Paulsen, J.; Kumakura, S.; Komaba, S. A New Electrode Material for Rechargeable Sodium Batteries: P2-Type Na2/3[Mg0.28Mn0.72]O2 with Anomalously High Reversible Capacity. J. Mater. Chem. A 2014, 2, 16851–16855. [Google Scholar] [CrossRef]
- Yabuuchi, N.; Kajiyama, M.; Iwatate, J.; Nishikawa, H.; Hitomi, S.; Okuyama, R.; Usui, R.; Yamada, Y.; Komaba, S. P2-Type Nax[Fe1/2Mn1/2]O2 Made from Earth-Abundant Elements for Rechargeable Na Batteries. Nat. Mater. 2012, 11, 512–517. [Google Scholar] [CrossRef] [PubMed]
- CATL: CATL Unveils Its Latest Breakthrough Technology by Releasing Its First Generation of Sodium-Ion Batteries. Available online: https://www.catl.com/en/news/665.html (accessed on 29 July 2021).



| 2023 | 2022 | 2021 | 2020 | 2019 | |
|---|---|---|---|---|---|
| China | 1300 | 1000 | 820 | 820 | 780 |
| Mozambique | 96 | 166 | 77 | 120 | 150 |
| Brazil | 73 | 73 | 82 | 92 | 90 |
| Canada | 3.5 | 13 | 42 | 40 | 35 |
| India | 11.5 | 11 | 10 | 10 | 39 |
| South Korea | 27 | 24 | 11 | ||
| Russia | 16 | 16 | 16 | 16 | 16 |
| Norway | 10 | 7 | 12 | 10 | 10 |
| Method | Graphitization Temp (°C) | Voltage Range (V) | Specific Capacity (mAhg−1) | Cycle Life | ICE | Ref. |
|---|---|---|---|---|---|---|
| Graphitization of needle coke | 2700 | 0.05–1.3 | ~325 at 0.1 C | 98.7% after 100 cycles at 0.1 C | >90% | [73] |
| Graphitization of bituminous coal | 2000–2800 | 0.001–2.0 | 310 at 0.1 C | 95.3% retention after 100 cycles at 0.1 C | ~87% | [78] |
| Graphitization of anthracite coupled with effective catalyst (boron oxide) | 2700 | 0.001–2.0 | ~320 at 0.5 C | 98% after 500 cycles at 0.5 C | ~81% | [79] |
| Catalytic graphitization | 2600 | 0.001–2.0 | 372 at 0.2 C | 89.2% after 500 cycles at 2 C | 85.88% | [80] |
| Low-temperature electrochemical graphitization of biomass-derived activated carbon from coconut waste in molten salts | 850 | 0.01–3.00 | 282 at 1 C~200 at 5 C | 92% after 1000 cycles at 5 C | ~65% | [81] |
| Graphitization of CO2-derived carbon | 2800 | 0.01–2.0 | 297–378.1 at 50 mAg−1 | ~100% after 300 cycles at 1 Ag−1 | 72.6–80.5% | [82] |
| Carbon Precursor | Advantages | Disadvantages | Reversible Capacity (mAhg−1) | Cycling Stability | Price (USD/kg) | Reference |
|---|---|---|---|---|---|---|
| Needle coke (10–15 μm) | High crystallinity, excellent structural stability, highly layered | High cost, limited pathways, lower rate capability | 360–370 at 0.1 Ag−1 | 92.6% after 100 cycles | 15–30 | [73] |
| Needle coke (2–5 μm) | Short ion diffusion distance, high charge/discharge capability | Low volumetric density, high porosity | 380–400 at 0.1 Ag−1 | 98.7% after 100 cycles | 15–30 | [73] |
| Porous activated carbon (PAC) from petroleum coke | Good graphitization potential, cost-effective, high surface area, great stability | Lower conductivity compared to needle coke | 330–350 at 0.1 Ag−1 | ~98% after 15,000 cycles | - | [107] |
| Coal tar pitch | High graphitization potential | Impurities require removal | 330–360 at 0.3 Ag−1 | ~95% after 100 cycles | 10–20 | [108] |
| Amorphous carbon (graphite derived from CO2 via LiAlH4 reaction) | Abundant, low cost, environmentally friendly, high purity | Poor conductivity, requires controlled pressure conditions | 320 after 1500 cycles at 1 Ag−1 | 99% retention after 100 cycles | - | [109] |
| Biomass-derived carbon | Eco-friendly, renewable | Low graphitization degree | 150–200 at 0.3 Ag−1 | ~90% after 150 cycles | 10–20 | [110] |
| Anode Material | Retention Capacity Rate | Number of Cycles |
|---|---|---|
| Natural graphite | 80–90% | 500 |
| Artificial graphite | 95–98% | 200 |
| Graphite powder | 94–96% | 100 |
| Carbon nanotubes | 94.6% | 50 |
| Silicon–graphene | 92.7% | 50 |
| Pre-lithiated hard carbon (PHC) | 80% | 300 |
| Anode Material | Initial Discharge Specific Capacity (mAhg−1) | C Rate | Voltage Range (V) | Retention Capacity | ICE (%) | Current Rate mAg−1 |
|---|---|---|---|---|---|---|
| Graphite | 372 | 1 | 0.5–3 | 97% after 100 cycles | 85.1 | - |
| Bituminous coal artificial graphite | 200–300 | 0.1 | 0–2 | 60–70% after 70 cycles | 69.7–87.5 | - |
| Modified artificial graphite | 250 | 1 | 0.01–2 | ~90% after 40 cycles | ~88.2 | 500 |
| Pitch drive hard carbon | 225.5 | 1 | 0–2 | 97% after 100 cycles | 81.3 | - |
| Soft carbon | 278 | 1 | 0–3 | 74.3% after 300 cycles | 70.2 | 600 |
| Silicon/carbon composite | 2900 | - | - | 37.9% after 600 cycles | - | 400 |
| Nitrogen-doped active carbon | 397.78 | 0.1 | 0–2 | ~ 90% after 100 cycles | 78.98 | 50 |
| Nitrogen-doped graphene nanosheet | 250 | 2.5 | 0.01–3 | ~100% after 10 cycles | ~100 | - |
| Sulfur-doped carbon | 170 | 1 | 0–3 | ~96.8% after 100 cycles | 60.6 | - |
| Nitrogen and fluorine co-doped carbon | 1075 | - | 0–3 | ~95% after 2000 cycles | 56.7 | 100 |
| PD-doped graphite | 230 | 0.5 | 0.005–1.5 | 69% after 100 cycles | - | 200 |
| Graphene nanosheet | 500 | 0.2 | 0–3 | 90% after 100 cycles | 56 | - |
| Boron-doped carbon | 310 | 0.1 | 0–2 | 88% after 50 cycles | - | 50 |
| Solvent | Intercalation Compound | Capacity (mAhg−1) | C Rate | Reversibility | Observations | Solvent Molecule Structure | References |
|---|---|---|---|---|---|---|---|
| Crown ether | Na(crown)2Cₙ | ~75 | 0.2 C | Moderate | Limited by rigid ring structure, resulting in lower capacity and slower kinetics |  | [224] |
| Propylene carbonate + Monoglyme(PC+G1) | Na(mgly)2Cₙ | ~80–100 | 1 C | Moderate | Shows reversible sodium intercalation but limited stability compared to longer glymes |  | [222] |
| Diglyme | Na(digl)2Cₙ | ~100–110 | 1 C | High | Stable plateau with excellent co-intercalation of sodium ions, forming stable graphite layers |  | [223] |
| Triglyme | Na(trigly)2Cₙ | ~110–120 | 1 C | High | Reduced efficiency at room temperature; performs better at higher temperatures | 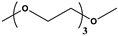 | [223] |
| Diethylene glycol dimethyl ether | Not applicable | ~150 | 1 C | High | High reversibility and great mobility | 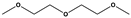 | [213] |
| Tetraglyme | Na(tetrag)2Cₙ | ~160–165 | 0.1 C | High | Stronger sodium-ion screening due to longer chains but at slightly higher intercalation voltages | 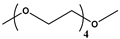 | [224] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nikgoftar, K.; Madikere Raghunatha Reddy, A.K.; Reddy, M.V.; Zaghib, K. Carbonaceous Materials as Anodes for Lithium-Ion and Sodium-Ion Batteries. Batteries 2025, 11, 123. https://doi.org/10.3390/batteries11040123
Nikgoftar K, Madikere Raghunatha Reddy AK, Reddy MV, Zaghib K. Carbonaceous Materials as Anodes for Lithium-Ion and Sodium-Ion Batteries. Batteries. 2025; 11(4):123. https://doi.org/10.3390/batteries11040123
Chicago/Turabian StyleNikgoftar, Koorosh, Anil Kumar Madikere Raghunatha Reddy, Mogalahalli Venkatashamy Reddy, and Karim Zaghib. 2025. "Carbonaceous Materials as Anodes for Lithium-Ion and Sodium-Ion Batteries" Batteries 11, no. 4: 123. https://doi.org/10.3390/batteries11040123
APA StyleNikgoftar, K., Madikere Raghunatha Reddy, A. K., Reddy, M. V., & Zaghib, K. (2025). Carbonaceous Materials as Anodes for Lithium-Ion and Sodium-Ion Batteries. Batteries, 11(4), 123. https://doi.org/10.3390/batteries11040123